Abstract
Postmenopausal longevity may have evolved in our lineage when ancestral grandmothers subsidized their daughters' fertility by provisioning grandchildren, but the verbal hypothesis has lacked mathematical support until now. Here, we present a formal simulation in which life spans similar to those of modern chimpanzees lengthen into the modern human range as a consequence of grandmother effects. Greater longevity raises the chance of living through the fertile years but is opposed by costs that differ for the sexes. Our grandmother assumptions are restrictive. Only females who are no longer fertile themselves are eligible, and female fertility extends to age 45 years. Initially, there are very few eligible grandmothers and effects are small. Grandmothers can support only one dependent at a time and do not care selectively for their daughters' offspring. They must take the oldest juveniles still relying on mothers; and infants under the age of 2 years are never eligible for subsidy. Our model includes no assumptions about brains, learning or pair bonds. Grandmother effects alone are sufficient to propel the doubling of life spans in less than sixty thousand years.
Keywords: human evolution, life history, sexual conflict
1. Introduction
Female fertility ends at similar ages in humans and the other great apes; all can have latest deliveries into the forties but not beyond [1]. But other ape females become frail in their thirties [2] and usually die during the cycling years. This is not true of humans. Even among hunter–gatherers, women past the childbearing years make up substantial fractions of human populations [3–6]. These comparisons suggest that the ancestral age when fertility ends has persisted among all great apes, while greater longevity evolved in our lineage. As W. D. Hamilton noted, the mismatch between human longevity and female fertility ‘inevitably suggests the special value of the old woman as mother or grandmother during a long ancestral period’ [7, p. 37]. Subsequent evidence from hunter–gatherers pointed to the special value of grandmothers supplying foods that just weaned juveniles cannot acquire effectively for themselves [8]. This economic productivity of older women prompted the Grandmother Hypothesis.
A verbal scenario begins with changing ecology. Increasingly arid and seasonal PlioPleistocene African savannahs constricted the distribution of foods ancestral juveniles could handle. This left ancestral mothers two choices: follow retreating foods and maintain diets their weanlings could manage or subsidize their offspring to older ages. Longer dependence would delay mothers' next birth, but also present a novel fitness opportunity to older females whose own fertility was declining. Elders could compensate for increased juvenile dependence by helping their grandchildren, allowing their daughters to have another baby sooner without risking the survival of previous offspring. Vigorous grandmothers could help more and leave more descendants. Consequently, longevity would have increased, expanding the fraction of female years lived past the fertile ages [9–11]. Or would it?
Researchers have looked for, and usually found, evidence of grandmother effects in contemporary and historical human populations (e.g. [12–14] but see [15]). But contributions by elders to the welfare of their younger kin might be consequences of postmenopausal life spans that evolved for other reasons. The question remains whether grandmother effects could transform a great ape-like life history in which adult females usually die during the cycling years into a human life history in which they usually do not.
Approaches to the mismatch between the end of female fertility and survival in humans often pose the question as the puzzle of menopause not increased longevity. This follows Williams' [16] influential formulation that assumed menopause to be unique to humans. This assumption is now known to be false [17,18]. Williams' focus on when to stop, generally taking observed rates of human ageing and population age structure as givens, has stimulated several treatments of the evolution of menopause, formal and otherwise (e.g. [4,19–21]).
Only recently has the evolution of postmenopausal longevity begun to receive formal attention. Lee [22] considered the effects of intergenerational transfers on selection against senescence. In contrast to Lee, we begin with an ancestral ape-like condition and include two sexes. Kachel et al. [23] used the Grandmother Hypothesis as their guide to construct an agent-based model of helpful grandmother effects on the evolution of lifespans. Our subsequent analysis of their model [24,25] set the foundation for the model we report here. In contrast to Kachel and others, we begin with a model population that is at an ape-like equilibrium for longevity without grandmothering, and ask whether weak grandmother effects could propel the evolution of increased longevity.
