Abstract
Mercury is a toxic heavy metal that is an environmental and industrial pollutant throughout the world. Mercury exposure leads to many physiopathological injuries in mammals. However, the precise toxicological effects of mercury on pancreatic islets in vivo are still unclear. Here, we investigated whether mercuric compounds can induce dysfunction and damage in the pancreatic islets of mice, as well as the possible mechanisms involved in this process. Mice were treated with methyl mercuric chloride (MeHgCl, 2 mg/kg) and mercuric chloride (HgCl2, 5 mg/kg) for more than 2 consecutive weeks. Our results showed that the blood glucose levels increased and plasma insulin secretions decreased in the mice as a consequence of their exposure. A significant number of TUNEL-positive cells were revealed in the islets of mice that were treated with mercury for 2 consecutive weeks, which was accompanied by changes in the expression of the mRNA of anti-apoptotic (Bcl-2, Mcl-1, and Mdm-2) and apoptotic (p53, caspase-3, and caspase-7) genes. Moreover, plasma malondialdehyde (MDA) levels increased significantly in the mice after treatment with mercuric compounds for 2 consecutive weeks, and the generation of reactive oxygen species (ROS) in the pancreatic islets also markedly increased. In addition, the mRNA expression of genes related to antioxidation, including Nrf2, GPx, and NQO1, were also significantly reduced in these islets. These results indicate that oxidative stress injuries that are induced by mercuric compounds can cause pancreatic islets dysfunction and apoptosis in vivo.
Keywords: mercuric compounds, pancreatic islets, oxidative stress, apoptosis
1. Introduction
Mercury, a toxic heavy metal and a widespread environmental pollutant, poses a serious health hazard [1,2]. Mercury is normally present in 3 forms-elemental mercury (Hg0), inorganic mercury (Hg2+ and Hg+), and organic mercury (methylmercury, MeHg)-all of which can produce varying degrees of toxic effects in many organs or systems. These effects include cardiovascular disease, endocrine system disruption, neurotoxicity, and immunotoxicity [3–5]. A previous study indicated that approximately 80% of mercury vapor (inorganic mercury) is inhaled through the lungs and then absorbed into the bloodstream, and remaining in the circulation for a long enough period to be distributed to other tissues. The organic form of mercury, MeHg, causes an irreversible neurotoxic disorder in mammals through biotransformation in the food chain, such as consumption of contaminated fish, seafood, and aquatic mammals [6,7]. The pancreatic islet cells destroyed and an increased incidence of diabetes mellitus (DM) was found in patients with Minamata disease (MeHg poisoning) in Japan [8,9] The study of Shigenaga [10] also found that repeated treatment of rats with MeHg induced a high blood glucose level that was accompanied by pancreatic islets injuries. Recently, Chen et al. [11,12] reported that mercuric compounds exposure can induce pancreatic β-cell dysfunction and death in vitro. However, the toxicological effects and possible mechanism by which mercuric compounds caused damage to the pancreatic islets in vivo remained to be clarified.
DM is part of a group of metabolic diseases that is characterized by hyperglycemia originating from defects of insulin secretion by the pancreatic β-cells and/or insulin action in the peripheral tissues. Many studies have reported that the death of pancreatic islet β-cells contributes to type 1 (insulin-dependent) diabetes, which is a prototype of organ-specific autoimmune diseases in which an immune-mediated inflammation results in the selective destruction and infiltration of islet β-cells, inhibits insulin secretion, and causes pancreatic β-cell death [13,14]. Some insults, such as lipoxygenases (expressed in human and rodent islets), can cause injury by inducing oxidative stress-regulated inflammatory damage and cell death in islet β-cells [15]. In addition, the production of reactive oxygen species (ROS) results in oxidative stress, which induces undesirable biological reactions and injuries to functional cells, including pancreatic islet β-cell dysfunction and apoptosis, that are caused by cytokines or autoimmune attack in type 1 DM. Pancreatic β-cells are reported to be vulnerable to oxidative stress damage [16,17]. Toxic metals, such as mercury and arsenic, can