Abstract
Social structures such as families emerge as outcomes of behavioural interactions among individuals, and can evolve over time if families with particular types of social structures tend to leave more individuals in subsequent generations. The social behaviour of interacting individuals is typically analysed as a series of multiple dyadic (pair-wise) interactions, rather than a network of interactions among multiple individuals. However, in species where parents feed dependant young, interactions within families nearly always involve more than two individuals simultaneously. Such social networks of interactions at least partly reflect conflicts of interest over the provision of costly parental investment. Consequently, variation in family network structure reflects variation in how conflicts of interest are resolved among family members. Despite its importance in understanding the evolution of emergent properties of social organization such as family life and cooperation, nothing is currently known about how selection acts on the structure of social networks. Here, we show that the social network structure of broods of begging nestling great tits Parus major predicts fitness in families. Although selection at the level of the individual favours large nestlings, selection at the level of the kin-group primarily favours families that resolve conflicts most effectively.
Keywords: social networks, sexual conflict, parent–offspring conflict, begging, social evolution
1. Introduction
Social networks analyses have advanced our understanding of the evolution of animal societies [1,2], cooperation [3–5], the transmission of disease [6] and human social [7] and socio-economic [8] systems. Taking a networks approach to the study of social behaviour shifts emphasis away from variation in behaviour among individuals per se to how interactions among individuals shape variation [9]. This more realistically reflects the behaviour of individuals as being both the cause and the effect of their social environment [10,11]. However, all previous research has focused on the importance of social position within a network on the fitness prospects of interacting individuals [12,13] rather than the structure of the network of interactions themselves. As a result, very little is known about the relationship between the structure of social networks and fitness in natural populations, despite the importance of such information in understanding the evolutionary and ecological significance of social networks [9,14].
One area where this is particularly notable concerns interactions among family members in species with parental care [15]. Communication among individuals in animal families involves a network of interactions, between male and female parents, between parents and offspring and among siblings [16]. Although they have not been modelled as such [17–19], the resolution of conflicts of interest over the provision of parental investment in families implicitly involves a network of multiple interactions rather than multiple independent dyadic interactions among individual members [18]. Quantifying the social network structure (SNS) of behavioural interactions among offspring during feeding by parents consequently provides a means to assess selection on these interactions among individuals acting at multiple levels [20,21], and therefore the fitness consequences of variation among families in how conflicts over parental investment are resolved.
Altricial bird nestlings, such as great tits (Parus major), interact with each other through begging competitions and by jockeying for favourable positions near the feeding parent [16,22–24]. Their begging displays and the dynamics of their movements in relation to each other and to their parents within the nest therefore reflect key components of behavioural interactions shaping brood social structure. Parent–offspring interactions in families of altricial species of birds provide an ideal study system to quantify between-group consequences of variation in SNS on fitness. There are no ‘gambit of the group’ issues (a common problem in social networks analyses where assumptions are made about social groupings of individuals based on their patterns of associations with one another; [1]) as all nestlings within each nest can be clearly and unambiguously assigned to a given group. In addition, because each individual within a brood interacts with all other individuals in the network and group membership is clearly defined, it is possible to make use of weighted networks metrics (i.e. incorporating the number of times or strength with which individuals interact with one another) as opposed to binary network metrics (where pairs of individuals in a network are simply classified as either associating with one another or not). This facilitates a measure of the strength of the behavioural interactions among network members (‘gregariousness’; [2]) and means no potentially important information is lost [2]. Finally, and perhaps most importantly, this form of study system allows the novel quantification of the fitness consequences of variation in social interactions among individuals at levels of selection higher than the individual through the use of replicated networks.
We used a nest-box population of great tits in the forests around Bern, Switzerland, to examine how the structure of social interactions among nestlings is related to the fitness of both offspring (recruitment into the population to breed) and parents (survival and breeding success in the year following the experiment). Sixty-three broods ranging in size from five to 10 nestlings were filmed during parental feeding events on day 10 post-hatch, when feeding rates are at their peak [25]. Variation in the hunger of nestlings (and, therefore their motivation to beg; [22]) in each brood was manipulated and the position and identity of the parent and the position, identity and begging intensity of all nestlings were recorded (see the electronic supplementary material for further details). Nestling positions were used to produce association matrices of begging nestlings. These matrices were then used to derive network metrics for each nestling within each network (social network position, SNP; which is an individual level trait) and summary metrics to describe the structure of each network (SNS, which is a group level trait). These were then used to relate SNP and SNS to measures of fitness and to quantify how SNS is related to interactions between parents and offspring (see §2).
