Abstract
Adaptation to divergent ecological niches can result in speciation. Traits subject to disruptive selection that also contribute to non-random mating will facilitate speciation with gene flow. Such ‘magic’ or ‘multiple-effect’ traits may be widespread and important for generating biodiversity, but strong empirical evidence is still lacking. Although there is evidence that putative ecological traits are indeed involved in assortative mating, evidence that these same traits are under divergent selection is considerably weaker. Heliconius butterfly wing patterns are subject to positive frequency-dependent selection by predators, owing to aposematism and Müllerian mimicry, and divergent colour patterns are used by closely related species to recognize potential mates. The amenability of colour patterns to experimental manipulation, independent of other traits, presents an excellent opportunity to test their role during speciation. We conducted field experiments with artificial butterflies, designed to match natural butterflies with respect to avian vision. These were complemented with enclosure trials with live birds and real butterflies. Our experiments showed that hybrid colour-pattern phenotypes are attacked more frequently than parental forms. For the first time, we demonstrate disruptive ecological selection on a trait that also acts as a mating cue.
Keywords: ecological speciation, Heliconius, magic trait, avian vision, mimicry, natural selection
1. Introduction
It is now widely accepted that adaptation to different ecological niches can result in the evolution of new species [1,2]. Disruptive selection on an ecological trait, where the external environment imposes selection against maladaptive hybrids, can impose a barrier to gene flow [3]. However, such extrinsic post-mating isolation alone may be insufficient for speciation and, if mating is random, then intermediate offspring will continue to be produced. Ecological speciation therefore commonly also involves the evolution of assortative mating, which may arise as a by-product of ecological divergence. Alternatively, selection acting against maladaptive hybrids may drive the evolution of assortative mating through reinforcement. This latter process is often invoked in the models of speciation where gene flow persists [4,5], but it relies on establishing linkage disequilibrium between genes under disruptive selection and those underlying assortative mating. Somehow the force of disruptive selection acting on the ecological trait needs to be transmitted to genes responsible for pre-mating isolation [6].
Hybridization between ecologically divergent populations can therefore simultaneously promote and inhibit speciation: on the one hand, it produces maladaptive intermediates, which may drive selection for assortative mating; on the other hand, subsequent recombination will break down associations between alleles underlying reproductive isolation [7]. One mechanism that has received considerable attention, and may resolve the antagonism between selection and recombination, involves traits under divergent selection that also influence assortative mating—the so-called magic [4,8] or multiple-effect [9] traits. These typically include mating cues (such as colour or body size), which are also under divergent ecological selection [8]. Although magic traits have often been invoked in theoretical and empirical studies of speciation, direct experimental evidence of mating cues under disruptive viability selection is limited. Some evidence exists that putative ecological traits are indeed used as mating cues [10–12]; however, evidence that these same traits are under divergent selection is considerably weaker, and to our knowledge reports of key manipulative experiments have yet to be published (see also Servedio et al. [8]). This probably reflects the empirical difficulties associated with demonstrating divergent selection on a particular trait rather than the rarity of magic traits in nature: following the fates of individual organisms and determining their fitness in the wild is difficult; and second, distinguishing selection acting on traits used as mating cues from that acting on other differences, which may be genetically correlated, often presents a considerable experimental challenge.
Mimicry in tropical butterflies has long been championed as a classical example of both adaptation and speciation [13]. The Neotropical genus Heliconius is famous for Müllerian mimicry, where two or more unpalatable species converge on the same bright warning-patterns to more efficiently advertise their distastefulness to predators [14]. Closely related taxa often belong to different mimicry rings and these are maintained by strong selection against non-mimetic patterns [15,16]. Five separate studies using model butterflies have now shown that male Heliconius use colour-pattern differences during mate selection [11,17–20]. Thus, divergence in colour pattern owing to mimicry also contributes to assortative mating. Hybrids display intermediate warning patterns that may not be recognized as distasteful [14]. As a result, we expect hybrids to be attacked more often by predators, but this has never been explicitly demonstrated.
