Abstract
Specialism is widespread in nature, generating and maintaining diversity, but recent work has demonstrated that generalists can be equally fit as specialists in some shared environments. This no-cost generalism challenges the maxim that ‘the jack of all trades is the master of none’, and requires evolutionary genetic mechanisms explaining the existence of specialism and no-cost generalism, and the persistence of specialism in the face of selection for generalism. Examining three well-described mechanisms with respect to epistasis and pleiotropy indicates that sign (or antagonistic) pleiotropy without epistasis cannot explain no-cost generalism and that magnitude pleiotropy without epistasis (including directional selection and mutation accumulation) cannot explain the persistence of specialism. However, pleiotropy with epistasis can explain all. Furthermore, epistatic pleiotropy may allow past habitat use to influence future use of novel environments, thereby affecting disease emergence and populations' responses to habitat change.
Keywords: antagonistic pleiotropy, directional selection, mutation accumulation, sign pleiotropy, magnitude pleiotropy, epistatic pleiotropy
1. Introduction
The existence of specialists—organisms that are more fit in some environments than in others (box 1)—influences taxonomic diversity [3], geographical variability [4] and complex community dynamics [5]. Developing a better understanding of why specialists evolve and persist will therefore result in a better understanding of what gives rise to the tremendous variability that exists in nature.
Box 1. Terminology.
Specialists and generalists. All definitions of specialism involve differences in the degree to which the environments used by some organisms are restricted relative to other organisms [1]. Herein, a specialist is defined as any genotype with asymmetric absolute fitness across environments and a generalist is defined as a genotype with equal absolute fitness across environments. Thus, specialists have narrow niche-breadths, and generalists have broader ones. In figure 1, phenotypes 1, 2 and 4 are specialists on blue; 6, 8 and 9 are specialists on red; and 3, 5 and 7 are generalists. For ease of discussion of the simple two-locus, two-allele, two-environment examples presented here, this definition is absolute, but could be expanded to include differences in degrees of specialism versus generalism by the inclusion of more continuous variation in fitness across environments.
Figure 1.

Phenotypes in two environments. All phenotypes existing under a simplified scenario in which three levels of fitness are possible in each of two environments (designated red and blue). Generalists with equal fitness in the two environments lie on the marked diagonal.
Cost of generalism. Costs associated with the range of habitats an organism can use have been defined in a number of ways. Herein, I focus on a definition of cost of generalism consistent with the phrase ‘the jack of all trades is the master of none’. The cost experienced by a jack of all trades is a consequence of a trade-off between equalizing performance across tasks and becoming a ‘master’ at any one. Thus, whether or not a generalist experiences a cost of generalism is defined relative to the specialist(s) with which it could share an environment. If the specialist has higher fitness than the generalist in the specialist's more permissive environment, then the generalist suffers a cost of generalism in that environment. In figure 1, generalist 3 is a no-cost generalist relative to all specialists; generalist 5 bears a cost relative to 1 and 2 in the blue environment, and relative to 6 and 9 in the red environment, but is a no-cost generalist relative to specialists 4 and 8. Generalist 7 bears a cost relative to all specialists among the 9 phenotypes shown, in both environments. Note that this cost is not dependent on the actual contribution of the environment to the habitat of the generalist or specialist.
Types of pleiotropy. Analogously to the distinction between no epistasis, magnitude epistasis and sign epistasis [2], the dependence of the effect of an allele on the physical environment can be classified as non-pleiotropy, magnitude pleiotropy or sign pleiotropy (traditionally called antagonistic pleiotropy). When there is no pleiotropy at a locus, a change in allele at that locus affects fitness equally across environments. In figure 1, such an allele change would move the phenotype diagonally from lower left to upper right (e.g. 4 versus 2, 7 versus 5). Magnitude pleiotropy occurs when a change in an allele at a locus increases or decreases fitness to different extents depending on the environment (e.g. 7 versus 2 in figure 1). Sign pleiotropy results in the sign of an allele's effect on fitness depending on the environment (e.g. 8 versus 4, 9 versus 5 in figure 1). Here I classify conditionally beneficial or conditionally deleterious alleles that are completely neutral in the other environment (e.g. 7 versus 4 or 7 versus 8 in figure 1) as cases of magnitude pleiotropy, to emphasize that the distinction between magnitude and sign involves the reversal of sign, resulting in a change in which allele is favoured by selection depending on the environment.
One major factor influencing whether specialism or generalism evolves is the diversity of environments that compose a population's habitat. Only two types of habitats are required for the evolution of both specialist and generalist populations. Habitats with one constant environment usually give rise to specialists [6–9], though this type of habitat can also give rise to generalists [10]. Temporally variable habitats in which the environment switches or the population migrates between two or more environments usually give rise to generalists. This review describes evolutionary genetic mechanisms that can explain the observed associations between specialism versus generalism and living in these two types of habitats.
MacArthur summarized an influential hypothesis for a mechanism explaining specialization in the phrase ‘the jack of all trades is the master of none’ [6]. The core of this idea is that generalists bear some cost in each environment they can use, such that a specialist on a given environment will always be able to outcompete a generalist sharing that environment (box 1). As a consequence, under equilibrium conditions, there would be no single genotype that has highest fitness in all environments [11–15], and habitat-use evolution would involve evolution of an unavoidable trade-off, in which increased fitness in one environment requires a loss in fitness in another [16]. However, there is accumulating evidence from experimental evolution studies that such a trade-off can in fact be avoidable. In these studies, generalist populations bearing no cost of generalism founded from the same common ancestor and evolving for the same number of generations as specialist populations achieve fitness equal to or higher than the specialists in their shared environment [11,17–21]. Taken together, this work shows that in some circumstances, the jack of all trades can be the master of all. If universal trade-offs do not exist, what are the possible evolutionary genetic mechanisms that can explain the existence of specialists?
The possible mechanisms explaining specialism described here differ in the type of pleiotropy for fitness in different environments involved, and the presence or absence of epistasis. Pleiotropy and epistasis have strong parallels because in both, the effect of an allele depends on its context: the context of the environment in pleiotropy for performance across environments and the context of the genetic background in epistasis. Weinreich et al. [2] have defined two types of epistasis (magnitude and sign) that have a clear parallel with respect to types of pleiotropy. For consistency, I will use this categorization for pleiotropy (box 1). In particular, I favour the term sign pleiotropy over antagonistic pleiotropy, which is traditionally used to describe this phenomenon, or negative pleiotropy, which has also been used in this context [22]. This will avoid potential confusion arising from the fact that antagonistic epistasis and negative epistasis refer to phenomena that are distinct from sign epistasis.
