Abstract
Most vertebrate groups exhibit eye shapes that vary predictably with activity pattern. Nocturnal vertebrates typically have large corneas relative to eye size as an adaptation for increased visual sensitivity. Conversely, diurnal vertebrates generally demonstrate smaller corneas relative to eye size as an adaptation for increased visual acuity. By contrast, several studies have concluded that many mammals exhibit typical nocturnal eye shapes, regardless of activity pattern. However, a recent study has argued that new statistical methods allow eye shape to accurately predict activity patterns of mammals, including cathemeral species (animals that are equally likely to be awake and active at any time of day or night). Here, we conduct a detailed analysis of eye shape and activity pattern in mammals, using a broad comparative sample of 266 species. We find that the eye shapes of cathemeral mammals completely overlap with nocturnal and diurnal species. Additionally, most diurnal and cathemeral mammals have eye shapes that are most similar to those of nocturnal birds and lizards. The only mammalian clade that diverges from this pattern is anthropoids, which have convergently evolved eye shapes similar to those of diurnal birds and lizards. Our results provide additional evidence for a nocturnal ‘bottleneck’ in the early evolution of crown mammals.
Keywords: vision, mammals, functional morphology
1. Introduction
The relationship between eye morphology and activity pattern in different vertebrate groups has been the subject of numerous recent comparative studies [1–12]. Activity pattern—the time of the day when an animal is awake and active—determines the amount of light available for vision, and as such is a key selective influence on the evolution of the vertebrate visual system. Prior research has demonstrated that the functional demands of vision under high-light (photopic) and low-light (scotopic) conditions select for divergent eye morphologies in nocturnal, cathemeral and diurnal species because the vertebrate eye cannot be optimized for high-quality vision under both photopic and scotopic conditions [13–17].
Theoretically, all vertebrates could benefit from having larger eyes regardless of their activity pattern. If other factors (e.g. photoreceptor size, spacing and retinal connectivity) are held constant, absolutely larger eyes have greater resolution than absolutely smaller eyes. Similarly, absolutely larger eyes may gather more light than smaller eyes. As a result, increased eye size may simultaneously benefit diurnal species by increasing visual acuity and nocturnal species by improving visual sensitivity. Axial eye length itself, a common measurement of overall eye size, does not always vary predictably with activity pattern among vertebrates. For example, both lizards [9] and non-primate mammals [6,18] exhibit little interspecific variation in axial eye length according to activity pattern. By comparison, the size of the cornea relative to eye length (henceforth ‘relative cornea size’) usually varies in a highly predictable manner with activity pattern. Among both birds and lizards, nocturnal species nearly always exhibit a larger corneal diameter than a diurnal species with the same axial eye length [7,9]. This configuration increases the light-gathering capacity of the eye at maximum pupillary dilation, thus improving visual sensitivity under scotopic light conditions [14,15,17–21]. Conversely, diurnal birds and lizards exhibit smaller relative cornea sizes compared with nocturnal species, and diurnal birds with smaller corneal diameters also consistently exhibit an absolutely longer axial length of the eye [7,9]. This configuration increases the posterior nodal distance of the eye, which increases retinal image size and enhances visual acuity [14,15,17–22]. Cathemeral birds exhibit an intermediate morphology between nocturnal and diurnal birds, with relative cornea sizes that are larger than those of diurnal birds but smaller than those of nocturnal birds [7]. Thus, in both birds and lizards, nocturnal species exhibit eye morphologies with relatively and absolutely larger corneas than diurnal species.
Previous studies of mammals [4,6] have also found significant differences in eye morphology between species of different activity patterns. Within some mammalian clades (e.g. strepsirrhine primates, artiodactyls, carnivorans, marsupials and xenarthrans), nocturnal species have larger relative cornea sizes than either diurnal or cathemeral species [4]. Within strepsirrhines, artiodactyls and carnivorans, cathemeral species also have eye morphologies that are intermediate between those of diurnal and nocturnal species. However, significantly more overlap in relative cornea size exists between mammal species of different activity patterns than between bird or lizard species of different activity patterns [4,6,7,9]. Additionally, when mammals are compared across clades, diurnal and cathemeral species have very similar relative cornea sizes [6]. Anthropoid primates are the only large mammalian clade that deviates substantially from this pattern. Diurnal anthropoids have significantly smaller corneas for their eye size than do other diurnal mammals [4,6,8,23], an important adaptation for increased visual acuity [15,17], and as such follow the more common vertebrate pattern seen in birds [7] and lizards [9]. Indeed, the great majority of non-anthropoid mammals have relative cornea sizes that overlap extensively with those of nocturnal birds and lizards, while diurnal anthropoids have relative cornea sizes that are most similar to those of diurnal birds and lizards [8,23,24]. These previous findings suggest that eye shape in non-anthropoid mammals varies with activity pattern in a manner that differs considerably from that observed in most other amniotes.
One possible explanation for the divergent eye morphologies of mammalian and non-mammalian amniotes lies with the hypothesis of a ‘nocturnal bottleneck’ in early mammalian evolution. According to this scenario, the ancestors of living mammals occupied nocturnal niches during the Mesozoic, possibly to avoid diurnal saurian predators [25]. During this time, mammals evolved a variety of characteristic traits as adaptations for life in a nocturnal setting [20] (but see [26]). These adaptations include major shifts in sensory anatomy and ecology, including the evolution of high-frequency hearing [27], tactile vibrissae [28] and high olfactory sensitivity [29]. At the same time, possibly as a result of relaxed selection, mammals lost many adaptations for photopic vision that were probably present in the last common ancestor of mammals and diapsids, including tetrachromatic colour vision and an active mechanism of visual accommodation [10].
