Abstract
The trade-off between lifespan and reproduction is commonly explained by differential allocation of limited resources. Recent research has shown that the ratio of protein to carbohydrate (P : C) of a fly's diet mediates the lifespan–reproduction trade-off, with higher P : C diets increasing egg production but decreasing lifespan. To test whether this P : C effect is because of changing allocation strategies (Y-model hypothesis) or detrimental effects of protein ingestion on lifespan (lethal protein hypothesis), we measured lifespan and egg production in Queensland fruit flies varying in reproductive status (mated, virgin and sterilized females, virgin males) that were fed one of 18 diets varying in protein and carbohydrate amounts. The Y-model predicts that for sterilized females and for males, which require little protein for reproduction, there will be no effect of P : C ratio on lifespan; the lethal protein hypothesis predicts that the effect of P : C ratio should be similar in all groups. In support of the lethal protein hypothesis, and counter to the Y-model, the P : C ratio of the ingested diets had similar effects for all groups. We conclude that the trade-off between lifespan and reproduction is mediated by the detrimental side-effects of protein ingestion on lifespan.
Keywords: Bactrocera tryoni, carbohydrate, geometric framework, nutrition, protein
1. Introduction
Costs of reproduction are generally accepted as a central pillar of life-history evolution [1–5]. In particular, increased reproduction rate has been associated with decreased lifespan in representatives from across a vast taxonomic spectrum [2,3]. This trade-off between the fundamental fitness characteristics of reproduction and lifespan has been the focus of substantial research but the proximate mechanisms linking reproduction and longevity remain poorly resolved [6–9].
The lifespan–reproduction (L–R) trade-off is most often interpreted as stemming from resource constraints [10–14]. In this view, reproduction and somatic maintenance are both costly processes and compete for limited resources. This is known as the Y-model, in which resources enter at the base of the ‘Y’ and are then allocated to reproduction and lifespan [12,14–16]. Under the Y-model it is not possible to maximize both lifespan and reproduction; increasing reproductive effort diverts essential resources away from lifespan extending somatic maintenance and repair. The physiological mechanisms that mediate resource allocation in the L–R trade-off strongly affect an organism's lifetime reproductive success and, accordingly, should be under strong selection pressures [1–5].
Tests of the Y-model traditionally have treated nutritional resources as a unitary entity (figure 1) [10,11,17–21]. However, recent advances using a nutrient-explicit approach that treats each macronutrient resource as distinct have promoted a new perspective on the role of nutrition in L–R trade-off of insects [22–26]. Increasing the ratio of protein to carbohydrate (P : C) for a given caloric intake increases egg production rates but also decreases lifespan (Drosophila melanogaster [26], Queensland fruit flies (Q-flies) [22,23] and crickets [25]). Furthermore, increasing total nutrient intake for a given P : C ratio increases both egg production rates and lifespan. These results suggest that it may be more appropriate to adopt a nutrient-explicit version of the Y-model, rather than treating different nutritional resources as unitary. Furthermore, a nutrient-explicit analysis can help explain a critical component of the Y-model; how is the allocation of resources to lifespan and reproduction determined? Although an ‘allocation strategy’ term (α) has been incorporated into the Y-model, this is a nebulous and general factor, without a clear mechanistic basis [27]. Recent studies that have taken a nutrient-explicit approach suggest that nutritional composition (P : C) may be a major determinant of allocation strategy [22,23,25,26].
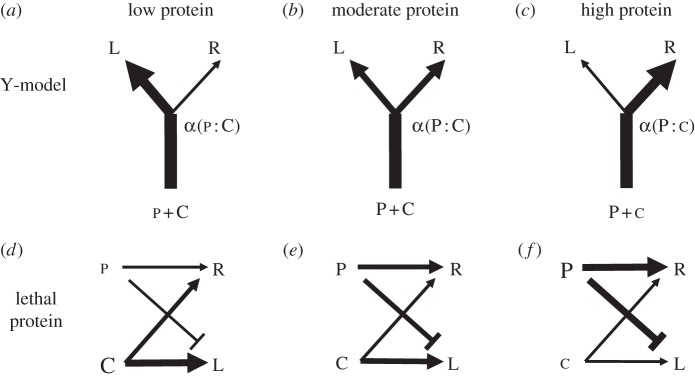
Figure 1.
Schematic of competing hypotheses to explain physiological trade-offs between lifespan (L) and reproduction (R). (a–c) The Y-model assumes a unitary resource (protein (P) + carbohydrate (C)) has positive effects on lifespan and reproduction, but changing the allocation strategies (α) by shifting P : C ratio can cause lifespan to decrease. (d–f) In contrast, the lethal protein assumes that protein has a detrimental effect on lifespan as well as a positive effect on reproduction.
Challenging the conventional Y-model, recent studies of how specific nutrients influence lifespan and reproduction have suggested an alternative interpretation, the lethal protein hypothesis [23,26,28]. In the Y-model, nutrient resources, especially macronutrients, are usually assumed to only have positive effects and hence more resources are considered beneficial. But emerging evidence suggests that macronutrients can also have negative effects [28,29] and that different macronutrients can have quite different effects on lifespan and reproduction. The lethal protein hypothesis uses a nutrient-explicit approach to explain the trade-off between lifespan and reproduction as arising from protein intake having a positive effect on egg production, but a negative effect on lifespan (figure 1). Thus, consuming more protein increased egg production rates in both flies and crickets [23,25,26], but this additional protein was detrimental to lifespan, possibly owing to toxicity of nitrogenous waste or enhanced production of mitochondrial radical oxygen species [30,31]. In contrast, carbohydrates may have positive effects on both lifespan and egg production rates. For instance, flies that consumed more carbohydrates for a given amount of protein had increased lifespan and egg production [23,26].