induce toxic effects via oxidative stress leading to apoptosis and pathophysiological injuries, which then cause to many disorders including DM [18–21]. Taken together, in this study, we sought to elucidate the toxicological effects induced by mercuric compounds (MeHg and mercuric chloride (HgCl2)) in the pancreatic islets of male mice (in vivo model) and to explore the hypothesis that mercuric compounds-induced oxidative stress damage leads to dysfunction and apoptosis in pancreatic islets. To examine these issues, we investigated the deleterious effects of exposure to MeHg (2 mg/kg/day) and HgCl2 (5 mg/kg/day) for 2 to 6 consecutive weeks in male mice by monitoring the changes in blood glucose, plasma insulin, and MDA levels, and by analyzing the Hg concentration of mouse whole blood samples. Moreover, we examined whether exposure to mercuric compounds could induce apoptosis and ROS generation while altering apoptotic- and antioxidant-related gene expression in the islets of treated mice at the end of 2 weeks.
2. Results and Discussion
2.1. Effects of Mercuric Compounds on Blood Glucose Regulation and Plasma Insulin Levels in Mice
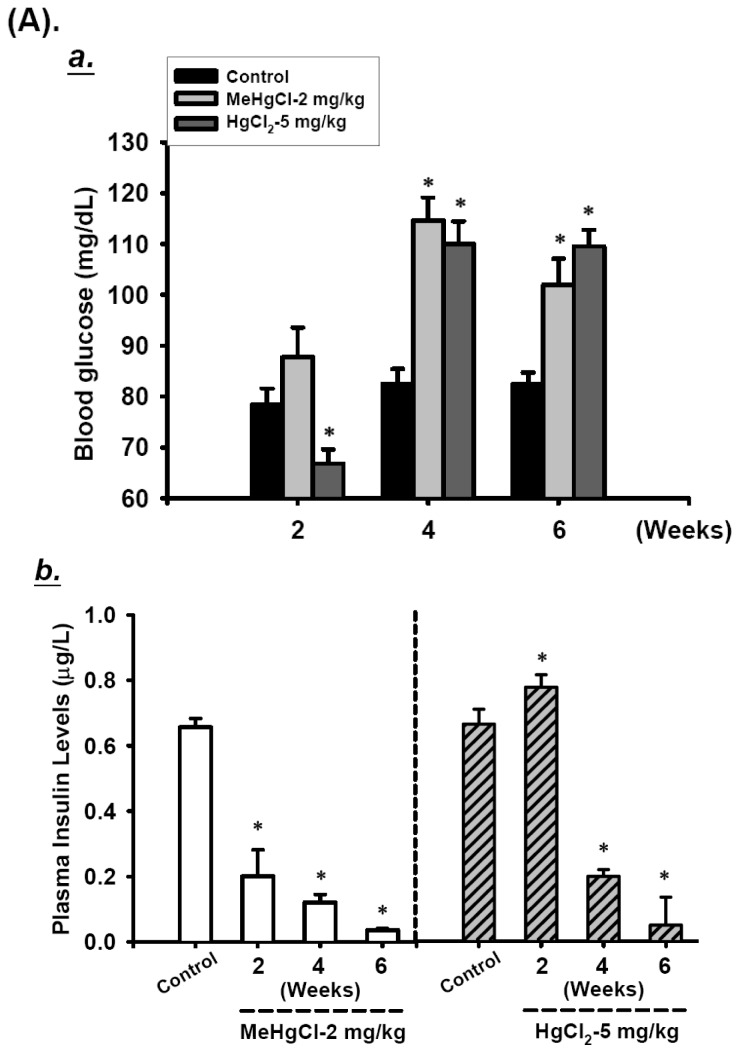
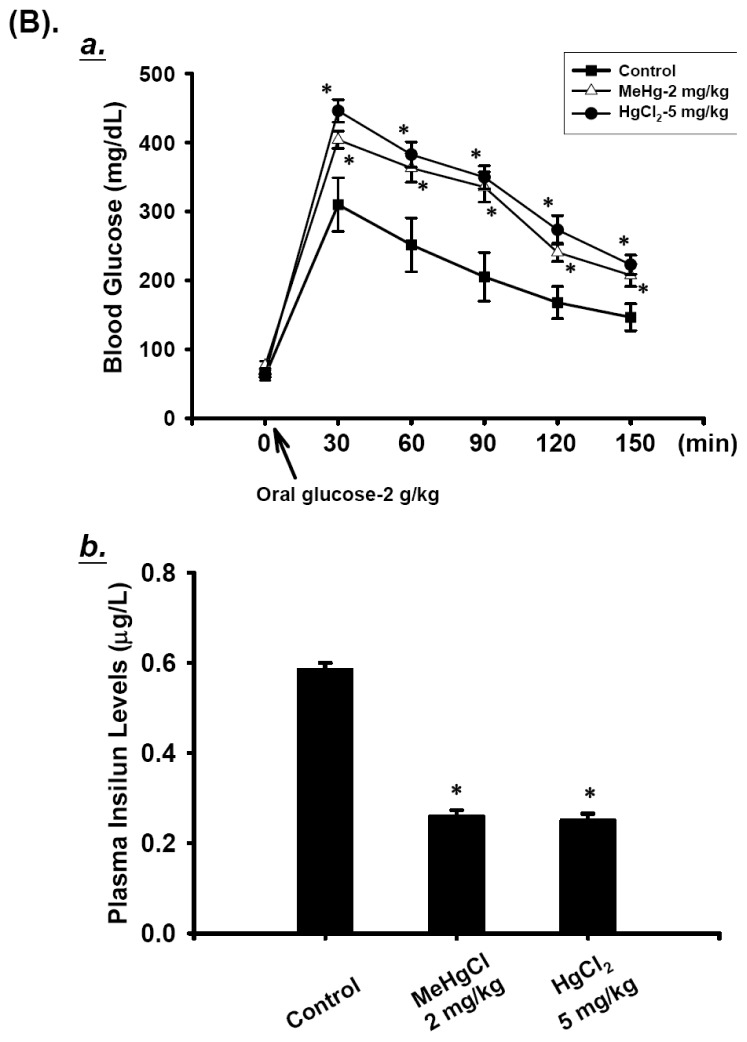
To investigate the effects of mercuric compounds on in vivo pancreatic islet function, we monitored the changes in blood glucose and plasma insulin levels in MeHgCl or HgCl2-exposed mice. Fasting blood glucose levels in mice showed a marked increase and the plasma insulin levels decreased after 4 or 6 consecutive weeks of exposure to MeHgCl (2 mg/kg/day) or HgCl2 (5 mg/kg/day) as compared with the control group (Figure 1A). After 2 consecutive weeks of exposure to MeHgCl, it was showed a light, but not statistically significant, increase in blood glucose levels, but there was a remarkable decrease in plasma insulin levels. By contrast, mice exposed to HgCl2 for 2 consecutive weeks were showed a significant decrease in blood glucose levels and increased plasma insulin levels (Figure 1A). To confirm that exposure to mercuric compounds can cause islet damage resulting in blood glucose dysregulation, we used the oral glucose tolerance test (OGTT). As shown in Figure 1B, both MeHgCl and HgCl2-exposed mice revealed an elevation in glucose intolerance (Figure 1B,a), and it was also a marked decrease in plasma insulin after glucose loading for 30 min following 2 consecutive weeks of exposure. Moreover, the mercury levels in the whole blood of mice exposed to mercuric compounds over a 2- to 6- consecutive weeks period were significantly elevated (MeHgCl group: 4970.8 ± 38.8 μg/L, 14827.6 ± 1938.7 μg/L, and 27741.4 ± 6747.1 μg/L at 2, 4, and 6 weeks, respectively; HgCl2 group: 432.0 ± 111.2 μg/L, 683.4 ± 47.9 μg/L, and 865.8 ± 222.5 μg/L at 2, 4, and 6 weeks, respectively; age-matched control group ranged from 2.4 ± 0.3 μg/L to 3.0 ± 0.5 μg/L) (Table 1). These results suggest that treatment with MeHgCl or HgCl2 destroys pancreatic islet function in mice.
Figure 1.
Effects of mercuric compounds on the regulation of blood glucose and plasma insulin levels in mice. (A) Mice were gavaged with 2 mg/kg/day MeHgCl or 5 mg/kg/day HgCl2 for 6 consecutive weeks. Fasting blood glucose was determined by SureStep blood glucose meter (A,a), and the plasma insulin levels were analyzed by insulin enzyme-linked immunosorbent assay (ELISA) assay kit (A,b) at 2, 4, and 6 weeks. (B) Oral glucose tolerance and insulin in fasting mice were determined as described in the Materials and Methods section. Oral glucose tolerance tests were carried out in mice given 2 mg/kg/day MeHgCl or 5 mg/kg/day HgCl2 for 2 consecutive weeks (B,a). Plasma insulin levels in mercuric compounds-treated mice after 2 g/kg glucose loading for 30 min were analyzed (B,b). All data are presented as means ± standard errors of the mean (SEM). (n = 16 mice for each group).* p < 0.05 compared with the control group.
Table 1.
Whole blood mercury levels in mercuric compounds-exposed mice.
| Weeks | Group | ||
|---|---|---|---|
|
| |||
| Control | MeHgCl-2 (mg/kg) | HgCl2-5 (mg/kg) | |
| 2 | 2.4 ± 0.3 | 4970.8 ± 38.8 * | 432.0 ± 111.2 * |
| 4 | 2.6 ± 0.4 | 14827.5 ± 1938.7 * | 683.4 ± 47.9 * |
| 6 | 3.0 ± 0.5 | 27741.4 ± 6747.1 * | 865.8 ± 222.5 * |
1. Hg content was expressed as μg/L; 2. Data are expressed as mean ± SEM (n = 16 mice for each group).
p < 0.05 as compared with control group.