Recent studies by Schmitz & Motani [11,12] argue that a new form of discriminant function analysis, the phylogenetic flexible discriminant function analysis (pFDA), allows them to use measurements of eye shape to accurately predict activity pattern for all terrestrial vertebrates, including non-anthropoid mammals. On the basis of an analysis including 37 extant mammals, Schmitz & Motani [11,12] assert that pFDA provides greater predictive power because it is a multivariate method, while previous studies used bivariate statistics, and this method includes statistical controls for phylogenetic non-independence, which are important for analysing differences between related species [30,31]. In addition, Schmitz & Motani argue that the pFDA method allows them to use eye-shape measurements to accurately predict species with cathemeral activity patterns, which previous studies were not able to resolve across mammal groups using bivariate statistics [4,6,8]. If Schmitz & Motani [11,12] are correct, then their results cast doubt on the nocturnal bottleneck hypothesis, and offer a perspective on the relationship between eye shape and activity patterns in mammals that is at odds with most prior studies.
The purpose of the current analysis is to provide a rigorous comparative examination of the influence of activity pattern on mammalian eye morphology. We use a large dataset of 266 mammal species, which represents a broader array of mammalian lineages than has been analysed in any previous comparative study of eye morphology. Using pFDA to analyse this expanded mammalian sample, we evaluate whether the activity pattern of most mammals can be predicted based on their eye morphology. We also evaluate the expectations of the nocturnal bottleneck hypothesis by investigating whether the pattern of eye shapes observed in mammals is similar to, or different from, those of two other groups of amniotes: the birds and the lizards.
2. Material and methods
(a). Data collection
We compiled eye-shape measurements for 266 mammal species representing 23 orders (see the electronic supplementary material, §S1). Species that are fully aquatic or primarily fossorial were excluded from this analysis. All eye measurements were taken from the published literature [4,6,18,32]. The measurements used in our analyses include (i) maximum corneal diameter, (ii) axial length of the eye and (iii) maximum transverse diameter of the eye. Corneal diameter is a proxy for maximum pupil diameter, which is directly correlated with visual sensitivity [15,21]. Axial eye length is a proxy for posterior nodal distance and is correlated with visual acuity [21]. Transverse eye diameter is a proxy for overall retinal size [33]. The ratio of corneal diameter to axial length of the eye is a useful measure of relative sensitivity and relative visual acuity that has been used in previous studies as a way to compare animals of disparate size [7,9].
We classified the diel activity pattern of each mammal species as either diurnal, cathemeral or nocturnal. Diurnal species are typically awake and active only by day under photopic conditions. Nocturnal species are typically awake and active only by night under mesopic and scotopic conditions. Cathemeral species are awake and active both by day and by night [33], and therefore may encounter a range of environmental light levels from photopic to scotopic. Mammalian activity pattern data follow in part the study of Ross & Kirk [6], which relied primarily on data published by Gittleman [34] and Nowak [35]. Modifications to some of the activity pattern classifications in Ross & Kirk [6] were based on the PanTHERIA database [36] and individual species reports in Mammalian Species (http://www.jstor.org/action/showPublication?journalCode=mammalianspecies). Our dataset contains 73 diurnal, 88 cathemeral and 105 nocturnal species. Of the diurnal species, 41 were anthropoid primates and 32 were non-anthropoid mammals.
(b). Data analysis
Comparative analyses have greatly benefited from advanced analytical techniques that account for the evolutionary history of the species [30,31,37]. In recent years, these methods have been extended to multivariate statistics, which enable the incorporation of phylogenetic information into these models [11,12]. We conducted a phylogenetically informed, flexible discriminant function analysis (pFDA) to examine the importance of three independent variables (axial length, corneal diameter and transverse diameter) for correctly predicting activity pattern in mammals. This is the same analytical method recently used in investigations of vertebrate visual evolution [11,12]. The phylogenetic discriminant function analysis is an extension of Hastie's [38] flexible discriminant function analysis (FDA). Our method should be an improvement over a non-phylogenetic model because the variance due to the shared evolutionary history of species is directly incorporated into the model's variance structure. In particular, the pFDA accounts for the non-independence of data due to phylogeny by incorporating a phylogenetic variance–covariance matrix that is scaled by Pagel's lambda. Pagel's lambda [39,40] is measured continuously from zero to one, and simultaneously quantifies and accounts for the phylogenetic relatedness of species in statistical models. A value of zero indicates that phylogeny has no importance in the model, therefore being equivalent to an analysis without phylogenetic information (i.e. a star phylogeny). A value of one indicates that phylogeny is an important component of the model, with the residuals following a Brownian motion model of evolution [41]. Using Pagel's lambda in phylogenetic comparative methods is becoming increasingly common in comparative biology research [42–44] and is an improvement over previous methods, such as phylogenetically independent contrasts [30], because the strength of phylogeny in the model is optimized via a maximum-likelihood approach rather than being fixed [6]. The phylogeny we used in the pFDA analysis was obtained from the mammal supertree presented by Bininda-Emonds et al. [45,46] (electronic supplementary material, §S2).