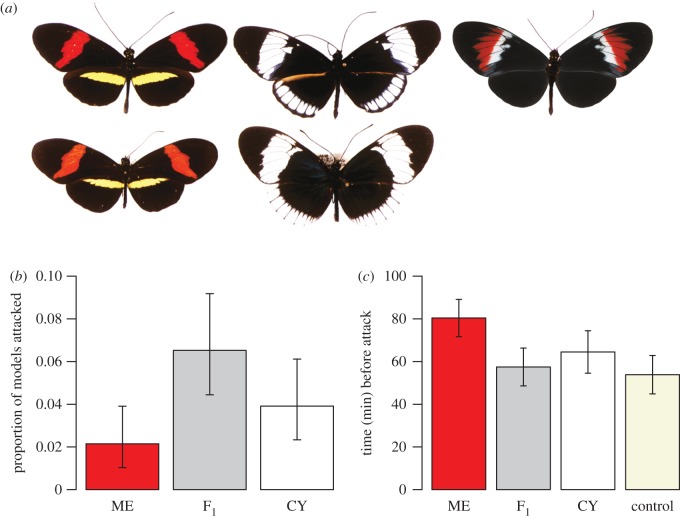
In central Panama, Heliconius melpomene is a near exact mimic of Heliconius erato and normally occurs in forest-edge habitats, whereas the closely-related Heliconius cydno mimics Heliconius sapho, and is more common in closed-forest habitats [21] (figure 1a). Despite these differences in habitat preference, the two species are often seen flying together, and hybrid individuals have been collected, albeit at very low frequencies (estimated at a frequency of just 0.001 [14]). Assortative mating between Heliconius melpomene and H. cydno is strong [11], and interspecific hybrids between H. melpomene and H. cydno are less attractive to males of either parental species [22]. Nevertheless, hybrids from crosses in one direction (H. cydno mother and H. melpomene father) can be produced, albeit with some difficulty, in the insectary. We had previously attempted to demonstrate disruptive mimicry selection in this system using live butterflies, either by field releases of laboratory reared parental and hybrid genotypes, or by manipulation of the phenotypes of field captured butterflies. Neither of these approaches were successful, in part due to low recapture rates of field released butterflies. Here, we take a different approach by using artificial butterflies, designed with consideration of bird vision [23], in addition to enclosure trials with live birds and butterflies, and demonstrate selection against non-mimetic hybrid phenotypes.
Figure 1.
Disruptive selection against non-mimetic hybrid colour patterns. (a) Helconius melpomene, Helconius cydno and their F1 hybrid (top row, left-to-right), and their co-mimics Helconius erato and Helconius sapho (bottom row). (b) Proportion of artificial butterflies attacked (± 95% CIs) after 72 h for each phenotype: H. melpomene (ME); F1 hybrid (F1); H. cydno (CY). (c) Mean time (±s.e.) survived before attack in enclosure trials with wild-caught birds for the four butterfly types: H. melpomene (ME); F1 hybrid (F1); H. cydno (CY); and the palatable butterfly Anartia fatima (control).
2. Material and methods
(a). Production and calibration of artificial butterflies
We produced models from photographs of dissected wings from 12 individuals each of H. cydno, H. melpomene and their F1 hybrids. Bird colour and luminance (‘lightness’) vision differs from human vision in a number of ways [24], and printouts of digital images often do not closely match real object colours, especially to non-human vision. Therefore, we needed to calibrate the appearance of the artificial prey to match the real butterfly colours to a bird's vision when printed [23]. Photographs of dissected wings were taken with a Fujifilm IS Pro UV-sensitive digital camera with a quartz CoastalOpt ultraviolet (UV) lens (Coastal Optical systems). To calibrate the appearance of the artificial prey to match the real butterfly colours, we first took reflectance spectra of the various colour patches of the wings of each of the butterfly forms, using an Ocean Optics USB4000 spectrometer (Dunedin, FL, USA) with illumination by a PX-2 pulsed xenon lamp, with a narrow-ended (1/8″) probe held at a constant distance and a 45° angle to the butterfly wings. Following this, we calculated the predicted photon catch values of a bird's four single cones (used in colour vision) and double cones (used in luminance vision) [25], based on the sensitivity of a blue tit's Cyanistes caeruleus receptors [26], and using irradiance spectra from deciduous woodland. Although there will be some error associated with not knowing the exact spectral sensitivity of the bird species found in our study site or having the corresponding habitat irradiance spectra, current modelling indicates the level of error from this should be minor [27]. To select appropriate colours to print, we then used an iterative process of printing different colours from the same printer onto the waterproof paper (HP LaserJet Tough Paper, Palo Alto, CA, USA using a Hewlett Packard LaserJet 2605dn printer at 300 dpi), and measuring the photon catches of these (as mentioned earlier). As with similar past work on camouflage [23], our criteria for selecting appropriate colours were that they produced photon catch values for each cone type that fell within the range of photon catch values from the corresponding colour patches on the real butterflies. This was generally achieved, with the exception that H. cydno and the hybrid had a white patch with relatively high ultraviolet reflectance. We were unable to find a substance to add to the models to recreate this without changing the other colour balances, and so our models for these two forms produced a lower photon catch value for the ultraviolet receptors (0.26 compared with a mean of 0.6, and 0.21 compared with a mean of 0.45, for the H. cydno and the hybrid models, respectively). However, the model values are not far outside the range we recorded for H. cydno (0.36–0.82) and within that recorded for the hybrid (0.19–0.67). Once we had selected appropriate colours in Photoshop CS4, we replaced the colours of the real butterfly wings in the images with these values before printing these as stimuli.
