Abstract
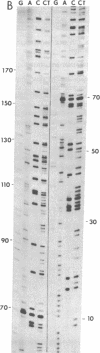
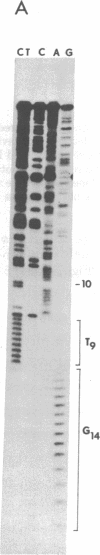
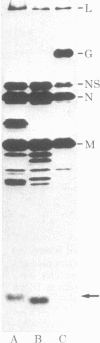
We have determined the COOH-terminal and NH2-terminal amino acid sequences of the vesicular stomatitis virus (VSV) glycoprotein (G). A sequence of 122 COOH-terminal amino acids was deduced from the complete sequence of a cloned DNA insert carrying 470 nucleotides derived from the 3′ end of the G mRNA. Evidence presented indicates that this portion of the polypeptide includes the domains of G that reside inside the virion and span the lipid bilayer of the virion. This seems clear because a partial amino acid sequence of a fragment of G that remains associated with the membrane of the virion after exhaustive proteolytic digestions can be located unambiguously in the predicted sequence. This predicted sequence contains an uninterrupted hydrophobic domain beginning 49 amino acids and ending 30 amino acids from the COOH terminus. This region presumably spans the lipid bilayer. The COOH-terminal portion of 29 amino acids contains a high proportion of basic residues and resides inside the virion. The COOH-terminal portion of the VSV G protein therefore resembles in structure that of glycophorin, an erythrocyte membrane protein well characterized previously. The configuration of G in the viral membrane demonstrated here is probably similar for other viral glycoproteins, although this has not been tested as directly in any other case. From the sequence of a DNA primer extended on the RNA genome from the adjacent M protein gene into the G protein gene, we have deduced an NH2-terminal G protein sequence of 53 amino acids, including the leader sequence of 16 amino acids. Our sequence confirms, extends, and corrects two partial amino acid sequences reported for this region previously.
Keywords: transmembrane protein sequence, signal sequence, cDNA cloning, DNA sequence determination
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Repik P., Obijeski J. F., Moore N. F., Wagner R. R. Restitution of infectivity to spikeless vesicular stomatitis virus by solubilized viral components. J Virol. 1975 Jul;16(1):75–84. doi: 10.1128/jvi.16.1.75-84.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F. Surface structure of vesicular stomatitis virus. J Gen Virol. 1969 Jul;5(1):1–10. doi: 10.1099/0022-1317-5-1-1. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Morrison T. G. Vesicular stomatitis virus glycoprotein is anchored to intracellular membranes near its carboxyl end and is proteolytically cleaved at its amino terminus. J Virol. 1979 Mar;29(3):957–963. doi: 10.1128/jvi.29.3.957-963.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison J. R., Robertson J. S., Summers D. F. Partial structural analysis of the oligosaccharide moieties of the vesicular stomatitis virus glycoprotein by sequential chemical and enzymatic degradation. Virology. 1977 May 15;78(2):375–392. doi: 10.1016/0042-6822(77)90115-5. [DOI] [PubMed] [Google Scholar]
- Garoff H., Söderlund H. The amphiphilic membrane glycoproteins of Semliki Forest virus are attached to the lipid bilayer by their COOH-terminal ends. J Mol Biol. 1978 Sep 25;124(3):535–549. doi: 10.1016/0022-2836(78)90186-9. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Irving R. A., Toneguzzo F., Rhee S. H., Hofmann T., Ghosh H. P. Synthesis and assembly of membrane glycoproteins: presence of leader peptide in nonglycosylated precursor of membrane glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):570–574. doi: 10.1073/pnas.76.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz F. N., Lodish H. F. Transmembrane biogenesis of the vesicular stomatitis virus glycoprotein. J Cell Biol. 1979 Feb;80(2):416–426. doi: 10.1083/jcb.80.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Rose J. K., Lodish H. F. Translation of individual species of vesicular stomatitis viral mRNA. J Virol. 1975 Apr;15(4):1004–1011. doi: 10.1128/jvi.15.4.1004-1011.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Katz F. N., Lodish H. F., Blobel G. A signal sequence for the insertion of a transmembrane glycoprotein. Similarities to the signals of secretory proteins in primary structure and function. J Biol Chem. 1978 Dec 25;253(24):8667–8670. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A. Glycoprotein fragment associated with vesicular stomatitis virus after proteolytic digestion. Virology. 1974 Dec;62(2):573–577. doi: 10.1016/0042-6822(74)90419-x. [DOI] [PubMed] [Google Scholar]
- Porter A. G., Barber C., Carey N. H., Hallewell R. A., Threlfall G., Emtage J. S. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. doi: 10.1038/282471a0. [DOI] [PubMed] [Google Scholar]
- Reading C. L., Penhoet E. E., Ballou C. E. Carbohydrate structure of vesicular stomatitis virus glycoprotein. J Biol Chem. 1978 Aug 25;253(16):5600–5612. [PubMed] [Google Scholar]
- Rose J. K. Complete intergenic and flanking gene sequences from the genome of vesicular stomatitis virus. Cell. 1980 Feb;19(2):415–421. doi: 10.1016/0092-8674(80)90515-2. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Iverson L. Nucleotide sequences from the 3'-ends of vesicular stomatitis virus mRNA's as determined from cloned DNA. J Virol. 1979 Nov;32(2):404–411. doi: 10.1128/jvi.32.2.404-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Nucleotide sequences of ribosome recgonition sites in messenger RNAs of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3672–3676. doi: 10.1073/pnas.74.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Association of vesicular stomatitis virus glycoprotein with virion membrane: characterization of the lipophilic tail fragment. J Virol. 1975 Aug;16(2):237–240. doi: 10.1128/jvi.16.2.237-240.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. The hydrophobic effect and the organization of living matter. Science. 1978 Jun 2;200(4345):1012–1018. doi: 10.1126/science.653353. [DOI] [PubMed] [Google Scholar]
- Tomita M., Furthmayr H., Marchesi V. T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry. 1978 Oct 31;17(22):4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. Synthesis and glycosylation in vitro of glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1516–1520. doi: 10.1073/pnas.74.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Sefton B. M. Two small virus-specific polypeptides are produced during infection with Sindbis virus. J Virol. 1979 Mar;29(3):1186–1195. doi: 10.1128/jvi.29.3.1186-1195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]