As in a typical discriminant function analysis, the pFDA allows the user to set the prior probabilities of group membership. We set our prior probabilities to be proportional to the size of each a priori activity pattern group. The flexible nature of the pFDA does not lend itself to producing a p-value (T. Hastie 2012, personal communication), yet does produce classification statistics. This output is sufficient because the ability of our model to classify species into their correct activity pattern is the main objective of our study and has been the main focus of recent studies using the same analytical approach [11,12]. All predictor variables were log10-transformed before analysis to better meet the assumptions of parametric statistics [47]. These analyses were performed in the R computing environment [48]. The code for the pFDA analysis was obtained from Schmitz & Motani [11], which was modified from the R package mda, based on the study of Hastie et al. [38]. The pFDA code also used functions from additional R packages, such as ape [49].
In order to compare mammal eye shapes with published bird and lizard eye shapes, we calculated ratios of corneal diameter: axial eye length for the mammals included in this study, and from the bird measurements reported by Hall & Ross [7] and the lizard measurements reported by Hall [9]. We used simple ratios for comparison because these previous studies do not report transverse eye diameters.
3. Results
Our pFDA correctly predicted the activity pattern of 67.7 per cent of mammal species in our dataset (table 1; electronic supplementary material, §S1). The percentage of correctly classified species varied greatly across activity patterns. Whereas 92.4 per cent of nocturnal species were correctly classified, only 57.5 per cent of diurnal species and 46.6 per cent of cathemeral species were correctly classified. These results are graphically illustrated by a plot of the two phylogenetic discriminant function axes (figure 1). Most nocturnal species have positive values on the x-axis, and diurnal species have negative values on this axis. Cathemeral species overlap both the diurnal and nocturnal species, and are not arranged in a discernibly separate cluster. Species in all three activity pattern groups overlap in the y-axis, yet the highest values tend to be nocturnal and cathemeral taxa. Inspecting the posterior probabilities of each species further emphasizes the variation in the model's ability to correctly predict activity pattern (table 1; electronic supplementary material, §S1). Only 17 of 88 cathemeral species exhibited posterior probability values greater than 50 per cent for correctly predicting their activity pattern (see the electronic supplementary material, §S3). By comparison, 30 of 73 diurnal species and 79 of 105 nocturnal species displayed posterior probabilities of greater than 50 per cent for correctly predicting their activity pattern (see the electronic supplementary material, §S3). It is important to note that phylogeny had a small effect in our pFDA, as indicated by the low value for Pagel's lambda (0.16). In fact, a standard, non-phylogenetic discriminant function analysis produced qualitatively similar results.
Table 1.
Classification table of mammal activity pattern based on phylogenetic flexible discriminant function analysis. Values in bold indicate the number of correctly predicted species.
|
a priori |
|||
|---|---|---|---|
| predicted | cathemeral | diurnal | nocturnal |
| cathemeral | 41 | 18 | 6 |
| diurnal | 13 | 42 | 2 |
| nocturnal | 34 | 13 | 97 |
| total species | 88 | 73 | 105 |
Figure 1.
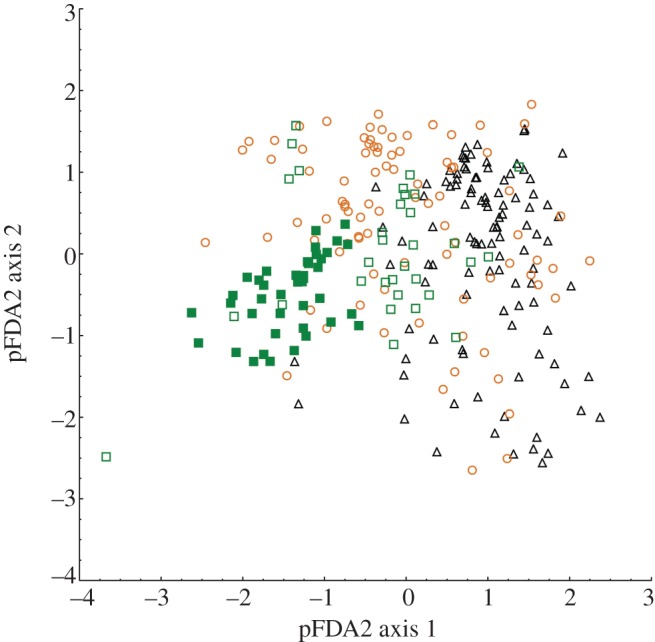
Scatter plot of pFDA results illustrating the degree of overlap between the activity pattern groups. Triangles, nocturnal; circles, cathemeral; open squares, diurnal; filled squares, diurnal anthropoid.
A closer examination of the individual mammalian orders reveals that the effectiveness of the pFDA varied considerably by clade (table 2). In the only two completely nocturnal orders, the Chiroptera and Didelphimorphia, all species were correctly classified by the model. Also, nearly 80.3 per cent of primate species were correctly classified, including 39 of 42 anthropoid species. By contrast, the activity patterns of most other mammalian orders were poorly predicted by the model, including less than 50 per cent of species in the Perissodactyla, Xenarthra and Rodentia.
Table 2.