2.2. Mercuric Compounds Caused Apoptosis in the Pancreatic Islets of Exposed Mice
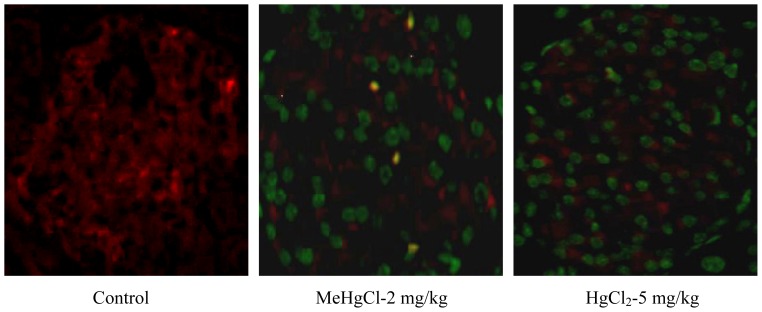
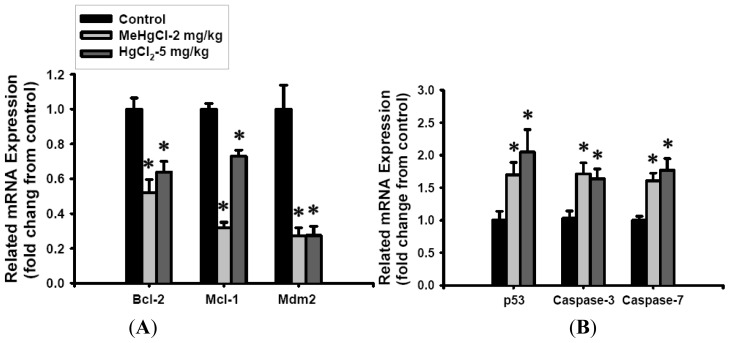
To investigate whether mercuric compounds induce dysfunction of pancreatic islets via an apoptotic mechanism, we performed terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and insulin dual staining, and were measured the expression of apoptosis-related genes (by real-time quantitative RT-PCR). As shown in Figure 2, the number of TUNEL-positive cells in the isolated islets of mice was significantly increased after exposure to MeHgCl (2 mg/kg/day) or HgCl2 (5 mg/kg/day) for 2 consecutive weeks, which only revealed a weak insulin immunoreactivity in comparison with the control group. In addition, the expression of Bcl-2, Mcl-1, and Mdm-2 (anti-apoptotic genes) were showed an obvious decreased (Figure 3A), while that of p53 (apoptotic gene) dramatically increased; these changes were accompanied by a marked up-regulation of caspase-3 and caspase-7 gene expression levels (approximately 1.5 to 2.0 fold; Figure 3B) in the isolated islets of mice exposed to MeHgCl or HgCl2. These results indicate that exposure to mercuric compounds in vivo can cause injury to pancreatic islets, leading to a pathophysiological state associated with apoptosis.
Figure 2.
Immunofluorescence analysis of islet apoptosis induction in mercuric compounds-exposed mice. Mice were gavaged with 2 mg/kg/day MeHgCl or 5 mg/kg/day HgCl2 for 2 consecutive weeks, and dual immunofluorescence staining of islet using anti-insulin (red) and TUNEL (green) was performed as described in the Materials and Methods section (400×).
Figure 3.
Mercuric compounds treatment regulated apoptotic related gene expression in the islets of mice. Mice were gavaged with 2 mg/kg/day MeHgCl or 5 mg/kg/day HgCl2 for 2 consecutive weeks, and the expression of anti-apoptotic (Bcl-2, Mcl-1, Mdm-2) (A) and apoptotic (p53, caspase-3 and caspase-7) (B) genes in the isolated islets were analyzed by real-time quantitative RT-PCR using SYBR Green. Target gene expression was normalized to β-actin, and the results are expressed as a fold change from the control. Results are expressed as mean ± SEM. (n = 8 mice for each group). * p < 0.05 as compared with control group.
2.3. Exposure to Mercuric Compounds Induced Oxidative Stress Damage in the Pancreatic Islets
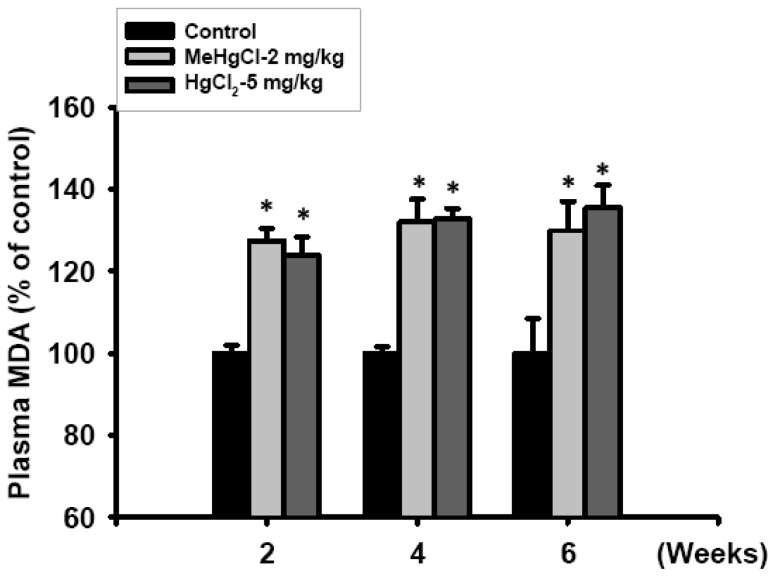
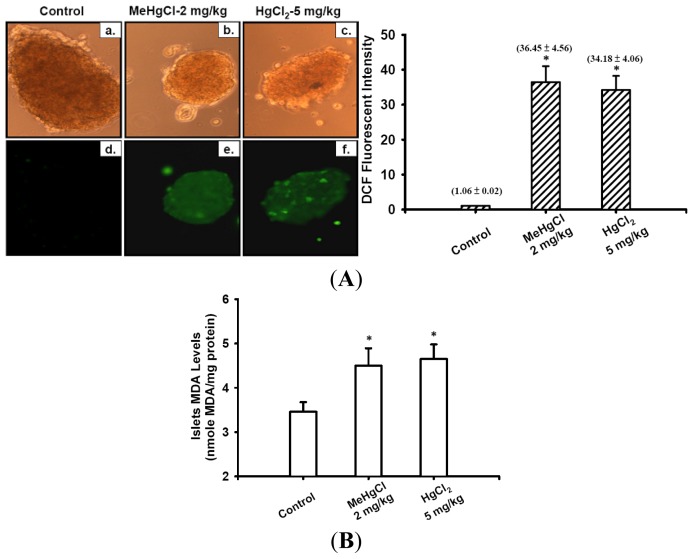
To further explore the involvement of oxidative stress damage in the mechanism underlying the toxicological effects induced by mercuric compounds in the islets of mice, we analyzed lipid peroxidation (LPO) production (as an indicator of oxidative stress damage) in the plasma and ROS generation in the islets of exposed mice. After the mice treated with MeHgCl (2 mg/kg/day) or HgCl2 (5 mg/kg/day) for 2 to 6 consecutive weeks, the plasma MDA levels were significantly increased at 2 weeks and continued to increase at 4 and 6 weeks (Figure 4). In addition, the results of 2′,7′-dichlorofluorescein (DCF) fluorescence probe intensity (as an indicator of ROS formation) and LPO assay also revealed that the intracellular ROS production (Figure 5A) and MDA levels (Figure 5B) in the isolated islets markedly increased after the mice were treated with MeHgCl or HgCl2 for 2 consecutive weeks.
Figure 4.
Effect of mercuric compounds on plasma lipid peroxidation (LPO) levels in mercuric compounds-exposed mice. Mice were gavaged with 2 mg/kg/day MeHgCl or 5 mg/kg/day HgCl2 for 2 to 6 consecutive weeks. Malondialdehyde (MDA) levels of the plasma were determined using the commercial manufacturer’s assay kit as described in the Materials and Methods section. All data are presented as means ± SEM. (n = 16 mice for each group). * p < 0.05 compared with control group.
Figure 5.
Mercuric compounds-triggered reactive oxygen species (ROS) generation in the islets of mice. Mice were treated (by oral gavaged) with 2 mg/kg/day MeHgCl or 5 mg/kg/day HgCl2 for 2 consecutive weeks, and the islets were isolated from mice. (A) The peroxide-sensitive fluorescent probe (DCFH-DA) was used to detect the ROS production in the islets. The upper panels were transmitted light images (a–c); staining with DTZ) and the lower panels were DCF fluorescence images (d–f) (200×). (B) Malondialdehyde (MDA) levels of the isolated islets were determined using the commercial manufacturer’s assay kit as described in the Materials and Methods section. Data in B are presented as means ± SEM. (n = 16 mice for each group). * p < 0.05 compared with control group.
