Abstract
Sulfate-reducing microbes utilize sulfate as an electron acceptor and produce sulfide that is depleted in heavy isotopes of sulfur relative to sulfate. Thus, the distribution of sulfur isotopes in sediments can trace microbial sulfate reduction (MSR), and it also has the potential to reflect the physiology of sulfate-reducing microbes. This study investigates the relationship between the availability of iron and reduced nitrogen and the magnitude of S-isotope fractionation during MSR by a marine sulfate-reducing bacterium, DMSS-1, a Desulfovibrio species, isolated from salt marsh in Cape Cod, MA. Submicromolar levels of iron increase sulfur isotope fractionation by about 50% relative to iron-replete cultures of DMSS-1. Iron-limited cultures also exhibit decreased cytochrome c-to-total protein ratios and cell-specific sulfate reduction rates (csSRR), implying changes in the electron transport chain that couples carbon and sulfur metabolisms. When DMSS-1 fixes nitrogen in ammonium-deficient medium, it also produces larger fractionation, but it occurs at faster csSRRs than in the ammonium-replete control cultures. The energy and reducing power required for nitrogen fixation may be responsible for the reverse trend between S-isotope fractionation and csSRR in this case. Iron deficiency and nitrogen fixation by sulfate-reducing microbes may lead to the large observed S-isotope effects in some euxinic basins and various anoxic sediments.
INTRODUCTION
Microbial sulfate reduction (MSR) is a process of anaerobic respiration that utilizes sulfate as an electron acceptor. This process remineralizes organic carbon in anoxic marine sediments and is the main driver of the global biogeochemical cycle of sulfur (8, 13). Of the four stable isotopes of sulfur in nature (32S, 33S, 34S, and 36S), MSR preferentially utilizes lighter S-isotopes, producing sulfide that is depleted in heavy isotopes relative to the reactant sulfate. This isotope fractionation between sulfate and sulfide has been widely used to trace the biogeochemical cycling of sulfur and carbon (43, 48). Numerous studies have characterized S-isotope fractionation in pure and mixed cultures of sulfate-reducing microbes, demonstrating a general correlation between sulfate reduction rates and the magnitude of S-isotope fractionation (6 and 62 and references therein). Most of these studies investigated the influence of terminal electron acceptor, sulfate, and electron donors (organic substrates or H2) on microbial S-isotope fractionation. These studies reported lower fractionations at sulfate concentrations below ∼200 μM (14, 27, 28, 32) and attributed higher fractionations to the limitation by organic electron donors or the presence of recalcitrant organic substrates (15, 40, 62, 63). However, links between environmental variables and physiological mechanisms that control sulfate reduction rates within cells deserve further investigation. In particular, sulfate-reducing microbes do not translocate electrons from the organic substrate directly to the terminal electron acceptor, sulfate. Instead, the rates of electron transfer depend on a number of enzymes and electron carriers (41, 54). Thus, any alterations in the electron transfer chain and changes in the flow of energy and reducing equivalents within the cell may ultimately influence the magnitude of S-isotope fractionation by changing the coupling between the electron donor and sulfate.
Importantly, these changes may arise from environmental limitations. For example, iron is a cofactor in many enzymes and electron carriers in microbial electron transfer chains, including hydrogenases, ferredoxin, or cytochromes (54, 58). Consequently, iron deficiency affects the synthesis and function of these enzymes and carriers in various microbes (17, 37), including sulfate-reducing bacteria (55), and likely affects the conservation of respiratory energy (31). The cellular energy and redox budgets also change if some of the reducing equivalents generated by the oxidation of organic substrates are diverted toward metabolic processes such as nitrogen fixation. In addition to the respiratory machinery, limitation of microbial growth by nitrogen is expected to affect general protein synthesis.
Here, we report the effect of Fe and N limitation on the physiology of and S-isotope fractionation by a marine sulfate-reducing bacterium, DMSS-1, a Desulfovibrio species, recently isolated from marine sediment (62). Although these two limitations are expected to affect different cellular processes, both influence S-isotope effects. The potential significance of both limitations and their accompanying S-isotope fractionations are discussed in an environmental context. To the best of our knowledge, previous studies have not investigated the effect of iron and other nutrient limitations on S-isotope fractionation during MSR.
MATERIALS AND METHODS
Organism and growth medium.
Desulfovibrio sp. strain DMSS-1, which is used in this study, was isolated from marine coastal sediments in Cape Cod, MA (62, 63). The nucleotide sequences of 16S rRNA and dsrAB genes of DMSS-1 have been deposited in GenBank under accession numbers JF968436 and JF968437, respectively. The culture is available upon request. DMSS-1 was grown in a chemically defined medium in batch culture. This medium consisted of (per liter) the following: NaHCO3, 9 g; KH2PO4, 0.2 g; NaCl 21g; MgCl2 · 6H2O, 3 g; KCl, 0.5 g; CaCl2 · 2H2O, 0.15 g; resazurin, 1 mg; 1 ml of trace element solution SL-10 without FeCl2 (68); 10 ml of vitamin solution described as a part of DSMZ medium 141 (catalogue of strains 1993; DSMZ, Braunschweig, Germany); and 1 ml of selenium stock solution (0.4 mg of Na2SeO3 per 200 ml of 0.01 N NaOH). Sodium ascorbate (1 g per liter) was added as a reducing agent. Cultures contained either lactate (20 mM) or malate (18 mM) as electron donors and carbon sources and 21 mM Na2SO4 as an electron acceptor. The medium was titrated to pH 7.5 and sterilized anaerobically under 80% N2-20% CO2 gas. The background concentration of iron in the basal medium, i.e., the concentration before the addition of iron stock solution, was 0.7 ± 0.2 μM, as measured by inductively coupled plasma-optical emission spectroscopy (ActLab, Ontario, Canada). The background concentration of ammonium in the basal medium before the addition of ammonium stock solution was 0.5 ± 0.2 μM, as measured by a fluorometric method (36). Regular (control) growth medium contained 10 ml of anoxic NH4Cl stock solution (3 g/100 ml water) and 1 ml of FeCl2 · 4H2O stock solution (150 mg FeCl2 · 4H2O in 100 ml of 0.25% HCl) per liter with final concentrations of 5.6 mM NH4Cl and 8 μM FeCl2, respectively. In growth limitation experiments, ammonium and iron stock solutions were serially diluted with anoxic water and 0.25% HCl, respectively, before they were added to the basal medium. The speciation of soluble iron in the medium was calculated at pH 7.5 and at a redox potential lower than −111 mV, as indicated by the conversion of resorufin (pink) to dihydroresorufin (colorless) (39). Calculations show that FeCO30 is the dominant form of dissolved iron in both control and iron-limited media and that the precipitation of Fe(OH)3(s), FeCO3(s), and Fe3(PO4)2(s) is not to be expected. Indeed, the fresh medium contained no observable precipitate. Concentrations of iron and ammonium used in different experiments are listed in Table 1.