Percentage of species with a correctly predicted activity pattern by order. Only orders with more than five species are included here.
| order | correctly classified species | total species | % correctly classified |
|---|---|---|---|
| Artiodactyla | 23 | 27 | 85.2 |
| Carnivora | 24 | 40 | 60.0 |
| Chiroptera | 20 | 20 | 100.0 |
| Didelphimorphia | 10 | 10 | 100.0 |
| Diprotodontia | 9 | 9 | 100.0 |
| Eulipotyphla | 6 | 8 | 75.0 |
| Perissodactyla | 2 | 6 | 33.3 |
| Primates | 53 | 66 | 80.3 |
| Rodentia | 14 | 39 | 35.9 |
| Xenarthra | 4 | 9 | 44.4 |
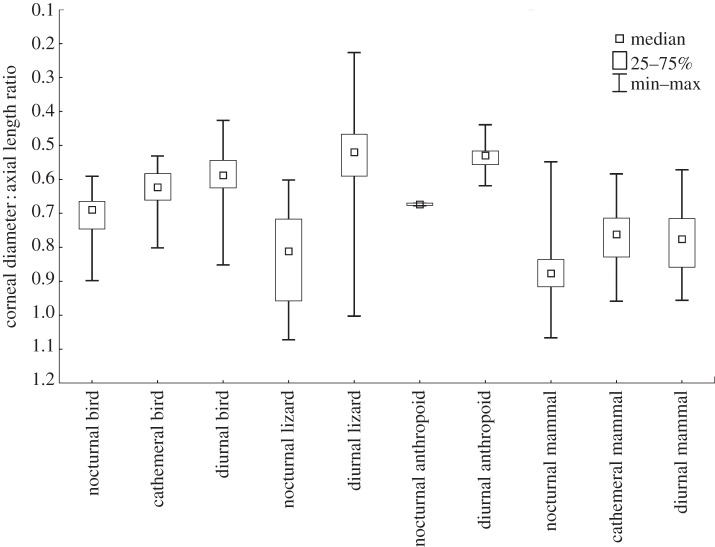
The ratios of corneal diameter to axial length of the eye demonstrate that diurnal birds and lizards have corneal diameters that, on average, measure just over half the size of their axial lengths (diurnal birds: ratio of 0.588; diurnal lizards: ratio of 0.522; table 3 and figure 2). Likewise, diurnal anthropoid primates exhibit a similar ratio of 0.532. By contrast, the mean ratio for diurnal non-anthropoid mammals is 0.773, which is most comparable with values for nocturnal birds and lizards. Furthermore, cathemeral mammals closely resemble diurnal non-anthropoid mammals in having a mean ratio of 0.772. This finding is consistent with the results of the multivariate pFDA, in which cathemeral mammals are not well differentiated from the other two activity patterns. Nocturnal mammals exhibit a mean corneal diameter : axial eye length ratio of 0.863. Nocturnal mammals thus have relative cornea sizes that are similar to those of nocturnal lizards, but are somewhat larger than those of nocturnal birds (table 3 and figure 2).
Table 3.
Ratios of corneal diameter : axial length of the eye.
| mean ratio | s.d. | lowest ratio | highest ratio | |
|---|---|---|---|---|
| diurnal anthropoids | 0.532 | 0.033 | 0.439 | 0.619 |
| diurnal non-anthropoid mammals | 0.773 | 0.097 | 0.571 | 0.956 |
| cathemeral mammals | 0.772 | 0.086 | 0.583 | 0.958 |
| nocturnal mammals | 0.863 | 0.084 | 0.548 | 1.067 |
| diurnal birds | 0.588 | 0.061 | 0.426 | 0.852 |
| cathemeral birds | 0.633 | 0.067 | 0.531 | 0.801 |
| nocturnal birds | 0.707 | 0.066 | 0.590 | 0.898 |
| diurnal lepidosaurs | 0.522 | 0.121 | 0.226 | 1.003 |
| nocturnal lepidosaurs | 0.825 | 0.139 | 0.602 | 1.072 |
Figure 2.
Boxplots showing the range of the corneal diameter : axial length ratios for birds, lizards, anthropoids and non-anthropoid mammals of varying activity patterns.
Within clades, most diurnal groups have mean corneal diameter : axial eye length ratios that partially overlap the range of ratios for nocturnal species (figure 2). However, the amount of overlap varies substantially by class. Whereas 19 per cent of diurnal lizards and 49 per cent of diurnal birds overlap the ranges of nocturnal lizards and birds (respectively), 100 per cent of diurnal non-anthropoid mammals overlap the range of nocturnal mammals. By comparison, only 30 per cent of diurnal anthropoids overlap the range of nocturnal mammals.
4. Discussion
Birds and lizards follow a consistent pattern of eye-shape variation with activity pattern, as predicted by the principles of dioptrics: nocturnal species consistently exhibit a larger relative corneal diameter than do diurnal species, probably as an adaptation for increased visual sensitivity [7,9]. Previous studies have suggested that there is a different pattern of eye-shape variation in mammals than is seen in these other amniote groups. Most mammals, regardless of activity pattern, have relative cornea sizes that are broadly comparable with those of nocturnal amniotes [6,8,10].