2. Model and results
We simulated the model in box 1, with parameters in table 1, using Matlab R (2011b). A key model parameter is L, the expected adult life span. Because we use the simplifying assumption that mortality is constant (see the electronic supplementary material for further discussion of the consequences of this assumption), L is the inverse of the annual mortality rate and does not change with age. Guided by demographic data from other great apes (see the electronic supplementary material), we specified expected adult life spans ranging from 16 to 27 years to represent the ancestral condition. Using findings from three well-known hunter–gatherer groups (Dobe !Kung [3], forest-dwelling Ache [4], Hadza [5]), we set an expected adult life span of 43 years as our human target. For starting populations, we assumed that the system begins with 1000 individuals, half male and half female, all of whom have the same expected adult life span, L. For convenience, we specified that the ages of individuals are distributed uniformly from τ1(L) to τ3. From this distribution, the population converges to a steady-state age distribution within several generations.
Box 1. Mathematical model.
Here, we describe a probabilistic agent-based model, which we will then convert to a deterministic difference equations model (see the electronic supplementary material). The agent-based model has the following features.
Mortality. For simplicity, we assume mortality rates are constant. Each individual has a lifetime mortality rate 1/L, where L is the individual's expected life span from any age, including the beginning of adulthood. We refer to L as the expected adult life span because this is our main interest (see the electronic supplementary material for discussion of the constant mortality assumption). In addition, the population is subject to an extrinsic, population-dependent death rate that affects everyone equally. Calculation of the population-dependent death rate is explained in the Agent-based model algorithm described in the electronic supplementary material.
Longevity trade-offs. Greater expected adult life span (L) always increases the chance of living through the fertile ages, but as is typical of mammals [26], females with greater longevity have offspring that take longer to reach independence. We assume age at independence to be L/6. Males with greater expected adult life spans are less successful at competing for paternities following Williams' [16] deduction that selection for reduced senescence should decrease youthful vigour. Our male fertility–longevity trade-off assigns each male a weighting factor α(L), where α is a decreasing function of the male's expected adult life span, L (see the electronic supplementary material).
Life histories. Each individual, male or female, passes through a period of nursing, weaned dependency, independent juvenility, fertility or eligibility, and females also reach an age of frailty. Individuals of age 0 to τ0 are still nursing, where τ0 is the age of weaning, and individuals of age τ0 to τ1(L) are weaned, but still dependent, where τ1(L) is the age of independence and is a function of expected adult life span, L. Age at maturity, τ2(L), is also a function of expected adult life span. This is the age at which females become fertile and eligible to conceive. Females of age τ2(L) to τ3 are fertile, where τ2(L) is the age of female maturity and τ3 is the end of fertility. Post-fertile females of age τ3 to τ4(L) are eligible to grandmother, where τ4(L) is the age of frailty. Males of age σ1 to σ2 can compete for paternities, where σ1 and σ2 specify the beginning and end of eligibility, respectively.
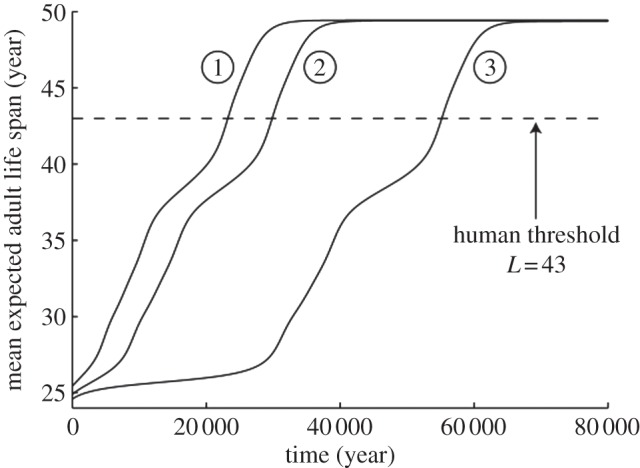
Mating, conception, and delivery. Only fertile females without dependents can conceive. For simplicity, we assume that females without dependents conceive and give birth at a constant rate throughout their fertile ages (see the electronic supplementary material). When a female is eligible to conceive, all eligible males compete for the paternity. A particular male's probability of success is α(L)/(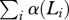 ) where L is the male's expected adult life span and the summation is taken over all eligible males at the current time. Offspring inherit the expected adult life spans of their parents with the possibility of a mutational shift up or down.
) where L is the male's expected adult life span and the summation is taken over all eligible males at the current time. Offspring inherit the expected adult life spans of their parents with the possibility of a mutational shift up or down.