Table 1.
Growth parameters and isotope fractionations in batch cultures containing different concentrations of iron and ammoniuma
| Organic substrate | Concn of: |
Growth yield (106 cells/μmol sulfate reduced) | csSRR (fmol/cell/day) | Sulfide produced (mM) | Isotopic ratio (‰): |
|||
|---|---|---|---|---|---|---|---|---|
| NH4Cl | FeCl2 (μM) | δ34Ssulfate | δ34Ssulfide | 34ε | ||||
| Lactate | 5.6 mM | 8 | 29 ± 5 | 28.1 ± 2.6 | 7.4 | 2.0 | −6.4 | 6.8 ± 0.2 |
| 5.6 mM | 8 | 32 ± 5 | 25.1 ± 2.5 | 8.7 | 3.1 | −5.9 | 7.0 ± 0.2 | |
| 5.6 mM | <1 | 15 ± 2 | 18.7 ± 1.4 | 9.3 | 5.5 | −9.9 | 11.7 ± 0.3 | |
| 5.6 mM | <1 | 18 ± 3 | 20.1 ± 1.7 | 8.2 | 4.8 | −8.4 | 10.4 ± 0.3 | |
| 112 μM | 8 | 7 ± 1 | 62.4 ± 5.2 | 8.5 | 3.3 | −7.4 | 8.4 ± 0.3 | |
| 56 μM | 8 | 9 ± 1 | 62.9 ± 5.7 | 8.6 | 3.4 | −6.7 | 7.8 ± 0.2 | |
| Malate | 5.6 mM | 8 | 114 ± 18 | 5.3 ± 0.7 | 6.8 | 5.5 | −14.6 | 16.7 ± 0.3 |
| 5.6 mM | 8 | 94 ± 15 | 4.9 ± 0.5 | 6.6 | 6.6 | −16.3 | 19.1 ± 0.3 | |
| 5.6 mM | 8 | 99 ± 16 | 4.3 ± 0.5 | 7.5 | 7.2 | −15.9 | 18.6 ± 0.3 | |
| 5.6 mM | 2 | 110 ± 17 | 4.1 ± 0.4 | 6.5 | 5.7 | −15.4 | 17.5 ± 0.3 | |
| 5.6 mM | <1 | 63 ± 10 | 2.9 ± 0.3 | 4.6 | 5.0 | −23.4 | 25.1 ± 0.3 | |
| 5.6 mM | <1 | 66 ± 10 | 2.2 ± 0.2 | 3.1 | 3.4 | −27.9 | 28.9 ± 0.3 | |
| 56 μM | 8 | 19 ± 3 | 6.5 ± 0.7 | 6.5 | 7.8 | −18.5 | 21.9 ± 0.4 | |
| 5.6 μM | 8 | 16 ± 3 | 8.3 ± 0.7 | 6.6 | 7.8 | −19.4 | 22.6 ± 0.4 | |
| 0.5 μM | 8 | 19 ± 3 | 8.5 ± 0.7 | 6.9 | 8.6 | −19.0 | 22.7 ± 0.4 | |
Each row represents a single independent experiment, and errors were propagated from analytic uncertainties of cell density, sulfide, and isotope measurements. Isotopic ratios were reported with respect to the laboratory working reference SF6.
Culture experiments.
DMSS-1 was incubated in batch cultures containing different concentrations of iron or ammonium (Table 1). Effects of limitations by iron and ammonium were reproducible and confirmed in multiple independent experiments (Fig. 1 and Table 1). Culture bottles (165 ml) containing sterile media (100 ml) were inoculated with washed cells. The cells were washed three times by anaerobic centrifugation and resuspension in fresh medium that lacked both iron and ammonium to minimize the carryover of sulfide, iron, and ammonium. Growth (optical density, cell counts, and protein) and sulfide concentrations were monitored every day in lactate-grown cultures and every other day in malate-grown cultures. Sulfide concentration was measured by a modified methylene blue assay (16) in 200-μl culture samples fixed by 1 ml of 0.05 M Zn-acetate solution. Growth was monitored by measuring the optical density at 630 nm using a Synergy2 Biotek microplate reader (Biotek, Winooski, VT) and by microscopic counts of cells stained by SYTOX-Green nucleic acid stain (S7020; Invitrogen, Paisley, United Kingdom) using a Zeiss Axio Imager M1 epifluorescence microscope (Carl Zeiss, Thornwood, NY). Microbial activity in cultures was terminated by the addition of 20 ml of 1 M Zn-acetate, and dissolved sulfide was precipitated as zinc sulfide. These samples were stored at 4°C until the extraction of sulfur and the subsequent isotope analysis. Preincubation experiments were conducted to determine the concentrations of sulfide and final cell densities produced under iron- and nitrogen-limited conditions. These cultures and control cultures contained the same amounts of sulfide at the onset of the stationary phase (not shown here).