Parental care is nearly always costly [26], and because individuals within families are not fully related to one another conflicts of interest are expected over the provision of parental investment [27–29]. The amount of parental investment provided depends on the outcome of such conflicts (the ‘resolution’), which are determined by how individuals interact with one another [18]. Great tit male and female parents do not differ to one another in feeding rate to nestlings at the population level but feed (predictably) from different positions in the nest and have different feeding rules to one another [22]. Mothers, but not fathers, respond to an increase in the begging calls of offspring by increasing their provisioning rate and preferentially feed hungry nestlings [22,23]. In contrast, fathers take longer to choose which offspring to feed and preferentially feed nestlings that jostle and compete for food most effectively [22,23]. These different feeding rules mean that the SNS of nestlings is predicted to be largely determined by whichever parent provides the most feeds and primarily controls the allocation of parental resources. Great tit nestlings respond more readily to greater female parental sensitivity to their state (i.e. they approach their mother when hungry and move or are displaced when fed; [22]). Broods where mothers, rather than fathers, primarily control the allocation of resources to nestlings are therefore expected to be composed of more strongly interacting (‘gregarious’) nestlings, as the higher responsiveness to variation in offspring state of mothers encourages nestlings to move around more in the nest in relation to the position of the feeding parent, reducing the variation in nestling state. In contrast, in broods where fathers provide most of the feeds, we expect a lower mean strength of interactions among nestlings because fathers preferentially feed the most competitive nestlings which can monopolize positions closest to where the male parent feeds from, so there will be less movement within the nest. The SNS of these broods is likely to be more clustered as more competitive nestlings can occupy the best positions near to the male parent [22] leading to increased variation in within-brood nestling state. If SNS is largely a consequence of variation in parental sensitivity to offspring state then the position of nestlings within the network (SNP) should be influenced by hunger and more strongly connected broods are expected to have a more uniform distribution of begging behaviour among offspring.
2. Methods
Information on video analysis and preparation of data for social network analysis is provided in Kölliker et al. [22] and also in the electronic supplementary material.
(a). Data collection and experimental manipulation of nestling hunger
Data collection has been previously described in detail by Kölliker et al. [22]. We provide a brief synopsis here. A nest-box population of great tits nesting in the Bremgarten forest near Bern, Switzerland, was used for the study. Nestlings at experimental nests were ringed with numbered aluminium rings 9 days post-hatching and a dummy camera was installed in the nest-box to habituate the birds to the presence of a camera. On day 10 post-hatch individual nestlings at each nest-box were weighed (± 0.1 g) and uniquely marked on the head with paint. Two intermediate-sized nestlings were then temporarily removed and randomly assigned to one of two treatments: food-deprived or fed to satiation for 2 h with bee larvae. This allowed us to assess the effects of hunger on SNP. After 2 h, the two nestlings were then replaced in an arbitrary position in the nest and parental provisioning and nestling begging behaviour filmed from above using a camera with an infrared light source for 45 min at 63 nests. Brood size at the time of the experiment ranged from five to 10 nestlings (the mean brood size is 7.4 nestlings in this population).
(b). Social network analysis
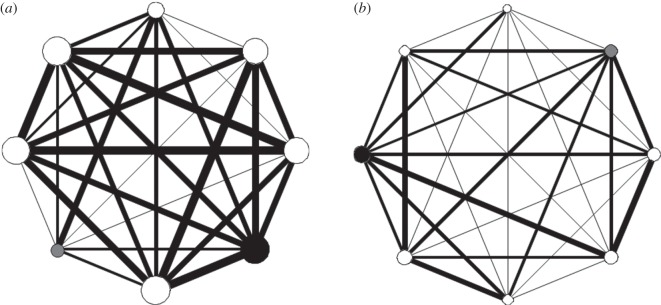
For each nestling at each feed, we established the direct social associates (i.e. the identity of the individuals immediately next to, or touching, the focal individual). These positions were calculated for each feed at each brood and the data used to produce association matrices for each brood. Association was measured as the proportion of feeding events in which individuals were immediate neighbours of each other member of the network; where a score of 1 between two given individuals means that they were always next to each other and a score of 0 means two individuals never associated. Data were extracted from data sheets involving feeding events at nests for calculation of matrices using MATLAB (MathWorks, Natick, MA, USA) and then UCINET [30] was used to derive our network metric, weighted degree (also known as node strength; [1]), for individuals within broods. Weighted degree is defined as the total weight of the edges (social associations) connected to a node (individual), and provides a measure of SNP for each individual (i.e. the number and strength of associations with other nestlings of a focal individual within the brood; [1]). Degree, whether unweighted or weighted, is a simple, robust metric that is widely used [1,2] and is a potentially important determinant of the evolution of cooperation [3,4]. We used weighted degree as our key network metric because individuals within broods all interacted with one another, so binary (unweighted) network metrics would be uninformative. In contrast, weighted degree allowed us to quantify the strength of interactions among individuals, which was our primary focus. Broods with higher mean weighted degree scores have greater overall strength of associations among interacting nestlings (i.e. nestlings moved around more so encountered other individuals more frequently; they were more gregarious, [2]) than broods with low mean weighted degree scores (SNS; figure 1).
Figure 1.
Representative social networks. (a) Social network for a brood of eight nestlings that interact with one another strongly. (b) Social network for a brood of eight nestlings that interact with one another weakly. Food deprived nestlings shown in black, satiated nestlings in grey and un-manipulated nestlings in white. The thickness of the lines (edges) indicates the strength of the connection between individuals (nodes). Node size is proportional to the weighted degree of the individual.
(c). Statistical data analysis
Statistical analysis was performed using R version 2.14.2 (Copyright © 2007 The R Foundation for Statistical Computing) and SPSS version 16 (SPSS Inc., Chicago, IL, USA). We accounted for correlations among continuous predictors by including terms as covariates in models, rather than using residuals or other forms of variance partitioning, as this has been shown to be the most effective method for dealing with collinearity [31,32]. During model simplification, we removed non-significant interactions, followed by lower-order terms in turn from the maximal model until no further terms could be dropped without significantly reducing the model fit (minimum adequate model, MAM; [33]). We used general and generalized linear mixed models (GLMMs) for analysis of individual level effects, with nest as a random term to account for non-independence of data from individuals in the same network. GLMM model simplification involved comparing maximum-likelihood (ML) models with and without each term. We took a term out of the model if its removal did not significantly increase the Akaike information criterion (AIC; [33]). For brood level effects we used general and generalized linear models (GLMs) and model simplification used analysis of deviance (a measure of the relative fit of the model compared to alternative models; [33]). Unless stated otherwise sample size for brood level analyses was 63 broods and for individual level analyses was 450 nestlings in 63 broods.
