Abstract
Phage therapy presents an alternative approach against the emerging methicillin-resistant Staphylococcus aureus (MRSA) threat. Some of the problems encountered during isolation of MRSA phages include the high prevalence of enteric phages in natural sources, nonspecific absorption of viable phage, and the formation of pinpoint or tiny plaques. The phage isolated in this study, MR-5, also formed tiny plaques against its host S. aureus ATCC 43300 (MRSA), making its detection and enumeration difficult. An improved method of increasing the plaque size of MRSA phage by incorporating sublethal concentrations of three different classes of antibiotics (inhibitors of protein synthesis) in the classical double-layer agar (DLA) method was investigated. The β-lactam and quinolone antibiotics commonly employed in earlier studies for increasing the plaque size did not show any significant effect on the plaque size of isolated MR-5 phage. Linezolid (oxazolidinone class), tetracycline, and ketolide antibiotics brought significant enhancements (3 times the original size) in the plaque size of MR-5 phage. Prior treatment with these antibiotics resulted in significant reductions in the time of adsorption and the latent period of MR-5 phage. To rule out whether the action of linezolid (which brought the maximum increase in plaque size) was specific for a single phage only, its effect on the plaque size of seven other S. aureus-specific phages was also assessed. Significant enhancements in the plaque size of these phages were observed. These results indicate that this modification can therefore safely be incorporated in the traditional DLA overlay method to search for new MRSA-virulent phages.
INTRODUCTION
Staphylococcus aureus is a leading cause of both community- and hospital-acquired infections, including skin and soft tissue infections, wound infections, endocarditis, osteomyelitis, and life-threatening bacteremia (6, 23). The emergence of high levels of penicillin resistance, followed by the development and spread of strains resistant to the action of nafcillin, oxacillin, methicillin, and now, even vancomycin has made therapy of staphylococcal disease a global challenge (23, 24, 34). More people in the United States now die from methicillin-resistant S. aureus (MRSA) infection than from AIDS (33). Hence, there is an unmet medical urgency and pressing need to develop novel antibacterials to tackle the staphylococcal menace. Phage therapy can serve as one of the stand-alone approaches for treating staphylococcal infections refractory to the action of commonly deployed antibiotics (6).
The first step in investigating the potential of therapy using phage or its products against MRSA infections involves the isolation and selection of a lytic bacteriophage covering a maximum number of MRSA strains, both from community and hospital sources. The difficulty encountered in the isolation of phages specific to staphylococcal strains is partly attributed to the prevalence of enteric phages in sewage samples and partly to the high level of nonspecific adsorption of viable phages to dead bacteria, body fluids, pus, serum, milk proteins, carbohydrate fractions, etc., present in various samples, leading to their inactivation (4, 5, 10, 19, 20). To further worsen the situation, staphylococcal phages tend to form pinpoint or tiny, small, or even turbid plaques that are difficult to enumerate or visualize. This decreases the probability of isolating an effective phage preparation (28). Hence, there is a need to devise methods of enhancing the size and visibility of the so-formed tiny plaques of staphylococcal phages in order to increase their chances of isolation, detection, and better enumeration. Plaque development occurs in four different phases: the primary adsorption event when phage searches for its host bacterium mainly through viral diffusion, the first few rounds of viral multiplication, the plaque enlargement phase, and the final phase, when phage multiplication ceases on maturation of the lawn to stationary phase (3). Phage growth characteristics, including phage adsorption rate, latent period, burst size, initial lawn density, bacterial division rates, and lawn maturation to stationary phase, greatly affect the plaque size, as the majority of plaque development occurs during the enlargement phase (1, 2, 3, 11). A high adsorption rate, short latent period, large burst size, high diffusion rate, lower initial lawn density, or delayed lawn maturation all seem to act positively toward the formation of a larger plaque. This study investigated the effects of protein synthesis-inhibiting antibiotics on the plaque size and morphology of staphylococcal phages by assessing their ability to affect different phases of phage growth (adsorption time, latent period, and burst size) and host growth characteristics favoring the formation of comparatively bigger plaques.
MATERIALS AND METHODS
Media and antibiotics.
Brain heart infusion broth (BHI) was used for bacterial growth. For use in the double-layer agar (DLA) method, the same medium was supplemented with Bacto agar (Becton, Dickinson Biosciences, Franklin Lakes, NJ) at final concentrations of 1.5% and 0.6% for the bottom and top layer, respectively. Ampicillin, ciprofloxacin, chloramphenicol, amikacin, linezolid, tetracycline, clarithromycin, and telithromycin were purchased from Sigma-Aldrich, Inc., India. The stock solutions (1 mg/ml) of these antibiotics were prepared in distilled water (except for telithromycin and clarithromycin, whose stocks were prepared in dimethyl sulfoxide), filtered through a 0.22-μm syringe filter, and stored at 4°C till further use.
Bacterial strains and susceptibility testing.
