Abstract
North American birds that feed on flying insects are experiencing steep population declines, particularly long-distance migratory populations in the northern breeding range. We determine, for the first time, the level of migratory connectivity across the range of a songbird using direct tracking of individuals, and test whether declining northern populations have higher exposure to agricultural landscapes at their non-breeding grounds in South America. We used light-level geolocators to track purple martins, Progne subis, originating from North American breeding populations, coast-to-coast (n = 95 individuals). We show that breeding populations of the eastern subspecies, P. s. subis, that are separated by ca. 2000 km, nevertheless have almost completely overlapping non-breeding ranges in Brazil. Most (76%) P. s. subis overwintered in northern Brazil near the Amazon River, not in the agricultural landscape of southern Brazil. Individual non-breeding sites had an average of 91 per cent forest and only 4 per cent agricultural ground cover within a 50 km radius, and birds originating from declining northern breeding populations were not more exposed to agricultural landscapes than stable southern breeding populations. Our results show that differences in wintering location and habitat do not explain recent trends in breeding population declines in this species, and instead northern populations may be constrained in their ability to respond to climate change.
Keywords: geolocator, songbird, South America
1. Introduction
Population dynamics of neotropical migratory songbirds are driven by the interaction of productivity on the breeding grounds and mortality, which occurs primarily during migration and the non-breeding season in the tropics [1–3]. Migratory birds are predicted to be more vulnerable to habitat disturbance and environmental change at their wintering grounds if they exhibit strong migratory connectivity, in which most members of a given breeding population migrate to the same region within the non-breeding range [4,5]. In contrast, species with weak migratory connectivity are buffered from habitat disturbance in specific non-breeding regions because reduced survival of individuals is spread diffusely across the breeding range [6]. However, understanding the population dynamics of long-distance migratory songbirds has been hampered by the difficulty in determining migratory connectivity [2,6] because until recently it was not possible to track migration of songbirds [7].
Connectivity patterns between breeding and wintering sites thousands of kilometres apart have been determined for migratory songbirds using large-scale band-recovery efforts [8–10], but for many species recovery records linking breeding and tropical ‘wintering’ sites are too infrequent. Stable isotope values of feathers, and to a lesser extent genetic markers, have been used to document migratory connectivity in several songbirds, but precision is generally restricted to large geographic regions [11–14]. Songbirds can exhibit coarse patterns of east–west parallel connectivity at a continental [10,12,14,15] or sub-continental [8,16] scale. Less common are patterns of leapfrog migration, where northern breeding populations have relatively southern non-breeding destinations [17], and crossover connectivity, in which western populations are connected to eastern non-breeding regions [18].
Many aerial insectivores in North America, particularly long-distance migrants, have experienced steep population declines since the mid-1980s [19,20]. There is a strong geographic pattern in declines, which are more prevalent towards the northeast of North America. One explanation for this pattern is that north-eastern regions receive relatively high levels of atmospheric pollutants, including acid precipitation, which in turn has negative effects on insect abundance and thus productivity of aerial insectivores [20]. Climate change is also expected to disproportionately affect populations in more seasonal habitats due to a phenological mismatch between food availability and timing of breeding in long-distance migrants [21]. Agricultural pesticide use may also influence aerial insectivore populations, through broad changes in their prey base [22]. Nebel et al. [20] suggested that northern populations may face higher mortality on the wintering grounds due to agricultural pesticides in South America, either through direct mortality or indirectly via reduction in food availability [23]. Associations with agricultural landscapes in the winter quarters could increase exposure to pesticides and their effects, potentially influencing year-round population dynamics of long-distance migratory birds. If this latter hypothesis is correct, then migratory connectivity should be strong in aerial insectivores and northern populations are predicted to have stronger associations with agricultural landscapes in the non-breeding season.
For the first time, using light-level geolocators, we tracked individual (n = 95) songbirds that originated from coast-to-coast breeding populations in North America and travelled to wintering sites in South America. Purple martins, Progne subis, are declining throughout much of their northern range [19,20] and their non-breeding range extends from the relatively undisturbed upper Amazon basin down to southern Brazil where the landscape has been converted almost entirely to agricultural use. Our primary objectives were to (i) measure the range-wide degree of migratory connectivity between breeding and non-breeding populations of purple martin and (ii) determine whether northern populations of this declining aerial insectivore may face distinct threats through greater association with agricultural habitat at their non-breeding grounds in South America.