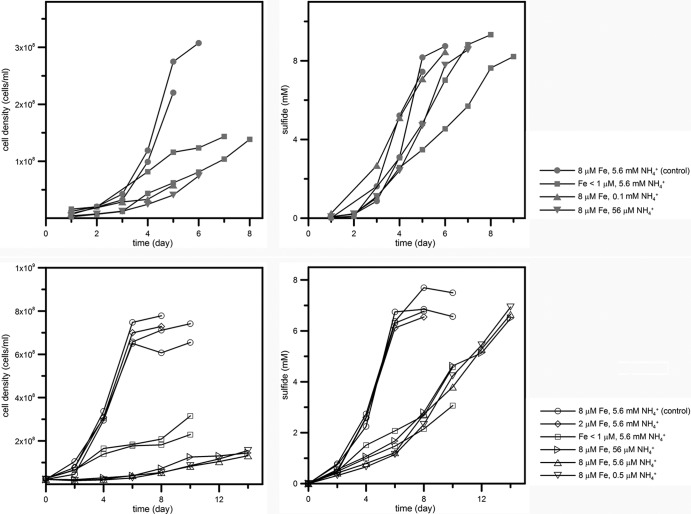
Fig 1.
Effect of iron and ammonium limitation on growth (left) and sulfide production (right) of DMSS-1 grown on lactate (upper) and malate (lower). Each line indicates a single independent experiment. Both control and nutrient-limited cultures produced similar amounts of sulfide at the end of the incubation, indicating that iron and nitrogen limitation caused a reduction of overall growth rate and not an extended lag phase followed by the same growth rate. For malate-grown iron-limited cultures, the incubation was stopped before the cessation of growth (see Materials and Methods). Concentrations of iron and ammonium were estimated at the beginning of the incubation. Cell density is subject to a ±15% error, and the analytical uncertainty of the measurement of sulfide concentrations is ±5%.
Nitrogenase activity.
Nitrogenase activity was determined in actively growing cultures of DMSS-1 using a modified acetylene reduction technique (21). Acetylene gas (20 ± 1 μmol) was injected into a set of culture bottles containing 10 ml of ammonium-free medium, and the bottles were subsequently inoculated with washed cells. One bottle was sacrificed every 2 days to monitor ethylene production from acetylene. H2S was removed from the headspace by adding 1 ml of 1 M zinc acetate solution, and 0.5 ml of the headspace gas was withdrawn using a gas-tight syringe. The gas sample was analyzed by a gas chromatograph (Shimadzu GC-2014) equipped with a flame ionization detector and a packed column (60/80 mesh; outer diameter, 1/8 in.; length, 4.6 m; Carboxen, Supelco). Oven temperature was held at 125°C for 5 min, increased to 225°C at a rate of 20°C/min, and held at 225°C for 5 min. Procedural and analytical reproducibility was 8%. Ethylene production was monitored in parallel control sterile media and cultures, both containing 5.6 mM ammonium.
Cytochrome spectra.
Reduced and oxidized cytochrome spectra and the differences between the two were obtained using the whole-cell method (37, 38). Cells were harvested from 45 ml of the culture by centrifugation at 3,000 × g for 15 min. The supernatant was discarded, and the cells were resuspended in 1 ml of phosphate-buffered saline solution (PBS; 50 mM phosphate buffer and 350 mM NaCl). Cell suspensions were transferred into 1.5-ml microcentrifuge tubes, washed twice by centrifugation (13,000 × g, 3 min), and resuspended in PBS. Protein was determined using a Pierce bicinchoninic acid protein assay kit (Pierce, Rockford, IL) with an albumin standard, and the cells were resuspended in PBS at ∼1.5 mg protein/ml. The resuspended cultures were transferred into flat-bottom 96-well plates (200 μl well volume) and oxidized or reduced by the addition of 20 μl of 30% H2O2 and 20 μl of freshly prepared 50 mg/ml sodium dithionite stock solution, respectively. After a 1-min incubation at room temperature, the cells were shaken in the Synergy2 microplate reader (BioTek, Winooski, VT) for 10 s, and optical absorption between 400 and 700 nm was measured in 2-nm intervals.
Isotope measurements.
Sulfide was extracted by acidifying the culture medium with 6 N HCl at 80°C under a flow of nitrogen gas for 2 h. Sulfide produced during the distillation was precipitated as ZnS in a Zn-acetate solution (0.18 M). After the extraction of sulfide, sulfate in the remaining medium was reduced to sulfide with 30 ml of the reducing agent (mixture of HI, H3PO2, and HCl) (66). Samples were boiled and purged by N2 gas for 2 h. Volatile products were passed through a condenser and a trap containing distilled water, and sulfide was subsequently collected in a Zn-acetate trap. ZnS was converted to Ag2S by the addition of AgNO3 and incubation at 70°C for 1 day. Ag2S was centrifuged, washed with distilled water three times, and dried at 70°C. The Ag2S samples reacted with an excess of fluorine gas for more than 5 h at 300°C, and the produced SF6 was purified by gas chromatography. Purified SF6 was transferred into an isotope-ratio mass spectrometer for sulfur isotope measurements in dual inlet mode (52).