3. Results
(a). Recruitment probability of the brood
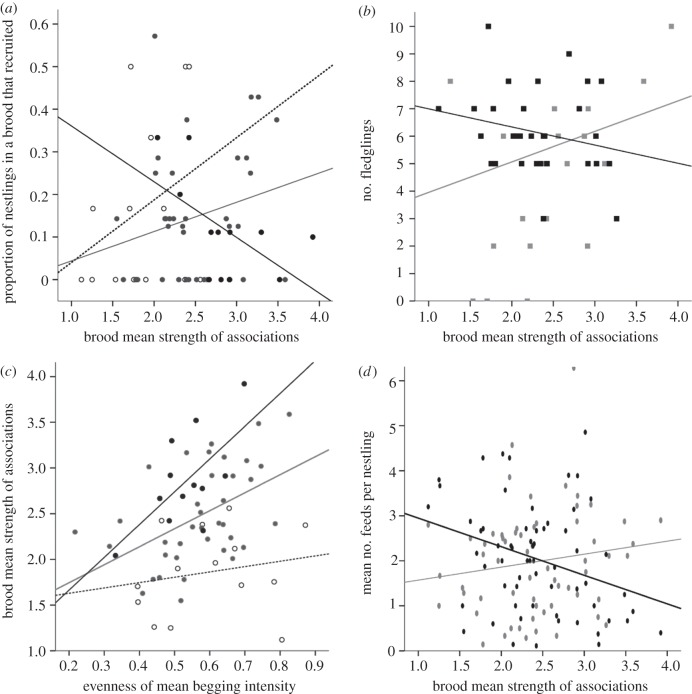
The first question we addressed was whether there was any evidence that selection acts on the SNS of great tit broods. In order to answer this, we examined whether SNS explained a significant amount of variation in the proportion of nestlings in a brood that survived to recruit into the breeding population the following year. The relationship between the strength of associations among nestlings and recruitment success of broods was dependent upon brood size; being positive in small (five or six nestlings) and medium (seven or eight nestlings) sized broods, but negative in large broods (nine or 10 nestlings; table 1a and figure 2a). In contrast to smaller broods, large broods with weaker networks of interactions were more successful than those with stronger nestling associations (table 1a). Variation in the strength of associations among nestlings within broods (CV of SNS) did not explain variation in recruitment success.
Table 1.
Analyses of nestling and parent fitness parameters. SNS, social network structure; SNP, social network position. Only significant interaction terms are shown. Parameter estimates are given with standard errors in brackets.
| model | response variable | terms in model | terms dropped | parameter estimates | test statistica | d.f. | p-value |
|---|---|---|---|---|---|---|---|
| (a) GLM with quasi-binomial errors (dispersion parameter = 1.34) | recruitment success of the brood | SNS × brood size | −0.52(0.26) | 5.00 | 1,59 | 0.029 | |
| SNS | 4.12(2.01) | 5.42 | 1,59 | 0.023 | |||
| brood size | 1.11(0.66) | 3.47 | 1,59 | 0.067 | |||
| mean mass | 3.07 | 1,58 | 0.085 | ||||
| sex-ratio | 2.18 | 1,57 | 0.145 | ||||
| CV of SNS | 0.04 | 1,56 | 0.837 | ||||
| intercept | −10.47(4.85) | ||||||
| (b) GLMM with binomial errors (nest as random effect; n = 450 nestlings in 63 broods) | recruitment probability of individual nestlings | SNS × brood size | −0.57(0.26) | 5.87 | 1 | 0.015 | |
| SNS | 4.72(2.02) | 6.85 | 1 | 0.009 | |||
| brood size | 1.22(0.66) | 4.24 | 1 | 0.040 | |||
| chick mass | 0.34(0.11) | 10.36 | 1 | 0.001 | |||
| SNP | 0.59 | 1 | 0.441 | ||||
| nestling sex | 0.53 | 1 | 0.466 | ||||
| mean mass | 0.89 | 1 | 0.346 | ||||
| intercept | −16.79(5.32) | ||||||
| (c) GLM with binomial errors | survival probability of parents | sex of parent | −0.80(0.38) | 4.54 | 1,119 | 0.033 | |
| SNS | 3.07 | 1,118 | 0.080 | ||||
| brood size | 3.11 | 1,117 | 0.078 | ||||
| intercept | 0.87(0.28) | ||||||
| (d) GLM with normal errors | number of fledglings raised in following year | SNS × sex ofparent | −1.74(0.83) | 4.45 | 1,69 | 0.039 | |
| new partner | −1.40(0.48) | 8.67 | 1,69 | 0.004 | |||
| SNS | 1.26(0.50) | 6.36 | 1,69 | 0.014 | |||
| sex of parent | 4.46(1.98) | 5.09 | 1,69 | 0.027 | |||
| brood size | 0.53 | 1,68 | 0.471 | ||||
| mean mass | 0.36 | 1,67 | 0.552 | ||||
| intercept | 3.16(1.20) |
aTest statistics given for models (a) and (d) are F-values and for models (b) and (c) are χ2 values.