(b). Field experiments with artificial butterflies
We conducted four experimental trials in Panama in September and October 2010. Two trials were conducted in closed-forest (H. cydno preferred) habitats along Pipeline Road in the Parque Nacional Soberanía, and two in forest-edge (H. melpomene preferred) habitats along Pipeline Road and nearby Gamboa [21]. Unlike our enclosure experiments with live butterflies (see below), we did not include models of a palatable butterfly as controls in this experiment. Designing an appropriate control is non-trivial because the attack frequency experienced will depend on contrast with the background and novelty to potential predators, among other factors. In particular, because palatable butterflies are often cryptic, it is unclear whether they would be attacked more often than the Heliconius models (because they are palatable) or attacked less (because they are less likely to be spotted by predators). Models were pinned to leaves, in random order at least 10 m apart, with a Plasticine ‘body’ on the upper side and secured with small ball of Plasticine on the other side of the leaf. Each experimental trial lasted 5 days: 180 models were placed on the first day, and 180 models were placed on the second day; these were then checked every 24 h for 3 days after placement for evidence of predation (a beak mark clearly visible, or part, or all, of the ‘body’ missing). Attacked models were photographed and taken down. These photographs were used to ‘blind’ score the model for beak marks. This was achieved by ‘removing’ the wing patterns in photographs of attacked models using the magic wand and cut tools in Photoshop CS4 (see the electronic supplementary material, figure S1). Models were excluded from analysis if there was any evidence of attack by insects, which were normally distinguished from beak marks due to the presence of multiple smaller marks (see the electronic supplementary material, figure S1). Similarly, models not recovered were excluded from analysis.
(c). Live butterfly experiments
In a second experiment, we presented wild-caught birds with live H. cydno, H. melpomene, and F1 hybrids during 2 h enclosure trials. To ensure individual birds were responsive to the experimental conditions, we additionally included the widespread palatable butterfly Anartia fatima as a control. Wild birds were caught for our enclosure experiments using mist-nets placed both in forest-edge (H. melpomene preferred) and closed-forest (H. cydno preferred) habitats around Gamboa and Pipeline Road between April and September 2001. However, of a total of 48 birds only six were caught in closed-forest habitats. After capture, birds were transferred to experiment cages (1.5 × 2 × 2.8 m), which contained a perch and a small tree. Experimental trials were performed early the morning after capture. We used 2 × T12 Paralite full-spectrum lights (colour rendering index (CRI) = 93, temperature of 5900 K), over a diffusion screen to provide lighting as close to natural light as possible (natural light is CRI = 100, temperature of 5500–6800 K, diffused light). For each bird, four butterflies were released simultaneously and the time of attack for each was recorded. Trials lasted for 2 h or until all the butterflies had been attacked. Birds were considered responsive, and included in subsequent analyses, if they attacked at least one of the butterflies during this 2 h period. Each bird was used only once and at the end of the experiment, was banded and released at the site of capture. Data were analysed with linear mixed models with time of attack as the response variable. Butterfly phenotype was fitted as a fixed effect and bird ID fitted as a random factor. Bird ID incorporates variation due to bird species (for which sample sizes were too small to incorporate as an additional factor), individual bird and trial. Reported p-values for post hoc pairwise analyses (performed by rerunning the mixed model with pairs of treatments) are reported after critical thresholds for the tests were adjusted according to tablewise sequential Bonferroni correction for multiple testing.
3. Results
In total, we placed 1440 artificial butterflies (480 H. cydno patterns, 480 H. melpomene patterns and 480 hybrid patterns) in lowland rainforest and nearby edge habitats in Panama. We successfully recovered 1386 artificial butterflies (96% of the total placed in the forest), and of these only 58 (4%) were attacked (table 1). This low frequency is perhaps unsurprising, as many birds will have learned to avoid the real distasteful butterflies. In addition, this is similar to previous studies of warning colouration and crypsis using Plasticine models to determine differential predation (these report attack frequencies of 1–10% of models attacked; [28,29–31]). Nevertheless, after 72 h, there were clear differences in the number of ‘predation events’ experienced by the three phenotypes (figure 1b; G = 10.60, d.f. = 2, p < 0.01). A greater proportion of hybrid models were attacked than those with parental phenotypes (30 of 460 recovered hybrid phenotype models compared to 28 of 926 parental phenotype models recovered; exact binomial test: p < 0.005). Tests of homogeneity revealed no evidence that the four trials differed in the relative number of predation events experienced by the three phenotypes (for all tests p > 0.9). Indeed, although models were split equally between H. melpomene (forest-edge) and H. cydno (closed-forest) habitats, we observed no difference in the number of ‘local’ versus ‘non-local’ parental phenotypes attacked. Exactly half of our 58 attacked models had missing bodies rather than beak marks. In these situations, it was impossible to determine whether a model was in fact attacked by a bird (or another visual predator). However, there is no evidence that the distribution of missing body data (4, 9 and 16 models for the H. melpomene, H. cydno and hybrid phenotypes, respectively) differs from those for beak marks (6, 9 and 14 models for the H. melpomene, H. cydno and hybrid phenotypes, respectively; G = 1.03, d.f. = 2, p = 0.30). If we remove the missing body data, the power of the experiment is greatly reduced: although the trend in the predicted direction remains, the numbers of ‘predation events’ experienced by the three phenotypes is no longer significantly different (G = 3.68, d.f. = 2, p = 0.16). However, a significantly greater proportion of hybrid phenotype models had beak marks than those with parental phenotype (14 of 444 recovered hybrid phenotype models compared with 15 of 913 parental phenotype models recovered; exact binomial test: p = 0.022). As such, our main prediction that models with hybrid phenotypes will be more frequently attacked is upheld even with this greatly reduced dataset.