Evolutionary genetic mechanisms that can cause specialism must be able to explain three phenomena: (i) the evolution of specialists in some habitats, such as one-environment habitats; (ii) the evolution of generalists that bear no cost of generalism in other habitats, such as temporally variable habitats; and (iii) evolutionary constraint prohibiting escape from specialism to no-cost generalism when habitat change favours the latter. §2 describes why in the absence of epistasis, sign pleiotropy for use of different environments (box 1) can explain the evolution of specialism but not of no-cost generalism. §3 presents two well-described mechanisms involving magnitude pleiotropy without epistasis: these are directional selection and mutation accumulation. These two can both explain evolution of specialism and no-cost generalism, but neither can prevent escape from specialism on its own. §4 explores how epistatic pleiotropy for use of different environments can give rise to both specialists and no-cost generalists, and can also cause a specialist population to be unable to escape to no-cost generalism.
This review focuses on examples of specialism versus generalism from viruses, with an emphasis on what has been learned from studies employing experimental evolution approaches, though the general principles will apply across taxa and contexts. Viruses, especially RNA viruses, are less probable than other taxa to be mutation-limited. Because viruses are fast-evolving and have small genomes, we can identify all genetic changes causing changes in host breadth in an experimental evolution, and thereby shed light on the relationship between ecological conditions and genetic architecture of adaptation [23,24]. In addition, from a practical perspective, identifying factors that contribute to specialism in pathogens could be useful for predicting disease emergence [24,25].
2. Non-epistastic sign pleiotropy
(a). Non-epistatic sign pleiotropy causes obligatory cost of generalism
Alleles involved in sign pleiotropy affect two traits in opposite ways: in the context of evolution of habitat use, they increase fitness in one environment but decrease it in another. Changes at loci displaying sign pleiotropy in their effects on fitness in different environments can drive the evolution of specialization because generalists bear a fitness cost relative to related specialists [8,11,26–29]. For example, in figure 2a, the generalist genotype a suffers a cost in the red environment relative to genotype A, but outperforms the specialist in the blue environment—the jack of all trades is the master of none. As a result, in a habitat made up of only the red environment, the specialist A is favoured.
Figure 2.
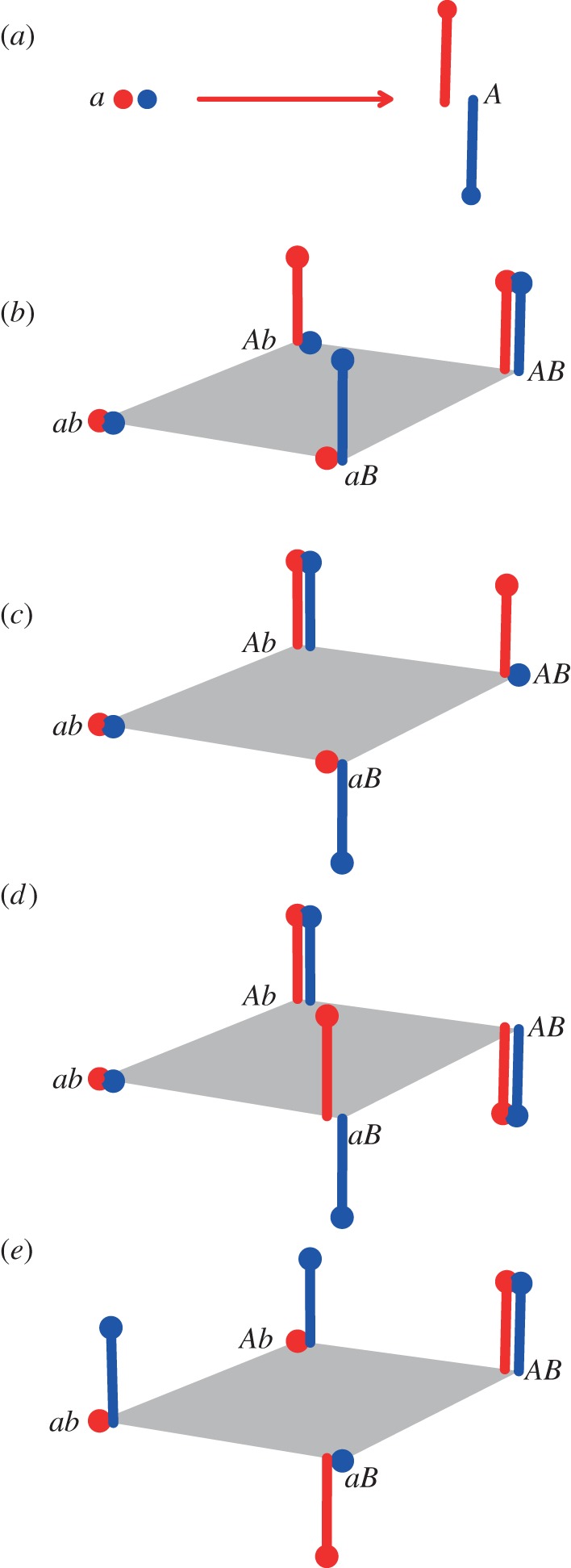
Genotype–phenotype maps. Fitness in two environments is depicted on the vertical axis (in red and blue) for (a) one-locus or (b–e) two-locus, two-allele genotype sequence spaces. (a) Under sign pleiotropy involving only one locus, cost-bearing generalist a is favoured in the blue environment or when exposure to the red and blue environments alternates, and A is favoured in the red environment. Two types of non-epistatic magnitude pleiotropy are shown. (b) Under directional selection, the B allele is favoured in the blue environment or alternating environments, the A allele is favoured under conditions of the red or alternating environments. (c) Under mutation accumulation, the A allele is favoured in both environments and in alternating environments, and in the red environment, the conditionally deleterious allele B can become fixed owing to drift or hitchhiking. (d) Epistatic pleiotropy with reciprocal sign epistasis. Here, the transition from specialism (aB) to no-cost generalism (Ab) requires a transient loss of fitness in the red environment and is therefore disfavoured in the red or alternating environments. (e) Epistatic pleiotropy facilitating escape from specialism (ab) to no-cost generalism (AB), via compensatory mutation for a deleterious mutation (aB), or via acquisition of cryptic variation (Ab).