2. Methods
(a). Geolocator deployment
Purple martins were captured and fitted with geolocators during the breeding season by trapping birds in nest boxes at eight breeding locations across the range (n = 421, electronic supplementary material, table S1). Geolocators (≤1.6 g; MK10s/12/12s/14s/20, British Antarctic Survey) were mounted using a leg-loop backpack harness [7,24] constructed with Teflon ribbon or, in some cases, polypropylene thread. Geolocators were retrieved (n = 120) at the same breeding sites in the year following deployment. Geolocator battery failure prior to arrival and residency at winter roosts reduced sample size to 95. At the Pennsylvania breeding site (2009–2011), the return rate of birds wearing geolocators was not lower than that of banded birds without geolocators (see the electronic supplementary material, table S2). Harness failure occurred for 10 per cent of birds when using a thread harness but only 3 per cent of birds using a Teflon harness.
(b). Analysis of light data from geolocators
Geolocators measured the intensity of visible light every 1 min and recorded the maximum reading within each 10 min interval (MK16, MK10) or each 2 min interval (MK12, MK20). Raw light data were corrected for clock drift (1–3 min lost during 10-month deployment) using BASTrak and analysed using TransEdit (British Antarctic Survey). We manually verified a sharp transition at each sunrise and sunset and ignored obvious shading events during the daytime. We used a light threshold level of 32 (MK16, MK10) or 5 (MK12, MK20) to define sunrise and sunset transitions, and used live calibration data (see below) from birds prior to migration to determine the average sun elevation that corresponded with this light threshold level at the breeding site. Transitions with light peaks or non-linear transitions before sunrise or after sunset were rejected from further analysis. Latitude was not determined for 15 days before and after the fall equinox when day length is similar everywhere.
Latitude and longitude coordinates were calculated with Locator software (British Antarctic Survey) using midnight locations because purple martins are diurnal migrants, and therefore midnight locations should be more accurate because birds are stationary during the night. No compensation for longitudinal movement was made when estimating latitude because birds were assumed to be stationary from sunset to sunrise. Locations that were clearly anomalous (i.e. >1000 km from previous location) were rejected as outliers.
All geolocators received a static pre-deployment and post-deployment calibration for 1 week in open habitat with a clear view of the horizon, to assess light sensitivity of units. Light sensitivity was virtually identical among units of similar model and was similar before and after deployment. Analysis of live locations for birds prior to migration was used to determine the average sun elevation (BASTrak, British Antarctic Survey) that corresponds to the light level (32 or 5, depending on geolocator model) that was used to define sunrise and sunset transitions. The sun elevation, in turn, is used in Locator (British Antarctic Survey) to determine location given the sun's position on a given date and time. Sun elevation was calculated separately for different geolocator models, after birds finished nesting but before migration, and averaged across individuals within each year to better represent average conditions for migrating birds at unknown locations.
(c). Estimating geolocator error
Geolocation accuracy was estimated for birds at each of the eastern breeding populations by averaging locations of individuals in late July and early August, prior to autumn migration. Average geolocator estimates closely matched breeding sites (see the electronic supplementary material, table S3), and geolocator positions for individuals mismatched breeding sites by an average of 49–60 km in latitude (range: 0–210 km) and 38–48 km in longitude (0–196 km). Differences in geolocation accuracy among individuals reflect individual pre-migration habitat at dawn and dusk and differing weather conditions at the time of live calibration. During the pre-migration period, the standard error of latitude for an individual ranged from 0.15 to 1.0 (avg. 0.3) and for longitude ranged from 0.06 to 0.36 (avg. 0.2).
Arrival at the wintering ground was considered to have occurred when the latitude and longitude ceased to shift in a direction consistent with autumn migration, fluctuated around a narrow range of values (less than 2° longitude), consistent with a stationary bird, and fluctuated around a similar value for at least 10 days within the winter range. During stationary periods at the wintering grounds, location was determined by calculating average latitude and longitude during the period. Most (63 of 95) martins shifted roost locations while at the wintering grounds, averaging ca. 700 km between sites.
(d). Band recovery data for connectivity
Band recovery data can be used to independently assess migratory connectivity, and to supplement geolocator tracking data [8]. Analysis of North American band recovery data (1921–2010) for purple martins banded at breeding sites (n = 2884 recoveries) identified 12 birds that were recovered in South America after autumn migration and before spring migration (see the electronic supplementary material, figure S1). However, 11 of 12 birds were banded as a nestling and therefore their first breeding site was unknown. Band recovery data were therefore not included in our analyses.