Two recent papers [11,12] have argued that these previous findings are a result of statistical limitations, and that pFDA allows mammal eye shapes to be confidently classified by activity pattern in the same manner as in other vertebrate groups. However, our analysis of a larger and more taxonomically diverse mammalian dataset yielded contrasting results that emphasize the poor relationship between eye shape and activity pattern in most mammalian clades compared with non-mammalian amniotes. As in previous studies using pFDA [11,12], we found a weak phylogenetic signal in the model predicting activity pattern from eye morphology. This result was expected because in most cases eye shape is driven in large part by functional demands, which in turn depend on the physics of light [15,16]. Although we agree that using a phylogenetically informed model is important for interspecific comparative analyses [31], we find that a phylogenetically corrected model has limited benefit in the analysis of mammalian eye shape and activity pattern. The phylogenetic signal in our model is probably further diminished by the fact that cathemeral mammals and diurnal non-anthropoid mammals have relatively similar eye shapes (figure 2).
As in prior discriminant function analyses of vertebrate eye shape [12,50], our pFDA demonstrates a substantial overlap between all of the activity pattern groups (figure 1). The only activity pattern that is well defined by the pFDA model is nocturnality. Indeed, the two largest mammalian clades that have 100 per cent correct classifications by this model are those that are represented here entirely by nocturnal species, the bats and the didelphid marsupials. The pFDA model identifies diurnal and cathemeral mammals much less accurately. Cathemeral mammals in particular are poorly classified by the pFDA, and completely overlap the combined nocturnal and diurnal mammal distributions (figure 1). Furthermore, the great majority of the correctly classified diurnal mammals are diurnal anthropoids. This result agrees well with previous studies showing that diurnal anthropoids have highly distinctive eye shapes compared with other mammals [3,6,8,23]. In fact, of the 32 non-anthropoid diurnal mammals included in this analysis, only four species are correctly classified by the model (see the electronic supplementary material, §S3).
These pFDA results are similar to those obtained by comparing the ratio of corneal diameter to axial eye length within mammals (figure 2). These comparisons reveal that the extant mammals in our sample fall into three basic groups: diurnal anthropoids, nocturnal mammals and day-active non-anthropoids (including both diurnal and cathemeral species). Diurnal anthropoids have smaller relative cornea sizes than most of the other mammals in our sample, and are closely comparable in this respect with diurnal birds and lizards. Nocturnal mammals have the largest relative cornea sizes in our mammalian sample, and only a small number of nocturnal mammal species have relative cornea sizes comparable with those of diurnal anthropoids. Day-active non-anthropoid mammals have mean relative cornea sizes that are intermediate between those of diurnal anthropoids and nocturnal mammals (table 3 and figure 2). However, the range of relative cornea sizes in day-active non-anthropoids completely overlaps that of nocturnal species (figures 1 and 2). The nearly identical relative cornea sizes of diurnal and cathemeral non-anthropoids, and the large amount of overlap with nocturnal species, partly explains the comparatively poor performance of our pFDA in classifying diurnal and cathemeral mammals.
Although our results indicate that it is difficult to predict mammalian activity patterns on the basis of eye morphology alone, our findings do provide new evidence supporting the nocturnal bottleneck hypothesis of early mammalian evolution. Diurnal and cathemeral non-anthropoid mammals have evolved eye morphologies that overlap entirely with nocturnal mammals, and also exhibit eye shapes that are most similar to those of nocturnal non-mammalian amniotes. By the same token, eye shape in nocturnal mammals is not fundamentally different from that in other nocturnal amniotes. If nocturnality is indeed the ancestral state for crown mammals, then our results have two important implications. First, our comparative data suggest that living nocturnal mammals may retain eye morphologies broadly similar to those of their Mesozoic predecessors. Second, our data suggest that diurnal and cathemeral non-anthropoid species (43% of our comparative sample) have diverged less from the ancestral condition than might be expected, on the basis of the eye morphologies of extant diurnal and cathemeral amniotes. Some precedent for the existence of such long-lasting phylogenetic effects is provided by the existence of ‘nocturnal’ eye shapes in other diurnal amniote groups [10]. Some diurnal owls and diurnal geckos retain relatively large corneas compared with diurnal birds and lizards generally [7,9] (but see [51]). Similarly, both owls and geckos probably diversified as predominantly nocturnal radiations [52,53]. In this respect, diurnal owls, geckoes and non-anthropoid mammals may represent three parallel instances of amniote lineages that have re-evolved diurnality but retain eye morphologies more typical of scotopically adapted species.
If these conclusions are correct, then they further raise the question of why most living day-active mammals have not re-evolved eye shapes that are better suited for function under photopic conditions. One possibility is that during the Mesozoic, mammals evolved keen non-visual senses that function well irrespective of environmental light levels [10,18]. For example, mammals have evolved excellent olfactory [29] and auditory sensitivity [27] compared with most other vertebrates, along with related traits such as the presence of finely tuned tactile vibrissae [28]. Indeed, of all extant bird species, the only group to routinely exhibit vibrissae-like structures are the nocturnal Caprimulgiformes (the nightjars and nighthawks) [54]. The evolution of such compensatory sensory adaptations may have diminished pressures to re-evolve eye morphologies associated with high visual acuity in most mammals that have re-invaded diurnal niches.