Standard strains of S. aureus, ATCC 43300 and ATCC 25923, from American Type Culture Collection (ATCC), Manassas, VA, were used in this study. The clinical isolates of S. aureus were procured from PGIMER, Chandigarh, India. These strains were identified on the basis of Gram reaction, growth on mannitol salt agar (MSA), catalase activity, and coagulase test. Disk diffusion assay was performed to confirm resistance to penicillin, methicillin, and oxacillin, followed by determination of MICs against oxacillin by broth microdilution assay as recommended by the Clinical and Laboratory Standards Institute (CLSI) (9). As stated in the CLSI standards, S. aureus isolates with oxacillin MICs of ≥4 μg/ml were taken as methicillin-resistant S. aureus (MRSA) and strains with MICs of ≤4 μg/ml as methicillin-sensitive S. aureus (MSSA). All clinical strains with MICs of ≥4 μg/ml were selected, numbered sequentially as MRSA 01 to 45, and stored in glycerol at −80°C.
The MICs of linezolid, tetracycline, telithromycin, and clarithromycin against S. aureus ATCC 43300 (MRSA) were determined by broth microdilution as recommended by CLSI (9) within an antibiotic working range of 0.25 to 32 μg/ml using cation-adjusted Mueller-Hinton broth (caMHB). The MIC was read manually after 16 to 18 h of incubation.
Phage isolation.
Phage isolation was carried out by the method of Cerveny et al. (7). Briefly, sewage samples, after centrifugation and filtration through a 0.45-μm syringe filter, were mixed with an equal volume of BHI broth (with 5 mM CaCl2) to which 3 to 5 ml of exponentially growing host strain was added, and the mixtures were incubated overnight at 37°C and 150 rpm. The next day, the samples were centrifuged, filtered again, and mixed with an equal volume of active host culture and then incubated overnight at 37°C and 150 rpm for amplification. The same procedure was followed the next day, and the final filtrate was spotted to check the lytic activity against its respective S. aureus host strain. To observe phage morphology, transmission electron microscopy (Hitachi H 7500 at 80 kV) was performed as described by Goodridge et al. (15).
Titration of phage by the DLA technique.
The titer of phage, expressed as PFU, was determined by using the DLA technique as described by Sambrook and Russell (30). Briefly, 100 μl of phage was added to 100 μl of a bacterial suspension grown overnight at 37°C and 120 rpm. This solution was added to 5 ml of the top agar (BHI broth with 0.6% Bacto agar), mixed gently, and poured into a 90-mm petri dish previously prepared with 25 ml of nutrient agar. The plates were gently swirled, dried for 10 min at room temperature, and then inverted and incubated at 37°C overnight.
Titration of phage by a modified DLA technique in the presence of antibiotics.
In the modified DLA method, various antibiotics at different working concentrations were added individually to 5 ml of top agar, along with MR-5 phage (100 μl) and bacterial suspension (100 μl). These were gently mixed and poured onto a nutrient agar plate. The subinhibitory concentrations of antibiotics giving the maximum increase in plaque size but not inhibitory for bacteria were selected. For comparison, pictures of plaques obtained with and without antibiotics were taken using a black background so as to provide equal light exposure and contrast. To obtain accurate dimensions, the diameter of the plaques was automatically determined from photographs at 4-fold magnification using the computer image analysis program Sigma Scan Pro, version 5.0.0, and each value was expressed as an average of the measurements of 20 different random plaques.
Adsorption of MR-5 phage in presence and absence of antibiotics.
The adsorption experiment was carried out as described by Kumari et al. (18). Briefly, to the culture of S. aureus 43300 (108 CFU/ml), CaCl2 (final concentration of 5 mM) and phage suspension (multiplicity of infection [MOI] of 0.01) were added, and the mixture incubated at 37°C. Aliquots (200 μl each) were taken out every minute up to 24 min, and the numbers of free infectious phage particles (free phage) were calculated by phage titration employing the DLA overlay technique. A control sample containing phage and no bacteria was also processed similarly to determine the phage titer.
In order to determine the effects on the adsorption rate of selected antibiotics showing significant enhancement of plaque size, the exponentially growing cells of S. aureus ATCC 43300 were pretreated with each of these four antibiotics separately; i.e., linezolid (1 μg/ml), tetracycline (0.25 μg/ml), clarithromycin (4 μg/ml), and telithromycin (4 μg/ml) at 37°C and 150 rpm for a period of 90 min. The adsorption experiment was carried out using these antibiotic-pretreated host culture cells (108 CFU/ml) following the same procedure as described above. The percentage of adsorbed phage was determined as follows: control − test/control × 100 = % adsorbed = 100 − % adsorbed = % free phage. The adsorption rate constant was calculated with the following mathematical formula: ka = (2.3/Bt) × log(Po/P), where ka is the adsorption rate constant in ml/min, B is the concentration of bacterial cells, and t is the time interval in which the titer falls from the original population, Po, to the final population, P.
Growth kinetics of MR-5 phage in the presence and absence of antibiotics.
A one-step growth curve was performed as described by Chhibber et al. (8). Briefly, MR-5 phage was added at an MOI of 0.01 to the cells of S. aureus 43300 and allowed to adsorb for 20 min at room temperature. The mixture was then centrifuged (10,000 rpm for 10 min at 4°C), and the pellet containing infected cells was suspended in 1 ml of BHI broth with CaCl2 (final concentration of 5 mM), followed by incubation at room temperature. Samples were taken in duplicate at 5-min intervals for a period of 1 h and immediately diluted and titrated by the double-layer agar technique. The first set of samples was immediately diluted before titration. The second set of samples was treated with 1% (vol/vol) chloroform to release intracellular phage in order to determine the eclipse period.