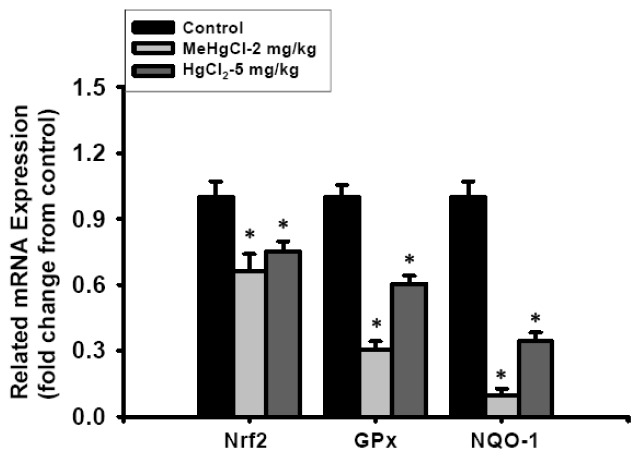
Furthermore, we analyzed the mRNA expression levels of Nrf2, GPX, and NQO1, which play an important role in the antioxidant system. A significant decrease in the expression of Nrf2, GPX, and NQO1 was revealed in the isolated islets of mice exposed to MeHgCl (2 mg/kg/day) or HgCl2 (5 mg/kg/day) for 2 consecutive weeks (Figure 6). These results indicate that exposure to mercuric compounds induces oxidative stress injury in islets in vivo.
Figure 6.
Related anti-oxidant gene expression in the isolated islets of mice exposed (by gavage) to 2 mg/kg/day MeHgCl or 5 mg/kg/day HgCl2 for 2 consecutive weeks. The expression of Nrf2, GPx, and NQO1 genes was determined by real-time quantitative RT-PCR using SYBR Green. Target gene expression was normalized to β-actin, and the results are expressed as a fold change from the control. Results are expressed as mean ± SEM. (n = 8 mice for each group). * p < 0.05 as compared with control group.
2.4. Discussion
Many in vivo studies have reported that exposure to high doses of mercury (4–40 mg/L in drinking water or 0.2–2 mg/kg/day for more than 7 consecutive days) can cause severe neuropathological injuries and neurophysiological disorders [22,23]. Recently, the growing studies have also shown that the toxicological effects of MeHgCl (2–26 mg/kg/day) or HgCl2 (5–10 mg/kg/day) induced by long-term exposure within the cerebral cortex, liver, kidney, and lung of experimental animals were accompanied by a significant production of ROS [24–27]. ROS, which include superoxide anion, hydrogen peroxide, and hydroxyl radicals, are highly reactive and can damage cell structure and function [28]. Many factors, such as ionizing radiation, xenobiotics, and toxic metals can promote ROS generation, which triggers cell death and implicates in the development of various disorders [19,20]. Mercury induces toxic effects by causing oxidative stress from ROS production, which oxidizes the membrane lipids of cells and causes the alteration of cellular function, and eventually results in cell death and pathophysiological injuries in mammals [19–21,26]. Moreover, it has been reported that oxidative stress plays a crucial role in inducing pancreatic islet β-cell injuries and the pathogenesis of DM, probably as a result of excessive levels of mitochondrial ROS production and the presence of fewer antioxidant enzymes in pancreatic β-cells [29,30]. For these reasons, it was supported that oxidative stress might contribute to the induction of the pancreatic islet injuries resulting from mercury intoxication. Recently, Chen et al. [11,12] reported that treatment with mercuric compounds can induce dysfunction and cell death in a pancreatic β-cell-derived cell line; the role of ROS in the toxicological effects induced by mercury on the pancreatic islets (in vivo), however, has not been understood. In this study, our results showed that exposure to MeHgCl (2 mg/kg/day) or HgCl2 (5 mg/kg/day) for more than 2 consecutive weeks caused a significant impairment in blood glucose regulation and decreased plasma insulin levels in mice, which was accompanied by a marked accumulation of mercury in the whole blood. In addition, the significant increase in the number of TUNEL-positive cells and changes to the expression of apoptosis-related genes in the islets of mice exposed to mercury were also revealed, that was along with an increase in plasma MDA levels, the induction of ROS generation, and the decrease in antioxidant-related mRNA expressions. Therefore, our results indicate that mercury causes oxidative stress-induced apoptosis in pancreatic islet cells, leading to deleterious effects on blood glucose regulation in vivo.
Our results in this study found that a significant increase in blood glucose and a decrease in plasma insulin levels were showed after treatment mice with MeHgCl (at 2 to 6 consecutive weeks) or HgCl2 (at 4 to 6 consecutive weeks). It is also interesting to note that after the mice treated with HgCl2 for 2 consecutive weeks, the suppression of blood glucose was associated with an increase of plasma insulin levels; but a significant elevation of blood glucose intolerance and a decrease in plasma insulin after glucose loading were also revealed. This effect might be related to the started induction of islet cell apoptosis in early stage by HgCl2, leading to the rupture the insulin secretory vesicle membranes and resulting in insulin release. Furthermore, blood glucose homeostasis is generally regulated by skeletal muscle and adipose tissues. Growing studies have focused on the mechanism by which metal-mediated glucose transport contributes to metal-induced pathologies [31–33]. It has been reported that HgCl2 can increase the levels of glucose uptake and transport in the adipocytes [34,35]. However, some studies found the opposite results [36,37]. On the basis of these reasons, we suggest that HgCl2 may be responsible for the disturbance of blood glucose homeostasis; however, the detailed mechanisms of this disturbance still require future investigation.
To ascertain whether MeHgCl- and HgCl2-induced islet dysfunction and apoptosis in vivo was mediated by a stress-related mitochondrial pathway, we analyzed the mRNA expressions of Bcl-2, Mcl-2, Mdm-2, p53, caspase-7, and caspase-3 in the islets of MeHgCl- or HgCl2-treated mice. Apoptosis, which is also known as programmed cell death, plays an important role in controlling the development of multicellular organisms and maintaining tissue homeostasis and in an increasing number of disease processes ranging from neurodegenerative diseases to the development of DM [30,38]. Caspases are cysteine aspartate proteases, which are represented the hallmark of the apoptotic process. Activation of the mitochondria (intrinsic)-regulated apoptosis pathway results in the activation of the BH3-only members of the Bcl-2 family (i.e., Bim, Bid, Bad, Puma, Noxa, Hrk, Bik, and Bmf) to initiate apoptosis signaling by binding to Bcl-2-like prosurvival proteins (i.e., Bcl-2, Bcl-xL, Bcl-w, and Mcl-1). The downstream apoptosis effector Bax or the Bax-related effector Bak is subsequently released, which induces a decrease in the mitochondrial outer membrane potential and the release of cytochrome c from mitochondria into the cytosol, leading to the activation of the caspase family of proteins [39,40]. In addition, the enhancement of Bak interaction with p53 also stimulates apoptotic effects [41]. Mdm2, an important negative regulator of the p53 tumor suppressor, binds to p53 and inhibits p53-mediated transactivation. Increased levels of mdm2 can inactivate the apoptotic and cell cycle arrest functions of p53 and regulate cell proliferation [42]. Recent studies have shown that the involvement of these apoptosis-related gene alterations in response to toxic metals (such as mercury and arsenic) can induce apoptosis [21,43,44]. Here, we found that the expression of anti-apoptosis-related mRNAs, including Bcl-2, Mcl-1, and mdm-2, were significantly decreased in the islets of mice treated with MeHgCl and HgCl2 for 2 consecutive weeks. Furthermore, the expression of apoptosis-related mRNAs, including p53, caspase-3, and caspase-7 were dramatically increased. These effects were accompanied by a significant increase in TUNEL-positive cells in the islets of mercuric compounds-treated mice.