Sign pleiotropy for performance across multiple environments can occur either in the absence or presence of epistasis, but only sign pleiotropy without epistasis always results in an obligatory cost to generalism. For a cost to be obligatory, all potential evolutionary paths that a generalist might take must lead to lower fitness than the specialist in the specialist's permissive environment. In addition, the cost of generalism must persist even when the population reaches an evolutionary equilibrium and all possible beneficial mutations with respect to that population's habitat are at a high frequency. In the presence of epistasis for differential fitness across environments (epistatic sign pleiotropy; figure 2d; §3c), a change at a second locus can alleviate the sign pleiotropy associated with the focal locus. By contrast, under non-epistatic sign pleiotropy, the presence of the cost of generalism associated with an allele at the focal locus is independent of genetic changes at other loci, and is therefore unavoidable and inescapable (figure 2a). Thus, non-epistatic sign pleiotropy can explain the evolution of specialism, but not the evolution of no-cost generalism.
Although a number of virus studies have identified alleles whose effects display sign pleiotropy with respect to performance in different hosts [14,30,31], most of these involve alleles that have not been tested in multiple genetic backgrounds [32]. The few studies that have addressed the degree to which pleiotropic alleles are also epistatic have shown that dependence on genetic background is common [32–34]. Thus, it is possible that even in the cases where sign pleiotropy involving host use has been demonstrated, this pleiotropy may be epistatic, and therefore may not actually generate an obligatory cost to generalism.
Despite its theoretical importance in explaining specialism, non-epistatic sign pleiotropy as a driver of habitat-use evolution is poorly supported. There are growing numbers of examples in which trade-offs do not evolve, and no cost to generalism exists [16–21]. It has been argued that populations far from equilibrium will fail to display a cost of generalism until variation at all non-pleiotropic and magnitude-pleiotropic loci has fixed [35], and that this could explain the absence of phenotypic costs of generalism in natural populations. However, this sort of non-equilibrium cannot explain the lack of trade-off demonstrated in the examples cited earlier, because all of them involve closely related populations that differ from one another at only a handful of loci.
(b). Evolutionary consequences of non-epistatic sign pleiotropy for escape from specialism
Non-epistatic sign pleiotropy can allow the long-term success of specialists because it does not allow escape from specialism to no-cost generalism. Specialists experience selection for greater generalism when the proportion of their habitat composed of their permissive environment changes such that their geometric mean fitness across environments is lower than that of a potential generalist. Alternation of environments, for example, due to changes in availability of environments or migration among them, can cause such selection. Under non-epistatic sign pleiotropy, the specialist may improve fitness in another environment by fixing an alternate allele at the locus in question, but only at the cost of loss of fitness in its original most permissive environment. This situation is represented in figure 2a by a transition from specialist A to generalist a.
In fast-evolving organisms such as viruses, changes in the host environment may result in rapid switching between alleles showing non-epistatic sign pleiotropy. There is strong evidence for host shifts involving single pleiotropic loci in viruses, but not for allele switching at loci involved in non-epistatic sign pleiotropy. For example, the host shift of a parvovirus from cats to dogs [36], and the expansion of chickungunya virus into a previously unused vector, Aedes albopictus [37], were each achieved through a change at a single locus, but in both cases the ancestral allele bears a cost in the new host, while the new allele is beneficial in the new host/vector and bears no cost in the original host/vector, and is therefore a no-cost generalist. A similar asymmetry in effects of alleles at a single locus was found in phage populations evolving in changing environments [13].
3. Non-epistatic magnitude pleiotropy
Two well-described evolutionary genetic mechanisms arise through magnitude pleiotropy for use of different environments without epistasis: directional selection and mutation accumulation. These can both produce specialists but can also result in the evolution of cost-free generalism (figure 2b,c).
(a). Evolution of specialism and no-cost generalism under directional selection
Under directional selection, sequential selective sweeps move a population towards an optimal phenotype. Alleles at some loci are conditionally beneficial (i.e. there is magnitude pleiotropy), but there is no sign pleiotropy and no epistasis for use of different environments. Under directional selection, habitat-use adaptation is considered a multi-locus trait and the number of possible beneficial mutations a population has acquired determines fitness in a given environment [38–40]. However, a simplified scenario in which two conditionally beneficial mutations each contribute to fitness in different environments is sufficient to generate specialists (figure 2b, genotypes Ab and aB), generalists bearing cost (ab) and no-cost generalists (AB).
Considering for clarity a multi-locus scenario with two environments, under directional selection specialism arises because once a more specialized sub-population arises for any reason, it will increase in fitness in its one environment more rapidly than would a generalist subpopulation that must be able to use both environments equally well. This is because a greater proportion of the specialist's adaptive response is to its more permissive environment, while the environment-specific beneficial alleles being fixed by the generalists are distributed across multiple environments [38,40]. Such specialism can arise either owing to limited access to different environments, or to habitat choice, and this difference in exposure to environments need only be transitory [41]. Because selection on alleles improving fitness on the permissive environment will on average be stronger, they will fix first, conferring higher fitness in one environment than in the other. This starts the adaptive walk towards specialism.
Temporal environmental variability can cause directional selection for increased performance in multiple environments to result in the evolution of generalists. However, if we consider the rate of fitness increase in populations evolving in parallel from the same common ancestor, but experiencing different habitats, we find that generalists and specialists increase in fitness in their shared environment at different rates. Under directional selection, generalist populations accumulate mutations that are beneficial in the permissive environment of a specialist more slowly than does the specialist because selection in the shared environment is diluted by the time spent in other environments (figure 3a,c). As a result, generalists may display a transitory cost of generalism relative to specialists because they have fixed a smaller proportion of the possible beneficial mutations in each of their many environments than the specialist has in its one environment. It has therefore been suggested that the failure to observe differences in rates of fitness gains in generalist and specialist virus populations sharing a common ancestor indicates that directional selection is not the primary force driving evolution of virus host range [21]. However, over time a specialist will reach equilibrium when all possible beneficial mutations in its environment are fixed, and the generalist will continue to improve across all environments. Habitat-use evolution by directional selection will therefore generate cost-free generalists as adaptation to the broad set of environments progresses towards the maximum number of beneficial alleles for all environments (e.g. figure 2b, AB genotype; figure 3a).
Figure 3.

Evolution from a common ancestor. (a,b) Trajectories of fitness in the blue environment for specialist populations evolving in the blue environment (blue lines) and generalist populations evolving in alternating environments (purple lines) under (a) directional selection and (b) mutation accumulation. (c,d) Genomes of replicate independently evolving specialists (blue lines) and generalists (purple lines) at time t as indicated in (a,b), under (c) directional selection and (d) mutation accumulation. Mutations conferring benefit in blue, red or both environments depicted with blue, red and purple vertical lines, respectively. Conditionally deleterious alleles reducing fitness in red depicted with red crosses. Vertical lines occurring in the same location in the genomes indicate parallel substitutions in independently evolving populations.