Previous studies have also suggested that once vertebrates evolve an eye shape suitable for enhancing visual sensitivity in a nocturnal setting, the functional anatomy of the vertebrate eye may ensure that a subsequent return to diurnal habits alone does not provide sufficient selection pressure to reduce relative corneal size [10,24]. As Martin [55,56] has argued, eyes with a large corneal diameter may function well across a range of ambient light levels because an animal can still freely increase or reduce the amount of light entering the eye simply by changing the size of the pupil. In other words, an animal can functionally reduce the size of the ocular aperture in response to too much light, but cannot increase the pupil beyond the size of the cornea in response to too little light. As a result, there are virtually no examples of secondarily nocturnal amniotes with relatively small corneas because cornea size sets an upper limit on the amount of light that the eye can gather at maximum pupil dilation. Conversely, there are a number of examples of secondarily diurnal amniotes with relatively large corneal sizes (e.g. diurnal geckoes, diurnal owls and most diurnal mammals) because pupillary constriction presumably allows the eyes of these species to function under photopic conditions [3,6–10,23].
As noted previously, the major outliers among day-active mammals in terms of eye morphology are diurnal anthropoids. Indeed, anthropoids, including humans, have converged on what is probably the primitive amniote condition of visual dependence with the evolution of a retinal fovea [22], small relative cornea sizes [3,6,8,22] and improved colour vision in some clades [57]. Diurnal anthropoids probably have eye and retinal morphologies that diverge from those of most other diurnal mammals owing to pervasive selection for high visual acuity in either haplorhine or anthropoid stem lineages [3,6,23,58–60]. This selection for high acuity probably occurred in the context of diurnal visual predation on arthropods and small vertebrates [22,58–61].
In summary, our results demonstrate that mammals exhibit the same pattern of eye-shape evolution found in other vertebrate groups: once an animal evolves large relative cornea size, there evidently is not a strong pressure to change eye shape with a change in activity pattern in the absence of selection for very high visual acuity. Therefore, because all mammals have descended from a nocturnal ancestor that had already evolved an eye shape that was well adapted for scotopic conditions, the majority of mammals retain eyes that deviate comparatively little from that ancestral morphology regardless of their modern activity pattern.
Acknowledgements
We thank Dr C. P. Heesy and Dr A. Grossman for helpful comments on earlier versions of this manuscript. We also thank two anonymous reviewers and the helpful comments of the editors.
References
- 1.Brooke M. d. L., Hanley S., Laughlin S. B. 1999. The scaling of eye size and body mass in birds. Proc. R. Soc. Lond. B 266, 405–412 10.1098/rspb.1999.0652 (doi:10.1098/rspb.1999.0652) [DOI] [Google Scholar]
- 2.Garamszegi L. Z., Møller A. P., Erritzøe J. 2002. Coevolving avian eye size and brain size in relation to prey capture and nocturnality. Proc. R. Soc. Lond. B 269, 961–967 10.1098/rspb.2002.1967 (doi:10.1098/rspb.2002.1967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirk E. C. 2004. Comparative morphology of the eye in primates. Anat. Rec. 281A, 1095–1103 10.1002/ar.a.20115 (doi:10.1002/ar.a.20115) [DOI] [PubMed] [Google Scholar]
- 4.Kirk E. C. 2006. Effects of activity pattern on eye size and orbital aperture size in primates. J. Hum. Evol. 51, 159–170 10.1016/j.jhevol.2006.02.004 (doi:10.1016/j.jhevol.2006.02.004) [DOI] [PubMed] [Google Scholar]
- 5.Kirk E. C. 2006. Visual influences on primate encephalization. J. Hum. Evol. 51, 76–90 10.1016/j.jhevol.2006.01.005 (doi:10.1016/j.jhevol.2006.01.005) [DOI] [PubMed] [Google Scholar]
- 6.Ross C. F., Kirk E. C. 2007. Evolution of eye size and shape in primates. J. Hum. Evol. 52, 294–313 10.1016/j.jhevol.2006.09.006 (doi:10.1016/j.jhevol.2006.09.006) [DOI] [PubMed] [Google Scholar]
- 7.Hall M. I., Ross C. F. 2007. Eye shape and activity pattern in birds. J. Zool. 271, 437–444 10.1111/j.1469-7998.2006.00227.x (doi:10.1111/j.1469-7998.2006.00227.x) [DOI] [Google Scholar]
- 8.Ross C. F., Hall M. I., Heesy C. P. 2007. Were basal primates nocturnal? Evidence from eye and orbit shape. In Primate origins: adaptations and evolution (eds Ravosa M. J., Dagosto M.), pp. 233–256 New York, NY: Springer [Google Scholar]
- 9.Hall M. I. 2008. Comparative analysis of the size and shape of the lizard eye. Zoology 111, 62–75 10.1016/j.zool.2007.04.003 (doi:10.1016/j.zool.2007.04.003) [DOI] [PubMed] [Google Scholar]