Table 1.
Cumulative number of attacks observed during field trials for the three artificial butterfly phenotypes: Heliconius melpomene; Heliconius cydno; and their F1 hybrids. Note that although 120 artificial butterflies of each phenotype were placed in the forest for each trial not all were recovered.
| phenotype | cumulative ‘predation’ events |
total recovered | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| (a) closed-forest habitats | ||||
| first trial | ||||
| H. melpomene | 2 | 2 | 2 | 118 |
| H. cydno | 1 | 2 | 3 | 117 |
| F1 | 1 | 4 | 6 | 112 |
| second trial | ||||
| H. melpomene | 0 | 0 | 1 | 117 |
| H. cydno | 2 | 3 | 4 | 116 |
| F1 | 1 | 3 | 6 | 119 |
| (b) forest-edge habitats | ||||
| first trial | ||||
| H. melpomene | 0 | 1 | 3 | 120 |
| H. cydno | 1 | 1 | 2 | 117 |
| F1 | 2 | 2 | 4 | 118 |
| second trial | ||||
| H. melpomene | 2 | 3 | 4 | 111 |
| H. cydno | 6 | 9 | 9 | 110 |
| F1 | 5 | 7 | 14 | 111 |
Of the 48 focal birds included in our live butterfly, experiments 22 attacked at least one of the four butterflies during the 2 h trial period (table 2). Unfortunately, and despite considerable effort, only three of the responsive birds were from closed-canopy habitats (of a total of six caught in these habitats) biasing the responsive birds towards those that may not have encountered H. cydno (or its comimic, H. sapho). Nevertheless, responsive birds most often attacked the palatable control A. fatima first (in nine of 22 trials), closely followed by the non-mimetic F1 hybrid (seven times). Heliconius melpomene and H. cydno individuals were attacked first in two and four of the trials, respectively. Overall, there was a significant difference in the time that the four butterfly phenotypes survived before attack (figure 1c; F = 2.82, d.f. = 3, p < 0.05). In agreement with the hypothesis that disruptive selection acts on mimetic colour pattern, responsive birds distinguished between H. melpomene and hybrids (mean ± s.e. time before attack: H. melpomene = 80 ± 9 min; F1 hybrid = 57 ± 9 min); F = 6.86, d.f. = 1, p < 0.05), but there were no significant differences between H. cydno (mean ± s.e. time before attack = 65 ± 10 min) and the other butterflies.
Table 2.
Focal bird species and time survived before attack during 2 h enclosure trials for the four butterfly types: Heliconius melpomene; Heliconius cydno; their F1 hybrids; and the palatable butterfly Anartia fatima (control). Only data for trials in which the focal bird was responsive are included.
| focal bird species | time of attack from start of trial (min) |
|||
|---|---|---|---|---|
| H. melpomene | H. cydno | F1 | control | |
| (a) closed-forest habitat species | ||||
| Trogon rufus | 120 | 120 | 33 | 13 |
| Eucometis penicillata | 50 | 120 | 120 | 120 |
| Elaenia chiriquensis | 120 | 120 | 120 | 36 |
| (b) forest-edge habitat species | ||||
| Momotus momota | 120 | 35 | 53 | 39 |
| Megarhynchus pitangua | 29 | 82 | 24 | 59 |
| Myiarchus crinitus | 75 | 50 | 10 | 55 |
| 37 | 120 | 29 | 5 | |
| Mionectes olivaceus | 120 | 120 | 39 | 120 |
| Myiarchus panamensis | 26 | 10 | 15 | 0 |
| 110 | 50 | 110 | 30 | |
| 120 | 95 | 111 | 120 | |
| 95 | 95 | 85 | 95 | |
| 55 | 9 | 3 | 29 | |
| 55 | 3 | 60 | 51 | |
| 2 | 9 | 29 | 4 | |
| Onychorhynchus coronatus | 40 | 34 | 39 | 30 |
| Myiozetetes texensis | 120 | 120 | 62 | 97 |
| 85 | 20 | 15 | 120 | |
| Myiodynastes maculates | 120 | 120 | 120 | 93 |
| Tyrannus melancholicus | 120 | 33 | 120 | 32 |
| 30 | 49 | 32 | 17 | |
| 120 | 5 | 35 | 20 | |
| mean (± s.e.) | 80 (±9) | 65 (±10) | 57 (±9) | 53 (±9) |
4. Discussion
Despite the prominent role of butterfly mimicry in shaping evolutionary discussion for over 150 years, our study is the first to demonstrate reproductive isolation due to mimetic selection against hybrids between species. Previous work in Heliconius has investigated selection on translocated phenotypes [15,16], or experimentally manipulated wing patterns [32], and has primarily focussed on intraspecific variation. Here, we show that interspecific hybrid phenotypes were more frequently attacked in a field experiment with artificial butterflies. A similar effect was demonstrated in our caged bird trials with live butterflies where hybrids were attacked more readily than H. melpomene. However, in the cage experiments, there was no evidence that birds in this second experiment differentiated between hybrid and H. cydno individuals. This likely reflects the fact that all but three of our 22 responsive experimental birds were caught in forest-edge (H. melpomene preferred) habitats, and so few had perhaps learnt about the unpalatability of H. cydno (or its co-mimic, H. sapho). Thus, selection against parental phenotypes in the ‘wrong’ habitat may additionally be important (and has been demonstrated elsewhere between Heliconius colour-pattern races [16]). This was not detected in our field experiment with artificial butterflies, but this could be due to a lack of power resulting from the low frequency of attacks in our study. Overall, our experiments suggest that selection against migrants between the two habitats is weaker than that against hybrids, which was strong in both experiments.