Oxidative stress, induced by toxic metals (including mercury), plays an important role in apoptosis and pathological injuries, which are accompanied by damage to antioxidant enzymes [20,21,44]. The Nrf2 pathway has been implicated in the cell’s response to pro-oxidant and electrophilic damage. Following the electrophilic attack, Nrf2 is released from Keap1-Nrf2 complex and translocated from the cytosol to the nucleus. Subsequently, the Nrf2 binds to antioxidant and electrophile response elements, and increases in the expression of many other antioxidant genes and proteins, such as heme oxygenase-1 (HO-1), glutathione S-transferase A2 (GSTA2), thioredoxin reductase, and NAD(P)H quinone oxidoreductase (NQO1) [45–47]. Enhancing these ROS-scavenging capacities is important in maintaining cellular redox homeostasis and decreasing oxidative stress [48]. Recently, Ni et al. [49] has reported that a knockdown of Nrf2 greatly increased microglial cell death during MeHg exposure. Furthermore, the encoded protein NQO1 is a member of the NAD(P)H dehydrogenase (quinone) family, which forms homodimers and reduces quinones to hydroquinones to prevent ROS production from the reduction of an electron in the quinone. Thus, NQO1 plays a classical direct antioxidant role in the detoxification of ROS, which can be induced by the overproduction of free radicals from toxic chemicals- induced oxidative stress [48,50,51]. Moreover, glutathione peroxidase (GPx) is an important antioxidant enzyme that catalyzes the reduction of hydrogen peroxide (H2O2) [52]. However, whether mercury-induced ROS production leading to apoptosis accompanied with the decrease in the gene expression level of antioxidants (including: Nrf2, GPx, and NQO1) in the islets of mercury-exposed mice remained to be clarified. The present work showed that DCF fluorescence intensity and MDA levels in the islets of mice treated with MeHgCl or HgCl2 for 2 consecutive weeks were dramatically increased. Furthermore, the mRNA expression of Nrf2, GPx, and NQO1 were remarkably decreased in the islets of mice treated with mercuric compounds. These results implicate that mercury-induced oxidative stress plays a key role in pancreatic islets dysfunction and death in vivo.
3. Experimental Section
3.1. Animal Preparation and Study Design
Male ICR mice (6 weeks old, 20–25 g) were obtained from the animal center of the BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan). Experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) and the care and use of laboratory animals were conducted in accordance with the guidelines of the Animal Research Committee of China Medical University. The mice were housed in groups of six per cage under standard laboratory conditions at a constant temperature (23 ± 2 °C), 50% ± 20% relative humidity, and given a solid diet and tap water available ad libidum, with 12 h:12 h light-dark cycles. The mice were acclimatized to the laboratory conditions prior to the experiments and all tests were carried out between 8:00 AM and 05:00 PM. The mice were randomly divided into 3 groups: MeHgCl (2 mg/kg/day), HgCl2 (5 mg/kg/day), and age-matched control (distilled water only) that were orally gavaged for 2 to 6 consecutive weeks. At each time point (2, 4, and 6 weeks; n = 16 mice for each group), the whole blood samples were collected from an eyehole vessel (under anesthesia), and whole blood mercury concentrations were detected. Morover, the whole blood samples were centrifuged at 3000g for 10 min, and plasma was obtained, and insulin and LPO levels were assayed immediately.
Prior to pancreatic islet isolation and purification, the mice were treated with or without mercuric compounds for 2 consecutive weeks (n = 8 mice for each group) and then sacrificed by decapitation under pentobarbital anesthesia (80 mg/kg, intra-peritoneal (i.p.)). The pancreas was quickly removed and the islets were isolated. After islets purification, the ROS levels, MDA levels, and apoptosis- and antioxidant-related genes expression were analyzed.
3.2. Blood Glucose Measurement and Oral Glucose Tolerance Test (OGTT)
Mice were treated with MeHgCl (2 mg/kg/day), HgCl2 (5 mg/kg/day), or distilled water (age-matched control group; n = 16 mice for each group) for 2 to 6 consecutive weeks. Blood samples were collected from mouse eyehole after an overnight fast, and blood glucose levels were measured using an OneTouch® SureStep® blood glucose meter (Lifescan, Milpitas, CA, USA). Oral glucose tolerance test (OGTT) was performed as previously detailed [43]. Mice were fed with D-glucose by gavage after an overnight fast. Blood was collected (from an eyehole) before treatment and 30, 60, 90, 120, and 150 min after glucose administration.
3.3. Plasma Insulin Level Detection
To measure the plasma insulin concentration, the whole blood of mice treated with MeHgCl (2 mg/kg/day), HgCl2 (5 mg/kg/day), or distilled water (age-matched control group; n = 16 mice for each group) for 2 to 6 consecutive weeks was collected and centrifuged at 3000g for 10 min to obtain plasma. Aliquots of samples were then subjected to insulin antiserum immunoassay according to the manufacturer’s instructions (Mercodia, Uppsala, Sweden).
3.4. Determination of Mercury Concentrations
To determine the Hg concentrations, 300 mg of whole blood from each mouse was placed in a 15 mL polyethylene tube, and 0.5–1 mL of a 3:1 mixture of hydrochloric acid (35%) and nitric acid (70%) was added. The tubes were capped and allowed to stand overnight in a 50 °C oven. After cooling, a suitable dilution buffer (0.3% nitric acid and 0.1% Triton X-100 in distilled water) was added to the digested material, and the total mercury content was determined by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). The detection limit for mercury was ~0.1 ppb (μg/L).
3.5. TUNEL and Insulin Double Staining
Mice were treated with MeHgCl (2 mg/kg/day), HgCl2 (5 mg/kg/day), or distilled water (age-matched control group) for 2 consecutive weeks, and their pancreases were isolated and fixed in 10% formaldehyde in PBS. To examine apoptosis in islets, TUNEL and insulin (to identify β-cells) double immunostaining was performed on 5-μm pancreas sections (onparaffin slide). After deparaffinization and rehydration, TUNEL staining was performed using a fluorometric transferase-mediated TUNEL assay kit (Promega Corporation, Madison, WI, USA) by following the manufacturer’s procedure. Following the TUNEL stain, the slide was rinsed in phosphate-buffer saline (PBS) and incubated with a rabbit polyclonal IgG anti-insulin antibody (Santa Cruz Biotechnology, Inc., CA, USA) for 1 h at room temperature. The slide was then washed four times with PBS and incubated with the secondary antibody labeled with Cy3 (Millipore Corporation, Billerica, MA, USA) for 1 h. After being washed twice with PBS, the slide was observed with a Leica DMIL inverted fluorescence microscope equipped with a charge-coupled device camera (400× magnification).
3.6. Lipid Peroxidation Detection
The formation of MDA, a substance produced during lipid peroxidation, was determined using a commercial LPO assay kit (Calbiochem, San Diego, CA, USA) according to the manufacturer’s instructions. Briefly, the isolated islets were homogenized separately in ice-cold 20 mM Tris-HCl buffer (pH 7.4), and then the homogenized samples were assayed immediately. Equal volumes of plasma and islet homogenates (n = 16 mice for each group) were added to 3.25 volumes of diluted R1 reagent (10.3 mM N-methyl-2-phenylindole in acetonitrile). After mixing, the mixture was added to a 0.75 volumes of 37% HCl and was then incubated at 45 °C for 60 min. After cooling, the absorbance of the clear supernatant was subjected to an enzyme-linked immunosorbent assay (ELISA) microplate and read at 586 nm. The linearity of the standard curve was confirmed with 0, 1, 2.5, 5, 10, 20, and 40 μM of MDA standard (1,1,3,3-tetramethoxypropane in Tris-HCl). The protein concentration was determined using a bicinchoninic acid protein assay kit with an absorption band of 570 nm. (Pierce, Rockford, IL, USA).