- 10.Heesy C. P., Hall M. I. 2010. The nocturnal bottleneck and the evolution of mammalian vision. Brain Behav. Evol. 75, 195–203 10.1159/000314278 (doi:10.1159/000314278) [DOI] [PubMed] [Google Scholar]
- 11.Schmitz L., Motani R. 2010. Morphological differences between the eyeballs of nocturnal and diurnal amniotes revisited from optical perspectives of visual environments. Vis. Res. 50, 936–946 10.1016/j.visres.2010.03.009 (doi:10.1016/j.visres.2010.03.009) [DOI] [PubMed] [Google Scholar]
- 12.Motani R., Schmitz L. 2011. Phylogenetic versus functional signals in the evolution of form–function relationships in terrestrial vision. Evolution 65, 2245–2257 10.1111/j.1558-5646.2011.01271.x (doi:10.1111/j.1558-5646.2011.01271.x) [DOI] [PubMed] [Google Scholar]
- 13.Land M. F. 1980. Optics and vision in invertebrates. In Handbook of sensory physiology VII/6B (ed. Antrum H.), pp. 471–592 Berlin, Germany: Springer [Google Scholar]
- 14.Ali M. A., Klyne M. A. 1985. Vision in vertebrates. New York, NY: Plenum Press [Google Scholar]
- 15.Land M. F., Nilsson D. E. 2012. Animal eyes, 2nd edn New York, NY: Oxford University Press [Google Scholar]
- 16.Johnsen S. 2012. The optics of life: a biologist’s guide to light in nature. Princeton, NJ: Princeton University Press [Google Scholar]
- 17.Warrant E. 2004. Vision in the dimmest habitats on Earth. J. Comp. Physiol. A 190, 765–789 10.1007/s00359-004-0546-z (doi:10.1007/s00359-004-0546-z) [DOI] [PubMed] [Google Scholar]
- 18.Heard-Booth A. N., Kirk E. C. 2012. Influence of maximum running speed on eye size: a test of Leukart's law in mammals. Anat. Rec. 295, 1053–1062 10.1002/ar.22480 (doi:10.1002/ar.22480) [DOI] [PubMed] [Google Scholar]
- 19.Kirk E. C. 2006. Eye morphology in cathemeral lemurids and other mammals. Folia Primatol. 77, 27–49 10.1159/000089694 (doi:10.1159/000089694) [DOI] [PubMed] [Google Scholar]
- 20.Walls G. L. 1942. The vertebrate eye and its adaptive radiation. New York, NY: Hafner [Google Scholar]
- 21.Hughes A. 1977. The topography of vision in mammals of contrasting life style: comparative optics and retinal organisation. In The visual system in vertebrates (ed. Crescitelli F.), pp. 613–756 Berlin, Germany: Springer [Google Scholar]
- 22.Lythgoe J. N. 1979. The ecology of vision. Oxford, UK: Clarendon Press [Google Scholar]
- 23.Ross C. F. 2000. Into the light: the origin of Anthropoidea. Annu. Rev. Anthropol. 29, 147–194 10.1146/annurev.anthro.29.1.147 (doi:10.1146/annurev.anthro.29.1.147) [DOI] [Google Scholar]
- 24.Hall M. I. 2005. The roles of function and phylogeny in the morphology of the diapsid visual system. PhD thesis, Stony Brook University, New York, NY, USA [Google Scholar]
- 25.Kielan-Jaworowska Z., Cifelli R. L., Zhe-Xi L. 2004. Mammals from the age of dinosaurs. New York, NY: Columbia University Press [Google Scholar]
- 26.Davies W. I. L., Collin S. P., Hunt D. M. 2012. Molecular ecology and adaptation of visual photopigments in craniates. Mol. Ecol. 38, 1–10 (doi:10.1111/j.1365–294X.2012.05617.x) [DOI] [PubMed] [Google Scholar]
- 27.Coleman M. N., Boyer D. M. 2012. Inner ear evolution in primates through the cenozoic: implications for the evolution of hearing. Anat. Rec. 295, 615–631 10.1002/ar.22422 (doi:10.1002/ar.22422) [DOI] [PubMed] [Google Scholar]
- 28.Muchlinski M. M. 2010. A comparative analysis of vibrissa count and infraorbital foramen area in primates and other mammals. J. Hum. Evol. 58, 447–473 10.1016/j.jhevol.2010.01.012 (doi:10.1016/j.jhevol.2010.01.012) [DOI] [PubMed] [Google Scholar]
- 29.Streidter G. F. 2005. Principles of brain evolution. Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- 30.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15 10.1086/284325 (doi:10.1086/284325) [DOI] [Google Scholar]
- 31.Nunn C. L. 2011. The comparative approach in evolutionary anthropology and biology. Chicago, IL: University of Chicago Press [Google Scholar]
- 32.Ritland S. 1982. The allometry of the vertebrate eye. PhD dissertation, Department of Biology, University of Chicago, Chicago, IL, USA
- 33.Rowe M. P. 2000. Inferring the retinal anatomy and visual capacities of extinct vertebrates. Palaeontol. Electron. 3, 43 [Google Scholar]
- 34.Gittleman J. 1986. Carnivore life history patterns: allometric, phylogenetic, and ecological associations. Am. Nat. 127, 744–771 10.1086/284523 (doi:10.1086/284523) [DOI] [Google Scholar]
- 35.Nowak R. M. 1991. Walker’s mammals of the world, 5th edn., vol. 1 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 36.Jones K. E., et al. 2009. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648. 10.1890/08-1494.1 (doi:10.1890/08-1494.1) [DOI] [Google Scholar]
- 37.Harvey P. H., Pagel M. D. 1991. The comparative method in evolutionary biology. Oxford, UK: Oxford University Press [Google Scholar]