Figure 2.
(a) Proportion of nestlings that recruited into the population the following year in relation to SNS. Broods are grouped by size for illustration only. Small broods (five to six nestlings) shown with open symbols and dotted line of best fit, medium broods (seven to eight nestlings) with grey symbols/line and large broods (9–10 nestlings) with black symbols/line. (b) Future reproductive success of parents (black symbols/lines are males, grey symbols/lines are females) in relation to SNS. (c) The relationship between SNS and evenness of brood begging intensity. Symbols and lines are as for figure 2a. (d) Mean number of feeds per nestling provided by male (black symbols/line) and female (grey symbols/line) parents respectively, in relation to the SNS.
(b). Recruitment probability of individual nestlings
We then examined whether recruitment probability at the level of the individual was best explained by individual level traits (SNP, nestling mass and nestling sex) or brood level traits (SNS, brood size, mean mass of nestlings). Nestling mass at day 10 was the only individual-level trait that explained a significant amount of the variation in the recruitment probability of individuals within a nest (table 1b; see also [34]), with larger nestlings more likely to recruit than smaller nestlings. With regard to brood-level traits, SNS also explained a significant amount of variation in individual recruitment probability (table 1b), with a significant interaction between SNS and brood size as also found in the brood level analysis of recruitment probability (figure 2a).
(c). Future reproductive success of parents
Does variation in SNS of broods also predict future parental success? Male parents had a lower probability of survival to the following year than females, but there was no significant effect of SNS on the survival of parents (table 1c). However, for parents that survived to breed there was a significant interaction between sex and brood mean strength of associations (SNS) on the number of fledglings reared (table 1d). Males that reared broods with strongly associating offspring fledged a lower number of nestlings in the following year than females that reared strongly associating broods of nestlings (figure 2b). In addition to the significantsex × SNS interaction, the number of fledglings produced by surviving parents was significantly affected by whether they had a new partner in the following year or not (in virtually all cases new partners were present because the previous partner did not apparently survive); parents with new partners had lower reproductive success. However, neither brood size nor the mean mass of the nestlings significantly affected reproductive output in the following year (table 1d).
(d). Social network position
As predicted, the SNP of experimental nestlings was significantly related to manipulated levels of hunger; nestlings that were food-deprived had stronger associations with other nestlings (i.e. were more gregarious) than satiated individuals (table 2a). Brood size was also positively related to SNP as expected (i.e. individuals had a greater number of associates in larger broods), and male nestlings were more gregarious than females, perhaps because males are larger than females, so became hungry more quickly. However, nestling mass at day 10 was dropped from the maximal model, as were all relevant interactions (table 2a).
Table 2.
Analyses of social network parameters of broods. Only significant interaction terms are shown. Parameter estimates are given with standard errors in brackets.
| model | response variable | terms in model | termsdropped | parameterestimates | test statistica | d.f. | p-value |
|---|---|---|---|---|---|---|---|
| (a) GLMM with normal errors(nest as random effect; n = 450 nestlings in 63 nests) | SNP of nestlings within broods (weighted degree) | hunger treatment | −0.66(0.11) −0.20(0.08) | 39.85 | 1 | <0.0001 | |
| brood size | 0.27(0.05) | 24.13 | 1 | <0.0001 | |||
| nestling sex | 0.21(0.06) | 11.47 | 1 | 0.0007 | |||
| nestling mass | 0.43 | 1 | 0.511 | ||||
| intercept | 0.59(0.38) | ||||||
| (b) GLM with normal errors | SNS of broods (brood mean weighted degree) | evenness of begging × brood size | −1.06(0.32) | 10.80 | 1,59 | <0.002 | |
| brood size | 0.75(0.14) | 26.82 | 1,59 | <0.0001 | |||
| mean brood begging intensity | 0.52(0.11) | 22.29 | 1,59 | <0.0001 | |||
| evenness of begging | 6.65(2.29) | 8.47 | 1,59 | 0.005 | |||
| evenness of nestling mass | 1.92 | 1,58 | 0.171 | ||||
| mean nestling mass | 0.01 | 1,57 | 0.912 | ||||
| sex-ratio | 0.01 | 1,56 | 0.932 | ||||
| intercept | −3.51(1.04) | ||||||
| (c) GLM with normal errors | brood mean begging intensity of nestlings | feeds by father | −0.16(0.05) | 13.07 | 1,61 | <0.001 | |
| feeds by mother | 0.01 | 1,60 | 0.961 | ||||
| brood size | 0.63 | 1,59 | 0.432 | ||||
| intercept | 2.07(0.11) | ||||||
| (d) GLM with normal errors | brood mean evenness of begging intensity of nestlings | feeds by mother | −0.04(0.01) | 8.67 | 1,61 | 0.004 | |
| feeds by father | 0.38 | 1,60 | 0.538 | ||||
| brood size | 0.51 | 1,59 | 0.480 | ||||
| intercept | 0.51(0.03) |
aTest statistics for model (a) are likelihood ratios and for models (b), (c) and (d) are F-values.