3.7. Pancreatic Islet Isolation and Purification Procedure
Isolation of the mouse islet of Langerhans was performed as previously described [53,54]. In brief, collagenase, prepared in Hank’s balanced salt solution with 25 mM Hepes, was infused into the main bile duct of each mouse with a 30-gauge needle connected to a 10 cc syringe. The whole pancreas was collected and digested at 37 °C. The islets were obtained using a Ficoll density gradient of 1.069–1.096 g/mL. The number of islets was counted by staining samples with dithizone (DTZ), and the islet equivalent (IEQ) range was 75–150 μm (where 1 IEQ is equivalent to an islet with a diameter of 150 μm). The islets were cultured in RPMI 1640 medium containing fetal bovine serum (10%), penicillin-streptomycin (100 unit/mL), L-glutamate (2 mM), and Hepes (25 mM).
3.8. Real-Time Quantitative Reverse-Transcribed Polymerase Chain Reaction (RT-PCR) Analysis
The expression of related genes was evaluated by the real-time quantitative RT-PCR as previously described [21,44]. Briefly, intracellular total RNA was extracted from 300 IEQ islets of each mouse (n = 16 mice for each group) using RN easy kits (Qiagen) according to the manufacturer’s instructions. Samples were heated to 90 °C for 5 min to remove any secondary structures, and then placed immediately on ice. Samples were reverse transcribed into cDNA using the AMV RTase system (Promega Corporation, Pty. Ltd., Madison, Wisconsin, USA) according to the manufacturer’s instructions. Each sample (2 μL) was tested with the Sybr Green Real-time PCR reagent (Invitrogen, Grand Island, NY, USA) using mouse-specific primers ((1) Bcl-2, Mcl-1, p53, Caspase-3, Caspase-7, glutathione peroxidase (GPx), NAD(P)H quinone oxidoreductase (NQO-1), and β-Actin as described in Lu et al. [21] and Yen et al. [44]; (2) murine double minute 2 (mdm2): forward: 5′-GGAGCGCAA AACGACACTTACA-3′ and Reverse: 5′-CTCGCTGCTGCTGCTGCTAC-3′ [55]; (3) nuclear factor erythroid-derived 2-related factor 2 (Nrf2) : forward: 5′-TGAAGCTCAGCTCGCATTGATCC-3′ and Reverse: 5′-AAGATACAAGGTGCTGAGCCGCC-3′ [56]) in a 25 μL reaction volume, and amplification and real-time fluorescence detection were performed using the ABI StepOnePlus sequence detection system (PE, Applied Biosystems, Carlsbad, CA, USA). The cycling conditions were as follows: 2 min at 50 °C, 10 min at 95 °C, 40 cycles of 92 °C for 30 s, and 1 min at 60 °C. Real-time fluorescence detection was performed during the 60 °C annealing/extension step of each cycle. A melt-curve analysis was performed on each primer set to ensure that no primer dimers or nonspecific amplification was present under the optimized cycling conditions. Data analysis was performed using StepOne™ software (Version 2.1, Applied Biosystems, Carlsbad, CA, USA, 2008). All amplification curves were analyzed with a normalized reporter (Rn: the ratio of the fluorescence emission intensity to the fluorescence signal of the passive reference dye) threshold of 0.2 to obtain the CT values (threshold cycle). The reference control genes were measured with four replicates from each PCR run, and the CT average was used for relative quantification analyses (the relative quantification method using real-time PCR efficiencies [57]). TF expression data were normalized by subtracting the mean of the reference gene CT values from their CT values (ΔCT). The fold change value was calculated using the expression 2−ΔΔC T, where ΔΔCT represents ΔCT-condition of interest − ΔCT-control. Prior to conducting statistical analyses, the fold change from the mean of the control group was calculated for each individual sample.
3.9. Detection of Intracellular ROS in Islets
Mice were treated with MeHgCl (2 mg/kg/day), HgCl2 (5 mg/kg/day), or distilled water (age-matched control group) for 2 consecutive weeks, and the islets were then isolated. After 15 min of incubation of 2′,7′-dichlorfluorescein diacetate (DCFH-DA), the islets were washed twice in PBS and the images were captured using a Leica DMIL inverted fluorescence microscope equipped with a charge-coupled device camera (with 200× magnification).
3.10. Statistical Analyses
Data are presented as means ± standard errors of the mean (SEM). Significant differences were evaluated using Student’s t-test. When more than one group was compared with the control, the significance was evaluated according to a one-way ANOVA, and the Duncan’s post hoc test was applied to identify group differences. The p value less than 0.05 was considered to be significant. The statistical package SPSS, version 11.0 for Windows (SPSS Inc., Chicago, IL, USA, 2001) was used for the statistical analyses.
4. Conclusions
Collectively, the present in vivo results provide evidence that mercuric compounds (MeHgCl and HgCl2) are capable of causing pancreatic islet dysfunction (elevated blood glucose levels and decreased plasma insulin secretion) and apoptosis (decreased anti-apoptotic (Bcl-2, Mcl-1, and mdm-2) and increased apoptotic (p53, caspase-3, and caspase-7) related gene expressions) in treated mice. More importantly, this study has demonstrated that mercuric compounds induce pancreatic islet apoptosis in vivo through ROS generation, which leads to the destruction of antioxidant enzyme function (decreased the mRNA expressions of Nrf2, GPx, and NQO1). These observations further clarify that mercuric compounds-induced oxidative stress injuries cause pancreatic islet dysfunction and apoptosis in vivo.