Selection against hybrids not only presents a barrier to gene flow, thereby allowing other traits that contribute to isolation to accumulate, but can also promote assortative mating through reinforcement [33]. In Heliconius, reproductive character displacement, consistent with reinforcement, has been demonstrated, including between the taxa studied here. Males sampled from populations of H. melpomene in French Guiana, where H. cydno is absent, are more likely to court (and mate with) H. cydno females than individuals collected in Panama, where the two species are sympatric [11] (R. M. Merrill 2012, unpublished data). In these species, a number of other factors may additionally contribute to reduced hybrid fitness [11,14,22,34]. In particular, F1 females are sterile, following Haldane's rule [35]. However, in the parapatric taxa H. cydno and H. pachinus, between which there are no known intrinsic incompatibilities, males from close to the zone of contact are similarly more resistant to courting heterospecifics than individuals from more distant populations [36]. Extrinsic post-zygotic isolation in Heliconius, where hybrids have intermediate phenotypes that fare poorly in both parental habitats, is likely an important component of ecological speciation [3,4,37].
Theoretical work suggests that traits under disruptive selection also used as mating cues (i.e. magic traits) may be especially effective in promoting speciation because they will form strong genetic associations with loci underlying premating isolation, and that use these cues as markers [4,5,8,38]. Despite this, it is perhaps often overlooked that the existence of magic traits does not make speciation automatic or inevitable [9]. Rather, in most cases, they will contribute to the associations between different components of reproductive isolation necessary for speciation. In Heliconius, for example, colour pattern likely interacts with additional cues such as behaviour and pheromones during both mate recognition and predator avoidance. Shifts in colour pattern must be accompanied by corresponding mate preferences to cause substantial reproductive isolation. Using genetic crosses between H. melpomene and H. cydno, we have previously demonstrated that a locus of major effect underlying male preference for red patterns is physically associated with the locus responsible for the red forewing band [39]. It seems likely that this and other associations observed between colour-pattern elements and additional traits that contribute to reproductive isolation [39,40] (R. M. Merrill 2012, unpublished data) have further facilitated speciation in these butterflies.
The antagonism between selection and recombination may be overcome in a number of ways. For example, learning may facilitate speciation because mating traits can arise without genetic divergence [41], as will situations where the same allele becomes fixed in diverging populations (a ‘one-allele’ mechanism [7]). Pleiotropy, where distinct traits are controlled by the same alleles [9,42], and genetic linkage, where alleles controlling ecological and mating traits are tightly associated in the genome [7] may also facilitate speciation with gene flow. Nevertheless, the extent to which tightly linked loci can act like magic traits remains unknown [8]. A key point is that, however tight associations that rely on separate loci may be, they will be eroded with time, even if they affect the expression of the same gene; for example, linkage disequilibrium declines to a low level within 10 kb in the H. melpomene genome [43]. Magic traits themselves, where the trait under divergent selection and that influencing assortative mating are one-in-the-same, may be widespread, but are difficult to prove. In a recent review, Servedio et al. [8] describe 18 putative case studies where the existence of magic traits seems likely, but assert that for each of these more work is required. A significant empirical problem lies in distinguishing magic traits from tightly linked genes, when testing for both divergent selection and the effects of a trait on non-random mating. The amenability of colour patterns to experimental manipulation using artificial models, independent of other traits is a considerable advantage in this regard.
A few studies in other species reveal similar disruptive selection on other traits that influence non-random mating [8]. In Darwin's finches, for example, competition for food selects against intermediate beak size [44]; and beak size can affect song, which acts as a mating cue [45]. In sympatric stickleback morphs, traits that evolved in response to benthic and limnetic habitats are used as mating cues [46,47]. Nonetheless, direct evidence of disruptive selection against traits used as mating cues, independent of other differences between hybridizing taxa, is lacking. Using clay models, Noonan & Comeault [48] demonstrate that novel colour patterns of the polymorphic poison arrow frog, Dendrobates tinctorius, in French Guiana are more likely to be attacked. However, the fate of hybrids remains unclear and colour pattern is known only to act as a mating cue among populations of the Panamanian species Dendrobates pumilio [12]. As such, our data provide the first experimental evidence of disruptive ecological selection acting on a trait that is also used during mate recognition. This contributes to an emerging body of work supporting the biological plausibility of mechanisms hypothesized to facilitate speciation with gene flow [39,49–52].