Data processing.
Cellular growth yield during the vegetative phase was calculated as the ratio of the increase in the number of cells and the amount of sulfide produced during the experiment. Because growth in iron- and ammonium-limited cultures was not exponential, the average cell-specific sulfate reduction rates (csSRR) during the vegetative phase were calculated as
| (1) |
where [H2S]1 and [H2S]N are sulfide concentrations at the first and the Nth sampling times, tn is the time of sampling, and Cn is the cell density at each sampling point. In previous studies, csSRR was calculated using the cell densities at two time points (20), but equation 1 used more cell density data to reflect growth more realistically. The form of equation 1 is the same as the one used in previous studies (20) if N is set to 2.
The isotope fractionation factor (α) in the batch culture experiment was calculated using the modified Rayleigh distillation equation as previously described (62):
| (2) |
where fr is the fraction of the remaining sulfate and δr and δp are sulfur isotope compositions of the remaining sulfate and produced sulfide, respectively. The isotope enrichment factor is defined as
| (3) |
In this definition, positive values represent the depletion of heavy isotopes in the product. For each calculation, errors from cell counts (±15%), sulfide concentration measurements (±5%), and isotope analyses (0.2‰ for δ34S) (51) were propagated according to Bevington and Robinson (5).
Nucleotide sequence accession numbers.
The nucleotide sequences of 16S rRNA and dsrAB genes of DMSS-1 have been deposited in GenBank under accession numbers JF968436 and JF968437, respectively.
RESULTS
DMSS-1 cultures grew and produced sulfide more slowly in the presence of <1 μM iron and <0.1 mM ammonium than iron- and ammonium-replete cultures (8 μM and 5.6 mM for iron and ammonium, respectively) (Fig. 1 and Table 1). These effects of iron and ammonium limitation could be observed in cultures that used either malate or lactate as the electron donor (Fig. 1 and 2). When cells grew on lactate, reduced concentrations of iron and ammonium reduced the cellular yield to one-half and one-fourth, respectively, relative to the control cultures (Fig. 2 and Table 1). Therefore, concentrations of iron and ammonium lower than 1 μM and 0.1 mM, respectively, limited the growth of DMSS-1. Cell-specific sulfate reduction rates (csSRRs) were lower in iron-limited cultures (19 to 20 fmol/cell/day) but higher in ammonium-limited cultures (62 to 63 fmol/cell/day) relative to the control cultures (25 to 28 fmol/cell/day) (Fig. 2 and Table 1) (P < 0.05). When DMSS-1 utilized malate as an electron donor for sulfate reduction, it attained higher cell densities (62) but exhibited slower csSRRs than during sulfate reduction coupled with the oxidation of lactate (Fig. 2 and Table 1). Iron limitation in malate-fed cultures diminished both the growth yield and the csSRR compared to the malate-fed control cultures (P < 0.05). Limitation by ammonium reduced the growth yield to 20% of the control cultures while accelerating the cell-specific respiration rate (Fig. 2 and Table 1).
Fig 2.
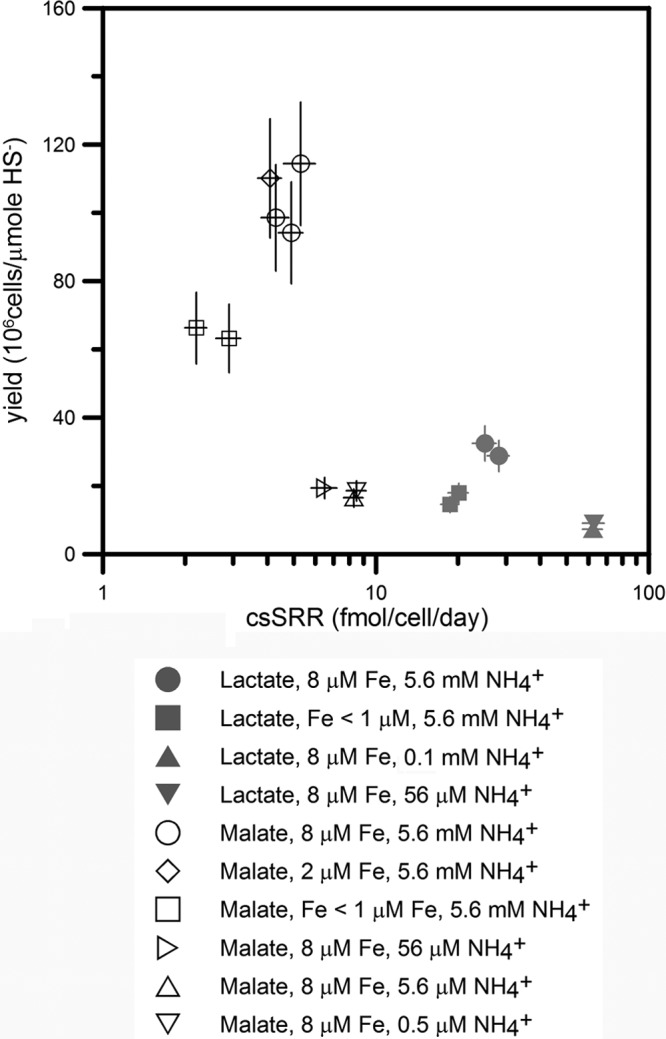
Effect of iron and ammonium limitation on DMSS-1 growth yields and cell-specific sulfate reduction rate (csSRR). Both limitations reduced the growth yield. Iron deficiency decreased csSRR, while cells limited by ammonium respired faster than control cultures. Errors were propagated from analytic uncertainties of cell density and sulfide measurements.