(e). Social network structure and begging behaviour
If SNS reflects variation in the response of male parents compared with female parents to offspring behaviour, we predicted that SNS would be related to the evenness of begging within broods, with a positive relationship indicating that mothers primarily controlled feeding. As expected SNS was significantly related to the begging behaviour of nestlings. Broods with more strongly interacting nestlings had higher mean begging intensity and were more uniform in their pattern of begging behaviour than broods with less strongly associating nestlings (table 2b), with larger broods having a stronger relationship between SNS and evenness (1-CV) of begging than smaller broods (table 2b; figure 1c). The mean begging intensity of broods was negatively related to the mean number of feeds provided by the male, but was not related to brood size or the number of female parent feeds (table 2c): the harder the male worked the less the nestlings in the brood begged (i.e. the less hungry they were). Conversely, variation in begging intensity within broods was primarily driven by how hard the female parent worked: the evenness of begging behaviour within broods was positively related to the number of feeds provided by the female and not to brood size or the number of male feeds (table 2d).
(f). Feeding behaviour
If SNS of broods is related to which parent primarily controls feeding we predicted that broods controlled by mothers would show a positive relationship between SNS and the number of feeds, and mothers would spend less time choosing which nestling to feed. As expected the number of feeds provided by parents to nestlings was negatively related to SNS (brood mean degree) for males, but positively related to SNS for females (figure 1d). Brood size dropped out of the model (table 3a). In contrast, females spent longer choosing which nestlings to feed when SNS was low, whereas males spent longer choosing when SNS was high (table 3b).
Table 3.
Analyses of parental feeding behaviour. Only significant interaction terms are shown. Parameter estimates are given with s.e. in brackets.
| model | response variable | terms in model | terms dropped | parameter estimates | test statistica | d.f. | p-value |
|---|---|---|---|---|---|---|---|
| (a) GLMM with normal errors (nest as random effect; n = 124 parents in 62 nests) | mean number of parental feeds to brood | SNS × sex of parent | −0.46(0.14) | 9.73 | 1 | 0.0018 | |
| SNS | −0.17(0.19) | 0.80 | 1 | 0.372 | |||
| sex of parent | 1.15(0.36) | 0.11 | 1 | 0.741 | |||
| brood size | 0.10 | 1 | 0.749 | ||||
| intercept | 2.43(0.49) | ||||||
| (b) GLMM with normal errors (nest as random effect; n = 124 parents in 62 nests) | mean time spent feeding brood | SNS × sex of parent | 0.60(0.24) | 6.04 | 1 | 0.014 | |
| SNS | 0.03(0.27) | 0.01 | 1 | 0.912 | |||
| sex of parent | −1.37(0.60) | 0.30 | 1 | 0.584 | |||
| brood size | 0.53 | 1 | 0.466 | ||||
| intercept | 3.37(0.68) |
aTest statistics for both models are likelihood ratios.
4. Discussion
Despite widespread interest in animal social networks [1,2,14], and the recognition that understanding the evolutionary and ecological importance of the structure of social networks requires information on the relationship between SNS and fitness [9], there have been only two previous studies examining fitness in relation to social network metrics [12,13]. Moreover, both of these studies were concerned with the relationship between the position of individual adults within networks and correlates of fitness. To our knowledge, our study is therefore the first to relate fitness to variation in the structure of whole, replicated, networks, not just position within a single network. We show that SNS predicts fitness in broods of great tits in the wild, but SNP does not. Selection acting at the level of the individual primarily favours large, well-nourished offspring, as might be expected, whereas selection acting at the level of the family depends upon how gregarious offspring are (SNS) in relation to the size of the network (brood) involved. Variation in social network attributes [35] or traits correlated with SNS, such as begging intensity or parental feeding behaviour, can be heritable [19,23,36,37]. If, as seems probable, SNS has a heritable basis, then family structure can evolve.
Selection acting at the level of the family emerges as a consequence of how interactions are distributed among nestlings during feeding by parents. The SNS represents the behavioural outcome of the resolution of within-family conflict over the provision of parental investment in terms of nestling positioning, which is a primary determinant of the probability of being fed in great tits [22]. Selection favours networks that are composed of strongly interacting (gregarious) individuals when broods are small and medium sized, with the evidence indicating that these patterns of associations are a result of offspring responding more readily to the feeding rules of mothers (who in turn are more responsive to variation in offspring state) than fathers [24]. Conversely, the finding that selection favours networks of interactions in large broods that are relatively weak indicates that nestling mobility may be constrained in large families owing to limited space, imposing costs on gregariousness when there are many mouths to feed. Thus, selection on network structure in great tit families depends on family size, a condition that may contribute to the maintenance of heritable variation in attributes of social networks or traits that correlate with SNS through genotype × family environment interactions.
As predicted, variation in the SNS of broods was related to differences in feeding behaviour by mothers and fathers. Broods with begging offspring that were more gregarious with one another were associated with mothers providing relatively more feeds than fathers, whereas broods with weakly interacting offspring were associated with fathers providing relatively more feeds. Consequently, the evidence indicates that resource allocation in broods of strongly associating nestlings is primarily controlled by mothers, not fathers. Furthermore, the mean time spent choosing before feeding nestlings showed the opposite pattern to the number of feeds. Female parents spent longer choosing which nestling to feed when the mean strength of interactions within broods was weak (i.e. when males provided more feeds), whereas male parents spent longer choosing nestlings when nestlings were highly gregarious (i.e. when females provided more feeds). Since it is expected that parents will take longer, on average, to decide how to allocate their resources when they have less information about brood need (i.e. when they provide a lower proportion of the number of feeds compared with their partner; [38]), this further supports the contention that high strength of interactions among nestlings (high gregariousness) in broods is associated with females primarily controlling feeding whereas weak interactions among nestlings are associated with greater male control.