To test the effects of antibiotics (sub-MIC levels) on the growth parameters of MR-5 phage, the exponentially growing cells of S. aureus 43300 (108 CFU/ml) pretreated with each of the four antibiotics as described above were used in a one-step growth curve experiment. The rest of the procedure was the same as described above.
Effects of subinhibitory concentrations of antibiotics on host morphology and physiology.
To study the effects of antibiotics on host morphology, microscopic observation of bacterial cells was made after growing them for a period of 3 h in BHI with one of the four antibiotics. BHI broth without the antibiotic acted as control. The bacterial suspension was then diluted and washed twice in saline, and smears were prepared and stained using a BacLight LIVE/DEAD bacterial viability kit (Molecular Probes, Invitrogen, Grand Island, NY) incorporating Syto 9 (for live cells) and propidium iodide (for dead cells) for a brief period of 30 min in the dark. Stained cells were visualized under an epifluorescence microscope (Nikon Eclipse 80i).
To study the effect on host physiology, growth was studied both in the absence and presence of linezolid (1 μg/ml), tetracycline (0.25 μg/ml), and clarithromycin and telithromycin (4 μg/ml) added to the BHI broth, and the mean growth rate constants (kg) of S. aureus 43300 in the absence and presence of antibiotics were calculated and compared by using the formula kg = [log(Xt) − log(Xo)]/0.301 × t, where Xo is the original population number, Xt is the population at time t, and t is the time between two points.
Assessment of modified DLA technique with other phage-host combinations.
An assessment of the modified DLA technique with other phage-host combinations was carried out in order to evaluate the potential of these antibiotics in the routine isolation of S. aureus-specific phages. Linezolid, which brought the maximum increase in the size of MR-5 phage at a concentration of 1 μg/ml, was used for isolating S. aureus-specific phages. In addition, seven other lytic phages active against MRSA strains were isolated in our laboratory and named MR-1, MR-2, MR-3, MR-4, MR-6, MR-7, and MR-10. Details of the phages, along with their primary hosts and susceptible ATCC strains, are listed in Table S1 in the supplemental material (http://researchdata.puchd.ac.in/aem02371.pdf). All of these phages were subjected to phage titration by using the classical DLA technique (without any antibiotic), as well as by using the modified DLA technique incorporating 1 μg/ml of linezolid, and any enhancement in the plaque size of these phages (in terms of plaque diameter) was noted.
Statistical analysis.
The data were analyzed by one-way analysis of variance. A P value of <0.05 was considered statistically significant. All statistical procedures were carried out using Sigma Stat Graph Pad Prism (Graph Pad Software, San Diego, CA).
RESULTS
With the objective of developing a novel alternative therapy for treating the ongoing threat posed by life-threatening MRSA infections, an attempt was made in our laboratory to isolate lytic phages with a broad host range from natural sources. A total of eight phages were isolated from different sewage samples. All of the isolated phages were virulent, forming clear though tiny plaques on their respective primary hosts. They were nonenveloped, double-stranded DNA, tailed phages, and therefore belonged to the order Caudovirales. The lytic spectrum of the isolated phages (denoted MR-1, MR-2, MR-3, MR-4, MR-5, MR-6, MR-7, and MR-10 phage) was determined against the standard ATCC strains S. aureus ATCC 25923 (MSSA) and S. aureus ATCC 43300 (MRSA), as well as a panel of 45 clinical MRSA isolates (denoted 01 to 45). MR-5 phage, which showed lytic activity against both of the ATCC strains, as well as against 18 of the 45 clinical MRSA isolates, was chosen for further characterization studies. MR-5 phage was found to be a nonenveloped phage with an icosahedral head (58 nm) and contractile tail (120 nm in length) possessing double-stranded DNA and morphology resembling that of members of the family Myoviridae (a transmission electron microscopy image of phage MR-5 is included in Fig. S1 in the supplemental material [http://researchdata.puchd.ac.in/aem02371.pdf]).
However, the phage formed tiny plaques on its host when grown by the classical DLA method, making counting difficult. To increase the size of the plaques of MR-5, different classes of antibiotics were incorporated at different concentrations (Table 1) in the modified DLA technique. The MICs of the four antibiotics showing maximum increases in plaque size were determined for S. aureus ATCC 43300 by broth microdilution assay. The MIC ranges obtained were 2 to 4 μg/ml for linezolid, 0.5 to 1.0 μg/ml for tetracycline, and 8 to 16 μg/ml for both clarithromycin and telithromycin.
Table 1.