Distinguishing between these hypotheses is a vital step towards understanding the role of nutrition in the L–R trade-off. Response to P : C ratio of diets is key to distinguishing between these hypotheses; the Y-model predicts that, for a given nutritional intake, elimination of reproduction should negate the L–R trade-off as P : C increases whereas the lethal protein model predicts that the L–R trade-off should remain despite the absence of reproduction. Traditionally, studies have modified nutrition by adjusting total caloric amount or by changing both caloric and nutritional composition of the diet [12,15,16,18], but since animals adjust nutrient intake in relation to diet concentration and composition [32–34], this approach can be difficult to interpret and even misleading [26,35,36]. Therefore, we adopt a robust experimental approach combining nutritional geometry (aka ‘Geometric Framework’; [34]) and modification of the reproductive capacities of female Q-flies: mated, virgin and sterile (irradiated). We also compare trends with male Q-flies, which lack the nutritional demands of egg production.
If the Y-model hypothesis is correct, we predict that mated females, which have the highest egg production, will have the steepest decrease in lifespan as P : C increases. Virgin females have reduced egg production compared with mated females and hence lifespan should decrease at a shallower rate as P : C increases. For sterile females, we predict that lifespan should not change with P : C ratios since these females do not produce any eggs. Finally, only minimal amounts of protein are required for reproduction in males [37–39] and thus we predict trends similar to those of sterile females. On the other hand, the lethal protein hypothesis predicts no differences across the treatment groups.
2. Material and methods
(a). Study animals and husbandry
We obtained Q-flies (Bactrocera tryoni) as pupae from the Fruit Fly Production Facility at Elizabeth Macarthur Agricultural Institute (EMAI, New South Wales, Australia), where they are maintained on a larval diet of lucerne chaff, sugar and torula yeast. Newly emerged flies (less than 24 h old) were sorted and grouped by sex and irradiation treatment into separate 5 l plastic cages, which contained a 70 ml container of distilled water with a cotton wick and two small food dishes containing granular sucrose and hydrolysed yeast. Temperature and humidity were maintained at approximately 24°C and approximately 82 per cent, respectively. Under these conditions, Q-flies reach sexually mature by day 10 [38,40].
(b). Experimental protocol
This experiment had five overarching experimental treatments: virgin females, virgin males, mated females, 40 Gy irradiated females and 70 Gy irradiated females. Guided by previous research on use of gamma radiation for sterility induction of Q-flies [40–45], we chose two sterilizing doses, 40 Gy and 70 Gy. The 40 Gy dose is the lowest dose that achieves nearly 100 per cent sterility and 70 Gy provides a metric of the potential effects of somatic damage in relation to the 40 Gy. Sterilizing doses of irradiation were administered to the pupae under hypoxia when 85 per cent of the pupal stage was complete. Non-irradiated flies experienced the same handling procedures as irradiated flies.
On the evening of day 11 after emergence, 120 non-irradiated females were mated by placing each female into a 70 ml container with a single male. Females were considered mated if they copulated for longer than 5 min (87% mated). On day 12, 90 flies from each treatment group were transferred to individual clear polystyrene containers (70 ml), which had 10 small (2 mm) holes drilled into the bottom and parafilm placed over the top. Containers housing mated and virgin females were then placed inverted on an ovipositing dish (i.e. the parafilm layer served as a floor and the holes were oriented upwards). The ovipositing dish comprised a plastic weigh-boat (35 × 35 mm) filled with 2.5 ml of 0.7 per cent lemon essence solution (Queen Fine Foods Pty Ltd, Alderly, Queensland, Australia) and covered with a single layer of parafilm that had been punctured several times within an insect pin [23]. All containers were then arranged evenly on a shelving unit to control for spatial effects.
A 200 µl pipette-tip filled with distilled water was inserted through one of the holes in the roof of each container. Except for mated females, each container also received one 50 µl microcapillary tube (Drummond) filled with 35 µl of diet (see below); mated females received two 50 µl microcapillary tubes owing to higher diet consumption than the other treatments. Mortality was checked daily and ovipositing dishes were replaced every 2 days. Eggs in the ovipositing dish were photographed and manually counted. Microcapillary tubes were refilled every 4 days, or sooner if depleted (checked regularly during the light phase).
As a measure of body size, we removed and photographed the right wing of each dead fly, and then using Adode Photoshop (v. 11.0.2, San Jose, CA, USA) we measured the distance from the intersection of the anal and median band to the margin of the costal band and the R4 + 5 vein [38].
(c). Experimental diets
We prepared 18 liquid diets varying in sucrose (S; Sigma no. 84100) and hydrolysed yeast content (Y; MP Biomedicals, Aurora, OH, USA, no. 103304: 45% protein, 24% carbohydrate, 21% indigestible fibre, 8% water and 2% other; electronic supplementary material, table S1). All diets were dissolved in distilled water. Diets differed in total diet concentrations (40, 120, 360 g l−1) and in Y : S ratios (0:1, 1:14.2, 1:7,1:3.4, 1:1.6 and 4.8:1), resulting in P : C ratios of 0:1, 1:32, 1:16, 1:8, 1:4 and 1:1. A recent study using a chemically defined diet demonstrated that the effect of varying Y : S ratios on lifespan and reproduction was owing to P : C ratios and not other nutrients in yeast [22].
(d). Measuring diet consumption
Diet consumption was measured by taking still pictures using an 8 MP Canon IXUS 80IS camera programmed to photograph the containers and microcapillary tubes every morning [33,46]. We corrected for barrel distortion using Adobe Photoshop and measured consumption as change in displacement of liquid in the microcapillary tubes using ImageTool (ImageJ v. 3.00; http://rsweb.nih.gov.ij). Correlation between photograph method and measurement using callipers was r = 0.99 (n = 84).