The growth of DMSS-1 in ammonium-limited medium suggested that this organism could fix nitrogen and use it in biosynthesis. Indeed, ammonium-limited cultures produced ethylene by acetylene reduction, but ethylene was not detected in ammonium-replete cultures and sterile controls (Fig. 3).
Fig 3.
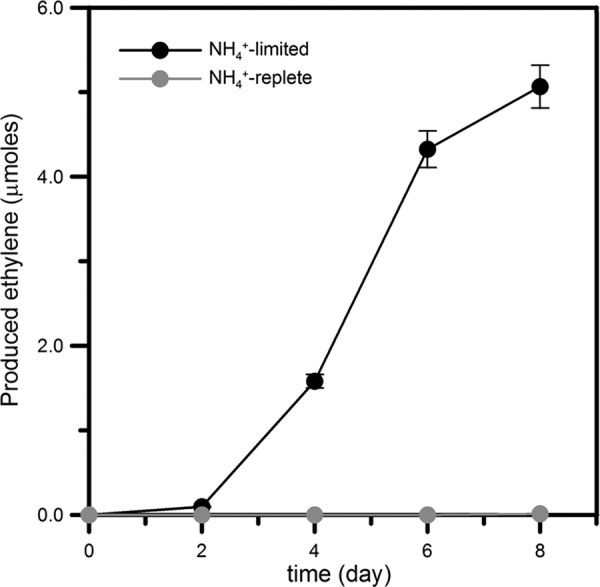
Production of ethylene by the reduction of acetylene in ammonium-limited medium (0.5 μM ammonium). Ethylene was not detected (<10 nmol) in cultures that contain 5.6 mM ammonium. Error bars indicate procedural and analytical reproducibility (±8%).
A slower respiration rate by DMSS-1 in iron-limited cultures indicated that this metal was impairing the function of respiratory proteins, among other processes. To test this further, we examined how iron limitation affected the content of cytochrome c, one of the essential proteins in electron transfer chains. Reduced minus oxidized spectra of iron-replete cells showed predominant peaks at 420 to 422, 523 to 524, and 552 to 553 nm, typical for c-type cytochromes (Fig. 4) (55, 57). These peaks were more than twice as large in cells cultured in the iron-replete medium than in the iron-limited medium (Fig. 4). Therefore, the abundance of cytochrome c (per cell protein) was lower in iron-limited cultures than in iron-replete cultures.
Fig 4.
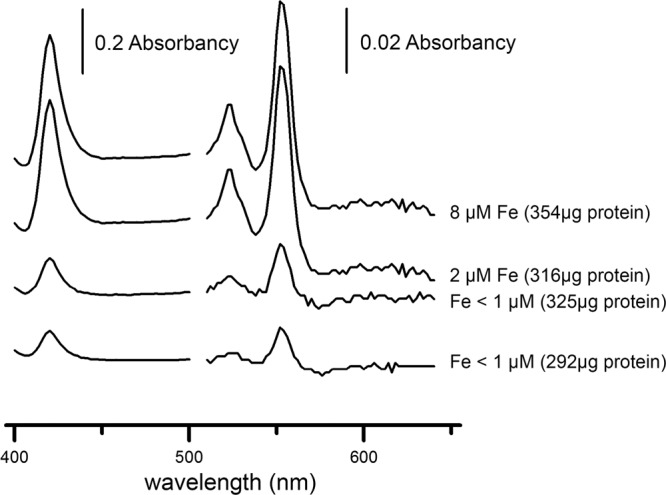
Reduced minus oxidized spectra of whole cells of DMSS-1 grown in media containing 18 mM malate, 5.4 mM ammonium, and different concentrations of iron. Three peaks typical for the absorption by cytochrome c were prominent in iron-replete cells. The ratios of these peaks to the background signal were smaller when DMSS-1 was grown in iron-deficient media. All samples contained similar amounts of total protein (values stated in the brackets).
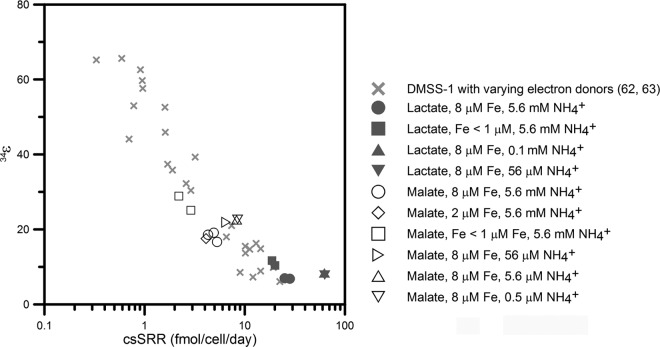
Analyses of S-isotope ratios of sulfates and sulfides in DMSS-1 cultures limited by iron or ammonium showed that both limitations increased the magnitude of sulfur isotope fractionation (Table 1). The calculated enrichment factor (34ε) in control cultures grown on lactate was 6.9‰ ± 0.2‰ (averages ± standard errors of the means; n = 2), as previously reported (62). Iron-deficient cells grown on lactate fractionated 34S/32S up to 12‰, while limitation by ammonium increased the enrichment factors in lactate-grown cultures by only 1‰ with respect to the control cultures (Fig. 5 and Table 1). Overall, larger fractionation factors associated with the growth on malate amplified the isotopic effects of limitations by iron and ammonium. Malate-fed, iron- and ammonium-replete cultures fractionated 34S at 18‰ ± 1‰ (averages ± SEM; n = 3), while limitations by ammonium and iron increased this enrichment factor to 23 and 29‰, respectively (Fig. 5 and Table 1).
Fig 5.