Plaque diameter in the presence of different concentrations of various antibiotics
| Antibiotic | Mean plaque diameter (mm) ± SD (fold increase over control) with indicated concn (μg/ml) of antibiotica |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | |
| DLAb | 1.12 ± 0.15 | ||||||
| Ampicillin | ND | ND | 1.12 ± 0.18 | 1.38 ± 0.19 | 1.51 ± 0.19 | 1.53 ± 0.21 | |
| Amikacin | ND | ND | 1.12 ± 0.18 | 1.31 ± 0.19 | 1.34 ± 0.16 | ND | |
| Telithromycin | ND | 1.14 ± 0.17 | 1.62 ± 0.16 (1.45) | 1.83 ± 0.14 (1.64) | 2.31 ± 0.10 (2.07) | 2.91 ± 0.28 (2.60) | |
| Clarithromycin | ND | 1.38 ± 0.29 | 2.16 ± 0.20 (1.93) | 2.57 ± 0.20 (2.23) | 3.07 ± 0.12 (2.75) | 3.25 ± 0.15 (2.91) | |
| Ciprofloxacin | ND | 1.21 ± 0 0.20 | 1.32 ± 0.19 | 1.50 ± 0.147 | ND | ND | |
| Chloramphenicol | ND | ND | ND | 1.29 ± 0.18 | 1.35 ± 0.17 | 1.53 ± 0.15 | |
| Tetracycline | 1.80 ± 0.14 (1.61) | 2.66 ± 0.14 (2.40) | 3.18 ± 0.28 (2.88) | ND | ND | ND | |
| Linezolid | 1.37 ± 0.10 (1.22) | 2.05 ± 0.18 (1.83) | 2.81 ± 0.13 (2.52) | 2.85 ± 0.17 (2.56) | 3.90 ± 0.20 (3.48) | ND | |
Plaque diameter values are the means ± standard deviations of 20 different plaques. ND, not done.
DLA, double-layer agar technique.
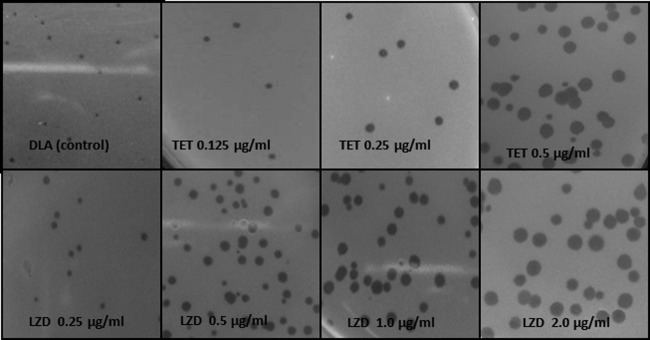
The results presented in Table 1 show that the maximum increase in plaque size in terms of diameter (mm) was seen with linezolid, which belongs to the oxazolidinone class of antibiotics that work by inhibiting the early steps of bacterial protein synthesis. This drug increased the plaque size by 1.2-fold at the minimal concentration of 0.125 μg/ml. This effect was concentration dependent, and a significant increase was seen in the plaque diameter, from 1.118 mm (in the classical DLA method) to 3.9 mm, giving an increase of ∼3.5-fold at 2 μg/ml. As shown by the data in Table 1, tetracycline was similarly effective in increasing the plaque diameter, to 3.18 mm at 0.5 μg/ml, a significant increase of ∼2.8-fold. Antibiotics belonging to the ketolide group, telithromycin and clarithromycin, also enhanced the plaque size, by 2.6- and 2.9-fold, respectively, at a concentration of 4 μg/ml. The results in Fig. 1 clearly depict significant enhancements in plaque size and in the overall contrast and visibility of plaques from using the modified DLA technique, incorporating four different antibiotics (including linezolid and tetracycline) at subinhibitory concentrations during the titration process. A figure showing visual enhancement in plaque size with both clarithromycin and telithromycin is included in the supplemental material (Fig. S2; http://researchdata.puchd.ac.in/aem02371.pdf).
Fig 1.
Visual comparison of plaques of MR-5 phage with normal DLA technique and in the presence of different concentrations of tetracycline (TET) and linezolid (LZD).
Effects of subinhibitory concentrations of antibiotics on adsorption time and adsorption rate constant of MR-5 phage.
The results presented in Table 2 indicate the total time taken by phage for its adsorption. The results showed that under normal conditions, the phage took an average of 22 min for complete adsorption of the phage population onto the host cell surface, and the adsorption rate constant (ka) was 1.05 × 10−9 ml/min. Linezolid significantly decreased the total time of adsorption, from 22 min to 14 min (P < 0.05). Phage treated with linezolid also showed significant decreases in the T50 (time to 50% adsorption) and T75 values (P < 0.05), which were almost half of the values for the control; the T50 value decreased from 3 min to 1.7 min and the T75 value from 7 min to 3.7 min, thus increasing the ka value to 1.43 × 10−8 ml/min. Similarly, in the presence of tetracycline, the adsorption time decreased by a significant amount, to 16 min (P < 0.05). Reductions in the T50 and T75 times, to 1.4 and 3.8 min, respectively, were also observed, comparable to those observed with linezolid. In the case of the ketolide antibiotics, clarithromycin and telithromycin, significant decreases in the time required for 75% adsorption (3.5 min and 4.0 min, respectively) were observed.
Table 2.