We included an additional 90 control containers to record evaporation rates on each diet with no flies present. High humidity levels helped us to minimize evaporation, but some evaporative loss did occur, especially in the lower concentration diets. To correct for evaporation, we measured evaporative loss from the control containers and fitted a regression model using initial yeast and sucrose concentration, temperature and humidity. We then corrected for evaporative loss each day by using the regression model to estimate water loss, recalculate the yeast and sucrose concentrations for this loss, and then using this new concentration to estimate nutrient consumption [47].
(e). Data analysis
For lifespan and daily egg production data, we used daily C and P amounts to predict lifespan for each treatment group and to predict daily egg production rate for mated and virgin females. Similar to other studies [22,23], mean daily C and P consumption for the first 7 days on the experimental diets (trends remain consistent for shorter or longer time frames) were used as a measure of nutritional intake. This 7-day window was chosen as all diet treatments still had multiple flies alive. For egg production rates, we took the average daily egg rate from day 12 to day 30, since egg production rates peaked for most individuals during this time period.
Y-model. To test the predictions of the Y-model, we need to describe how the amount of resources consumed and the allocation strategy affect lifespan and reproduction. Therefore, we performed a general linear mixed model using daily intake (P + C) as an estimate of resource amount and proportion of protein in the diet (P : C) as the allocation term. We also included the interaction between these predictor variables. For mated and virgin groups, we included both egg production rate and lifespan in the model as predictor variables and modelled the covariance structure assuming an unstructured design within each individual [48]. Wing size was added to control for possible body size effects. For the other treatment groups, which do not produce eggs, the response variable was just lifespan. Post hoc pairwise comparisons of parameter estimates among the treatment groups were then conducted using a general linear model assuming unequal variance.
Lethal protein model. As results from the Y-model analysis indicated that protein may have a negative effect on lifespan, we re-analysed lifespan and egg production patterns assuming a nutrient-explicit approach in which protein and carbohydrates are treated as individual predictor variables. This allowed us to separately estimate the effects of protein and carbohydrate on lifespan and egg production rates. For this analysis, we fitted separate response surface regressions using daily C and P consumption to predict lifespan for each treatment group (male, irradiated 40/70 Gy, mated, virgin) and egg production rates for mated and virgin. For both analyses, daily P and C consumptions were centred and all second-order effects of P and C were included [49]. Additionally, wing size was added to the model to control for possible body size effects.
We then compared each surface regression for all treatments in a pairwise manner. For this analysis, we compared the fit of (i) surface regression models with separate regression coefficients for both treatments with (ii) the simpler model in which data were grouped across the two treatments. We compared −2 log likelihoods using a likelihood-ratio test (ΔL) to determine statistical significance.
All statistical analyses were conducted in SAS (v. 9.1). All final models satisfied homoscedasticity and normality assumptions. For all surface analyses, we fitted surface plots to the predicted data using the FIELDS package in r (v. 2.9.0) to facilitate visualization of the results. Data were deposited in the Dryad repository (doi:10.5061/dryad.2d7j0).
3. Results
(a). Egg production patterns
Manipulation of female Q-fly reproductive capacity was a key element of this study. No females irradiated at 70 Gy laid any eggs and only two of 90 females irradiated at 40 Gy laid eggs. For these two females, eggs were laid at older ages (>40 days) and each laid fewer than 30 eggs in total. Mated females had roughly twice the daily egg production of virgin females (table 1; figure 2).
Table 1.
Parameter estimates from the Y-model regression for mean lifespan (days) and daily egg production rates. For this regression, total resources (protein (P) + carbohydrate (C) consumption), protein:carbohydrate (P : C) ratio and body size were included as predictor variables (see text). Significant terms are listed in bold.
| effect | mated | virgin | irradiated 40 Gy | irradiated 70 Gy | male |
|---|---|---|---|---|---|
| lifespan | |||||
| P : C ratio | −5.45 | −5.71 | −6.33 | −4.85 | −4.25 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| resource (P + C) | 0.50 | 1.43 | 0.10 | 1.93 | −0.32 |
| p = 0.48 | p = 0.009 | p = 0.83 | p = 0.001 | p = 0.48 | |
| resource by P : C | −1.27 | −4.01 | −0.51 | −3.99 | 1.08 |
| p = 0.75 | p = 0.25 | p = 0.90 | p = 0.38 | p = 0.67 | |
| body size | −0.38 | −1.65 | 0.68 | −1.50 | 0.51 |
| p = 0.76 | p = 0.19 | p = 0.62 | p = 0.31 | p = 0.60 | |
| egg rate | |||||
| P : C ratio | 6.05 | −0.93 | |||
| p = 0.003 | p = 0.64 | ||||
| resources (P + C) | 6.80 | 4.04 | |||
| p < 0.001 | p < 0.001 | ||||
| resource by P : C | 5.80 | 6.54 | |||
| p = 0.49 | p = 0.40 | ||||
| body size | −1.64 | 0.64 | |||
| p = 0.49 | p = 0.78 | ||||
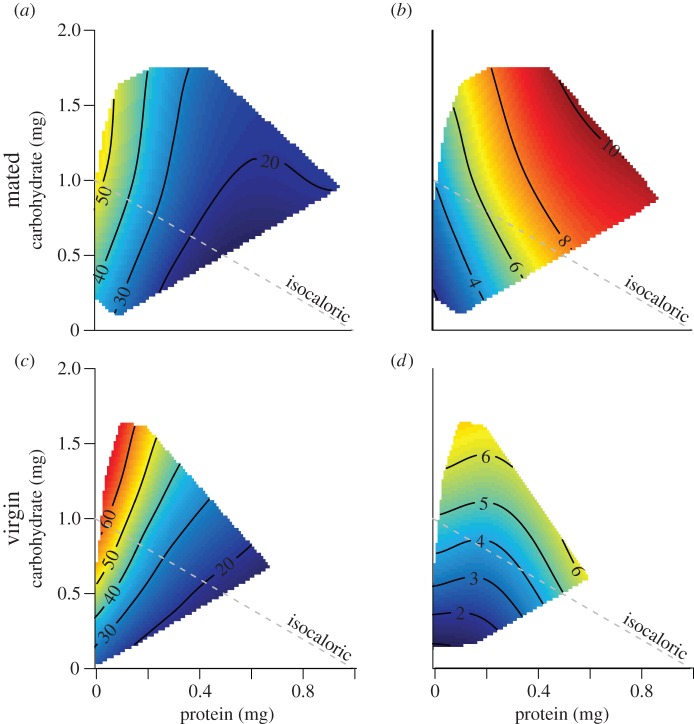
Figure 2.