(e). GIS data and analysis
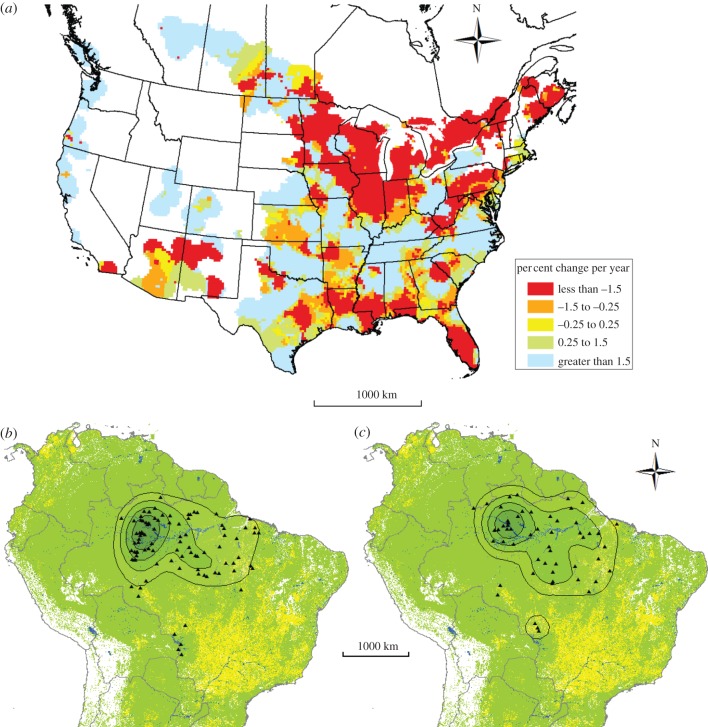
Northern breeding populations of purple martins (P. s. subis) are declining more severely than central or southern populations (figure 2a). For all roost sites in South America occupied for ≥ 30 days, we used kernel density analysis to test whether the core wintering region of northern breeding populations was associated with more extensive agricultural land use than breeding populations from central and southern regions. We measured winter roost density of northern (SD, MN, PA, NJ, n = 59 individuals, 97 roosts) versus southern and central (VA, OK, TX, n = 30 individuals, 55 roosts) breeding populations based on points of 1–3 roosts per individual bird using fixed kernel densities in the program ArcGIS 10 [25]. We determined kernel densities at 20, 40, 60 and 80 per cent of the total density using a sample radius of 50 km (or 0.45 decimal degrees) and a cell size of 1 km2. We derived land-cover data for the purple martin wintering range in South America from Eva et al. [26]. We calculated per cent of agricultural land-cover versus forest and other vegetated, non-agricultural cover (hereafter called forest) within a 50 km radius (which corresponds to longitudinal geolocator error) around each winter roost and compared roosts of northern versus south-central breeding birds using a t-test.
Figure 2.
(a) Population trends based on the BBS data (1966–2010). Kernel density of all winter roost locations occupied for ≥30 days by martins (P. s. subis) from (b) northern breeding populations (Minnesota, South Dakota, Pennsylvania, New Jersey; n = 59 individuals, 97 roosts) and (c) central/southern breeding populations (Oklahoma, Texas, Virginia; n = 30 individuals, 55 roosts). Maps show kernels of 20, 40, 60 and 80% of the total density. Green shading represents forest and non-agricultural vegetated cover; yellow shading represents agricultural lands.