Comparison of phage adsorption and adsorption rate constant of control with those of antibiotic-pretreated S. aureus ATCC 43300
| Pretreatment (μg/ml) | Mean phage adsorption time (min) ± SDa |
ka (ml/min) | ||
|---|---|---|---|---|
| T50 | T75 | T100 | ||
| Control | 3.0 ± 0.52 | 7.0 ± 0.64 | 21.3 ± 1.15 | 1.05 × 10−9 |
| Linezolid (1) | 1.7 ± 0.20b | 3.7 ± 0.21b | 14.6 ± 1.12b | 1.43 × 10−8 |
| Tetracycline (0.25) | 1.4 ± 0.31b | 3.8 ± 0.25b | 15.3 ± 1.15b | 0.63 × 10−8 |
| Clarithromycin (4) | 2.0 ± 0.50b | 3.5 ± 0.25b | 17.4 ± 1.11 | 0.59 × 10−8 |
| Telithromycin (4) | 2.0 ± 0.28b | 4.0 ± 0.36b | 18.6 ± 1.12 | 0.28 × 10−8 |
Values are means ± standard deviations of three values. T50, T75, T100, times to 50%, 75%, and 100% adsorption.
Data have P values of <0.05, which is considered statistically significant.
Effects of subinhibitory concentrations of antibiotics on latent period, plaque enlargement period, and burst size of MR-5 phage.
As shown in Table 3, a prolonged latent period of 33 min (the latent period encompasses the process of nucleic acid translocation into host cytoplasm, progeny virion assembly, and finally, the burst, with release of newly formed phage) was observed when phage MR-5 was exposed to untreated cells of S. aureus 43300.
Table 3.
Phage growth parameters with or without antibiotic pretreatment of S. aureus ATCC 43300
| Antibiotic (μg/ml) | Growth curve parameter (mean ± SD) |
|||
|---|---|---|---|---|
| Time (min) for: |
Burst size (no. of phage/cell) | |||
| Adsorption | Latent period | Plaque enlargement period | ||
| Control | 21.3 ± 1.15 | 33.3 ± 2.8 | 21.7 ± 2.80 | 51.7 ± 1.23 |
| Linezolid (1) | 14.6 ± 1.12b | 16.6 ± 2.6b | 26.6 ± 2.5b | 58.6 ± 1.10b |
| Tetracycline (0.25) | 15.3 ± 1.15b | 18.3 ± 2.90b | 26.6 ± 2.60b | 57.4 ± 1.05b |
| Clarithromycin (4) | 17.4 ± 1.11b | 23.4 ± 2.89b | 28.3 ± 2.2b | 54.7 ± 0.45b |
| Telithromycin (4) | 18.6 ± 1.12b | 23.4 ± 2.82b | 28.3 ± 2.30b | 54.1 ± 0.8b |
Values are means ± standard deviations of three values.
Data have P values of <0.05, which is considered statistically significant.
The average burst size was 52 phage released per host cell, with an average plaque enlargement period of 22 min. In contrast, linezolid significantly reduced the latent period, to 16.6 min (P < 0.01), accompanied by an increase in the burst size to an average value of 59 phage/cell. Similarly, a decrease in the latent period from 33.3 min to 18.3 min (P < 0.05) was observed with tetracycline as well. In the case of the ketolide antibiotics, the effects of clarithromycin and telithromycin were almost comparable with each other, as both of these drugs significantly reduced the latent period, by 27%.
Effects of subinhibitory doses of antibiotics on host morphology and physiology.
As shown in Table 4, the growth rate constant, kg (number of generations per minute), and generation time, tgen (time required to double the population), for control cells were calculated to be 0.059 generations/min and 17.1 min/generation. In the case of antibiotic-stressed cells, tgen was increased from 17.1 to 45.2 min/generation (for linezolid) and 50.4 min/generation (for tetracycline), respectively. Both clarithromycin and telithromycin also produced significant increases in generation time compared to the times in control cells.
Table 4.
Effects of subinhibitory concentrations of antibiotics on growth rate of S. aureus ATCC 43300
| Treatment (μg/ml) | Mean kg (generations/min) | Mean tgen (min/generation) |
|---|---|---|
| Untreated cells | 0.059 | 17.1 |
| Linezolid (1) | 0.022 | 45.2 |
| Tetracycline (0.25) | 0.020 | 50.4 |
| Telithromycin (4) | 0.044 | 22.9 |
| Clarithromycin (4) | 0.032 | 31.7 |
Microscopic observation of the fluorescence-stained host cells (shown in Fig. S3 in the supplemental material [http://researchdata.puchd.ac.in/aem02371.pdf]) showed no change in the overall cell morphology in comparison to that of control cells on exposure to antibiotics.
Assessment of modified DLA technique in different phage-host systems.
Linezolid not only increased the plaques of the MR-5–S. aureus ATCC 43300 phage host system but also significantly increased the plaque size of all of the other seven phage-host combinations. Visual enhancement of the plaque size of other phages with their respective primary hosts, as well as with the ATCC strains, was observed (see Fig. S4 in the supplemental material [http://researchdata.puchd.ac.in/aem02371.pdf]). This increase in plaque size made their detection and enumeration easier and simpler at the isolation step itself.
DISCUSSION
Different strategies have been attempted in the past to increase the visualization of tiny plaques formed by different bacteriophages, including S. aureus-specific phages (21, 26, 27, 28, 31). Most of the researchers have focused on the use of β-lactam antibiotics or quinolone antibiotics for increasing the size of staphylococcal phages (14, 17, 31). These antibiotics may work very well in increasing the plaque size of phages which are active on antibiotic-sensitive staphylococcal strains but may fail to influence the plaques of phages attacking those bacterial populations that have become fully resistant and do not respond to β-lactam or quinolone groups of drugs even at high concentrations, i.e., the MRSA phages. Hence, in order to increase the plaque size of MRSA phages, there is a need to look for newer classes of drugs that are effective in increasing the plaque size of such phages. We report for the first time here the use of antibiotics that are protein synthesis inhibitors to improve the plaque size by a significant amount, leading to increased chances of isolation, detection, and enumeration of MRSA phages, which at the moment appears to be of great clinical importance.