(b). Evolution of specialism and no-cost generalism under mutation accumulation
The process of specialization through mutation accumulation involves magnitude pleiotropy through conditionally deleterious mutations and no epistasis for loci affecting adaptation to different environments. It occurs when populations experiencing only one or a few environments fix mutations that are deleterious in environments to which the population has not been exposed, either through genetic drift or through hitchhiking. Unlike under directional selection, under mutation accumulation all adaptive changes at habitat-use loci are universally beneficial [39,40,42]. In figure 2c, which depicts a simplified case of mutation accumulation, the cost-bearing generalist is the genotype ab. Populations that acquire the A allele, for example through selection in the red environment, increase fitness in both environments; here Ab is a no-cost generalist. However, the acquisition of the B allele, through drift or hitchhiking in the absence of purifying selection against it, reduces fitness relative to genotype Ab in the blue environment, and genotypes aB and AB are specialists in the red environment.
Under mutation accumulation, cost-free generalists can arise in two ways. First they arise in populations exposed to temporally variable habitats, when purifying selection can act to purge environment-specific deleterious mutations. Second, they arise in populations evolving in one or a few environments when conditionally deleterious mutations fail to arise stochastically [10]. Unlike under directional selection, where generalists have a slower sojourn time to high fitness in the environment shared with the specialist [38] (figure 3a), under mutation accumulation there need not be a difference in the rate of fitness gains in the shared environment for parallel evolving populations of specialists and generalists because the adaptive alleles being fixed in the two types of populations are the same (figure 3b,d).
There is both indirect and direct evidence of specialization by mutation accumulation in the context of experimental evolution. In phage, alleles derived in mutation accumulation experiments have been shown to conditionally affect fitness [43]. This indicates that some random mutations arising in simple, one-environment habitats have deleterious effects in unselected environments, as would be necessary to generate phenotypic trade-off via this mechanism. Specialism via mutation accumulation has been documented directly in digital organisms, where specialists suffered fitness declines owing to the acquisition of new alleles that are neutral in the selected environment and deleterious in an unselected environment [44].
(c). Directional selection and mutation accumulation can allow escape to no-cost generalism
While mutation accumulation is often invoked as the alternative to sign pleiotropy in explaining phenotypic costs of generalism across environments [44–48], directional selection and mutation accumulation cause some similar patterns of genetic changes and similar consequences for habitat-use evolution. Because of the absence of a true genetically based cost of environment-specific increases in fitness for the specialist, under both these mechanisms specialists selected for expansion of the range of environments used can improve in a new environment while maintaining high fitness in the original. Directional selection specialists can acquire additional conditionally beneficial mutations that increase their fitness in a new environment, and mutation accumulation specialists can achieve higher generalism simply by purging conditionally deleterious alleles.
Nevertheless, transient specialism arising by these mechanisms can become permanent. If alleles that confer preferential use of one environment arise in a population with variation at loci affecting performance in that environment, the development of genetic linkage between these two types of loci will be favoured [39,40]. Such linkage would decrease a population's ability to respond evolutionarily to opportunities for access to new environments. As a result, both directional selection and mutation accumulation can be important contributors to long-term specialism.
(d). Comparing evolution under directional selection and mutation accumulation
Two similarities between habitat-use evolution under directional selection and mutation accumulation become apparent when considering the evolution of populations founded from a common ancestor evolving in parallel, but differing in habitat. First, under both directional selection and mutation accumulation, parallel populations of specialists evolving in one environment and generalists evolving in temporally variable environments can fix parallel underlying beneficial habitat-use alleles (figure 3c, blue alleles; figure 3d, purple alleles). This occurs because under both directional selection and mutation accumulation, the mutations that confer increased fitness in one environment are not selected against in the other environment because these mutations involve magnitude pleiotropy, rather than sign pleiotropy. Second, if one of these specialist populations experiences a change in its habitat resulting in selection for use of the second environment, it can increase in fitness in the second environment while retaining adaptive alleles acquired in its specialist phase. This ability to retain high fitness in an environment to which a specialist was previously well adapted while expanding into another is the reason that non-epistatic magnitude pleiotropy does not constrain escape from specialism to no-cost generalism.
Directional selection and mutation accumulation differ in pattern of genetic changes expected to arise in specialist populations escaping towards greater generalism (for example, due to exposure to a new or additional environment), when compared with specialist populations evolving from a common ancestor but remaining in a one-environment habitat. Under directional selection, populations shifting from specialism to greater generalism will acquire new parallel alleles conferring a benefit in the new environments (figure 2b, genotypes Ab or aB to genotype AB). These alleles will not be shared with specialists remaining in a one-environment habitat (figure 3c). By contrast, under mutation accumulation, specialist populations escaping towards generalism will revert at the sites of deleterious mutations (figure 2c, genotype AB to genotype Ab), rather than gain new parallel adaptive alleles (figure 3d). This difference in the nature of the genetic changes required to achieve greater generalism is the reason that these two processes differ in the relative rate of fitness increase in the shared environment of parallel evolving specialist and generalist populations (figure 3a,b).
4. Epistatic pleiotropy
(a). Evolution of specialism and no-cost generalism under epistatic pleiotropy
Epistatic pleiotropy occurs when genetic backgrounds differ in how the effect of an allele depends on the environment. Under epistatic pleiotropy, populations may achieve either specialism or no-cost generalism, depending on the habitat in which they evolve. Importantly, no-cost generalism can evolve despite the existence of true genetic trade-offs in genotype space.
In a simple example (figure 2d), imagine an evolving population beginning at the cost-bearing generalist genotype ab. If allele B arises under selection in the red environment, aB would be favoured, resulting in specialization. By contrast, if A arises in the ab background Ab is favoured regardless of which environment the population experiences (red, blue or both). This Ab genotype experiences no cost of the new allele in either environment—it is a cost-free generalist. In this example, specialism will evolve under selection in the red environment, provided mutation b to B arises before a to A does.
In this example of epistatic pleiotropy, cost-free generalists can arise in three ways, depending on the environments to which populations are exposed, and on the order in which mutations arise [10]. First, in populations beginning at genotype ab in a temporally variable habitat, purifying selection will purge mutations to aB, maintaining ab in the population until the cost-free generalist Ab arises and can become fixed. Second, cost-free generalists can evolve by chance in ab populations experiencing only the red environment, but in which A is the first beneficial mutation to sweep through the population. Finally, ab populations experiencing the blue environment will become cost-free generalists through an indirect response to selection, because adaptation to this environment (via fixation of genotype Ab) comes with a pleiotropic benefit on the unselected red environment.