3. Results
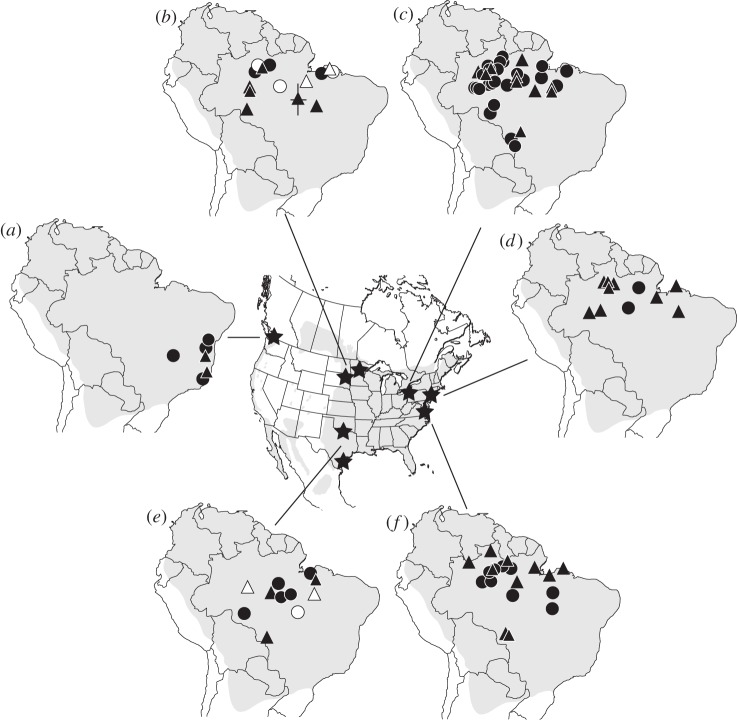
Migratory connectivity was very weak, and there was extensive overlap in non-breeding sites within South America for martins originating from across eastern North America (figure 1). Individuals from breeding sites up to 2000 km apart in both latitude and longitude were often mapped to within 100 km of each other in South America, the limit of geolocator accuracy. Birds from the western subspecies, P. s. arboricola (n = 6), appear to have a distinct wintering region in southeastern Brazil, whose core area is ca. 3000 km from the core wintering region of the eastern subspecies.
Figure 1.
Migratory connectivity of purple martin breeding populations tracked with geolocators to South America; range shown with grey shading. The site with the longest winter residency (124 ± 5.4 days, n = 95) is shown for each individual (triangles are males, circles are females) from (a) British Columbia (n = 6), (b) Minnesota (white, n = 5) and South Dakota (black, n = 9), (c) Pennsylvania (n = 34), (d) New Jersey (n = 11), (e) Oklahoma (white, n = 3) and Texas (black, n = 8), and (f) Virginia (n = 19). British Columbia birds are Progne subis arboricola and all other populations are P. s. subis. Error bars for roost location in (b) shows typical standard deviation in latitude and longitude for estimated winter locations. Map of North America shows the breeding range in grey and stars indicate the location of geolocator deployments.
Most individuals (76%; 68 of 89) of the eastern subspecies roosted for the longest period in northern Brazil (ca. 6° S to 1° N) from the Rio Negro region eastward to the mouth of the Amazon River (64° to 47° W). Many individuals had multiple roost sites that they occupied for at least 30 days (1 roost: 26%; 2 roosts: 61%; 3 roosts: 11%). Individuals moved an average of 700 km between roost sites (up to 1400 km in some cases), but all these additional roosts were within the same geographic region as defined by the longest occupied roosts. The average occupancy of the longest roost (124 days) was more than double that of other roost sites (2nd longest roost: 55 days, 3rd longest: 43 days). For the longest occupied roosts, there was no significant correlation between breeding and wintering latitude (rs = −0.12, p = 0.80, n = 89), or between breeding and wintering longitude (rs = −0.04, p = 0.77, n = 89) (see the electronic supplementary material, figure S2) and sex was not a significant factor in winter roost location (t = −1.46, df = 90, p = 0.15).
Individuals from a discrete breeding population had a broad distribution at the wintering grounds. For the Pennsylvania population, for instance, the average distance between individuals, for the longest occupied roost sites, was 903 km (±23 km SE, n = 34 individuals, 561 comparisons) with an average nearest neighbour distance of 140 ± 20 km and average farthest neighbour distance of 1787 ± 336 km.
Birds originating from northern and south-central North American breeding populations shared a similar and mostly overlapping over-wintering area centred in northern Brazil near the Amazon River (figure 2b). The distance between the centre of northern (lat. −2.51°, long. −62.19°) and south-central (lat. −1.34°, long. −61.6°) wintering ranges was just 144 km, within a region dominated by relatively undisturbed evergreen tropical forest (figure 2b). Ground cover within 50 km of overwintering roosts was mostly forest (average for all birds 91% ± 1 forest and 4% ± 0.8 agriculture). Northern breeding birds did not have greater per cent agricultural ground cover at winter roosts than birds from southern and central breeding sites (t = −0.59, df = 109, p = 0.55).