Of the antibiotics tested in this study, linezolid and tetracycline, both of which are inhibitors of protein synthesis, produced significant increases in plaque size (∼ 4-fold) at lower concentrations of antibiotics. In contrast, the other two antibiotics, clarithromycin and telithromycin, belonging to the ketolide family, showed positive effects on the plaque size, but plaques were visible at comparatively higher concentrations of these two antibiotics. Researchers in the past have shown larger plaques in the presence of tetracycline than were obtained in the presence of the β-lactam group of antibiotics (31). Similarly, Loś et al. showed that both tetracycline and kanamycin were able to improve the plaque size for λ phage and phage T4 (Escherichia coli phages) (22). These researchers explained the underlying mechanism by reporting that, at low concentrations, these antibiotics may partially slow down or impair but not fully halt the protein synthesis of the host bacteria, and host cells in this altered or suboptimal state may then be easily accessible to attack by phages.
In the present study, an attempt was made to study the effects of four different antibiotics on the growth of phage MR-5. It was observed that phage MR-5 had a long adsorption time of 22 min, and by the time the majority of the phage entered the lytic cascade to produce enough progeny virions to feed the growing plaque, host overgrowth might have hindered the process of diffusion, leading to the formation of tiny plaques. In addition, due to the active growth of the host cells, the diffusing phage is more likely to encounter dead cells and cell debris in the vicinity, thus hindering the rate of diffusion and impairing the formation of bigger plaques. A similar observation was made by Loś and coworkers (22), who stated that plaque growth kinetics is limited mostly due to high host cell density that leads to increased depletion of virus particles by adsorption onto host cells and fragments of lysed cells.
However, pretreatment of actively growing host cells with linezolid (1 μg/ml) and tetracycline (0.25 μg/ml) for 90 min resulted in significant reductions in adsorption time. With antibiotic-pretreated cells, 75% of the phage population was easily adsorbed onto host cells in less than 4 min, thus entering the lytic cycle more quickly to produce progeny phage. This helped phage form bigger plaques before being overshadowed by host cell growth. A phage-antibiotic synergy (PAS) effect was also seen with ketolide antibiotics. Ketolide antibiotics, though, did not decrease the total time of adsorption by an appreciable amount, but they definitely reduced the T50 and T75 values, so that the majority of the phage population entered the lytic cycle at a higher rate than under normal conditions.
Most of the β-lactam and quinolone antibiotics induce filamentation and increase the cell size (11, 22, 31). Bigger cells lead to a higher phage adsorption rate and larger burst size, leading to an increase in plaque size. In order to study whether the higher adsorption rate seen with antibiotic pretreatment was due to an increase in cell size or not, S. aureus 43300 cells grown in the absence and presence of antibiotics were observed under an epifluorescence microscope after staining with fluorescent dyes. Since Syto 9 stains only the live cells, which appear green, while propidium iodide stains the dead cells, the use of the two different dyes allowed observation of the effect of antibiotic treatment on the cell size of live cells. Cells treated with antibiotics did not show any appreciable change in cell morphology in comparison to the morphology of control (untreated) cells. Therefore, unlike β-lactam and quinolone antibiotics, these antibiotics were not able to bring filamentation or cell elongation. This is in agreement with the findings of Santos et al. (31), who stated that filamentation (or cell size elongation) is not the only determinant of plaque size increase and found no correlation between plaque size and cell size.
The observed effect may be due to the altered growth rate of bacterial cells in the presence of subinhibitory concentrations of antibiotics, as under these conditions, the host cells are under stress due to impaired protein synthesis. To experimentally verify this finding, the effects of sublethal doses of all four antibiotics on the host growth rate were determined. The experiment clearly proved that all four antibiotics significantly reduced the growth rate of S. aureus 43300 (as measured by the mean kg value) and increased the doubling time (tgen) by 3-fold.
This effect was especially seen with tetracycline- and linezolid-treated cells. The bulk of plaque development occurs during the enlargement phase (3). However, as the host physiology shifts from the active exponential phase to the eventual stationary phase, most of the phage is unable to sustain productive infections, and plaque development ceases upon lawn maturation. This length of productive time can be manipulated either by using a lower initial host density or by slowing the host growth rate (3, 14). For example, in the case of phage ϕ6, the phage made a larger plaque when plated with a lower initial host density (12, 16). In the present study, the use of sublethal concentrations of all four antibiotics delayed the onset of lawn maturation to stationary phase by slowing the overall bacterial growth rate, thereby giving more time to phage particles to diffuse freely and, thus, to participate in plaque production for a longer time. Also, due to lower host density, less hindrance was encountered by the diffusing phage, which further facilitated the spreading of phage MR-5 during the plaque enlargement phase.