Variations in 34ε and cell-specific sulfate reduction rate (csSRR) in DMSS-1 cultures limited by iron or ammonium (this study) or various electron donors (62, 63). Iron deficiency reduced csSRR and led to increasing fractionation factors. A similar relationship between csSRR and 34ε is observed when the csSRR rates in DMSS-1 are changed by changing the concentration of a single electron donor or changing the type of organic electron donor (62, 63). In contrast, nitrogen limitation increased both csSRR and 34ε.
DISCUSSION
Iron limitation.
At the end of the incubation experiments using lactate-grown cultures, sulfide concentration increased up to 8.5 mM. Our calculations indicate that in the presence of high concentrations of sulfide, most of the dissolved iron forms complexes (e.g., FeHS+) (60). Under these conditions, the concentration of free ferrous ion is around 3 nM and 0.3 nM in control and iron-limited media, respectively. The corresponding ion activity product (IAP) values ([Fe2+][HS−]/[H+]) in control and iron-limited media are 10−2.9 and 10−3.9, respectively. These values are comparable to the equilibrium solubility products of amorphous FeS (10−2.95) and mackinawite (10−3.6) (18). Iron in the form of amorphous FeS is considered to be bioavailable (e.g., see reference 30). The media should be supersaturated with respect to pyrrhotite and pyrite (18), but the precipitation of these crystalline sulfides requires longer incubation times than those used in our study (33). More importantly, DMSS-1 grew slower and fractionated more in media that initially contained less iron than in control cultures. Hence, the contrast between control and iron-limited cultures can be attributed to different availabilities of iron regardless of the precipitation of poorly crystalline iron sulfide minerals.
Iron deficiency reduced the growth rate and cell-specific sulfate reduction rate (csSRR) of DMSS-1 and led to increasing fractionation factors. This inverse relationship between csSRR and 34ε was previously reported for DMSS-1 cultures that grew in the presence of different electron donors (Fig. 5) (62) but were not limited by nitrogen or iron. The similarities between the effects of organic limitation and iron on the relationship between csSRR and 34ε are consistent with a central role of iron in the MSR pathway, given that both iron and electron donors can dictate the rate at which electrons are supplied to the sulfate reduction system (9, 62, 63). In contrast to previous reports, which considered the effect of electron donors and acceptors on the relationship between csSRR and 34ε (15, 28, 32, 40, 62), to the best of our knowledge, this is the first study that considers the effect of other limitations on S isotope fractionation by a sulfate-reducing microbe.
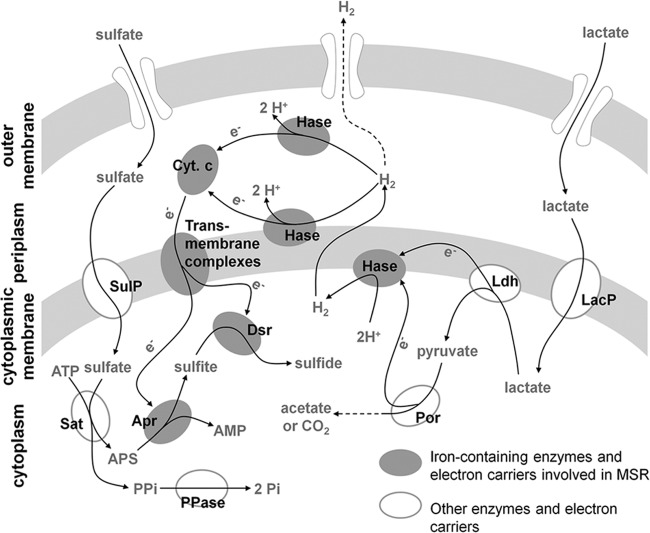
Although the mechanism of energy conservation by sulfate-reducing bacteria is not fully understood, H2 cycling between cytoplasm and periplasm is thought to be involved in this process (Fig. 6) (49). In the H2-cycling model, electrons generated by the oxidation of organic compounds react with protons to form H2 in reactions catalyzed by various Fe-containing cytoplasmic hydrogenases. H2 then diffuses across the cytoplasmic membrane into the periplasm, where it is split into protons and electrons by periplasmic hydrogenases. Electrons return to the cytoplasm through cytochromes and transmembrane protein complexes and are delivered to terminal reductases and used to reduce sulfate. The protons remaining in periplasm generate proton motive force. This model requires both cytoplasmic and periplasmic hydrogenases, enzymes that invariably contain iron in their active site (54). The key electron carrier involved in H2 cycling, a cytochrome c, is also a metalloprotein containing a heme prosthetic group (55). Increasing genetic and biochemical evidence points to the presence of redundant electron transport chains, suggesting that hydrogen cycling is not essential for the energy conservation in sulfate-reducing bacteria (41). Nonetheless, because most components involved in alternative electron transport pathways contain iron in their active sites (54), iron limitation would conceivably affect the function of electron transfer pathways. Our experiments strongly support a role of iron in the electron transfer chain that couples sulfate reduction and oxidation of lactate or malate in DMSS-1 cells. In particular, the ratio of cytochrome c to total protein decreases in iron-starved DMSS-1 cells (Fig. 4). This decrease likely impairs the flow of electrons from organic electron donors to sulfur through H2 cycling and slows down the respiration. This measurable effect is one of the many potential changes in the activities and concentrations of components involved in MSR during iron limitation. These changes can be explored by future transcriptomic, proteomic, and biochemical studies.
Fig 6.