This simple example shows two important points about epistatic pleiotropy. First, unlike either sign or magnitude pleiotropy in the absence of epistasis, it allows for the evolution of either specialists or no-cost generalists depending on the population's habitat. Second, when the epistasis is in the form of reciprocal sign epistasis, the ruggedness of the fitness landscape inhibits a population's ability to escape from specialism to no-cost generalism [49,50]. In figure 2d, reciprocal sign epistasis occurs in the red environment, in which the specialist aB and no-cost generalist Ab each occupy a fitness peak. The specialist cannot evolve towards no-cost generalism without entering a fitness valley. This ‘brake’ slowing the evolution of generalism is in contrast to non-epistatic magnitude pleiotropy, where a single mutation can carry a specialist to higher fitness in its previously less permissive environment, resulting in no-cost generalism (Ab or aB to AB in figure 2b; AB to Ab in figure 2c).
In §3, the difference in the pattern of expected fitness increases under directional selection and mutation accumulation were described (figure 3). This difference suggests that support for one or the other of these mechanisms of specialization might be obtained by comparing fitness trajectories of experimentally evolved specialists from one environment and generalists from temporally fluctuating environments [11,17–21]. However, epistatic pleiotropy can result in fitness trajectories that could be confused either with those generated by directional selection or by mutation accumulation, depending on conditions.
Like directional selection, epistatic pleiotropy can cause generalist populations evolving in a temporally variable habitat to have slower rates of fitness increase than specialist populations founded from a common ancestor and evolving in a single environment (figure 3a). Under epistatic pleiotropy, these dissimilar fitness trajectories arise when pleiotropic mutations cause greater fitness gains than non-pleiotropic mutations, or when a majority of the available beneficial mutations are pleiotropic. Specialism arises because of the benefit conferred by these pleiotropic mutations, and generalism because a fluctuating environment purges all but the non-pleiotropic mutations. Like mutation accumulation, epistatic pleiotropy can also cause evolving generalists and specialists to have the same rates of fitness increase (figure 3b). When this pattern occurs under epistatic pleiotropy, mutations that are beneficial in the focal environment do not differ in the magnitude of the benefit conferred depending on whether they are non-pleiotropic, magnitude pleiotropic or sign pleiotropic, and specialization results from random factors such as the order and timing of mutations.
There are a number of lines of empirical evidence for the potential importance of epistatic pleiotropy in the evolution of habitat-use diversity. Work in bacterial systems has shown that epistasis among loci involved in adaptive evolution can be extremely common [51–53], and analogous studies in multiple environments are needed. When epistatic loci that also have pleiotropic effects are explicitly sought, they are found to be common [32–34]. Evidence for the importance of epistatic pleiotropy also comes from studies demonstrating the role of compensatory mutations in correcting environment-dependent costs [13,54–56]. The ability of cryptic variation to facilitate adaptation after an environmental change also demonstrates evolution involving epistatic pleiotropy [57]. Furthermore, epistatic pleiotropy causing environment-specific fitness variability has been shown to arise in evolving populations [13,32,33,58].
(b). Epistatic pleiotropy can inhibit escape from specialism to no-cost generalism
When selection for use of the original environment persists, specialist populations that arise through epistatic pleiotropy can be less able to escape specialism and achieve no-cost generalism than specialists arising through directional selection or mutation accumulation. In the simple example in figure 2d, a population that has specialized (genotype aB) is now one additional mutational step away from higher fitness in the blue environment than is the ancestor, ab. In a more complex fitness landscape involving pleiotropy with reciprocal sign epistasis at more loci, evolution in a single environment could result in an adaptive walk towards an environment-specific fitness peak involving a number of loci. Upon exposure to a broader range of environments, such a population could find itself many mutational steps through an adaptive valley away from achieving a generalist phenotype. By contrast, for specialists arising through directional selection and mutation accumulation, the need to maintain high fitness on the original environment does not create a ‘brake’ on improvement in the new environment (e.g. figure 2b, Ab or aB to AB, and figure 2c, AB to Ab). Still, specialist populations constrained through epistatic pleiotropy differ from those evolved under non-epistatic sign pleiotropy, because of the existence of the possibility of escape to no-cost generalism via fixation of multiple mutations.
Notably, epistatic pleiotropy for use of different environments does not always slow escape from specialism, because it does not always result in a fitness valley between the specialist and the no-cost generalist. In fact, epistasis and epistatic pleiotropy have been shown to sometimes speed adaptation, including adaptation towards use of more environments. Escape from specialism to no-cost generalism can be facilitated by epistatic pleiotropy during recovery from mutation accumulation caused by genetic drift, where compensatory mutations are more common than reversions [59] (figure 2e, blue specialist ab to generalist AB via aB), or when cryptic variation speeds adaptation after an environmental change [57] (figure 2e, blue specialist ab to generalist AB via Ab).
(c). Epistatic pleiotropy may facilitate response to new environments
Epistatic pleiotropy may be able to help generalist populations arising in variable environments to use novel environments, and even to respond to new environments evolutionarily. The first of these phenomena, an increased ability to use novel environments, arises when a generalist population evolves environmental robustness. Any population evolving towards greater generalism will improve and/or equalize fitness in the environments to which it is exposed through fixation of alleles with pleiotropic effects, and therefore experience changes in the correlation among fitnesses in different environments. However, the presence of epistasis affecting pleiotropy allows an additional kind of change: the correlation in performance across multiple environments can itself respond to selection, rather than changing only as a consequence of response to selection for performance in multiple environments. This is analogous to how epistatic pleiotropy allows trait modularity to evolve in response to selection, rather than changing only as a consequence of evolution of the individual component traits [60,61]. Evolution of environmental robustness is supported by the observation that vesicular stomatitis virus populations selected for greater generalism replicate better than do specialist populations on novel hosts never yet experienced by either type of population [10].
It is also possible that greater environmental robustness, such as what could potentially arise in evolving generalist populations under epistatic pleiotropy, could give rise to a greater ability to respond evolutionarily to challenges experienced in a new environment. The evolution of environmental robustness may give rise to greater genetic robustness, as has been shown to be the case in models of RNA folding [61,62]. High but not complete genetic robustness can allow populations to explore a broader area of genotype space than can less robust ones in that more single mutations will be neutral, allowing for exploration of more potentially beneficial epistatic double and triple mutants [63–65].
There is some support for the hypothesis that greater environmental robustness causes greater genetic robustness, and therefore greater evolvability. Studies in phage have shown that evolution of greater thermotolerance resulted in greater genetic robustness [66], and vice versa [67]. It has, however, been noted in these cases that the correlation may be due to a direct effect of thermotolerance on genetic robustness rather than an indirect effect of selection for environmental robustness [65]. Also, models of gene regulatory networks showed a correlation between environmental variability and evolvability, where alternating environments caused evolution towards an area in the fitness landscape where most mutations were neutral and the population had access to a small set of beneficial mutations [68].