Acknowledgments
This work was supported by research grants form the China Medical University Hospital, Taichung, Taiwan (DMR-100-069), the National Science Council of Taiwan (NSC 100-2314-B-039-023-, NSC 98-2320-B-039-014-MY3), the Changhua Christian Hospital, Changhua, Taiwan (101-CCH-IRP-06 and 100-CCH-IRP-53), the Buddhist Tzu Chi General Hospital, Taichung Branch, Taiwan (TTCRD100-20 and TTCRD101-13), and also supported in part by Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH101-TD-B-111-004).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Karlsson K., Viklander M., Scholes L., Revitt M. Heavy metal concentrations and toxicity in water and sediment from stormwater ponds and sedimentation tanks. J. Hazard Mater. 2010;178:612–618. doi: 10.1016/j.jhazmat.2010.01.129. [DOI] [PubMed] [Google Scholar]
- 2.Ratcliffe H.E., Swanson G.M., Fischer L.J. Human exposure to mercury: A critical assessment of the evidence of adverse health effects. J. Toxicol. Environ. Health. 1996;49:221–270. doi: 10.1080/713851079. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D. Fish, mercury, selenium and cardiovascular risk: Current evidence and unanswered questions. Int. J. Environ. Res. Public. Health. 2009;6:1894–1916. doi: 10.3390/ijerph6061894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iavicoli I., Fontana L., Bergamaschi A. The effects of metals as endocrine disruptors. J. Toxicol. Environ. Health B Crit. Rev. 2009;12:206–223. doi: 10.1080/10937400902902062. [DOI] [PubMed] [Google Scholar]
- 5.Michalke B., Halbach S., Nischwitz V. JEM spotlight: Metal speciation related to neurotoxicity in humans. J. Environ. Monit. 2009;11:939–954. doi: 10.1039/b817817h. [DOI] [PubMed] [Google Scholar]
- 6.Clarkson T.W., Magos L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 7.Ishitobi H., Stern S., Thurston S.W., Zareba G., Langdon M., Gelein R., Weiss B. Organic and inorganic mercury in neonatal rat brain after prenatal exposure to methylmercury and mercury vapor. Environ. Health Perspect. 2010;118:242–248. doi: 10.1289/ehp.0900956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi T., Eto K. Pathology and Pathogenesis of Minamata Disease. In: Tsubaki T., Irukayama K., editors. Minamata Diseases Methyl Mercury Poisoning in Minamata and Niigata, Japan. Kodansya; Tokyo, Japan: 1997. pp. 103–141. [Google Scholar]
- 9.Uchino M., Tanaka Y., Ando Y., Yonehara T., Hara A., Mishima I., Okajima T., Ando M. Neurologic features of chronic minamata disease (organic mercury poisoning) and incidence of complications with aging. J. Environ. Sci. Health B. 1995;30:699–715. doi: 10.1080/03601239509372961. [DOI] [PubMed] [Google Scholar]
- 10.Shigenaga K. Pancreatic islet injury induced by methyl mercuric chloride light and electron microscopic studies. Kumamoto Med. J. 1976;29:67–81. [PubMed] [Google Scholar]
- 11.Chen Y.W., Huang C.F., Tsai K.S., Yang R.S., Yen C.C., Yang C.Y., Lin-Shiau S.Y., Liu S.H. Methylmercury induces pancreatic β-cell apoptosis and dysfunction. Chem. Res. Toxicol. 2006;19:1080–1085. doi: 10.1021/tx0600705. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y.W., Huang C.F., Yang C.Y., Yen C.C., Tsai K.S., Liu S.H. Inorganic mercury causes pancreatic β-cell death via the oxidative stress-induced apoptotic and necrotic pathways. Toxicol. Appl. Pharmacol. 2010;243:323–331. doi: 10.1016/j.taap.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Chatenoud L., You S., Okada H., Kuhn C., Michaud B., Bach J.F. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Immune therapies of type 1 diabetes: New opportunities based on the hygiene hypothesis. Clin. Exp. Immunol. 2010;160:106–112. doi: 10.1111/j.1365-2249.2010.04125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson J.D., Luciani D.S. Mechanisms of pancreatic β-cell apoptosis in diabetes and its therapies. Adv. Exp. Med. Biol. 2010;654:447–462. doi: 10.1007/978-90-481-3271-3_19. [DOI] [PubMed] [Google Scholar]
- 15.Ma K., Nunemaker C.S., Wu R., Chakrabarti S.K., Taylor-Fishwick D.A., Nadler J.L. 12-lipoxygenase products reduce insulin secretion and β-cell viability in human islets. J. Clin. Endocrinol. Metab. 2010;95:887–893. doi: 10.1210/jc.2009-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou N., Torii S., Saito N., Hosaka M., Takeuchi T. Reactive oxygen species-mediated pancreatic β-cell death is regulated by interactions between stress-activated protein kinases, p38 and c-Jun N-terminal kinase, and mitogen-activated protein kinase phosphatases. Endocrinology. 2008;149:1654–1665. doi: 10.1210/en.2007-0988. [DOI] [PubMed] [Google Scholar]
- 17.Kaneto H., Kawamori D., Matsuoka T.A., Kajimoto Y., Yamasaki Y. Oxidative stress and pancreatic β-cell dysfunction. Am. J. Ther. 2005;12:529–533. doi: 10.1097/01.mjt.0000178773.31525.c2. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y.W., Yang C.Y., Huang C.F., Hung D.Z., Leung Y.M., Liu S.H. Heavy metals, islet function and diabetes development. Islets. 2009;1:169–176. doi: 10.4161/isl.1.3.9262. [DOI] [PubMed] [Google Scholar]
- 19.Jomova K., Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Jomova K., Vondrakova D., Lawson M., Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 21.Lu T.H., Hsieh S.Y., Yen C.C., Wu H.C., Chen K.L., Hung D.Z., Chen C.H., Wu C.C., Su Y.C., Chen Y.W., et al. Involvement of oxidative stress-mediated ERK1/2 and p38 activation regulated mitochondria-dependent apoptotic signals in methylmercury-induced neuronal cell injury. Toxicol. Lett. 2011;204:71–80. doi: 10.1016/j.toxlet.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho M.C., Franco J.L., Ghizoni H., Kobus K., Nazari E.M., Rocha J.B., Nogueira C.W., Dafre A.L., Muller Y.M., Farina M. Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on methylmercury-induced locomotor deficits and cerebellar toxicity in mice. Toxicology. 2007;239:195–203. doi: 10.1016/j.tox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Goulet S., Dore F.Y., Mirault M.E. Neurobehavioral changes in mice chronically exposed to methylmercury during fetal and early postnatal development. Neurotoxicol. Teratol. 2003;25:335–347. doi: 10.1016/s0892-0362(03)00007-2. [DOI] [PubMed] [Google Scholar]
- 24.De Freitas A.S., Funck V.R., Rotta Mdos S., Bohrer D., Morschbacher V., Puntel R.L., Nogueira C.W., Farina M., Aschner M., Rocha J.B. Diphenyl diselenide, a simple organoselenium compound, decreases methylmercury-induced cerebral, hepatic and renal oxidative stress and mercury deposition in adult mice. Brain Res. Bull. 2009;79:77–84. doi: 10.1016/j.brainresbull.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Glaser V., Nazari E.M., Muller Y.M., Feksa L., Wannmacher C.M., Rocha J.B., de Bem A.F., Farina M., Latini A. Effects of inorganic selenium administration in methylmercury-induced neurotoxicity in mouse cerebral cortex. Int. J. Dev. Neurosci. 2010;28:631–637. doi: 10.1016/j.ijdevneu.2010.07.225. [DOI] [PubMed] [Google Scholar]
- 26.Lu T.H., Chen C.H., Lee M.J., Ho T.J., Leung Y.M., Hung D.Z., Yen C.C., He T.Y., Chen Y.W. Methylmercury chloride induces alveolar type II epithelial cell damage through an oxidative stress-related mitochondrial cell death pathway. Toxicol. Lett. 2010;194:70–78. doi: 10.1016/j.toxlet.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J.Q., Wen Y.F., Bhadauria M., Nirala S.K., Sharma A., Shrivastava S., Shukla S., Agrawal O.P., Mathur R. Protective effects of propolis on inorganic mercury induced oxidative stress in mice. Indian J. Exp. Biol. 2009;47:264–269. [PubMed] [Google Scholar]