Schematic representation of electron flow during MSR through hydrogen cycling in Gram-negative sulfate-reducing bacteria that oxidize lactate (41, 49, 54). Malate is also oxidized via pyruvate, but the transfer of reducing equivalents from the first oxidation step of malate may differ from that of lactate oxidation (44). Many enzymes and electron carriers involved in this process, from the cytoplasm to the periplasm, contain iron in their active sites (45, 58). Iron limitation impairs the synthesis of cytochromes and hydrogenases (55), reducing the flow of electrons to the sulfate reduction system. Abbreviations: LacP, lactate permease; Ldh, lactate dehydrogenase; Por, pyruvate-ferredoxin oxidoreductase; Hase, hydrogenase; Cyt. c, cytochrome c; SulP, sulfate permease; Sat, ATP sulfurylase; Apr, APS reductase; Dsr, dissimilatory sulfite reductase; PPase, pyrophosphate phosphatase.
The reduced flow of electrons to the sulfate reducing system would affect S-isotope fractionation. According to the reaction and isotope fractionation scheme for MSR initially developed by Rees (59) and further modified by Brunner and Bernasconi (9) and Farquhar et al. (23) (referred to as the RBF model), the MSR pathway consists of several enzymatic steps that are thought to be reversible. These reversible steps include ATP sulfurylation, reduction of adenosine-5′-phosphosulfate (APS), and reduction of sulfite. The RBF model explains the overall S-isotope fractionation by considering both the intrinsic isotope effects of these enzymatic steps and the ratios between forward and backward fluxes (reversibility) at each step, predicting the increase in S-isotope fractionation when reversibilities approach unity. Isotope effects at individual enzymatic steps depend on the difference between ground-state and transition-state structures in each reaction (50) and are unlikely to be altered by nutrient availability. Instead, larger S-isotope effects in iron-limited cultures suggest MSR operating in a more reversible manner. Given that electrons are essential for sulfate reduction, a slower flow of electrons to terminal reductases could slow down the forward reactions and increase the reversibility, thereby increasing the overall isotope fractionation. Our results suggest that this slowing down can be triggered by a deficiency in iron-dependent components of the respiratory chain. Limitations by other metals required for MSR, such as Co, Zn, Ni, and Se (25, 54), on S-isotope effects remain to be explored in future studies.
Ammonium limitation.
Acetylene reduction assay confirms that DMSS-1 can fix nitrogen in ammonium-limited media (Fig. 3), an ability that DMSS-1 shares with other Desulfovibrio species (56). Nitrogen limitation diminishes the growth yield of DMSS-1 in spite of increased csSRR (Fig. 2). The higher respiratory rates likely support the energetically costly N2 fixation, a process that requires 8 electrons and 16 ATP molecules (56). In other words, if a larger portion of energy generated by the respiratory activity is spent on nitrogen fixation rather than biosynthesis, the growth yield decreases. Unlike the limitation by iron or organic compounds, which decreased csSRR and increased 34ε, nitrogen limitation increased both csSRR and 34ε in DMSS-1 cultures (Fig. 5). The RBF model described above ascribes the overall isotope fractionation during MSR to the reversibility and the intrinsic isotope effect of each enzymatic step, predicting that MSR operates in a more reversible manner during diazotrophic growth of DMSS-1. At this point, it is unclear how this model prediction can account for the intracellular physiology, given that N2 fixation acts as a sink for reducing power but the csSRR is higher. In other words, N2 fixation should draw electrons out of the MSR pathway, increasing S-isotope fractionation, but the higher csSRR reflects the increased net flow of electrons through the MSR pathway during diazotrophic growth of DMSS-1.
Interestingly, both iron- and ammonium-limited cultures of DMSS-1 exhibit slow and nonexponential growth kinetics, consistent with the density-dependent limitation by a substrate that is present in the initial medium (69). Not only are cell densities at the end of growth lower than those in iron- and ammonium-replete cultures but the growth rates are also much slower, suggesting different growth physiologies in these cultures. These differences between nutrient-limited and nutrient-replete cultures cannot be attributed to the production of observable extracellular polymers, cell clumping, or measurable changes in cell size. Instead, relevant examples of similar, slow growth kinetics occur in cultures of syntrophic organisms limited by energy supply (22) and microbes that produce intracellular granules (53). Namely, both nitrogen fixation and the lack of iron in critical energy-generating enzymes are expected to reduce the energy yield available for biosynthesis of new cellular material and cell doubling. In the presence of abundant carbon and reducing equivalents, these limitations can promote the formation of intracellular polyglucose granules at the expense of other cellular material. The link between low iron and ammonium availability and the synthesis of abundant intracellular polyglucose granules is observed in various Desulfovibrio species and Desulfobulbus propionicus (64). In addition, given that nitrogenases contain an iron cofactor (10), iron limitation can aggravate the effects of nitrogen limitation in nature. One could expect larger fractionations in the media that contain low concentrations of iron and nitrogen, although our experiments did not directly test this possibility.
Environmental and geological significance.
Effects of iron and nitrogen limitation on S-isotope fractionation can inform interpretations of sulfur isotope data in modern and ancient environments. Porewaters in the natural habitat of DMSS-1, salt marsh sediments, are characterized by dissolved Fe concentrations that range from 2 to 60 μM (26), i.e., they are higher than the submicromolar iron concentrations limiting the growth of DMSS-1 under our experimental conditions. It is thus unlikely that these bacteria are limited by iron in their natural habitat. In the oxic water column, iron oxyhydroxides accumulate in the oxic surface sediment and are subsequently reduced and dissolved during early diagenesis. Consequently, in most marine environments, the concentration of dissolved iron (II) in the porewaters of the sulfate reduction zone is usually several to several hundred μM (7, 35, 42, 67). Under these conditions, iron is not the key factor controlling the magnitude of microbial S-isotope fractionation in the subsurface zone of sulfate reduction. However, the situation may be different in euxinic basins, where iron oxyhydroxides dissolve and form FeS and pyrite while settling through a sulfidic water column (11). For example, in the euxinic part of the Black Sea, iron concentrations are usually lower than 0.3 μM in the water column (2, 46) and lower than 1 μM in the porewater (11). The submicromolar concentrations of dissolved iron may limit MSR and contribute to the production of sulfides depleted up to 60‰ (65). The same mechanism may have been relevant in the past, particularly during times of oceanic euxinia when sulfidic conditions would have expanded from subsurface sediments into the water column (47). This expansion, accompanying the syngenetic formation of pyrite in the water column (34), might have diminished the overall availability of iron for marine sulfate-reducing microbes, thereby increasing the magnitude of S-isotope fractionation. This hypothesis may explain some of the >20‰ increase in S-isotopic difference between sedimentary sulfide and sulfate at the onset of the Cretaceous Oceanic Anoxic Event 2 (1, 34).