5. Conclusion
The traditional notion that ‘the jack of all trades is the master of none’ implies unavoidable fitness trade-offs from changing environments consistent with sign pleiotropy without epistasis. But accumulating examples of no-cost generalism call for new explanations. Directional selection and mutation accumulation, which arise from magnitude pleiotropy without epistasis, can account for no-cost generalism. However, because they allow expansion into new environments without disruption of the alleles conferring high fitness in the old, neither mechanism explains the persistence of specialism. Pleiotropy with epistasis, however, can create rugged fitness landscapes, which can lead to either specialists or no-cost generalists, and also impede escape from specialism to no-cost generalism. To clarify the prevalence of these mechanisms, new studies should explicitly address the degree to which pleiotropic alleles are also epistatic. Past habitat use may also affect both a population's ability to immediately use and to adapt to novel environments. This phenomenon is of particular potential importance, because it could elucidate disease emergence and, more generally, population responses to habitat change.
Acknowledgements
I thank Tom Hundley, Ben Kerr, Paul Turner and Dan Weinreich for stimulating conversations and comments, two anonymous reviewers for constructive comments, and Tom Hundley and Lindsey Willett for assistance with figure preparation. This work was supported by NSF DEB-0950361.
References
- 1.Devictor V., Clavel J., Julliard R., Lavergne S., Mouillot D., Thuiller W., Venail P., Villeger S., Mouquet N. 2010. Defining and measuring ecological specialization. J. Appl. Ecol. 47, 15–25 10.1111/j.1365-2664.2009.01744.x (doi:10.1111/j.1365-2664.2009.01744.x) [DOI] [Google Scholar]
- 2.Weinreich D. M., Watson R. A., Chao L. 2005. Perspective: sign epistasis and genetic constraint on evolutionary trajectories. Evolution 59, 1165–1174 10.1111/j.0014-3820.2005.tb01768.x (doi:10.1111/j.0014-3820.2005.tb01768.x) [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson G. E. 1959. Homage to Santa Rosalia, or why are there so many kinds of animals? Am. Nat. 93, 145–159 10.1086/282070 (doi:10.1086/282070) [DOI] [Google Scholar]
- 4.Thompson J. N. 1994. The coevolutionary process. Chicago, IL: University of Chicago Press [Google Scholar]
- 5.Pandit S. N., Kolasa J., Cottenie K. 2009. Contrasts between habitat generalists and specialists: an empirical extension to the basic metacommunity framework. Ecology 90, 2253–2262 (doi:10.1890/08–0851.1) [DOI] [PubMed] [Google Scholar]
- 6.MacArthur R. H. 1972. Geographical ecology: patterns in the distribution of species. New York, NY: Harper & Row [Google Scholar]
- 7.Roughgarden J. 1972. Evolution of niche width. Am. Nat. 106, 683–718 10.1086/282807 (doi:10.1086/282807) [DOI] [Google Scholar]
- 8.Futuyma D. J., Moreno G. 1988. The evolution of ecological specialization. Annu. Rev. Ecol. Syst. 19, 207–233 10.1146/annurev.ecolsys.19.1.207 (doi:10.1146/annurev.ecolsys.19.1.207) [DOI] [Google Scholar]
- 9.Kassen R. 2002. The experimental evolution of specialists, generalists, and the maintenance of diversity. J. Evol. Biol. 15, 173–190 (doi:10.1046/j.1420–9101.2002.00377.x) [DOI] [Google Scholar]
- 10.Turner P. E., Morales N. M., Alto B. W., Remold S. K. 2010. Role of evolved host breadth in the initial emergence of an RNA virus. Evolution 64, 3273–3286 10.1111/j.1558-5646.2010.01051 (doi:10.1111/j.1558-5646.2010.01051) [DOI] [PubMed] [Google Scholar]
- 11.Elena S. F., Agudelo-Romero P., Lalic J. 2009. The evolution of viruses in multi-host fitness landscapes. Open Virol. J. 2009, 1–6 10.2174/1874357900903010001 (doi:10.2174/1874357900903010001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffey L. L., Vasilakis N., Brault A. C., Powers A. M., Tripet F., Weaver S. C. 2008. Arbovirus evolution in vivo is constrained by host alternation. Proc. Natl Acad. Sci. USA 105, 6970–6975 10.1073/pnas.0712130105 (doi:10.1073/pnas.0712130105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crill W. D., Wichman H. A., Bull J. J. 2000. Evolutionary reversals during viral adaptation to alternating hosts. Genetics 154, 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffy S., Turner P. E., Burch C. L. 2006. Pleiotropic costs of niche expansion in the RNA bacteriophage Φ6. Genetics 172, 751–757 10.1534/genetics.105.051136 (doi:10.1534/genetics.105.051136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agudelo-Romero P., de la Iglesia F., Elena S. F. 2008. The pleiotropic cost of host-specialization in Tobacco etch potyvirus. Infect. Genet. Evol. 8, 806–814 10.1016/j.meegid.2008.07.010 (doi:10.1016/j.meegid.2008.07.010) [DOI] [PubMed] [Google Scholar]
- 16.Smith-Tsurkan S. D., Wilke C. O., Novella I. S. 2010. Incongruent fitness landscapes, not tradeoffs, dominate the adaptation of vesicular stomatitis virus to novel host types. J. Gen. Virol. 91, 1484–1493 10.1099/vir.0.017855-0 (doi:10.1099/vir.0.017855-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novella I. S., Hershey C. L., Escarmis C., Domingo E., Holland J. J. 1999. Lack of evolutionary stasis during alternating replication of an arbovirus in insect and mammalian cells. J. Mol. Biol. 287, 459–465 10.1006/jmbi.1999.2635 (doi:10.1006/jmbi.1999.2635) [DOI] [PubMed] [Google Scholar]