Acknowledgements
We are very grateful to K. Marshall and P. J. Merrill for assistance in producing the artificial butterflies, and to T. Thurman, S. Montgomery, J. Mavárez and two anonymous referees for helpful comments on earlier versions of the manuscript. R.M.M. is supported by a Junior Research Fellowship at King's College, Cambridge, UK. M.S. was supported by a Biotechnology and Biological Sciences Research Council David Phillips Research Fellowship (BB/G022887/1). We are also grateful to the BBSRC, Leverhulme Trust, Grupo Santander, Clare College and the Hanne and Torkel Weis-Fogh Fund for funding; and to the Smithsonian Tropical Research Institute, and in particular W. O. McMillan, for logistical assistance.
References
- 1.Nosil P. 2012. Ecological speciation. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Schluter D. 2009. Evidence for ecological speciation and its alternative. Science 323, 737–741 10.1126/science.1160006 (doi:10.1126/science.1160006) [DOI] [PubMed] [Google Scholar]
- 3.McBride C. S., Singer M. C. 2010. Field studies reveal strong postmating isolation between ecologically divergent butterfly populations. PLoS Biol. 8, e1000529. 10.1371/journal.pbio.1000529 (doi:10.1371/journal.pbio.1000529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gavrilets S. 2004. Fitness landscapes and the origin of species. Princeton, NJ: Princeton University Press [Google Scholar]
- 5.Weissing F. J., Edelaar P., van Doorn G. S. 2011. Adaptive speciation theory: a conceptual review. Behav. Ecol. Sociobiol. 65, 461–480 10.1007/s00265-010-1125-7 (doi:10.1007/s00265-010-1125-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkpatrick M., Ravigné V. 2002. Speciation by natural and sexual selection: models and experiments. Am. Nat. 158, S22–S35 10.1086/338370 (doi:10.1086/338370) [DOI] [PubMed] [Google Scholar]
- 7.Felsenstein J. 1981. Skepticism towards Santa Rosalia, or why are there so few kinds of animals? Evolution 35, 124–138 10.2307/2407946 (doi:10.2307/2407946) [DOI] [PubMed] [Google Scholar]
- 8.Servedio M. R., Van Doorn G. S., Kopp M., Frame A. M., Nosil P. 2011. Magic traits in speciation: ‘magic’ but not rare? Trends Ecol. Evol. 26, 389–397 10.1016/j.tree.2011.04.005 (doi:10.1016/j.tree.2011.04.005) [DOI] [PubMed] [Google Scholar]
- 9.Smadja C. M., Butlin R. K. 2011. A framework for comparing processes of speciation in the presence of gene flow. Mol. Ecol. 20, 5123–5140 10.1111/j.1365-294X.2011.05350.x (doi:10.1111/j.1365-294X.2011.05350.x) [DOI] [PubMed] [Google Scholar]
- 10.Feulner P. G. D., Plath M., Engelmann J., Kirschbaum F., Tiedemann R. 2009. Electrifying love: electric fish use species-specific discharge for mate recognition. Biol. Lett. 5, 225–228 10.1098/rsbl.2008.0566 (doi:10.1098/rsbl.2008.0566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiggins C., Naisbit R., Coe R., Mallet J. 2001. Reproductive isolation caused by colour pattern mimicry. Nature 411, 302–305 10.1038/35077075 (doi:10.1038/35077075) [DOI] [PubMed] [Google Scholar]
- 12.Reynolds R. G., Fitzpatrick B. M. 2007. Assortative mating in poison-dart frogs based on an ecologically important trait. Evolution 61, 2253–2259 10.1111/j.1558-5646.2007.00174.x (doi:10.1111/j.1558-5646.2007.00174.x) [DOI] [PubMed] [Google Scholar]
- 13.Bates H. W. 1862. Contributions to an insect fauna of the Amazon valley (Lepidoptera: Heliconidae). Trans. Linn. Soc. 23, 495–566 10.1111/j.1096-3642.1860.tb00146.x (doi:10.1111/j.1096-3642.1860.tb00146.x) [DOI] [Google Scholar]
- 14.Jiggins C. D. 2008. Ecological speciation in mimetic butterflies. Bioscience 58, 541–548 10.1641/B580610 (doi:10.1641/B580610) [DOI] [Google Scholar]
- 15.Kapan D. D. 2001. Three-butterfly system provides a field test of müllerian mimicry. Nature 409, 338–340 10.1038/35053066 (doi:10.1038/35053066) [DOI] [PubMed] [Google Scholar]
- 16.Mallet J., Barton N. 1989. Strong natural selection in a warning color hybrid zone. Evolution 43, 421–431 10.2307/2409217 (doi:10.2307/2409217) [DOI] [PubMed] [Google Scholar]