Response surfaces showing the effects of protein and carbohydrate consumption on lifespan (days) and egg production rates (eggs/day) for mated and virgin females. (a,c) Show mean lifespans and (b,d) show egg production rates. Redder colours indicate higher responses and blue colours lower. Protein and carbohydrate intakes are the mean daily intakes for the first 7 days. Grey dotted line represents an isocaloric line.
(b). Y-model analysis
For mated females, the composition of nutrient resources (the P : C ratio) appears to alter the resource allocation strategy. With increasing P : C ratio, mated flies increased egg production and decreased lifespan, suggesting a resource trade-off. Consuming more resources (P + C) increased daily egg production, but had no effect on lifespan (table 1). Therefore, as intake increases, mated females appear to allocate the additional resources almost entirely to reproduction.
Despite considerable differences in reproductive effort (figure 2b,d), lifespan patterns of virgin females were very similar to those of mated females (table 1). The Y-model analysis found a nearly identical effect of P : C ratio on lifespan, suggesting that virgin females alter their allocation strategy in a manner very similar to that seen in mated females (t429 = −0.3, p = 0.76; table 1). However, since egg production rates were nearly half those of mated females (figure 2; table 1; t159 = 9.3, p < 0.001), we expected the effect of P : C ratio on lifespan to be significantly less. Additionally, unlike mated females, P : C ratio had no significant effect on egg production rates in virgin females (table 1). Instead, egg production rate depended only on total resource consumed (caloric intake) (table 1). Thus, the trade-off between lifespan and egg production rate does not hold.
Patterns observed for irradiated females and males are also discordant with predictions of the Y-model. As irradiation eliminated egg production in females, irradiated females did not lay any eggs, except for two females from the 40 Gy group that laid fewer than 30 eggs in total. Males, on the other hand, require only small amounts of protein for reproduction. However, despite the absence of egg production, increasing P : C ratio had a negative effect on lifespan of irradiated females (40 and 70 Gy) and males in a manner that was strikingly similar to that of mated fertile females (table 1; versus mated: t429 =−1.0, p = 0.52; t429 = 0.42, p = 0.78; t429 = −1.5, p = 0.32, respectively).
(c). Lethal protein model
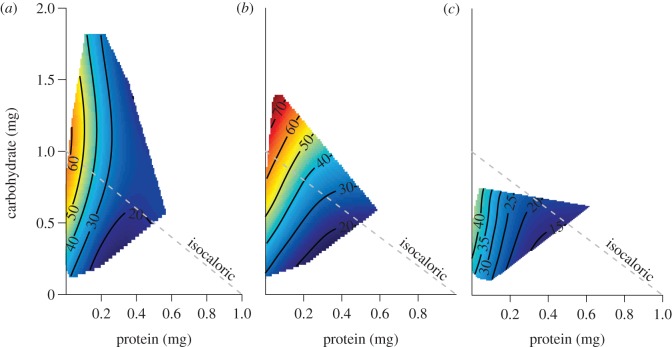
The nutrient-explicit analysis treats nutrients as separate entities and estimates their effects on lifespan and egg production rates independently. The effect of protein and carbohydrate consumption on mean lifespan was consistent across the treatment groups (figures 2 and 3; table 2, electronic supplementary material, S4). For each female group, carbohydrate intake had a significant positive effect on lifespan, but protein had a stronger negative effect (table 2). For males, the negative effect of protein on lifespan was similar to that observed in females, but the effect of carbohydrate was not significant. Absence of a significant carbohydrate effect in males probably reflects low leverage associated with the restricted surface area available to estimate the regression; males ate substantially less than females and thus samples became clumped for each P : C ratio.
Figure 3.
Effects of protein and carbohydrate consumption on lifespan for (a) irradiated 40 and (b) 70 Gy females and (c) for virgin males.
Table 2.