4. Discussion
For the first time, we determined the degree of range-wide migratory connectivity between northern breeding populations and corresponding non-breeding areas in the tropics using direct tracking of individual songbirds. We show that seven breeding populations of the eastern subspecies of purple martin (Progne s. subis) exhibit very weak connectivity, and share a broad, overlapping non-breeding region along the Amazon River in northern Brazil. This is remarkable, considering that breeding populations were separated by up to 2000 km, and other songbird species have exhibited sub-continental patterns of connectivity [8,16]. This shared core area encompasses only about 20 per cent of the entire wintering distribution of the species, yet supports an estimated 80 per cent of the eastern subspecies. Threats to the core overwintering areas in the upper Amazon could therefore influence population dynamics across the eastern breeding range of purple martin.
Long-distance migratory songbirds that feed on aerial insects, as a group, show strong breeding population declines, with steeper declines in more northern populations of several species [20]. Direct tracking of individual Swainson's hawk (Buteo swainsonii) using satellite tags revealed that breeding population declines in North America were likely a result of intensive spraying of organophosphate insecticides and associated mortality (approx. 1% of global population) at wintering sites in South America [23,27]. Similarly, range-wide declines in dickcissel (Spiza americana) were attributed to associations with intensive agriculture and persecution at their South American wintering sites [28]. The use of agricultural habitats in the non-breeding season could expose purple martins to pesticides, causing either direct mortality or reduced food availability with subsequent fitness costs, potentially contributing to declines observed at breeding areas [20]. However, we found no significant difference between declining and stable breeding populations in the use of agricultural non-breeding habitat. The core ‘wintering’ region for the eastern subspecies of purple martin, including declining northern breeding populations, is dominated by largely undisturbed tropical rainforest (figure 2b). Our kernel density analyses show that the centre of the core over-wintering area of northern and south-central birds was only 144 km apart. Owing to this overlap, eastern breeding populations likely experience similar conditions at their overwintering sites in South America, and wintering ground events are unlikely to be the cause of differential population declines in breeding populations. Thus we conclude that exposure to agricultural landscapes does not appear to be the cause of declines of northern breeding populations.
Proposed alternative explanations for population declines of purple martins (and other aerial insectivores) include the effects of acid rain on prey abundance at breeding sites, which is greatest in the northeast, and climate change [20]. For the latter, constrained migratory schedules can limit adaptive responses to climate change, and can result in severe population declines of long-distance migratory songbirds [21,29,30], and these patterns are expected to be stronger for birds in more seasonal habitats [21], such as more northern breeding sites of purple martin.
Populations of long-distance migratory birds may be most limited during the overwintering and migratory period, thus it is important to determine connections between different periods of the annual cycle in order to better understand, and mitigate, population declines [2]. Determining continent-wide connectivity using direct tracking provided the surprising result that breeding populations separated by up to 2000 km share a broad overwintering region in South America, suggesting that future habitat disturbance in this region would have a broad influence on population dynamics across the range. We also quantified habitat use thousands of kilometres away from breeding sites to show that the patterns of population decline in this long-distance migratory aerial insectivore are not associated with different threats on the wintering grounds. The dramatic increase in direct tracking of songbirds will soon establish the levels of migratory connectivity in a wide range of species, allowing for better conservation and management of declining songbirds.
Acknowledgements
This was a large collaborative project that involved dozens of private and public funding sources and scores of volunteers (see the electronic supplementary material, additional acknowledgements). In particular, we thank R. Aeppli of the PMCA for extensive fieldwork. E. A. McKinnon provided comments on the manuscript and S. Barretto contributed GIS analysis and mapping. This work was funded by the Natural Sciences and Engineering Research Council of Canada, National Geographic Society, proceeds from Silence of the Songbirds (Stutchbury 2007, Walker & Co.), Coastal Bend Audubon Society, National Audubon Society, North American Bluebird Society, Shell Canada Environment Fund, TD Friends of the Environment, Canadian Wildlife Foundation, Monmouth County Audubon Society, United States Golf Association, Purple Martin Society of Collier County, Wildlife Diversity Small Grant Program of the South Dakota Department of Game, Fish & Parks and Purple Martin Conservation Association. Vancouver Island University Biology Dept. staff provided administrative, scientific and field support for the BC study. We thank Eli Bridge and Jeff Kelley for sharing their geolocator data from Oklahoma; their geolocator design was funded by the National Science Foundation (NSF: EAGER 0946685).