- 17.Jiggins C. D., Estrada C., Rodrigues A. 2004. Mimicry and the evolution of premating isolation in Heliconius melpomene Linnaeus. J. Evol. Biol. 17, 680–691 10.1111/j.1420-9101.2004.00675.x (doi:10.1111/j.1420-9101.2004.00675.x) [DOI] [PubMed] [Google Scholar]
- 18.Kronforst M. R., Young L. G., Kapan D. D., McNeely C., O'Neill R. J., Gilbert L. E. 2006. Linkage of butterfly mate preference and wing color preference cue at the genomic location of wingless. Proc. Natl Acad. Sci. USA 103, 6575–6580 10.1073/pnas.0509685103 (doi:10.1073/pnas.0509685103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mavarez J., Salazar C. A., Bermingham E., Salcedo C., Jiggins C. D., Linares M. 2006. Speciation by hybridization in Heliconius butterflies. Nature 441, 868–871 10.1038/nature04738 (doi:10.1038/nature04738) [DOI] [PubMed] [Google Scholar]
- 20.Muñoz A. G., Salazar C., Castaño J., Jiggins C. D., Linares M. 2010. Multiple sources of reproductive isolation in a bimodal butterfly hybrid zone. J. Evol. Biol. 23, 1312–1320 10.1111/j.1420-9101.2010.02001.x (doi:10.1111/j.1420-9101.2010.02001.x) [DOI] [PubMed] [Google Scholar]
- 21.Estrada C., Jiggins C. D. 2002. Patterns of pollen feeding and habitat preference among Heliconius species. Ecol. Entomol. 27, 448–456 10.1046/j.1365-2311.2002.00434.x (doi:10.1046/j.1365-2311.2002.00434.x) [DOI] [Google Scholar]
- 22.Naisbit R. E., Jiggins C. D., Mallet J. 2001. Disruptive sexual selection against hybrids contributes to speciation between Heliconius cydno and Heliconius melpomene. Proc. R. Soc. Lond. B 268, 1849–1854 10.1098/rspb.2001.1753 (doi:10.1098/rspb.2001.1753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuthill I. C., Stevens M., Sheppard J., Maddocks T., Parraga C. A., Troscianko T. S. 2005. Disruptive coloration and background pattern matching. Nature 434, 72–74 10.1038/nature03312 (doi:10.1038/nature03312) [DOI] [PubMed] [Google Scholar]
- 24.Cuthill I. C. 2006. Color perception. In Bird coloration: mechanisms and measurement (eds Hill G. E., McGraw K. J.), pp. 3–40 Cambridge, MA: Harvard University Press [Google Scholar]
- 25.Endler J., Meike P. 2005. Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 86, 405–431 10.1111/j.1095-8312.2005.00540.x (doi:10.1111/j.1095-8312.2005.00540.x) [DOI] [Google Scholar]
- 26.Hart N. S., Partridge J., Cuthill I. 2000. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus, L.) and the blackbird (Turdus merula, L.). Comp. Physiol. A 186, 375–387 10.1007/s003590050437 (doi:10.1007/s003590050437) [DOI] [PubMed] [Google Scholar]
- 27.Langmore N. E. 2009. Are dark cuckoo eggs cryptic in host nests? Anim. Behav. 78, 461–468 10.1016/j.anbehav.2009.06.003 (doi:10.1016/j.anbehav.2009.06.003) [DOI] [Google Scholar]
- 28.Brodie E. D., III 1993. Differential avoidence of coral snake banded patterns by free-ranging avian predators in Costa Rica. Evolution 47, 227–235 10.2307/2410131 (doi:10.2307/2410131) [DOI] [PubMed] [Google Scholar]
- 29.Vignieri S. N., Larson J. G., Hoekstra H. E. 2010. The selective advantage of crypsis in mice. Evolution 64, 2153–2158 [DOI] [PubMed] [Google Scholar]
- 30.Valkonen J., Niskanen M., Björklund M., Mappes J. 2011. Disruptive or aposematism? Significance of dorsal zigzag pattern of European vipers. Evol. Ecol. 25, 1047–1063 10.1007/s10682-011-9463-0 (doi:10.1007/s10682-011-9463-0) [DOI] [Google Scholar]
- 31.Comeault A. A., Noonan B. P. 2011. Spatial variation in the fitness of divergent aposematic phenotypes of the poisen frog, Dendrobates tinctorius. J. Evol. Biol. 24, 1374–1379 10.1111/j.1420-9101.2011.02258.x (doi:10.1111/j.1420-9101.2011.02258.x) [DOI] [PubMed] [Google Scholar]
- 32.Benson W. 1972. Natural selection for Müllerian mimicry in Heliconius erato Costa Rica. Science 176, 936–939 10.1126/science.176.4037.936 (doi:10.1126/science.176.4037.936) [DOI] [PubMed] [Google Scholar]
- 33.Servedio M. R., Noor M. A. 2003. The role of reinforcement in speciation: theory and data. Annu. Rev. Ecol. Syst. 34, 339–364 10.1146/annurev.ecolsys.34.011802.132412 (doi:10.1146/annurev.ecolsys.34.011802.132412) [DOI] [Google Scholar]