- 38.Hastie T., Tibshirani R., Buja A. 1994. Flexible discriminant analysis by optimal scoring. J. Am. Stat. Assoc. 89, 1255–1270 10.1080/01621459.1994.10476866 (doi:10.1080/01621459.1994.10476866) [DOI] [Google Scholar]
- 39.Freckleton R. P., Harvery P. H., Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726 10.1086/343873 (doi:10.1086/343873) [DOI] [PubMed] [Google Scholar]
- 40.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884 10.1038/44766 (doi:10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 41.Revell L. J. 2010. Phylogenetic signal and linear regression on species data. Meth. Ecol. Evol. 1, 319–329 10.1111/j.2041-210X.2010.00044.x (doi:10.1111/j.2041-210X.2010.00044.x) [DOI] [Google Scholar]
- 42.Heesy C. P., Kamilar J. M., Willms J. 2011. Retinogeniculostriate pathway components scale with orbit convergence only in Primates and not in other mammals. Brain Behav. Evol. 77, 105–115 10.1159/000324860 (doi:10.1159/000324860) [DOI] [PubMed] [Google Scholar]
- 43.Kamilar J. M., Bradley B. J. 2011. Interspecific variation in primate coat color supports Gloger's rule. J. Biogeog. 38, 2270–2277 [Google Scholar]
- 44.Pointer M. A., Kamilar J. M., Warmuth V., Chester S. G. B., Delsuc F., Mundy N. I., Asher R. J., Bradley B. J. 2012 Runx2 tandem repeats and the evolution of facial length in placental mammals. BMC Evol. Biol. 12, 103. 10.1186/1471-2148-12-103 (doi:10.1186/1471-2148-12-103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bininda-Emonds O. R. P., et al. 2007. The delayed rise of present-day mammals. Nature 446, 507–512 10.1038/nature05634 (doi:10.1038/nature05634) [DOI] [PubMed] [Google Scholar]
- 46.Bininda-Emonds O. R. P., et al. 2008. Corrigendum. The delayed rise of present-day mammals. Nature 456, 274. 10.1038/nature07347 (doi:10.1038/nature07347) [DOI] [PubMed] [Google Scholar]
- 47.Quinn G. P., Keough M. J. 2002. Experimental design and data analysis for biologists. Cambridge, UK: Cambridge University Press [Google Scholar]
- 48.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 49.Paradis E., Claude J., Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 10.1093/bioinformatics/btg412 (doi:10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 50.Hall M. I., Kirk E. C., Kamilar J., Carrano M. 2011. Comment on ‘Nocturnality in dinosaurs inferred from scleral ring and orbit morphology’. Science 334, 1641. 10.1126/science.1208489 (doi:10.1126/science.1208489) [DOI] [PubMed] [Google Scholar]
- 51.Lisney T. J., Iwaniuk A. N., Bandet M. V., Wylie D. R. 2012. Eye shape and retinal topography in owls (Aves: Strigiformes). Brain Behav. Evol. 79, 218–236 10.1159/000337760 (doi:10.1159/000337760) [DOI] [PubMed] [Google Scholar]
- 52.Donnellan S. C., Hutchinson M. N., Saint K. M. 1999. Molecular evidence for the phylogeny of Australian gekkonid lizards. Biol. J. Linnean Soc. 67, 97–118 10.1111/j.1095-8312.1999.tb01932.x (doi:10.1111/j.1095-8312.1999.tb01932.x) [DOI] [Google Scholar]
- 53.Wink M., Sauer-Gurth H., Elsayed A.-A., Gonzales J. 2008. Molecular phylogeny and systematics of owls (Strigiformes). In Owls of the world (eds Konig C., Weick F.), pp. 42–63 London, UK: Christopher Helm [Google Scholar]
- 54.del Hoyo J., Elliott A., Sargatal J. (eds) 1999. Handbook of the birds of the world, vol. 5 Barcelona, Spain: Lynx Ediciones [Google Scholar]
- 55.Martin G. R. 1982. An owl's eye: schematic optics and visual performance in Strix aluco. J. Comp. Physiol. 145, 341–349 10.1007/BF00619338 (doi:10.1007/BF00619338) [DOI] [Google Scholar]
- 56.Martin G. R. 1990. Birds by night. San Diego, CA: Academic Press [Google Scholar]
- 57.Jacobs G. H. 2009. Evolution of colour vision in mammals. Phil. Trans. R. Soc. B 364, 2957–2967 10.1098/rstb.2009.0039 (doi:10.1098/rstb.2009.0039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kay R. F., Kirk E. C. 2000. Osteological evidence for the evolution of activity pattern and visual acuity in primates. Am. J. Phys. Anthropol. 113, 235–262 (doi:10.1002/1096-8644(200010)113:2<235::AID-AJPA7>3.0.CO;2-9) [DOI] [PubMed] [Google Scholar]
- 59.Williams B. A., Kay R. F., Kirk E. C. 2010. New perspectives on anthropoid origins. Proc. Natl Acad. Sci. USA 107, 4797–4804 10.1073/pnas.0908320107 (doi:10.1073/pnas.0908320107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heesy C. P., Ross C. F. 2001. Evolution of activity patterns and chromatic vision in primates: morphometrics, genetics and cladistics. J. Hum. Evol. 40, 111–149 10.1006/jhev.2000.0447 (doi:10.1006/jhev.2000.0447) [DOI] [PubMed] [Google Scholar]
- 61.Yamashita N., Stoner K. E., Riba-Hernandez P., Dominy N. J., Lucas P. W. 2005. Light levels used during feeding by primate species with different color vision phenotypes. Behav. Ecol. Sociobiol. 58, 618–629 10.1007/s00265-005-0936-4 (doi:10.1007/s00265-005-0936-4) [DOI] [Google Scholar]