References
- 1.Sillett T. S., Holmes R. T. 2002. Variation in survivorship of a migratory songbird throughout its annual cycle. J. Anim. Ecol. 71, 296–308 10.1046/j.1365-2656.2002.00599.x (doi:10.1046/j.1365-2656.2002.00599.x) [DOI] [Google Scholar]
- 2.Faaborg J., et al. 2010. Conserving migratory land birds in the New World: do we know enough? Ecol. Appl. 20, 398–418 10.1890/09-0397.1 (doi:10.1890/09-0397.1) [DOI] [PubMed] [Google Scholar]
- 3.Sherry T. W., Holmes R. T. 1995. Summer versus winter limitation of populations: conceptual issues and evidence. In Ecology and management of Neotropical migratory birds: a synthesis and review of critical issues (eds Martin T., Finch D.). New York: Oxford University Press [Google Scholar]
- 4.Sheehy J., Taylor C. M., McCann K. S., Norris D. R. 2010. Optimal conservation planning for migratory animals: integrating demographic information across seasons. Conserv. Lett. 3, 192–202 10.1111/j.1755-263x.2010.00100.x (doi:10.1111/j.1755-263x.2010.00100.x) [DOI] [Google Scholar]
- 5.Marra P. P., Norris D. R., Haig S. M., Webster M. S., Royle J. A. 2006. Migratory connectivity. In Connectivity conservation (eds Crooks K. R., Sanjayan M.), pp. 157–183 New York: Cambridge University Press [Google Scholar]
- 6.Webster M. S., Marra P. P., Haig S. M., Bensch S., Holmes R. T. 2002. Links between worlds: unraveling migratory connectivity. Trends Ecol. Evol. 17, 76–83 10.1016/S0169-5347(01)02380-1 (doi:10.1016/S0169-5347(01)02380-1) [DOI] [Google Scholar]
- 7.Stutchbury B. J. M., Tarof S. A., Done T., Gow E., Kramer P. M., Tautin J., Fox J. W., Afanasyev V. 2009. Tracking long-distance songbird migration by using geolocators. Science 323, 896. 10.1126/science.1166664 (doi:10.1126/science.1166664) [DOI] [PubMed] [Google Scholar]
- 8.Ryder T. B., Fox J. W., Marra P. P. 2011. Estimating migratory connectivity of Gray Catbirds (Dumetella carolinensis) using geolocator and mark-recapture data. Auk 128, 448. 10.1525/auk.2011.128.4.810 (doi:10.1525/auk.2011.128.4.810) [DOI] [Google Scholar]
- 9.Ambrosini R., Moller A. P., Saino N. 2009. A quantitative measure of migratory connectivity. J. Theor. Biol. 257, 203–211 10.1016/j.jtbi.2008.11.019 (doi:10.1016/j.jtbi.2008.11.019) [DOI] [PubMed] [Google Scholar]
- 10.Boulet M., Norris D. R. 2006. Patterns of migratory connectivity in two Nearctic-neotropical songbirds: new insights from intrinsic markers, vii, pp. 88. Washington, DC: American Ornithologists’ Union [Google Scholar]
- 11.Clegg S. M., Kelly J. F., Kimura M., Smith T. B. 2003. Combining genetic markers and stable isotopes to reveal population connectivity and migration patterns in a Neotropical migrant, Wilson's warbler (Wilsonia pusilla). Mol. Ecol. 12, 819–830 10.1046/j.1365-294X.2003.01757.x (doi:10.1046/j.1365-294X.2003.01757.x) [DOI] [PubMed] [Google Scholar]
- 12.Irwin D. E., Irwin J. H., Smith T. B. 2011. Genetic variation and seasonal migratory connectivity in Wilson's warblers (Wilsonia pusilla): species-level differences in nuclear DNA between western and eastern populations. Mol. Ecol. 20, 3102–3115 10.1111/j.1365-294x.2011.05159.x (doi:10.1111/j.1365-294x.2011.05159.x) [DOI] [PubMed] [Google Scholar]
- 13.Chabot A. A., Hobson K. A., Van Wilgenburg S. L., McQuat G. J., Lougheed S. C. 2012. Advances in linking wintering migrant birds to their breeding-ground origins using combined analyses of genetic and stable isotope markers. PLoS ONE 7, e43627. 10.1371/journal.pone.0043627 (doi:10.1371/journal.pone.0043627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norris D. R., Marra P. P., Bowen G. J., Ratcliffe L. M., Royle J. A., Kyser T. K. 2006. Migratory connectivity of a widely distributed Nearctic-Neotropical songbird, the American Redstart. Ornithol. Monogr. 61, 14–28 10.2307/40166836 (doi:10.2307/40166836) [DOI] [Google Scholar]