Grandmothering. We consider a more generalized form of allomaternal care than literal grandmothering, in which females who are eligible to grandmother can assume care of any weaned dependent in the population, not only direct matrilineal descendants. This generalization weakens grandmother effects, but it allows us to easily rewrite our agent-based model as a deterministic system, since we do not keep track of matrilineal lineages.
For convenience, we still use the term grandmothering to refer to general transfers of dependents between fertile and post-fertile females. In our model, grandmothering occurs whenever a female who is no longer fertile and has no current dependent adopts a weaned dependent from a female of fertile age, freeing the fertile female for another conception. When a grandmother adopts a child, she functions thereafter as though she were the child's mother.
We further weaken potential grandmother effects by restricting eligibility to females who are past the fertile ages, but have not reached frailty, with the age of frailty varying as a function of expected adult life span (min {2L, 75}). Since the end of fertility is fixed at 45 years (as suggested by the empirical pattern for humans and great apes), the frailty constraint ensures that there are no eligible grandmothers when expected adult life span is less than 22.5 years. At L = 23, only 45 year olds without dependents are eligible, so less than 1 per cent of the caring females are eligible to grandmother. Our frailty constraint reduces the effects of having females grandmother at unreasonably old ages (see the electronic supplementary material for further discussion).
The deterministic system (see the electronic supplementary material) can be much more efficiently simulated than the agent-based model, allowing us to investigate a wide range of scenarios rapidly. The conclusions of our analysis appear sufficiently general and robust that they should hold for a more sophisticated model that tracks separate lineages.
Parameter estimates for the model are summarized in table 1. See the electronic supplementary material for further discussion.
Table 1.
Model parameters, descriptions and estimated values. (The variable L denotes expected adult life span. See the electronic supplementary material for discussion of parameter values.)
| parameter | description | estimate |
|---|---|---|
| τ0 | weaning age (youngest age eligible for adoption) | 2 years |
| τ1(L) | age of independence | L/6 |
| τ2(L) | age of female sexual maturity | L/2.5 + τ0 |
| τ3 | age female fertility ends | 45 |
| τ4(L) | age of frailty (ineligibility to adopt dependents) | min {2L, 75} |
| σ1 | age of beginning of male eligibility period | 20 years |
| σ2 | age of beginning of male ineligibility period | 25 years |
| c | female conception and delivery rate | 1/year |
| α(L) | male weighting factor for mating | decreasing function of L |
| μ | probability of mutation in L at birth | 5% |
| K | population carrying capacity | 1000 individuals |
| Δt | time step of simulation | 1/3 year |
For populations with fixed expected adult life spans, L, we numerically calculated the net growth rates, r, at a steady-state age distribution with and without grandmothering and plotted the results in figure 1. Peaks on this plot correspond to local optima of reproductive output for females. They are not population-level equilibria since the effects of male trade-offs are absent, but they would be equilibria in a one-sex version of our model.
Figure 1.
Female reproductive output as a function of expected adult life span. Plots of net growth rate, r, versus expected adult life span, L, for populations with and without grandmothering. (In this simulation, the time step, Δt, was taken to be 1/12 years to generate a smoother plot. Such a small time step proved to be too computationally demanding for other simulations.)
Figure 1 shows that except at the optimum, L = 18.2, the population grows at a rate of less than 0.7 per cent per year. Since females cannot grandmother before age τ3 = 45, or after τ4(L) = 2L, none are eligible to grandmother in populations with L ≤ 22.5, so growth rates with and without grandmothering coincide in this region. Without grandmothering, the growth rate decreases past L = 18.2 until it falls below 0, at which point the population cannot sustain itself. The decrease in r results from increasing ages at first birth, decreasing fertile periods and increasing ages of independence; but with grandmothering, the growth rate rises gradually from L = 22.5 to 37 and stays above 0 up to approximately L = 60 because mothers can transfer dependents to grandmother care, allowing birth intervals to remain steady (see the electronic supplementary material, for further analysis).