Social network position of individual nestlings was primarily determined by hunger, and the consequent increased motivation to beg [22]. SNS was therefore strongly related to begging behaviour. Broods of highly gregarious nestlings had higher overall begging intensity and greater evenness of begging behaviour across the brood. Begging intensity was more evenly distributed across the brood when females provided more food, indicating that females are more responsive to nestling hunger than males [22,23]. However, the negative relationship between the number of feeds provided by males and mean brood begging intensity shows that attending to offspring demands is also dependent on providing sufficient food.
A probable explanatory scenario for the different relationships between SNS and the feeding behaviour of males and females is as follows: hunger drives offspring motivation to position themselves with respect to feeding parents [22]. Mothers are more responsive to variation in hunger than fathers, so nestlings move about more as they become hungry to gain access to feeds provided by mothers. Broods composed of strongly associating (gregarious) nestlings are therefore characterized by female control of resources to offspring (i.e. higher feeding rate by the female compared with the male parent, a more even distribution of begging among nestlings and higher overall begging intensity). In contrast, broods characterized by relatively weak patterns of associations among nestlings had higher feeding rates by fathers relative to mothers, a more skewed distribution of begging behaviour and lower overall intensity of begging. Fathers are less responsive than mothers to offspring demands [22,23], so in broods where the feeds are primarily controlled by male parents it does not pay hungry nestlings to preferentially move towards fathers, who have feeding rules that primarily favour more competitive, not necessarily hungrier, offspring. Relatively high mean strength of interactions among nestlings is selectively advantageous in small- and medium-sized broods, whereas relatively weak interactions among nestlings are favoured when broods are large. The results indicate that fathers that put relatively more effort into provisioning appeared to gain more control over resource allocation, reflected in the SNS of the brood (lower gregariousness of nestlings), perhaps because they obtain more information about the need or quality of their brood [38]. However, the higher provisioning effort of these males may be offset by the increased efficiency of the allocation of their parental investment because males rearing broods with weakly interacting networks of offspring had higher future reproductive success than males with broods of strongly interacting nestlings.
Recent theoretical analyses have shown that the controllability of networks depends upon the distribution of behavioural interactions within networks [39]. Networks with dense, relatively homogeneous interactions among individuals are easier to control than sparse, heterogeneous networks [39]. In our population of great tits dense, homogeneous networks (i.e. those with highly gregarious individuals) are characteristics of broods where mothers feed more than fathers. This provides further support that, in the most common sized broods, it is mothers that primarily control SNS not fathers. However, perhaps because it is more difficult for mothers to attend to offspring demands and/or the simpler feeding rules of fathers are more effective with many nestlings, in large broods selection favours weaker interactions among nestlings and greater relative male control of feeding. This fits with theory showing that the benefits of male parental care to females are expected to co-evolve with clutch size; the larger the clutch the greater the benefit of increased male care [40]. Our results suggest a mechanism for how this might be maintained in great tits: the use of different feeding rules by mothers and fathers.
SNS can affect average group performance so it can shape the structure of social interactions within groups, and, therefore, social evolution [1,2,10,14,41], including the evolution of family life [19]. Further work is needed to determine causality in the relationship between parent–offspring interactions and brood SNS, and the mechanistic basis of the effect of nestling SNS on recruitment probability. However, the current study shows that selection can act at the level of the family on variation in parent–offspring behaviours that affect kin-group structure. How individuals interact with each other may be at least as important as the phenotypic characteristics of the interacting individuals in determining how selection acts on families. These results lend some support to a recent study on cooperation in humans by Fehl et al. [42] showing that coevolutionary relationships between behaviour and SNS can increase cooperation beyond direct reciprocity itself. In great tits, associations between feeding behaviours of parents and SNS affects how conflicts over investment are resolved, which may lead to selection on families that are most efficient at resolving conflicts (i.e. ‘cooperative families’). Our results are also applicable to any network of individuals whose behaviour is influenced by how they are ‘managed’ and whose success depends on group performance. In our study, selection acts on parent–offspring relationships in birds, but there are parallels in how individual humans interact in business and in team sports structures that would repay further investigation using our approach.
Acknowledgements
N.J.R. was supported by NERC Fellowship NE/C002199/1 and M.K. by Swiss NSF Assistant Professor Fellowship PP00A_119190. This work also forms part of the ‘Laboratoire d'Excellence (LABEX)’ entitled TULIP (ANR-10-LABX-41). The idea for the project was conceived by N.J.R. and M.K. All authors contributed to the further development of the project. Planning of the experimental design was by M.K., P.H. and H.R. and the field data were collected by M.K. and P.H. Data were analysed by N.J.R. and T.W.P. All authors contributed to writing the manuscript. We thank Angus Buckling, Sasha Dall, Dave Hosken, Kate Lessells, Joël Meunier, Allen Moore, Dustin Rubenstein Tom Tregenza and two anonymous referees for discussions and comments on previous drafts of this paper. The authors declare no competing financial interests.