Parameter estimates for the effects of body size and protein and carbohydrate consumption on lifespan for flies varying in reproductive capacity. Mean lifespan is the estimated lifespan at the mean carbohydrate and protein intake for all flies. Significant terms are listed in bold.
| mated | virgin | irradiated 40 Gy | irradiated 70 Gy | male |
|---|---|---|---|---|
| mean lifespan | ||||
| 35.37 | 39.29 | 39.04 | 37.75 | 32.10 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| body size | ||||
| −15.94 | −26.49 | 9.10 | −23.62 | −0.60 |
| p = 0.37 | p = 0.16 | p = 0.65 | p = 0.30 | p = 0.96 |
| carbohydrate (C) | ||||
| 26.00 | 38.20 | 36.90 | 41.12 | 13.71 |
| p = 0.043 | p = 0.001 | p = 0.008 | p = 0.002 | p = 0.18 |
| protein (P) | ||||
| −151.32 | −128.13 | −167.15 | −76.68 | −121.82 |
| p < 0.001 | p < 0.001 | p < 0.001 | p = 0.05 | p = 0.007 |
| C by C | ||||
| −3.80 | −10.36 | −28.30 | −1.94 | −14.80 |
| p = 0.94 | p = 0.56 | p = 0.14 | p = 0.94 | p = 0.21 |
| P by P | ||||
| 304.47 | 190.04 | 315.09 | 87.71 | 201.58 |
| p = 0.05 | p = 0.07 | p = 0.038 | p = 0.50 | p = 0.11 |
| C by P | ||||
| −79.99 | −161.31 | −40.17 | −36.07 | −91.51 |
| p = 0.59 | p = 0.17 | p = 0.65 | p = 0.76 | p = 0.53 |
Carbohydrate consumption increased egg-laying rates in both virgin and mated females, but, interestingly, protein only increased egg production significantly in mated females (see the electronic supplementary material, table S5). In mated females, the magnitude of the protein effect was estimated at nearly five times the effect of carbohydrate (see the electronic supplementary material, table S5).
4. Discussion
The limited resource allocation model, or Y-model, is by far the most prevalent explanation posed for the often-reported trade-off between lifespan and reproduction [2,3,12,14,16]. As a robust approach to testing the Y-model, we combined a nutrient-explicit experimental design with experimental modifications of female reproductive capacity. Our results showed that the Y-model could potentially explain changes in lifespan and egg production in mated female Q-flies. As predicted by the Y-model, egg production increased and lifespan decreased as P : C ratio increased for a given caloric intake. However, the Y-model does not explain patterns for virgin females, irradiated females, or males. The Y-model predicts that in the absence of resource demand for reproduction, lifespan should remain constant across P : C ratios. In contrast, we found that as P : C increased lifespan decreased at a similar rate across all treatment groups.
The lethal protein hypothesis provides a far more compelling explanation for our results. This hypothesis postulates that protein consumption has a positive effect on egg production, but a negative effect on lifespan. Therefore, we predicted that as P : C ratio increased all treatment groups would exhibit a similar decrease in lifespan. Our results correspond closely with this prediction. Analysing protein and carbohydrate as distinct resources revealed a strong negative effect of protein on lifespan for all treatment groups and moderate positive effect of carbohydrate on lifespan for all groups except males.
Our results also do not support the hypothesis that reproduction inflicts direct physiological damage on the soma (‘reproductive damage’ hypothesis; [8,15]). According to this hypothesis, reproductive effort causes somatic damage and reduces lifespan. Therefore, elimination of reproductive effort should eliminate the decrease in lifespan with increasing P : C (i.e. the same predictions as the Y-model). However, the negative effect of protein on lifespan persisted in irradiated females and males. Our findings constitute evidence against a direct physiological cost of reproduction on lifespan ([15]; reviewed in [50]).
While the lethal protein hypothesis is far more consistent with our findings than the conventional Y-model interpretation, which takes reproductive output as the primary or sole metric of reproductive effort, there are less conventional interpretations of the Y-model that come closer. One possibility is that protein consumption induces costly non-gonadal reproductive processes [6,8,51]. Although irradiation effectively curtails gonadal development it may not completely negate other costs normally associated with reproduction. The main weakness of this hypothesis for our results is that, despite having much lower protein requirements for reproductive maturation and mating [38,52], male Q-flies expressed patterns that were very similar to those of females. Similarly, male crickets require carbohydrate-rich diets to maximize calling effort (a proxy of mating success), but male crickets also have reduced lifespan as P : C ratio increases [25].
Another potential explanation is that metabolic signalling pathways associated with lifespan and reproduction trade-offs may still be activated in the virgin and irradiated females [53–55]. Recent research into the signalling pathways of Drosophila spp. and Caenorhabditis elegans have highlighted that reproduction and lifespan can be decoupled, suggesting that direct competition for resources does not explain L–R trade-offs [53–55]. For example, ablation of the germline extends lifespan in C. elegans, but ablation of the germline and somatic gonads does not [54,56]. Assuming no difference in nutrient uptake, lifespan in C. elegans is affected by tissue presence rather than resource availability. Furthermore, nutrient sensing pathways, such as insulin-like/IGF-1 (IIS) and Target of Rapamycin (TOR) pathways can have potent effects on lifespan and reproduction [57]. P : C ratio might still drive activation of these pathways in virgin and sterilized females, signalling for decreased lifespan and attempts to increase egg production. But such signalling can provide only a very limited explanation for our results. First, if the signalling pathways are independent of reproductive potential, then we would have expected virgin females to have egg production rates and patterns similar to those of mated females. However, virgins laid significantly fewer eggs and were less affected by protein. Second, similar to females, male lifespan decreased with increasing P : C ratios, even though nutritional requirements for male reproduction are vastly lower.
Our results illustrate that a nutrient-explicit approach to investigating mechanisms underlying L–R trade-offs can provide substantial insights that would not be evident with a unitary resource approach. While breaking nutrition down into the two major macronutrient groups of protein and carbohydrate has proved to be highly instructive, recent research indicates that substantial additional insights may be gained by introducing yet finer resolution. Within the broad macronutrient class of protein, individual amino acids can have quite different effects on lifespan and reproduction. For instance, methionine increases egg production in Drosophila melanogaster with little or no negative effect on lifespan, whereas the other essential amino acids increase reproduction but cause lifespan to decrease and non-essential amino acids have no effect on either reproduction or lifespan [58]. As hydrolysed yeast contains all of the essential amino acids, our results are in accord with these Drosophila findings.