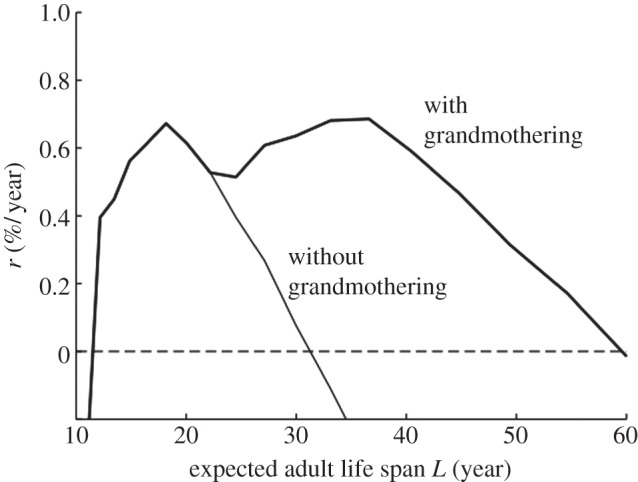
Male trade-offs also play a role in determining population equilibria in our model (see the electronic supplementary material). We consider three male trade-off functions shown in figure 2. The competitiveness of males decreases with L.
Figure 2.
Three male weighting functions, α(L), versus expected adult life span, L.
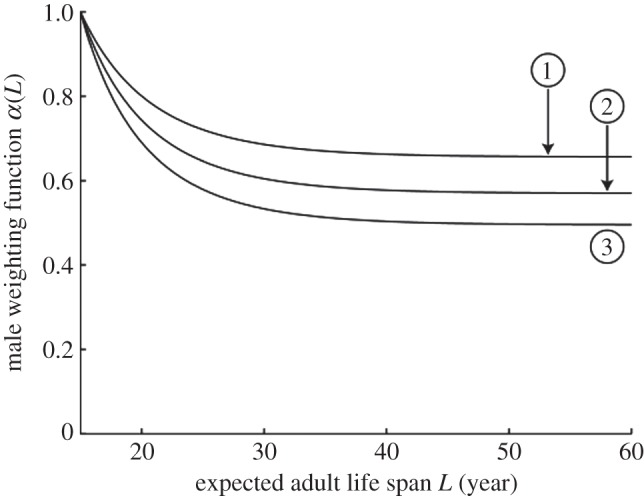
With the female trade-offs that arise from the parameter values in table 1 and give the female reproductive output plotted in figure 1, the male trade-off curves in figure 2 push the geometric mean of expected adult life spans in the population to equilibrium values of (1) 25.4, (2) 24.9, and (3) 24.6 years in the absence of grandmothering (see the electronic supplementary material, figure S2). (We use the geometric mean, since we represent expected adult life spans on a logarithmic scale as discussed in the electronic supplementary material.) To investigate the effect of grandmothering, we start at those equilibria and introduce grandmothering. Figure 3 shows the evolution of the geometric mean of expected adult life spans in the population from equilibria without grandmothering to equilibria with grandmothering. Sexual conflict pushes the population to an equilibrium L that is an inevitable compromise, neither sex achieving the L that would maximize its reproductive output in the absence of net effects on the opposite sex.
Figure 3.
Evolution of populations from lower to higher expected adult life spans in the presence of grandmothering. The starting points (1) 25.4, (2) 24.9 and (3) 24.6 years correspond to equilibria without grandmothering of the three male trade-off curves in figure 2. Mean expected adult life spans over the population converge to (1) 49.43, (2) 49.40 and (3) 49.37 years in the presence of grandmothering. The population crosses the human threshold of L = 43 within (1) 24 000, (2) 30 000 and (3) 56 000 years.
3. Discussion
Our model population moves from chimpanzee-like life spans into the human longevity range as grandmothers allow mothers to have their next baby sooner without reducing the survival chances of previous offspring. Longer adult life spans (resulting from lower adult mortality) always confer an increased chance of living through the fertile years. But longer-lived females have later ages of first birth and their longer-lived offspring remain dependent to older ages (as holds for mammals generally [26]). Without grandmothering, the longevity that maximizes female lifetime reproductive success depends on the sum of these effects. Grandmothering alters the equilibrium. Grandmothering also alters the longevity that maximizes male reproductive success (see the electronic supplementary material for further discussion). Our simulations show that by altering the payoffs for both sexes, even weak grandmothering drives the evolution of longevity from an ape-like value into the human range.