In summary, the events that favored the formation of bigger plaques due to the alteration of host physiology (growth rate) by antibiotics included the increase in time for more progeny phage to be added onto the growing plaque before being dominated by host overgrowth. In addition, spread of phage was facilitated due to faster and easier diffusion, since less hindrance from cell debris and dead cells was encountered, due to lower cell density. A similar explanation has been provided by researchers in the past (35, 36). These studies reported that no hindrance is seen when host concentration is low because of the low fraction of volume they occupy. However, at high host concentrations, the balance shifts and the host density acts as a barrier to diffusion, hindering the process of viral spread in the surrounding medium (32, 35, 36). Also, as mentioned, antibiotics actually lengthened the productive/plaque enlargement phase (during which the bulk of plaque enlargement occurs), as bacterial lawns take longer to mature under these conditions, thus delaying the onset of lawn maturation and giving more time to progeny virions to add onto the growing plaque during this period. Some phages display larger plaques during anaerobic growth (25). Similarly, Eisenstark showed that lawn bacteria pretreated with UV tend to delay their growth, resulting in increased plaque size due to delays in lawn maturation (13).
In the intracellular phase of phage growth, the phage uses the host machinery for its own progeny production. Plaque size seems to be dependent on the duration of the latent period. Too long a latent period means that phage is spending more time inside the host cytoplasm and less time in viral diffusion, thus leading to the formation of smaller plaques. Similarly, if the latent period is too short, it may give rise to a faster burst with smaller burst size, resulting in fewer progeny virions to feed the growing plaque (14). Under normal conditions, MR-5 spent a total of 33 min to complete the process till burst occurred. This further adds to one of the reasons why this phage formed tiny plaques on its host cell, as it spent comparatively more time inside the host cell, with less time available for its diffusion outside. In contrast, monitoring of bacterial growth in the presence of all four antibiotics showed a significant (P < 0.05) decrease in the latent period. This means that antibiotic-pretreated cells are under a state of physiological stress, and this situation appears advantageous for the attacking phage once it injects its genetic material into the stressed host. This observation suggests that antibiotics optimally reallocate the time for the intracellular and extracellular phases, leading to optimization of the latent period. No appreciable effect on the burst size of this phage was observed with any of the ketolide antibiotics, although significant increases (P < 0.05) in the burst size were observed with linezolid and tetracycline. Researchers in the past have found that antibiotics influence phage growth characteristics (17). Sublethal concentrations of aztreonam and cefixime influenced the growth curve of phage T4, resulting in increased burst sizes and reduced latent periods (11). This antimicrobial synergy was later assessed for eradicating biofilms of the T4 host strain (E. coli) in the presence of both phage and sublethal concentrations of antibiotics (29).
In order to establish whether the observed effect of antibiotics on the plaque morphology of phage MR-5 is a universal phenomenon or not, another experiment was conducted with seven other MRSA phages. Linezolid, which produced the maximum change in the morphology of phage MR-5, was incorporated at a concentration of 1 μg/ml in agar. Linezolid not only enhanced the plaque size of phage MR-5 but also significantly improved the plaque sizes of seven other lytic S. aureus-specific phages, indicating that these antibiotics can be routinely incorporated in the isolation step for improving the plaque size of MRSA phages.
The present study supports the use of subinhibitory concentrations of newer class of drugs (other than β-lactam and quinolone class) that work by inhibiting bacterial protein synthesis, such as linezolid, tetracycline, clarithromycin, and telithromycin, to increase the size as well as the overall visibility and contrast of the growing plaques without any suppression of phage titer. Plaque growth kinetics is limited mostly by a high host cell density, since the presence of fragments of lysed cells results in physical blockade of diffusing phage (14, 22).This effect is partially overcome by using sublethal concentrations of these drugs that slow down the growth rate in comparison to the normal growth of bacterial cells (control). Moreover, incorporation of these antibiotics at subinhibitory concentrations makes the environment more conducive for phage growth, leading to increased enhancement in plaque size. This modification can safely be incorporated in the traditional DLA overlay method to search for newer MRSA-virulent phages that can participate in the ongoing battle against the MRSA menace.
ACKNOWLEDGMENT
The financial assistance provided by the Council of Scientific and Industrial Research (CSIR), India, in the form of a research fellowship is gratefully acknowledged.