We tested competing hypotheses for explaining the trade-off between reproduction and lifespan in Q-flies, adopting a novel approach that incorporates advances in nutritional geometry [34–36] and standard approaches [12,15,16,18] for exploring L–R tradeoffs. Only by creating these nutritional surfaces was a consistent negative effect of protein consumption on lifespan across all treatment groups apparent. The lethal protein hypothesis provides the most parsimonious explanation for our results, whereas the more known Y-model is generally unsupported. Our findings highlight that specific nutrients may have pleiotropic effects on a suite of traits and some of these effects may be negative.
Acknowledgements
This work was supported by the Macquarie University Research Excellence Scholarship to BGF. We thank the staff of New South Wales Department of Primary Industries for providing Queensland fruit flies. We also thank S. Yap and S. Collins for experimental assistance. The manuscript was greatly improved by comments from Russell Bonduriansky and two anonymous reviewers. We declare that we have no competing interests.
References
- 1.Houston A. I., McNamara J. M., Barta Z., Klasing K. C. 2007. The effect of energy reserves and food availability on optimal immune defence. Proc. R. Soc. B 274, 2835–2842 10.1098/rspb.2007.0934 (doi:10.1098/rspb.2007.0934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stearns S. C. 1992. The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- 3.Roff D. A. 1992. The evolution of life histories: theory and analysis. New York, NY: Chapman and Hall [Google Scholar]
- 4.Charlesworth B. 1973. Selection in populations with overlapping generations. V. Natural selection and life histories. Am. Nat. 107, 303–311 10.1086/282832 (doi:10.1086/282832) [DOI] [Google Scholar]
- 5.Fisher R. A. 1930. The genetical theory of natural selection. Oxford, UK: Clarendon [Google Scholar]
- 6.Flatt T., Heyland A. 2011. Mechanisms of life history evolution: the genetics and physiology of life history traits and trade-offs. Oxford, UK: Oxford University Press [Google Scholar]
- 7.Harshman L. G., Zera A. J. 2007. The cost of reproduction: the devil in the details. Trends Ecol. Evol. 22, 80–86 10.1016/j.tree.2006.10.008 (doi:10.1016/j.tree.2006.10.008) [DOI] [PubMed] [Google Scholar]
- 8.Barnes A. I., Partridge L. 2003. Costing reproduction. Anim. Behav. 66, 199–204 10.1006/anbe.2003.2122 (doi:10.1006/anbe.2003.2122) [DOI] [Google Scholar]
- 9.Reznick D., Nunney L., Tessier A. 2000. Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol. Evol. 15, 421–425 10.1016/S0169-5347(00)01941-8 (doi:10.1016/S0169-5347(00)01941-8) [DOI] [PubMed] [Google Scholar]
- 10.Gadgil M., Bossert W. H. 1970. Life historical consequences of natural selection. Am. Nat. 104, 1–24 10.1086/282637 (doi:10.1086/282637) [DOI] [Google Scholar]
- 11.Kirkwood T. B. L. 1977. Evolution of ageing. Nature 270, 301–304 10.1038/270301a0 (doi:10.1038/270301a0) [DOI] [PubMed] [Google Scholar]
- 12.van Noordwijk A. J., de Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142 10.1086/284547 (doi:10.1086/284547) [DOI] [Google Scholar]
- 13.Williams G. C. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 10.2307/2406060 (doi:10.2307/2406060) [DOI] [Google Scholar]
- 14.Worley Anne C., Houle D., Barrett Spencer C. 2003. Consequences of hierarchical allocation for the evolution of life history traits. Am. Nat. 161, 153–167 10.1086/345461 (doi:10.1086/345461) [DOI] [PubMed] [Google Scholar]
- 15.Tatar M., Carey J. R. 1995. Nutrition mediates reproductive trade-offs with age-specific mortality in the beetle Callosobruchus maculatus. Ecology 76, 2066–2073 10.2307/1941681 (doi:10.2307/1941681) [DOI] [Google Scholar]
- 16.Zera A. J., Harshman L. G. 2001. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 32, 95–126 10.1146/annurev.ecolsys.32.081501.114006 (doi:10.1146/annurev.ecolsys.32.081501.114006) [DOI] [Google Scholar]
- 17.Bell G. 1980. The costs of reproduction and their consequences. Am. Nat. 116, 45–76 10.1086/283611 (doi:10.1086/283611) [DOI] [Google Scholar]
- 18.King E. G., Roff D. A., Fairbairn D. J. 2011. Trade-off acquisition and allocation in Gryllus firmus: a test of the Y-model. J. Evol. Biol. 24, 256–264 10.1111/j.1420-9101.2010.02160.x (doi:10.1111/j.1420-9101.2010.02160.x) [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood T. B., Holliday R. 1979. The evolution of ageing and longevity. Proc. R. Soc. Lond. B 205, 531–546 10.1098/rspb.1979.0083 (doi:10.1098/rspb.1979.0083) [DOI] [PubMed] [Google Scholar]
- 20.Kirkwood T. B. L., Rose M. R. 1991. Evolution of senescence: late survival sacrificed for reproduction. Phil. Trans. R. Soc. Lond. B 332, 15–24 10.1098/rstb.1991.0028 (doi:10.1098/rstb.1991.0028) [DOI] [PubMed] [Google Scholar]