We have made no assumptions about sex-biased dispersal, an issue often raised as a problem for the Grandmother Hypothesis (see the electronic supplementary material). Rather than grandmothers helping only their own daughters, our model distributes grandmothering to any eligible dependents in the population. It may seem that this would not only weaken selection for grandmothering, but undercut it altogether. Those with shorter life spans spend little or no time grandmothering and have higher rates of offspring production. If females with lower L free-ride on grandmothering supplied by others, this should halt the spread of grandmothering and the evolution of increasing life spans.
Selection will lead to increased longevity only if grandmothers disproportionately favour their own fitness. Our simulations show that they do, even without a bias toward daughters. Increasing L raises the number of grandmothering years and grandmothering gives greater benefits to females with higher L because their offspring probably have higher L as well. The latter point matters because offspring with higher L have higher survival and are dependent longer, making them more likely to be adopted. In addition, grandmothers take oldest dependents first, disproportionately accepting those with higher L. Although adoption does not benefit the dependents themselves, it does benefit their mothers. This differential benefit that mothers with higher L gain from grandmothering drives the evolution of increased longevity.
Examination of our model populations underlines how little grandmothering it takes to produce the longevity change. At the initial ape-like equilibrium, birth intervals are just over 5 years—shorter than those of orangutans, longer than gorillas' and close to the empirical value for chimpanzees [27]. At the grandmothering equilibrium where L = 49, age at independence is 8.2. Since children are eligible to leave mothers for grandmothers at 2 years, this nursing period plus a year to conceive and deliver the next baby would make birth intervals 3 years—again close to the empirical value for humans [1]. But all mothers could do that only if grandmothers cared for children during 6.2 of the 8.2 years of dependency. Given our assumptions that only females past 45 years are eligible and can only care for one dependent at a time, there are not enough grandmothers to make birth intervals that short.
With our assumptions, eligible grandmothers initially make up less than 1 per cent of caring females, but that proportion steadily increases to 43 per cent at the grandmothering equilibrium. In other words, at the equilibrium age distribution, grandmothers care for 43 per cent of the dependent years (43% of 8.2 = 3.5), leaving mothers responsible for the remaining 4.7 of the 8.2 dependent years. If mothers hand off dependent juveniles at an average age of 4.7 years, and then take another year to conceive and deliver the next baby, their intervals are 5.7 years—even longer than those at the initial non-grandmothering equilibrium. Although grandmothering shortens intervals, the weak grandmothering in this model can push the population to human longevities without the very short birth intervals that distinguish humans from other great apes.
Of course in the real world mothers get help from sources not included in our model [28]. For example, fathers sometimes help [29] as do older siblings [30], and stronger grandmother effects could also play a role. However, in our model, we have only allowed females past the age of 45 years to grandmother and diluted the effects by distributing their help throughout the population, restricting subsidies to one dependent at a time, and ignoring probable economies of scale and the decreasing amounts of help required by older dependents. We have fixed the end of fertility at 45 years on grounds that this feature is little changed in humans compared with the other great apes. We leave investigation of ‘why 45?’ to future work, here demonstrating only that given that end to fertility, grandmothering can account for the evolution of increased longevity.
Other hypotheses for the evolution of human longevity appeal to our large brains [31]. Kaplan et al.'s [32,33] embodied capital model links the evolution of larger human brains to increased skill learning that allowed ancestral hunters to be productive enough to provision their mates and offspring. Kaplan et al. argue that these skills take a long time to learn, with benefits fully realized only well into adulthood. This increases the payoffs for living to older ages and so favours increased somatic maintenance and longer life spans. Our model, in contrast, assumes nothing about the larger brains, hunting, skill learning or pair bonds that distinguish modern humans from the other great apes. It shows that very weak grandmothering can move life spans from the great ape to the human range without any of those features. Our model is also silent on social capacities that others have associated with reliance on allomaternal care across the mammals including humans [28,34]. Hrdy's [28] synthesis flags especially important selection pressures on both mothers and offspring that accompanied the ancestral switch from an ape pattern of independent rearing to the human pattern of reliance on help. Grandmothers were the probable initial source of that rearing help. As our model shows, selection for grandmothering alone could have propelled the evolution of our post-menopausal longevity, amplifying interdependencies and setting the social context for many other features that subsequently evolved in our lineage.
Acknowledgements
We thank Earle Keefe for compiling data on alpha male chimpanzee tenures, Fred Adler, Adrian Bell, Brett Kennedy, and two anonymous reviewers for clarifying suggestions, and the National Science Foundation, grant no. 0717886, and the Australian Research Council, Discovery Early Career Research Award for support.