- 15.Ruegg K., Smith T. B. 2002. Not as the crow flies: a historical explanation for the circuitous migration in Swainson's thrush (Catharus ustulatus). Proc. R. Soc. Lond. B 269, 1375–1381 10.1098/rspb.2002.2032 (doi:10.1098/rspb.2002.2032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones J., Norris D. R., Girvan M. K., Barg J. J., Kyser T. K., Robertson R. J. 2008. Migratory connectivity and rate of population decline in a vulnerable songbird. Condor 110, 538–544 10.1525/cond.2008.8563 (doi:10.1525/cond.2008.8563) [DOI] [Google Scholar]
- 17.Bell C. P. 1997. Leap-frog migration in the fox sparrow: minimizing the cost of spring migration. Condor 99, 470–477 10.2307/1369953 (doi:10.2307/1369953) [DOI] [Google Scholar]
- 18.Rubenstein D. R., Chamberlain C. P., Holmes R. T., Ayres M. P., Waldbauer J. R., Graves G. R., Tuross N. C. 2002. Linking breeding and wintering ranges of a migratory songbird using stable isotopes. Science 295, 1062–1065 10.1126/science.1067124 (doi:10.1126/science.1067124) [DOI] [PubMed] [Google Scholar]
- 19.Sauer J. R., Hines J. E., Fallon J. E., Pardieck K. L., Ziolkoski D. J., Jr, Link W. A. 2011. The North American breeding bird survey, results and analysis 1966–2009. Laurel: USGS Patuxent Wildlife Research Center [Google Scholar]
- 20.Nebel S., Mills A., McCracken J. D., Taylor P. D. 2010. Declines of aerial insectivores in North America follow a geographic gradient. Avian Conserv Ecol 5, 1. 10.5751/ACE-00391-050201 (doi:10.5751/ACE-00391-050201) [DOI] [Google Scholar]
- 21.Both C., Van Turnhout C. A., Bijlsma R. G., Siepel H., Van Strien A. J., Foppen R. P. 2010. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. R. Soc. B 277, 1259–1266 10.1098/rspb.2009.1525 (doi:10.1098/rspb.2009.1525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nocera J. J., et al. 2012. Historical pesticide applications coincided with an altered diet of aerially foraging insectivorous chimney swifts. Proc. R. Soc. B 279, 3114–3120 10.1098/rspb.2012.0445 (doi:10.1098/rspb.2012.0445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein M. I., et al. 1999. Monocrotophos-induced mass mortality of Swainson's Hawks in Argentina. Ecotoxicology 8, 201–214 10.1023/A:1026496331396 (doi:10.1023/A:1026496331396) [DOI] [Google Scholar]
- 24.Stutchbury B. J. M., Gow E. A., Done T., MacPherson M., Fox J. W., Afanasyev V. 2011. Effects of post-breeding moult and energetic condition on timing of songbird migration into the tropics. Proc. R. Soc. B 278, 131–137 10.1098/rspb.2010.1220 (doi:10.1098/rspb.2010.1220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ESRI. 2011. ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute [Google Scholar]
- 26.Eva H. D., et al. 2002. A vegetation map of South America (ed. Centre E. C. J. R.). Ispra, Italy: Office for Official Publications of the European Communities [Google Scholar]
- 27.Woodbridge B., Finley K. K., Seager S. T. 1995. An investigation of Swainson's hawk in Argentina. J. Raptor Res. 29, 202–204 [Google Scholar]
- 28.Basili G. D., Temple S. A. 1999. Dickcissels and crop damage in Venezuela: defining the problem with ecological models. Ecol. Appl. 9, 732–739 10.1890/1051-0761(1999)009[0732:DACDIV]2.0.CO;2 (doi:10.1890/1051-0761(1999)009[0732:DACDIV]2.0.CO;2) [DOI] [Google Scholar]
- 29.Both C., Bouwhuis S., Lessells C. M., Visser M. E. 2006. Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83 10.1038/nature04539 (doi:10.1038/nature04539) [DOI] [PubMed] [Google Scholar]
- 30.Both C., Visser M. E. 2001. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298 10.1038/35077063 (doi:10.1038/35077063) [DOI] [PubMed] [Google Scholar]