- 21.Williams G. C. 1966. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 100, 687–690 10.1086/282461 (doi:10.1086/282461) [DOI] [Google Scholar]
- 22.Fanson B. G., Taylor P. W. In press Protein:carbohydrate ratios explain lifespan patterns found in Queensland fruit fly on diets varying in yeast:sugar ratios. Age. 10.1007/s11357-011-9308-3 (doi:10.1007/s11357-011-9308-3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanson B. G., Weldon C. W., Perez-Staples D., Simpson S. J., Taylor P. W. 2009. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni). Aging Cell 8, 514–523 10.1111/j.1474-9726.2009.00497.x (doi:10.1111/j.1474-9726.2009.00497.x) [DOI] [PubMed] [Google Scholar]
- 24.O'Brien D. M., Min K. J., Larsen T., Tatar M. 2008. Use of stable isotopes to examine how dietary restriction extends Drosophila lifespan. Curr. Biol. 18, R155–R156 10.1016/j.cub.2008.01.021 (doi:10.1016/j.cub.2008.01.021) [DOI] [PubMed] [Google Scholar]
- 25.Maklakov A. A., Simpson S. J., Zajitschek F., Hall M. D., Dessmann J., Clissold F., Raubenheimer D., Bonduriansky R., Brooks R. C. 2008. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr. Biol. 18, 1062–1066 10.1016/j.cub.2008.06.059 (doi:10.1016/j.cub.2008.06.059) [DOI] [PubMed] [Google Scholar]
- 26.Lee K. P., Simpson S. J., Clissold F. J., Brooks R., Ballard J. W. O., Taylor P. W., Soran N., Raubenheimer D. 2008. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl Acad. Sci. USA 105, 2498–2503 10.1073/pnas.0710787105 (doi:10.1073/pnas.0710787105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Jong G., van Noordwijk A. J. 1992. Acquisition and allocation of resources: genetic (co)variances, selection, and life histories. Am. Nat. 139, 749–770 10.1086/285356 (doi:10.1086/285356) [DOI] [Google Scholar]
- 28.Simpson S. J., Raubenheimer D. 2009. Macronutrient balance and lifespan. Aging 1, 875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raubenheimer D., Lee K. P., Simpson S. J. 2005. Does Bertrand's rule apply to macronutrients? Proc. R. Soc. B 272, 2429–2434 10.1098/rspb.205.3271 (doi:10.1098/rspb.205.3271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayala V., Naudi A., Sanz A., Caro P., Portero-Otin M., Barja G., Pamplona R. 2007. Dietary protein restriction decreases oxidative protein damage, peroxidizability index, and mitochondrial complex I content in rat liver. J. Gerontol. A Biol. Sci. Med. Sci. 62, 352–360 10.1093/gerona/62.4.352 (doi:10.1093/gerona/62.4.352) [DOI] [PubMed] [Google Scholar]
- 31.Sanz A., Caro P., Barja G. 2004. Protein restriction without strong caloric restriction decreases mitochondrial oxygen radical production and oxidative DNA damage in rat liver. J. Bioenerg. Biomembr. 36, 545–552 10.1007/s10863-004-9001-7 (doi:10.1007/s10863-004-9001-7) [DOI] [PubMed] [Google Scholar]
- 32.Cheng K., Simpson S. J., Raubenheimer D. 2008. A geometry of regulatory scaling. Am. Nat. 172, 681–693 10.1086/591686 (doi:10.1086/591686) [DOI] [PubMed] [Google Scholar]
- 33.Fanson B. G., Yap S., Taylor P. W. 2012. Geometry of compensatory feeding and water consumption in Drosophila melanogaster. J. Exp. Biol. 215, 766–773 10.1242/jeb.066860 (doi:10.1242/jeb.066860) [DOI] [PubMed] [Google Scholar]
- 34.Simpson S., Raubenheimer D. 1999. Assuaging nutritional complexity, a geometrical approach. Proc. Nutr. Soc. 58, 779–789 10.1017/S0029665199001068 (doi:10.1017/S0029665199001068) [DOI] [PubMed] [Google Scholar]
- 35.Piper Matthew D. W., Partridge L., Raubenheimer D., Simpson Stephen J. 2011. Dietary restriction and aging: a unifying perspective. Cell Metab. 14, 154–160 10.1016/j.cmet.2011.06.013 (doi:10.1016/j.cmet.2011.06.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson S. J., Raubenheimer D. 2007. Caloric restriction and aging revisited: the need for a geometric analysis of the nutritional bases of aging. J. Gerontol. A Biol. Sci. Med. Sci. 62, 707–713 10.1093/gerona/62.7.707 (doi:10.1093/gerona/62.7.707) [DOI] [PubMed] [Google Scholar]
- 37.Perez-Staples D., Harmer A. M. T., Collins S. R., Taylor P. W. 2008. Potential for pre-release diet supplements to increase the sexual performance and longevity of male Queensland fruit flies. Agric. Entomol. 10, 255–262 10.1111/j.1461-9563.2008.00385.x (doi:10.1111/j.1461-9563.2008.00385.x) [DOI] [Google Scholar]
- 38.Perez-Staples D., Prabhu V., Taylor P. W. 2007. Post-teneral protein feeding enhances sexual performance of Queensland fruit flies. Physiol. Entomol. 32, 225–232 10.1111/j.1365-3032.2007.00568.x (doi:10.1111/j.1365-3032.2007.00568.x) [DOI] [Google Scholar]
- 39.Taylor P., Perez-Staples D., Weldon C., Collins S., Fanson B., Smallridge C., Yap S. In press. Post-teneral nutrition as an influence on longevity, reproduction and sexual performance of Queensland fruit flies: a review of current evidence. J. Appl. Entomol. 10.1111/j.1439-0418.2011.01644.x (doi:10.1111/j.1439-0418.2011.01644.x) [DOI] [Google Scholar]