References
- 1.Robson S. L., van Schaik C. P., Hawkes K. 2006. The derived features of human life history. In The evolution of human life history (eds Hawkes K., Paine R.), pp. 17–44 Santa Fe, NM: SAR Press [Google Scholar]
- 2.Finch C. E. 2010. Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc. Natl Acad. Sci. USA 107, 1718–1724 10.1073/pnas.0909606106 (doi:10.1073/pnas.0909606106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howell N. 1979. Demography of the Dobe !Kung. New York, NY: Academic Press [Google Scholar]
- 4.Hill K., Hurtado A. M. 1996. Ache life history: the ecology and demography of a foraging people. New York, NY: Aldine de Gruyter [Google Scholar]
- 5.Blurton Jones N. G., Hawkes K., O'Connell J. F. 2002. Antiquity of postreproductive life: are there modern impacts on hunter–gatherer postreproductive life spans? Am. J. Hum. Biol. 14, 184–205 10.1002/ajhb.10038 (doi:10.1002/ajhb.10038) [DOI] [PubMed] [Google Scholar]
- 6.Hawkes K., Blurton Jones N. G. 2005. Human age structures, paleodemography, and the grandmother hypothesis. In Grandmotherhood: the evolutionary significance of the second half of female life (eds Voland E., Chasiotis A., Schiefenhovel W.), pp. 118–140 New Brunswick, Canada: Rutgers University Press [Google Scholar]
- 7.Hamilton W. D. 1966. The moulding of senescence by natural selection. J. Theoret. Biol. 12, 12–45 10.1016/0022-5193(66)90184-6 (doi:10.1016/0022-5193(66)90184-6) [DOI] [PubMed] [Google Scholar]
- 8.Hawkes K., O'Connell J. F., Blurton Jones N. G. 1989. Hardworking Hadza grandmothers. In Comparative socioecology: the behavioural ecology of humans and other mammals (eds Standen V., Foley R. A.), pp. 341–366 Oxford, UK: Blackwell Scientific [Google Scholar]
- 9.Hawkes K., O'Connell J. F., Blurton Jones N. G., Alvarez H. P., Charnov E. L. 1998. Grandmothering, menopause, and the evolution of human life histories. Proc. Natl Acad. Sci. USA 95, 1336–1339 10.1073/pnas.95.3.1336 (doi:10.1073/pnas.95.3.1336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connell J. F., Hawkes K., Blurton Jones N. G. 1999. Grandmothering and the evolution of Homo erectus. J. Hum. Evol. 36, 461–485 10.1006/jhev.1998.0285 (doi:10.1006/jhev.1998.0285) [DOI] [PubMed] [Google Scholar]
- 11.Hawkes K. 2003. Grandmothers and the evolution of human longevity. Am. J. Hum. Biol. 15, 380–400 10.1002/ajhb.10156 (doi:10.1002/ajhb.10156) [DOI] [PubMed] [Google Scholar]
- 12.Voland E., Chasiotis A., Schiefenhovel W. (eds) 2005. Grandmotherhood: the evolutionary significance of the second half of female life. New Brunswick, Canada: Rutgers University Press [Google Scholar]
- 13.Sear R., Mace R. 2008. Who keeps children alive? A review of the effects of kin on child survival. Evol. Hum. Behav. 29, 1–18 10.1016/j.evolhumbehav.2007.10.001 (doi:10.1016/j.evolhumbehav.2007.10.001) [DOI] [Google Scholar]
- 14.Sear R., Coall D. 2011. How much does family matter? Cooperative breeding and the demographic transition. Pop. Dev. Rev. 37, 81–112 10.1111/j.1728-4457.2011.00379.x (doi:10.1111/j.1728-4457.2011.00379.x) [DOI] [PubMed] [Google Scholar]
- 15.Hill K., Hurtado A. M. 2009. Cooperative breeding in South American hunter–gatherers. Proc. R. Soc. B 276, 3863–3870 10.1098/rspb.2009.1061 (doi:10.1098/rspb.2009.1061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams G. C. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 10.2307/2406060 (doi:10.2307/2406060) [DOI] [Google Scholar]
- 17.Paul A. 2005. Primate predispositions for human grandmaternal behavior. In Grandmotherhood: the evolutionary significance of the second half of female life (eds Voland E., Chasiotis A., Schiefenhovel W.), pp. 21–37 New Brunswick, Canada: Rutgers University Press [Google Scholar]