References
- 1.Croft D. P., James R., Krause J. 2008. Exploring social networks. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Whitehead H. 2008. Analyzing animal societies. Chicago, IL: Chicago University Press [Google Scholar]
- 3.Ohtsuki H., Hauert C., Lieberman E., Nowak M. A. 2006. A simple rule for the evolution of cooperation on graphs and social networks. Nature 441, 502–505 10.1038/nature04605 (doi:10.1038/nature04605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos F. C., Pacheco J. M., Lanaerts T. 2006. Cooperation prevails when individuals adjust their social ties. PLoS Compt. Biol. 2, 1284–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rand D. G., Arbesman S., Christakis N. A. 2011. Dynamic social networks promote cooperation in experiments with humans. Proc. Natl Acad. Sci. USA 108, 19 193–19 198 10.1073/pnas.1108243108 (doi:10.1073/pnas.1108243108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamede R. K., Bashford J., McCallum H., Jones M. 2009. Contact networks in a wild Tasmanian devil (Sarcophilus harrisii) population: using social networks analysis to reveal seasonal variation in social behaviours and its implications for transmission of devil facial tumour disease. Ecol Lett. 12, 1147–1157 10.1111/j.1461-0248.2009.01370.x (doi:10.1111/j.1461-0248.2009.01370.x) [DOI] [PubMed] [Google Scholar]
- 7.Centola D. 2010. The spread of behavior in an online social network. Science 329, 1194–1197 10.1126/science.1185231 (doi:10.1126/science.1185231) [DOI] [PubMed] [Google Scholar]
- 8.Eagle N., Macy M., Claxton R. 2010. Network diversity and economic development. Science 328, 1029–1031 10.1126/science.1186605 (doi:10.1126/science.1186605) [DOI] [PubMed] [Google Scholar]
- 9.Fewell J. H. 2003. Social insect networks. Science 301, 1867–1870 10.1126/science.1088945 (doi:10.1126/science.1088945) [DOI] [PubMed] [Google Scholar]
- 10.Moore A. J., Brodie E. D., Wolf J. B. 1997. Interacting phenotypes and the evolutionary process: I. Direct and indirect genetic effects of social interactions. Evolution 51, 1352–1362 10.2307/2411187 (doi:10.2307/2411187) [DOI] [PubMed] [Google Scholar]
- 11.Bleakley B. H., Parker D. J., Brodie E. D. 2007. Nonadditive effects of group membership can lead to additive group phenotypes for anti-predator behavior of guppies Poecilia reticulata. J. Evol. Biol. 20, 1375–1384 10.1111/j.1420-9101.2007.01342.x (doi:10.1111/j.1420-9101.2007.01342.x) [DOI] [PubMed] [Google Scholar]
- 12.McDonald D. B. 2007. Predicting fate from early connectivity in a social network. Proc. Natl Acad. Sci. USA 104, 10 910–10 914 10.1073/pnas.0701159104 (doi:10.1073/pnas.0701159104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh K. P., Badyaev A. V. 2010. Structure of social networks in a passerine bird: consequences for sexual selection and the evolution of mating strategies. Am. Nat. 176, E80–E90 10.1086/655216 (doi:10.1086/655216) [DOI] [PubMed] [Google Scholar]
- 14.Sih A., Hanser S. F., McHugh K. A. 2009. Social network theory: new insights and issues for behavioral ecologists. Behav. Ecol. Sociobiol. 63, 975–988 10.1007/s00265-009-0725-6 (doi:10.1007/s00265-009-0725-6) [DOI] [Google Scholar]
- 15.Royle N. J., Smiseth P. T., Kölliker M. 2012. The evolution of parental care. Oxford, UK: Oxford University Press [Google Scholar]
- 16.Horn A. G., Leonard M. L. 2005. Nestling begging as a communication network. In Animal communication networks (ed. McGregor P. K.), pp. 170–190 Cambridge, UK: Cambridge University Press [Google Scholar]
- 17.Godfray H. C. J., Johnstone R. A. 2000. Begging and bleating: the evolution of parent–offspring signaling. Phil. Trans. R. Soc. Lond. B 355, 1581–1591 10.1098/rstb.2000.0719 (doi:10.1098/rstb.2000.0719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker G. A., Royle N. J., Hartley I. R. 2002. Intrafamilial conflict and parental investment: a synthesis. Phil. Trans. R. Soc. Lond. B 357, 295–307 10.1098/rstb.2001.0950 (doi:10.1098/rstb.2001.0950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kölliker M. 2005. Ontogeny in the family. Behav. Genet. 35, 7–18 10.1007/s10519-004-0852-9 (doi:10.1007/s10519-004-0852-9) [DOI] [PubMed] [Google Scholar]
- 20.Wade M. J., et al. 2010. Multilevel selection and kin selection in a connected world. Nature 463, E8–E9 10.1038/nature08809 (doi:10.1038/nature08809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wild G., Gardner A., West S. A. 2010. Reply to Wade et al. Nature 463, E9–E10 10.1038/nature08810 (doi:10.1038/nature08810) [DOI] [Google Scholar]
- 22.Kölliker M., Richner H., Werner I., Heeb P. 1998. Begging signals and biparental care: nestling choice between parental feeding locations. Anim. Behav. 55, 215–222 10.1006/anbe.1997.0571 (doi:10.1006/anbe.1997.0571) [DOI] [PubMed] [Google Scholar]