- 28.Zhang Q., Pi J., Woods C.G., Andersen M.E. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol. Appl. Pharmacol. 2010;244:84–97. doi: 10.1016/j.taap.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drews G., Krippeit-Drews P., Dufer M. Oxidative stress and β-cell dysfunction. Pflugers Arch. 2010;460:703–718. doi: 10.1007/s00424-010-0862-9. [DOI] [PubMed] [Google Scholar]
- 30.Rains J.L., Jain S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bazuine M., Ouwens D.M., Gomes de Mesquita D.S., Maassen J.A. Arsenite stimulated glucose transport in 3T3-L1 adipocytes involves both Glut4 translocation and p38 MAPK activity. Eur. J. Biochem. 2003;270:3891–3903. doi: 10.1046/j.1432-1033.2003.03771.x. [DOI] [PubMed] [Google Scholar]
- 32.Xue P., Hou Y., Zhang Q., Woods C.G., Yarborough K., Liu H., Sun G., Andersen M.E., Pi J. Prolonged inorganic arsenite exposure suppresses insulin-stimulated AKT S473 phosphorylation and glucose uptake in 3T3-L1 adipocytes: Involvement of the adaptive antioxidant response. Biochem. Biophys. Res. Commun. 2011;407:360–365. doi: 10.1016/j.bbrc.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walton F.S., Harmon A.W., Paul D.S., Drobna Z., Patel Y.M., Styblo M. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: possible mechanism of arsenic-induced diabetes. Toxicol. Appl. Pharmacol. 2004;198:424–433. doi: 10.1016/j.taap.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Barnes D.M., Hanlon P.R., Kircher E.A. Effects of inorganic HgCl2 on adipogenesis. Toxicol. Sci. 2003;75:368–377. doi: 10.1093/toxsci/kfg195. [DOI] [PubMed] [Google Scholar]
- 35.Barnes D.M., Kircher E.A. Effects of mercuric chloride on glucose transport in 3T3-L1 adipocytes. Toxicol. In Vitro. 2005;19:207–214. doi: 10.1016/j.tiv.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Kasahara T., Kasahara M. Characterization of rat Glut4 glucose transporter expressed in the yeast Saccharomyces cerevisiae: Comparison with Glut1 glucose transporter. Biochim. Biophys. Acta. 1997;1324:111–119. doi: 10.1016/s0005-2736(96)00217-9. [DOI] [PubMed] [Google Scholar]
- 37.Miller D.S., Shehata A.T., Lerner J. HgCl2 inhibition of D-glucose transport in jejunal tissue from 2 day and 21 day chicks. J. Pharmacol. Exp. Ther. 1980;214:101–105. [PubMed] [Google Scholar]
- 38.Loh K.P., Huang S.H., De Silva R., Tan B.K., Zhu Y.Z. Oxidative stress: Apoptosis in neuronal injury. Curr. Alzheimer Res. 2006;3:327–337. doi: 10.2174/156720506778249515. [DOI] [PubMed] [Google Scholar]
- 39.Willis S.N., Fletcher J.I., Kaufmann T., van Delft M.F., Chen L., Czabotar P.E., Ierino H., Lee E.F., Fairlie W.D., Bouillet P., et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 40.McKenzie M.D., Jamieson E., Jansen E.S., Scott C.L., Huang D.C., Bouillet P., Allison J., Kay T.W., Strasser A., Thomas H.E. Glucose induces pancreatic islet cell apoptosis that requires the BH3-only proteins Bim and Puma and multi-BH domain protein Bax. Diabetes. 2010;59:644–652. doi: 10.2337/db09-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu C., Smith S.D., Pang L., Sadovsky Y., Nelson D.M. Enhanced basal apoptosis in cultured term human cytotrophoblasts is associated with a higher expression and physical interaction of p53 and Bak. Placenta. 2006;27:978–983. doi: 10.1016/j.placenta.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Iwakuma T., Lozano G. MDM2, an introduction. Mol. Cancer Res. 2003;1:993–1000. [PubMed] [Google Scholar]
- 43.Lu T.H., Su C.C., Chen Y.W., Yang C.Y., Wu C.C., Hung D.Z., Chen C.H., Cheng P.W., Liu S.H., Huang C.F. Arsenic induces pancreatic β-cell apoptosis via the oxidative stress-regulated mitochondria-dependent and endoplasmic reticulum stress-triggered signaling pathways. Toxicol. Lett. 2011;201:15–26. doi: 10.1016/j.toxlet.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Yen C.C., Ho T.J., Wu C.C., Chang C.F., Su C.C., Chen Y.W., Jinn T.R., Lu T.H., Cheng P.W., et al. Inorganic arsenic causes cell apoptosis in mouse cerebrum through an oxidative stress-regulated signaling pathway. Arch. Toxicol. 2011;85:565–575. doi: 10.1007/s00204-011-0709-y. [DOI] [PubMed] [Google Scholar]
- 45.Jaiswal A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 46.Ade N., Leon F., Pallardy M., Peiffer J.L., Kerdine-Romer S., Tissier M.H., Bonnet P.A., Fabre I., Ourlin J.C. HMOX1 and NQO1 genes are upregulated in response to contact sensitizers in dendritic cells and THP-1 cell line: role of the Keap1/Nrf2 pathway. Toxicol. Sci. 2009;107:451–460. doi: 10.1093/toxsci/kfn243. [DOI] [PubMed] [Google Scholar]
- 47.Simmons S.O., Fan C.Y., Yeoman K., Wakefield J., Ramabhadran R. NRF2 oxidative stress induced by heavy metals is cell type dependent. Curr. Chem. Genomics. 2011;5:1–12. doi: 10.2174/1875397301105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pi J., Freeman M.L., Yamamoto M. Nrf2 in toxicology and pharmacology: The good, the bad and the ugly? Toxicol. Appl. Pharmacol. 2010;244:1–3. doi: 10.1016/j.taap.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ni M., Li X., Yin Z., Jiang H., Sidoryk-Wegrzynowicz M., Milatovic D., Cai J., Aschner M. Methylmercury induces acute oxidative stress, altering Nrf2 protein level in primary microglial cells. Toxicol. Sci. 2010;116:590–603. doi: 10.1093/toxsci/kfq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pi J., Zhang Q., Woods C.G., Wong V., Collins S., Andersen M.E. Activation of Nrf2-mediated oxidative stress response in macrophages by hypochlorous acid. Toxicol. Appl. Pharmacol. 2008;226:236–243. doi: 10.1016/j.taap.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 51.Siegel D., Gustafson D.L., Dehn D.L., Han J.Y., Boonchoong P., Berliner L.J., Ross D. NAD(P)H:quinone oxidoreductase 1: Role as a superoxide scavenger. Mol. Pharmacol. 2004;65:1238–1247. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- 52.Kudin A.P., Augustynek B., Lehmann A.K., Kovacs R., Kunz W.S. The contribution of thioredoxin-2 reductase and glutathione peroxidase to H(2)O(2) detoxification of rat brain mitochondria. Biochim. Biophys. Acta. 2012;1817:1901–1906. doi: 10.1016/j.bbabio.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 53.Huang C.F., Chen Y.W., Yang C.Y., Lin H.Y., Way T.D., Chiang W., Liu S.H. Extract of lotus leaf (Nelumbo nucifera) and its active constituent catechin with insulin secretagogue activity. J. Agric. Food Chem. 2011;59:1087–1094. doi: 10.1021/jf103382h. [DOI] [PubMed] [Google Scholar]
- 54.Huang G.C., Zhao M., Jones P., Persaud S., Ramracheya R., Lobner K., Christie M.R., Banga J.P., Peakman M., Sirinivsan P., et al. The development of new density gradient media for purifying human islets and islet-quality assessments. Transplantation. 2004;77:143–145. doi: 10.1097/01.TP.0000100401.62912.B2. [DOI] [PubMed] [Google Scholar]
- 55.Valenzuela M.T., Guerrero R., Nunez M.I., Ruiz de Almodovar J.M., Sarker M., de Murcia G., Oliver F.J. PARP-1 modifies the effectiveness of p53-mediated DNA damage response. Oncogene. 2002;21:1108–1116. doi: 10.1038/sj.onc.1205169. [DOI] [PubMed] [Google Scholar]
- 56.Barve A., Khor T.O., Nair S., Reuhl K., Suh N., Reddy B., Newmark H., Kong A.N. γ-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int. J. Cancer. 2009;124:1693–1699. doi: 10.1002/ijc.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pfaffl M.W., Horgan G.W., Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36–e45. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]