- 34.Mallet J. 2006. What does Drosophila genetics tell us about speciation? Trends Ecol. Evol. 21, 386–393 10.1016/j.tree.2006.05.004 (doi:10.1016/j.tree.2006.05.004) [DOI] [PubMed] [Google Scholar]
- 35.Naisbit R. E., Jiggins C. D., Linares M., Salazar C., Mallet J. 2002. Hybrid sterility, Haldane's rule and speciation in Heliconius cydno and, H. melpomene. Genetics 161, 1517–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kronforst M. R., Young L. G., Gilbert L. E. 2007. Reinforcement of mate preference among hybridizing Heliconius butterflies. J. Evol. Biol. 20, 278–285 10.1111/j.1420-9101.2006.01198.x (doi:10.1111/j.1420-9101.2006.01198.x) [DOI] [PubMed] [Google Scholar]
- 37.Merrill R. M., Gompert Z., Dembeck L. M., Kronforst M. R., McMillan W. O., Jiggins C. D. 2011. Mate preference across the speciation continuum in a clade of mimetic butterflies. Evolution 65, 1489–1500 10.1111/j.1558-5646.2010.01216.x (doi:10.1111/j.1558-5646.2010.01216.x) [DOI] [PubMed] [Google Scholar]
- 38.Servedio M. R. 2009. The role of linkage disequilibrium in the evolution of premating isolation. Heredity 102, 51–56 10.1038/hdy.2008.98 (doi:10.1038/hdy.2008.98) [DOI] [PubMed] [Google Scholar]
- 39.Merrill R. M., Van Schooten B., Scott J. A., Jiggins C. D. 2011. Pervasive genetic associations between traits causing reproductive isolation in Heliconius butterflies. Proc. R. Soc. B 278, 511–518 10.1098/rspb.2010.1493 (doi:10.1098/rspb.2010.1493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naisbit R., Jiggins C., Mallet J. 2003. Mimicry: developmental genes that contribute to speciation. Evol. Dev. 5, 269–280 10.1046/j.1525-142X.2003.03034.x (doi:10.1046/j.1525-142X.2003.03034.x) [DOI] [PubMed] [Google Scholar]
- 41.Servedio M. R., Saether S. A., Saetre G.-P. 2009. Reinforcement and learning. Evol. Ecol. 23, 109–123 10.1007/s10682-007-9188-2 (doi:10.1007/s10682-007-9188-2) [DOI] [Google Scholar]
- 42.Maynard Smith J. 1966. Sympatric speciation. Am. Nat. 100, 637–650 10.1086/282457 (doi:10.1086/282457) [DOI] [Google Scholar]
- 43.Heliconius Genome Consortium 2012 Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487, 94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hendry A. P., Huber S. K., De Leon L. F., Herrel A., Podos J. 2009. Disruptive selection in a bimodal population of Darwin's finches. Proc. R. Soc. B 276, 753–759 10.1098/rspb.2008.1321 (doi:10.1098/rspb.2008.1321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Podos J. 2001. Correlated evolution of morphology and vocal signal structure in Darwin's finches. Nature 409, 185–188 10.1038/35051570 (doi:10.1038/35051570) [DOI] [PubMed] [Google Scholar]
- 46.Nagel L., Schuter D. 1998. Body size, natural selection, and speciation in sticklebacks. Evolution 52, 209–218 10.2307/2410936 (doi:10.2307/2410936) [DOI] [PubMed] [Google Scholar]
- 47.Boughman J. W., Rundle H., Schluter D. 2005. Parallel evolution of sexual isolation in sticklebacks. Evolution 59, 361–373 [PubMed] [Google Scholar]
- 48.Noonan B. P., Comeault A. A. 2009. The role of predator selection on polymorphic aposematic poison frogs. Biol. Lett. 5, 51–54 10.1098/rsbl.2008.0586 (doi:10.1098/rsbl.2008.0586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ortíz-Barrientos D., Noor M. A. F. 2005. Evidence for a one-allele assortative mating locus. Science 310, 1467. 10.1126/science.1121260 (doi:10.1126/science.1121260) [DOI] [PubMed] [Google Scholar]
- 50.Wiley C., Shaw K. L. 2010. Multiple genetic linkages between female preference and male signal in rapidly speciating Hawaiian crickets. Evolution 64, 2238–2245 [DOI] [PubMed] [Google Scholar]
- 51.Pryke S. R. 2010. Sex chromosome linkage of mate preference and color signal maintains assortative mating between interbreeding finch morphs. Evolution 64, 1301–1310 [DOI] [PubMed] [Google Scholar]
- 52.Saether S. A., et al. 2007. Sex chromosome-linked species recognition and evolution of reproductive isolation in flycatchers. Science 318, 95–97 10.1126/science.1141506 (doi:10.1126/science.1141506) [DOI] [PubMed] [Google Scholar]