- 18.Cooper L. A., Scott T. W. 2001. Differential evolution of eastern equine encephalitis virus populations in response to host cell type. Genetics 157, 1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner P. E., Elena S. F. 2000. Cost of host radiation in an RNA virus. Genetics 156, 1465–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver S. C., Brault A. C., Kang W. L., Holland J. J. 1999. Genetic and fitness changes accompanying adaptation of an arbovirus to vertebrate and invertebrate cells. J. Virol. 73, 4316–4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elena S. F. 2002. Restrictions to RNA virus adaptation: an experimental approach. Anton. Leeuw. Int. J. G 81, 135–142 10.1023/A:1020589929125 (doi:10.1023/A:1020589929125) [DOI] [PubMed] [Google Scholar]
- 22.de Visser J. A. G. M., Cooper T. F., Elena S. F. 2011. The causes of epistasis. Proc. R. Soc. B 278, 3617–3624 10.1098/rspb.2011.1537 (doi:10.1098/rspb.2011.1537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domingo E., Escarmis C., Sevilla N., Moya A., Elena S. F., Quer J., Novella I. S., Holland J. J. 1996. Basic concepts in RNA virus evolution. FASEB J. 10, 859–864 [DOI] [PubMed] [Google Scholar]
- 24.Dennehy J. J. 2009. Bacteriophages as model organisms for virus emergence research. Trends Microbiol. 17, 450–457 10.1016/j.tim.2009.07.006 (doi:10.1016/j.tim.2009.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woolhouse M. E. J., Taylor L. H., Haydon D. T. 2001. Population biology of multihost pathogens. Science 292, 1109–1112 10.1126/science.1059026 (doi:10.1126/science.1059026) [DOI] [PubMed] [Google Scholar]
- 26.Jaenike J. 1990. Host specialization in phytophagous insects. Annu. Rev. Ecol. Syst. 21, 243–273 10.1146/annurev.ecolsys.21.1.243 (doi:10.1146/annurev.ecolsys.21.1.243) [DOI] [Google Scholar]
- 27.Via S. 1990. Ecological genetics and host adaptation in herbivorous insects—the experimental study of evolution in natural and agricultural systems. Annu. Rev. Entomol. 35, 421–446 10.1146/annurev.ento.35.1.421 (doi:10.1146/annurev.ento.35.1.421) [DOI] [PubMed] [Google Scholar]
- 28.Bell G. 1997. The basics of selection. New York, NY: Chapman & Hall [Google Scholar]
- 29.Elena S. F., Lenski R. E. 2003. Evolution experiments with microorganisms: the dynamics and genetic bases of adaptation. Nat. Rev. Genet. 4, 457–469 10.1038/nrg1088 (doi:10.1038/nrg1088) [DOI] [PubMed] [Google Scholar]
- 30.Hanley K. A., Manlucu L. R., Gilmore L. E., Blaney J. E., Hanson C. T., Murphy B. R., Whitehead S. S. 2003. A trade-off in replication in mosquito versus mammalian systems conferred by a point mutation in the NS4B protein of dengue virus type 4. Virology 312, 222–232 10.1016/S0042-6822(03)00197-1 (doi:10.1016/S0042-6822(03)00197-1) [DOI] [PubMed] [Google Scholar]
- 31.Ferris M. T., Joyce P., Burch C. L. 2007. High frequency of mutations that expand the host range of an RNA virus. Genetics 176, 1013–1022 10.1534/genetics.106.064634 (doi:10.1534/genetics.106.064634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepin K. M., Wichman H. A. 2007. Variable epistatic effects between mutations at host recognition sites in ΦX174 bacteriophage. Evolution 61, 1710–1724 10.1111/j.1558-5646.2007.00143.x (doi:10.1111/j.1558-5646.2007.00143.x) [DOI] [PubMed] [Google Scholar]
- 33.Bohannan B. J. M., Travisano M., Lenski R. E. 1999. Epistatic interactions can lower the cost of resistance to multiple consumers. Evolution 53, 292–295 10.2307/2640942 (doi:10.2307/2640942) [DOI] [PubMed] [Google Scholar]
- 34.Remold S. K., Lenski R. E. 2004. Pervasive joint influence of epistasis and plasticity on mutational effects in Escherichia coli. Nat. Genet. 36, 423–426 10.1038/ng1324 (doi:10.1038/ng1324) [DOI] [PubMed] [Google Scholar]
- 35.Joshi A., Thompson J. N. 1995. Trade-offs and the evolution of host specialization. Evol. Ecol. 9, 82–92 10.1007/BF01237699 (doi:10.1007/BF01237699) [DOI] [Google Scholar]
- 36.Hueffer K., Parker J. S. L., Weichert W. S., Geisel R. E., Sgro J.-Y., Parrish C. R. 2003. The natural host range shift and subsequent evolution of canine parvovirus resulted from virus-specific binding to the canine transferrin receptor. J. Virol. 77, 1718–1726 10.1128/jvi.77.3.1718-1726.2003 (doi:10.1128/jvi.77.3.1718-1726.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsetsarkin K. A., Vanlandingham D. L., McGee C. E., Higgs S. 2007. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 3, e201. 10.1371/journal.ppat.0030201 (doi:10.1371/journal.ppat.0030201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitlock M. C. 1996. The red queen beats the jack-of-all-trades: the limitations on the evolution of phenotypic plasticity and niche breadth. Am. Nat. 148, S65–S77 10.1086/285902 (doi:10.1086/285902) [DOI] [Google Scholar]
- 39.Kawecki T. J. 1994. Accumulation of deleterious mutations and the evolutionary cost of being a generalist. Am. Nat. 144, 833–838 10.1086/285709 (doi:10.1086/285709) [DOI] [Google Scholar]
- 40.Kawecki T. J. 1998. Red queen meets Santa Rosalia: arms races and the evolution of host specialization in organisms with parasitic lifestyles. Am. Nat. 152, 635–651 10.1086/286195 (doi:10.1086/286195) [DOI] [PubMed] [Google Scholar]
- 41.Fry J. D. 1996. The evolution of host specialization: are trade-offs overrated? Am. Nat. 148, S84–S107 10.1086/285904 (doi:10.1086/285904) [DOI] [Google Scholar]
- 42.Kawecki T. J., Barton N. H., Fry J. D. 1997. Mutational collapse of fitness in marginal habitats and the evolution of ecological specialisation. J. Evol. Biol. 10, 407–429 10.1007/s000360050032 (doi:10.1007/s000360050032) [DOI] [Google Scholar]
- 43.Burch C. L., Chao L. 2000. Evolvability of an RNA virus is determined by its mutational neighbourhood. Nature 406, 625–628 10.1038/35020564 (doi:10.1038/35020564) [DOI] [PubMed] [Google Scholar]
- 44.Ostrowski E. A., Ofria C., Lenski R. E. 2007. Ecological specialization and adaptive decay in digital organisms. Am. Nat. 169, E1–E20 10.1086/510211 (doi:10.1086/510211) [DOI] [PubMed] [Google Scholar]