- 18.Walker M. L., Herndon J. G. 2008. Menopause in nonhuman primates? Biol. Reprod. 79, 398–406 10.1095/biolreprod.108.068536 (doi:10.1095/biolreprod.108.068536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers A. R. 1993. Why menopause? Evol. Ecol. 7, 406–420 10.1007/bf01237872 (doi:10.1007/bf01237872) [DOI] [Google Scholar]
- 20.Shanley D. P., Sear R., Mace R., Kirkwood T. B. L. 2007. Testing evolutionary theories of menopause. Proc. R. Soc. B 274, 2943–2949 10.1098/rspb.2007.1028 (doi:10.1098/rspb.2007.1028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cant M. A., Johnstone R. A. 2008. Reproductive conflict and the separation of reproductive generations in humans. Proc. Natl Acad. Sci. USA 105, 5332–5336 10.1073/pnas.0711911105 (doi:10.1073/pnas.0711911105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee R. D. 2003. Rethinking the evolutionary theory of aging: transfers, not births, shape social species. Proc. Natl Acad. Sci. USA 100, 9637–9642 10.1073/pnas.1530303100 (doi:10.1073/pnas.1530303100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kachel A. F., Premo L. S., Hublin J. J. 2011. Grandmothering and natural selection. Proc. R. Soc. B 278, 384–391 10.1098/rspb.2010.1247 (doi:10.1098/rspb.2010.1247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkes K., Kim P. S., Kennedy B., Bohlender R., Hawks J. A. 2011. Reappraisal of grandmothering and natural selection. Proc. R. Soc. B 278, 1936–1938 10.1098/rspb.2010.2720 (doi:10.1098/rspb.2010.2720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kachel A. F., Premo L. S., Hublin J. J. 2011. Grandmothering and natural selection revisited reply. Proc. R. Soc. B 278, 1939–1941 10.1098/rspb.2011.0472 (doi:10.1098/rspb.2011.0472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charnov E. L. 1993. Life history invariants: some explorations of symmetry in evolutionary ecology. Oxford, UK: Oxford University Press [Google Scholar]
- 27.Knott C. 2001. Female reproductive ecology of the apes: implications for human evolution. In Reproductive ecology and human evolution (ed. Ellison P. T.), pp. 429–463 New York, NY: Aldine de Gruyter [Google Scholar]
- 28.Hrdy S. B. 2009. Mothers and others: the evolutionary origins of mutual understanding. Cambridge, MA: Belknap Press of Harvard University Press [Google Scholar]
- 29.Grey P., Anderson K. G. 2010. Fatherhood: evolution and human paternal behavior. Cambridge, MA: Harvard University Press [Google Scholar]
- 30.Kramer K. 2011. The evolution of human parental care and recruitment of juvenile help. Trends Ecol. Evol. 26, 533–540 10.1016/j.tree.2011.06.002 (doi:10.1016/j.tree.2011.06.002) [DOI] [PubMed] [Google Scholar]
- 31.Isler K., van Schaik C. P. 2009. The expensive brain: a framework for explaining evolutionary changes in brain size. J. Hum. Evol. 57, 392–400 10.1016/j.jhevol.2009.04.009 (doi:10.1016/j.jhevol.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 32.Kaplan H., Hill K., Lancaster J., Hurtado A. M. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185 (doi:10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7) [DOI] [Google Scholar]
- 33.Kaplan H., Gurven M., Winking J., Hooper P., Stieglitz J. 2010. Learning, menopause and the human adaptive complex. Ann. NY Acad. Sci. 1204, 30–42 10.1111/j.1749-6632.2010.05528.x (doi:10.1111/j.1749-6632.2010.05528.x) [DOI] [PubMed] [Google Scholar]
- 34.Burkart J., van Schaik C. 2010. Cognitive consequences of cooperative breeding in primates? Anim. Cogn. 13, 1–19 10.1007/s10071-009-0263-7 (doi:10.1007/s10071-009-0263-7) [DOI] [PubMed] [Google Scholar]