Footnotes
Published ahead of print 21 September 2012
REFERENCES
- 1. Abedon ST, Culler RR. 2007. Bacteriophage evolution given spatial constraint. J. Theor. Biol. 248:111–119 [DOI] [PubMed] [Google Scholar]
- 2. Abedon ST, Culler RR. 2007. Optimizing bacteriophage plaque fecundity. J. Theor. Biol. 249:582–592 [DOI] [PubMed] [Google Scholar]
- 3. Abedon ST, Yin J. 2009. Bacteriophage plaques: theory and analysis. Methods Mol. Biol. 501:161–174 [DOI] [PubMed] [Google Scholar]
- 4. Burnet FM, Freeman M. 1937. A comparative study of the inactivation of a bacteriophage by immune serum and by bacterial polysaccharides. Aust. J. Exp. Biol. Med. Sci. 15:49 [Google Scholar]
- 5. Burnet FM. 1934. Phage-inactivating agent of bacterial extracts. J. Pathol. Bacteriol. 38:285–299 [Google Scholar]
- 6. Capparelli R, Parlato M, Borriello G, Salvatore P, Lannelli D. 2007. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob. Agents Chemother. 51:2765–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cerveny KE, DePaola A, Duckworth DH, Gulig PA. 2002. Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice. Infect. Immun. 70:6251–6262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chhibber S, Kaur S, Kumari S. 2008. Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice. J. Med. Microbiol. 57:1508–1513 [DOI] [PubMed] [Google Scholar]
- 9. CLSI 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10. Colvin MG. 1932. Behavior of bacteriophage in body fluids and in exudates. J. Infect. Dis. 51:527–541 [Google Scholar]
- 11. Comeau AM, Tetart F, Trojet SN, Prere MF, Krisch HM. 2007. Phage-antibiotic synergy (PAS): β-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS One 2:e799 doi:10.1371/journal.pone.0000799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dennehy JJ, Abedon ST, Turner PE. 2007. Host density impacts relative fitness of bacteriophage ϕ6 genotypes in structured habitats. Evolution 61:2516–2527 [DOI] [PubMed] [Google Scholar]
- 13. Eisenstark A. 1967. Bacteriophage techniques. Methods Virol. 1:449–524 [Google Scholar]
- 14. Gallet R, Kannoly S, Wang IN. 2011. Effects of bacteriophage traits on plaque formation. BMC Microbiol. 11:181 doi:10.1186/1471-2180-11-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goodridge L, Gallacci A, Griffiths MW. 2003. Morphological, host range and genetic characterization of two coliphages. Appl. Environ. Microbiol. 69:5364–5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koch AL. 1964. The growth of viral plaques during the enlargement phase. J. Theor. Biol. 6:413–431 [DOI] [PubMed] [Google Scholar]
- 17. Krueger AP, Cohn T, Smith PN, Mcguire CD. 1948. Observations on the effect of penicillin on the reaction between phage and staphylococci. J. Gen. Physiol. 31:477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumari S, Harjai K, Chhibber S. 2010. Isolation and characterization of Klebsiella pneumoniae specific bacteriophages from sewage samples. Folia Microbiol. (Praha) 55:221–227 [DOI] [PubMed] [Google Scholar]
- 19. Levine P, Frisch AW. 1934. On absorption of phage by bacilli. J. Immunol. 26:321–325 [Google Scholar]
- 20. Levine P, Frisch AW. 1934. On specific inhibition of bacteriophage action by bacterial extracts. J. Exp. Med. 59:213–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lillehaug D. 1997. An improved plaque assay for poor plaque-producing temperate lactococcal bacteriophages. J. Appl. Microbiol. 83:85–90 [DOI] [PubMed] [Google Scholar]
- 22. Loś JM, Golec P, Wegrzyn G, Wegrzyn A, Loś M. 2008. Simple method for plating Escherichia coli bacteriophages forming very small plaques or no plaques under standard conditions. Appl. Environ. Microbiol. 74:5113–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532 [DOI] [PubMed] [Google Scholar]
- 24. Maranan MC, Moreira B, Boyle-Vavra S, Daum RS. 1997. Antimicrobial resistance in staphylococci. Epidemiology, molecular mechanisms, and clinical relevance. Infect. Dis. Clin. North Am. 11:813–849 [DOI] [PubMed] [Google Scholar]
- 25. McConnell M, Wright A. 1975. An anaerobic technique for increasing bacteriophage plaque size. Virology 65:588–590 [DOI] [PubMed] [Google Scholar]
- 26. McLaughlin MR, Balaa MF. 2006. Enhanced contrast of bacteriophage plaques in Salmonella with ferric ammonium citrate and sodium thiosulfate (FACST) and tetrazolium red (TZR). J. Microbiol. Methods 65:318–323 [DOI] [PubMed] [Google Scholar]
- 27. McLaughlin MR. 2006. Factors affecting iron sulfide-enhanced bacteriophage plaque assays in Salmonella. J. Microbiol. Methods 67:611–615 [DOI] [PubMed] [Google Scholar]
- 28. Pattee PA. 1966. Use of tetrazolium for improved resolution of bacteriophage plaques. J. Bacteriol. 92:787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ryan EM, Alkawareek MY, Donnelly RF, Gilmore BF. 2012. Synergistic phage-antibiotic combinations for the control of Escherichia coli biofilms in vitro. FEMS Immunol. Med. Microbiol. 65:395–398 [DOI] [PubMed] [Google Scholar]
- 30. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 31. Santos SB, et al. 2009. The use of antibiotics to improve phage detection and enumeration by the double-layer agar technique. BMC Microbiol. 9:148 doi:10.1186/1471-2180-9-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shao Y, Wang IN. 2008. Bacteriophage adsorption rate and optimal lysis time. Genetics 180:471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stein R. 17 October 2007. Drug-resistant staph germ's toll is higher than thought. Washington Post, Washington, DC: Accessed 18 October 2007 [Google Scholar]
- 34. Struelens MJ, Mertens R. 1994. National survey of methicillin-resistant Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 13:56–63 [DOI] [PubMed] [Google Scholar]
- 35. Yin J, McCaskill JS. 1992. Replication of viruses in a growing plaque: a reaction-diffusion model. Biophys. J. 61:1540–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. You L, Yin J. 1999. Amplification and spread of viruses in a growing plaque. J. Theor. Biol. 200:365–373 [DOI] [PubMed] [Google Scholar]