- 45.Remold S. K., Rambaut A., Turner P. E. 2008. Evolutionary genomics of host adaptation in vesicular stomatitis virus. Mol. Biol. Evol. 25, 1138–1147 10.1093/molbev/msn059 (doi:10.1093/molbev/msn059) [DOI] [PubMed] [Google Scholar]
- 46.Presloid J. B., Ebendick-Corpus B. E., Zárate S., Novella I. S. 2008. Antagonistic pleiotropy involving promoter sequences in a virus. J. Mol. Biol. 382, 342–352 10.1016/j.jmb.2008.06.080 (doi:10.1016/j.jmb.2008.06.080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reboud X., Bell G. 1997. Experimental evolution in Chlamydomonas. III. Evolution of specialist and generalist types in environments that vary in space and time. Heredity 78, 507–514 10.1038/hdy.1997.79 (doi:10.1038/hdy.1997.79) [DOI] [Google Scholar]
- 48.Cooper V. S., Lenski R. E. 2000. The population genetics of ecological specialization in evolving Escherichia coli populations. Nature 407, 736–739 10.1038/35037572 (doi:10.1038/35037572) [DOI] [PubMed] [Google Scholar]
- 49.Poelwijk F. J., Kiviet D. J., Weinreich D. M., Tans S. J. 2007. Empirical fitness landscapes reveal accessible evolutionary paths. Nature 445, 383–386 10.1038/nature05451 (doi:10.1038/nature05451) [DOI] [PubMed] [Google Scholar]
- 50.Poelwijk F. J., Tanase-Nicola S., Kiviet D. J., Tans S. J. 2011. Reciprocal sign epistasis is a necessary condition for multi-peaked fitness landscapes. J. Theor. Biol. 272, 141–144 10.1016/j.jtbi.2010.12.015 (doi:10.1016/j.jtbi.2010.12.015) [DOI] [PubMed] [Google Scholar]
- 51.Weinreich D. M., Delaney N. F., DePristo M. A., Hartl D. L. 2006. Darwinian evolution can follow only very few mutational paths to fitter proteins. Science 312, 111–114 10.1126/science.1123539 (doi:10.1126/science.1123539) [DOI] [PubMed] [Google Scholar]
- 52.Khan A. I., Dinh D. M., Schneider D., Lenski R. E., Cooper T. F. 2011. Negative epistasis between beneficial mutations in an evolving bacterial population. Science 332, 1193–1196 10.1126/science.1203801 (doi:10.1126/science.1203801) [DOI] [PubMed] [Google Scholar]
- 53.Chou H. H., Chiu H. C., Delaney N. F., Segre D., Marx C. J. 2011. Diminishing returns epistasis among beneficial mutations decelerates adaptation. Science 332, 1190–1192 10.1126/science.1203799 (doi:10.1126/science.1203799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borman A. M., Paulous S., Clavel F. 1996. Resistance of human immunodeficiency virus type 1 to protease inhibitors: selection of resistance mutations in the presence and absence of the drug. J. Gen. Virol. 77, 419–426 10.1099/0022-1317-77-3-419 (doi:10.1099/0022-1317-77-3-419) [DOI] [PubMed] [Google Scholar]
- 55.Cuevas J. M., Sanjuán R., Moya A., Elena S. F. 2005. Mode of selection and experimental evolution of antiviral drugs resistance in vesicular stomatitis virus. Infect. Genet. Evol. 5, 55–65 10.1016/j.meegid.2004.06.006 (doi:10.1016/j.meegid.2004.06.006) [DOI] [PubMed] [Google Scholar]
- 56.Nielsen H. S., Oleksiewicz M. B., Forsberg R., Stadejek T., Bøtner A., Storgaard T. 2001. Reversion of a live porcine reproductive and respiratory syndrome virus vaccine investigated by parallel mutations. J. Gen. Virol. 82, 1263–1272 [DOI] [PubMed] [Google Scholar]
- 57.Hayden E. J., Ferrada E., Wagner A. 2011. Cryptic genetic variation promotes rapid evolutionary adaptation in an RNA enzyme. Nature 474, U92–U120 10.1038/nature10083 (doi:10.1038/nature10083) [DOI] [PubMed] [Google Scholar]
- 58.Wichman H. A., Scott L. A., Yarber C. D., Bull J. J. 2000. Experimental evolution recapitulates natural evolution. Phil. Trans. R. Soc. Lond. B 355, 1677–1684 10.1098/rstb.2000.0731 (doi:10.1098/rstb.2000.0731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burch C. L., Chao L. 1999. Evolution by small steps and rugged landscapes in the RNA virus φ6. Genetics 151, 921–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheverud J. M. 2001. The genetic architecture of pleiotropic relations and differential epistasis. In The character concept in evolutionary biology (ed. Wagner G. P.), pp. 411–433 San Diego, CA: Academic Press [Google Scholar]
- 61.Wagner G. P., Pavlicev M., Cheverud J. M. 2007. The road to modularity. Nat. Rev. Genet. 8, 921–931 10.1038/nrg2267 (doi:10.1038/nrg2267) [DOI] [PubMed] [Google Scholar]
- 62.Ancel L. W., Fontana W. 2000. Plasticity, evolvability, and modularity in RNA. J. Exp. Zool. 288, 242–283 (doi:10.1002/1097-010X(20001015)288:3<242::AID-JEZ5>3.0.CO;2-O) [DOI] [PubMed] [Google Scholar]
- 63.Wagner A. 2008. Robustness and evolvability: a paradox resolved. Proc. R. Soc. B 275, 91–100 10.1098/rspb.2007.1137 (doi:10.1098/rspb.2007.1137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Draghi J. A., Parsons T. L., Wagner G. P., Plotkin J. B. 2010. Mutational robustness can facilitate adaptation. Nature 463, 353–355 10.1038/nature08694 (doi:10.1038/nature08694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Masel J., Trotter M. V. 2010. Robustness and evolvability. Trends Genet. 26, 406–414 10.1016/j.tig.2010.06.002 (doi:10.1016/j.tig.2010.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Domingo-Calap P., Pereira-Gómez M., Sanjuán R. 2010. Selection for thermostability can lead to the emergence of mutational robustness in an RNA virus. J. Evol. Biol. 23, 2453–2460 10.1111/j.1420-9101.2010.02107.x (doi:10.1111/j.1420-9101.2010.02107.x) [DOI] [PubMed] [Google Scholar]
- 67.McBride R. C., Ogbunugafor C. B., Turner P. E. 2008. Robustness promotes evolvability of thermotolerance in an RNA virus. BMC Evol. Biol. 8, 231. 10.1186/1471-2148-8-231 (doi:10.1186/1471-2148-8-231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crombach A., Hogeweg P. 2008. Evolution of evolvability in gene regulatory networks. PLoS Comput. Biol. 4, e1000112. 10.1371/journal.pcbi.1000112 (doi:10.1371/journal.pcbi.1000112) [DOI] [PMC free article] [PubMed] [Google Scholar]