- 23.Kölliker M., Brinkhof M. W. G., Heeb P., Fitze P. S., Richner H. 2000. The quantitative genetic basis of offspring solicitation and parental response in a passerine bird with biparental care. Proc. R. Soc. B 267, 2127–2132 10.1098/rspb.2000.1259 (doi:10.1098/rspb.2000.1259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinde C. A., Johnstone R. A., Kilner R. M. 2010. Parent–offspring conflict and coadaptation. Science 327, 1373–1376 10.1126/science.1186056 (doi:10.1126/science.1186056) [DOI] [PubMed] [Google Scholar]
- 25.Barba E., Atiénzar F., Marín M., Monrós J. S., Gil-Delgado J. A. 2009. Patterns of nestling provisioning by a single-prey loader bird, great tit Parus major. Bird Study 56, 187–197 10.1080/00063650902792049 (doi:10.1080/00063650902792049) [DOI] [Google Scholar]
- 26.Alonso-Alvarez C., Velando A. 2012. Benefits and costs of parental care. In The evolution of parental care (eds Royle N. J., Smiseth P. T., Kölliker M.), pp. 40–61 Oxford, UK: Oxford University Press [Google Scholar]
- 27.Lessells C. M. 2012. Sexual conflict. In The evolution of parental care (eds Royle N. J., Smiseth P. T., Kölliker M.), pp. 150–170 Oxford, UK: Oxford University Press [Google Scholar]
- 28.Kilner R. M., Hinde C. A. 2012. Parent–offspring conflict. In The evolution of parental care (eds Royle N. J., Smiseth P. T., Kölliker M.), pp. 119–132 Oxford, UK: Oxford University Press [Google Scholar]
- 29.Roulin A., Dreiss A. 2012. Sibling conflict and cooperation over parental care. In The evolution of parental care (eds Royle N. J., Smiseth P. T., Kölliker M.), pp. 133–149 Oxford, UK: Oxford University Press [Google Scholar]
- 30.Borgatti S. P., Everett M. G., Freeman L. C. 2002. UCINET for Windows: Software for social networks analysis. Harvard, MA: Analytic Technologies [Google Scholar]
- 31.Freckleton R. P. 2002. On the misuse of residuals in ecology: regression of residuals versus multiple regression. J. Anim. Ecol. 71, 542–545 10.1046/j.1365-2656.2002.00618.x (doi:10.1046/j.1365-2656.2002.00618.x) [DOI] [Google Scholar]
- 32.Smith A. C., Koper N., Francis C. M., Fahrig L. 2009. Confronting collinearity: comparing methods for disentangling the effects of habitat loss and fragmentation. Landscape Ecol. 24, 1271–1285 10.1007/s10980-009-9383-3 (doi:10.1007/s10980-009-9383-3) [DOI] [Google Scholar]
- 33.Crawley M. J. 2007. The R book. Chichester, UK: John Wiley & Sons [Google Scholar]
- 34.Heeb P., Werner I., Mateman A. C., Kölliker M., Brinkhof M. W. G., Lessells C. M., Richner H. 1999. Ectoparasite infestation and sex-biased local recruitment of hosts. Nature 400, 63–65 10.1038/21881 (doi:10.1038/21881) [DOI] [PubMed] [Google Scholar]
- 35.Fowler J. H., Dawes C. T., Christakis N. A. 2009. Model of genetic variation in human social networks. Proc. Natl Acad. Sci. USA 106, 1720–1724 10.1073/pnas.0806746106 (doi:10.1073/pnas.0806746106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dor R., Lotem A. 2010. Heritability of nestling begging intensity in the house sparrow (Passer domesticus). Evolution 63, 738–748 10.1111/j.1558-5646.2008.00598.x (doi:10.1111/j.1558-5646.2008.00598.x) [DOI] [PubMed] [Google Scholar]
- 37.Dor R., Lotem A. 2010. Parental effort and response to nestling begging in the house sparrow: repeatability, heritability and parent–offspring co-evolution. J. Evol. Biol. 23, 1605–1612 10.1111/j.1420-9101.2010.02023.x (doi:10.1111/j.1420-9101.2010.02023.x) [DOI] [PubMed] [Google Scholar]
- 38.Johnstone R. A., Hinde C. A. 2006. Negotiation over offspring care—how should parents respond to each other's efforts? Behav. Ecol. 17, 818–827 10.1093/beheco/arl009 (doi:10.1093/beheco/arl009) [DOI] [Google Scholar]
- 39.Liu Y-Y, Slotine J-J, Barabási A-L. 2011. Controllability of complex networks. Nature 473, 167–173 10.1038/nature10011 (doi:10.1038/nature10011) [DOI] [PubMed] [Google Scholar]
- 40.Smith H. G., Härdling R. 2000. Clutch size evolution under sexual conflict enhances the stability of mating systems. Proc. R. Soc. Lond. B 267, 2163–2170 10.1098/rspb.2000.1264 (doi:10.1098/rspb.2000.1264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.West-Eberhard M. J. 1983. Sexual selection, social competition and speciation. Q. Rev. Biol. 58, 155–183 10.1086/413215 (doi:10.1086/413215) [DOI] [Google Scholar]
- 42.Fehl K., van der Post D. J., Semman D. 2011. Co-evolution of behaviour and social network structure promotes human cooperation. Ecol. Lett. 14, 546–551 10.1111/j.1461-0248.2011.01615.x (doi:10.1111/j.1461-0248.2011.01615.x) [DOI] [PubMed] [Google Scholar]