- 40.Meats A., Leighton S. M. 2004. Protein consumption by mated, unmated, sterile and fertile adults of the Queensland fruit fly, Bactrocera tryoni and its relation to egg production. Physiol. Entomol. 29, 176–182 10.1111/j.1365-3032.2004.00383.x (doi:10.1111/j.1365-3032.2004.00383.x) [DOI] [Google Scholar]
- 41.Collins S. R., Weldon C. W., Banos C., Taylor P. W. 2008. Effects of irradiation dose rate on quality and sterility of Queensland fruit flies, Bactrocera tryoni (Froggatt). J. Appl. Entomol. 132, 398–405 10.1111/j.1439-0418.2008.01284.x (doi:10.1111/j.1439-0418.2008.01284.x) [DOI] [Google Scholar]
- 42.Collins S. R., Weldon C. W., Banos C., Taylor P. W. 2009. Optimizing irradiation dose for sterility induction and quality of Bactrocera tryoni. J. Econ. Entomol. 102, 1791–1800 10.1603/029.102.0509 (doi:10.1603/029.102.0509) [DOI] [PubMed] [Google Scholar]
- 43.Hooper G. H. S. 1989. The effect of ionizing radiation on reproduction. In Fruit flies: their biology, natural enemies and control, World crop pests (eds Robinson A. S., Hooper G. H. S.), pp. 153–164 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 44.Monro J., Bailey P. T. 1965. Influence of radiation on ovarian maturation and histolysis of pupal fat body in Diptera. Nature 207, 437–438 10.1038/207437a0 (doi:10.1038/207437a0) [DOI] [PubMed] [Google Scholar]
- 45.Monro J., Osborn A. W. 1967. The use of sterile males to control populations of Queensland fruit fly, Dacus tryoni (Frogg.) (Diptera: Tephritidae). 1. Method of mass rearing, transporting, irradiating and releasing sterile flies. Aust. J. Zool. 15, 461–473 10.1071/ZO9670461 (doi:10.1071/ZO9670461) [DOI] [Google Scholar]
- 46.Fanson B. G., Taylor P. W. 2012. Additive and interactive effects of nutrient classes on longevity, reproduction, and diet consumption in the Queensland fruit fly (Bactrocera tryoni). J. Insect Physiol. 58, 327–334 10.1016/j.jinsphys.2011.11.002 (doi:10.1016/j.jinsphys.2011.11.002) [DOI] [PubMed] [Google Scholar]
- 47.Meats A., Kelly G. L. 2008. Relation of constant, daily fluctuating, and ambient feeding temperature to daily and accumulated consumption of yeast autolysate and sucrose by female Queensland fruit fly. Entomol. Exp. Appl. 129, 87–95 10.1111/j.1570-7458.2008.00755.x (doi:10.1111/j.1570-7458.2008.00755.x) [DOI] [Google Scholar]
- 48.Littell R. C., Milliken G. A., Stroup W. W., Wolfinger R. D., Schabenberger O. 2006. SAS System for Mixed Models. Cary, NC: SAS Institute Inc [Google Scholar]
- 49.Myers R. H., Montgomery D. C., Anderson-Cook C. M. 2009. Response surface methodology: process and product optimization using designed experiments. Hoboken, NJ: Wiley [Google Scholar]
- 50.Edward D. A., Chapman T. 2011. Mechanisms underlying reproductive trade-offs: costs of reproduction. In Mechanisms of life history evolution: the genetics and physiology of life history traits and trade-offs (eds Flatt T., Heyland A.), pp. 137–152 Oxford, UK: Oxford University Press [Google Scholar]
- 51.Lessells K., Colegrave N. 2001. Molecular signals or the Loi de Balancement? Trends Ecol. Evol. 16, 284–285 10.1016/s0169-5347(01)02162-0 (doi:10.1016/s0169-5347(01)02162-0) [DOI] [PubMed] [Google Scholar]
- 52.Pérez-Staples D., Weldon C. W., Taylor P. W. 2011. Sex differences in developmental response to yeast hydrolysate supplements in adult Queensland fruit fly. Entomol. Exp. Appl. 141, 103–113 10.1111/j.1570-7458.2011.01173.x (doi:10.1111/j.1570-7458.2011.01173.x) [DOI] [Google Scholar]
- 53.Flatt T. 2011. Survival costs of reproduction in Drosophila. Exp. Gerontol. 46, 369–375 10.1016/j.exger.2010.10.008 (doi:10.1016/j.exger.2010.10.008) [DOI] [PubMed] [Google Scholar]
- 54.Kenyon C. 2010. A pathway that links reproductive status to lifespan in Caenorhabditis elegans. Ann. NY Acad. Sci. 1204, 156–162 10.1111/j.1749-6632.2010.05640.x (doi:10.1111/j.1749-6632.2010.05640.x) [DOI] [PubMed] [Google Scholar]
- 55.Leroi A. M. 2001. Molecular signals versus the Loi de Balancement. Trends Ecol. Evol. 16, 24–29 10.1016/S0169-5347(00)02032-2 (doi:10.1016/S0169-5347(00)02032-2) [DOI] [PubMed] [Google Scholar]
- 56.Flatt T., Min K.-J., D'Alterio C., Villa-Cuesta E., Cumbers J., Lehmann R., Jones D. L., Tatar M. 2008. Drosophila germ-line modulation of insulin signaling and lifespan. Proc. Natl Acad. Sci. USA 105, 6368–6373 10.1073/pnas.0709128105 (doi:10.1073/pnas.0709128105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Partridge L. 2011. Ageing in Drosophila: the role of the insulin/Igf and TOR signalling network. Exp. Gerontol. 46, 376–381 10.1016/j.exger.2010.09.003 (doi:10.1016/j.exger.2010.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grandison R. C., Piper M. D. W., Partridge L. 2009. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462, 1061–1064 10.1038/nature08619 (doi:10.1038/nature08619) [DOI] [PMC free article] [PubMed] [Google Scholar]