By exploring the effect of N2 fixation on S-isotope fractionation by sulfate reducers, our observations indicate that nitrogen limitation increase the already large S-isotope fractionation in areas characterized by low productivity, such as deep sea sediments (e.g., see reference 61). Organic compounds limit the growth and MSR in these settings, but the nitrogen requirement of microbial communities in deep sea sediments may further increase 34ε depending on the C/N ratio of organic substrates used for growth (3). More surprisingly, recent studies also have correlated nitrogen fixation with the activity of sulfate reducers in porewaters characterized by ammonium concentrations as high as 1 mM (4) and in organic-rich estuarine sediments (24). Nitrogen fixation thus occurs in sediments under hypoxic water columns even though the porewater ammonium concentrations are higher than 0.1 mM (4). These concentrations are comparable to those limiting the growth of DMSS-1 in our laboratory cultures (Fig. 1). Nitrogen fixation by sulfate reducers in organic-rich areas and its isotopic signatures have yet to be explored in detail, but our data predict higher S-isotope fractionations even in zones with rather high rates of sulfate reduction if sulfate reducers also fix N2. These conditions may be met in various estuarine sediments (24), oxygen minimum zones, hypoxic fjords (4), and, possibly, in Lake Cadagno, a meromictic lake in Switzerland. In this lake, dense populations of anoxygenic phototrophic bacteria at the chemocline enrich the underlying monimolimnion and sediments with organic compounds accessible to sulfate-reducing microbes (19), and sulfate-reducing Deltaproteobacteria there fix N2 (29), likely contributing to a portion of the S-isotope difference between dissolved sulfate and sulfide, which is often as large as 45‰ (12). These hypotheses can be tested experimentally by parallel measurements of the expression of nitrogenases, sulfate-reducing rates, and S-isotope fractionations.
Emerging constraints on the physiological coupling among S, C, Fe, N, and other cycles show that controls on natural S-isotope signatures are complex and may involve limitations by various nutrients. In particular, the effects of iron and nitrogen on S-isotope fractionation described by this study underscore the need to better understand nutrient and metal requirements of sulfate-reducing microbes in natural settings. While this may complicate current interpretations of the S-isotope record, it will also draw more realistic and better informed links between microbial physiology and sedimentary biogeochemistry.
ACKNOWLEDGMENTS
This work was supported by grants from the NASA Astrobiology Institute and NSF (EAR-1159318) to S.O. and T.B.
We are grateful to all members of our laboratory for their support. We thank the four anonymous reviewers for constructive suggestions that improved the manuscript.
Footnotes
Published ahead of print 21 September 2012
REFERENCES
- 1. Adams DD, Hurtgen MT, Sageman BB. 2010. Volcanic triggering of a biogeochemical cascade during Oceanic Anoxic Event 2. Nat. Geosci. 3:201–204 [Google Scholar]
- 2. Alkan N, Tüfekçi M. 2009. Distribution of dissolved forms of manganese and iron in the water column of the southeastern Black Sea. Turk. J. Fish. Aquat. Sci. 9:159–164 [Google Scholar]
- 3. Anderson TR, Pondaven P. 2003. Non-redfield carbon and nitrogen cycling in the Sargasso Sea: pelagic imbalance and export flux. Deep Sea Res. I 50:573–591 [Google Scholar]
- 4. Bertics VJ, et al. 2012. Occurrence of benthic microbial nitrogen fixation coupled to sulfate reduction in the seasonally hypoxic Eckernförde Bay, Baltic Sea. Biogeosciences 9:6489–6533 [Google Scholar]
- 5. Bevington PR, Robinson DK. 2003. Data reduction and error analysis for the physical sciences, 3rd ed McGraw-Hill, New York, NY [Google Scholar]
- 6. Bolliger C, Schroth MH, Bernasconi SM, Kleikemper J, Zeyer J. 2001. Sulfur isotope fractionation during microbial sulfate reduction by toluene-degrading bacteria. Geochim. Cosmochim. Acta 19:3289–3298 [Google Scholar]
- 7. Böttcher ME, et al. 2000. The biochemistry, stable isotope geochemistry, and microbial community structure of a temperate intertidal mudflat: an integrated study. Cont. Shelf Res. 20:1749–1769 [Google Scholar]
- 8. Bottrell SH, Newton R. 2006. Reconstruction of change in global sulfur cycling from marine sulfate isotopes. Earth Sci. Rev. 75:59–83 [Google Scholar]
- 9. Brunner B, Bernasconi SM. 2005. A revised isotope fractionation model for dissimilatory sulfate reduction in sulfate reducing bacteria. Geochim. Cosmochim. Acta 69:4759–4771 [Google Scholar]
- 10. Burgess BK. 1990. The iron-molybdenum cofactor of nitrogenase. Chem. Rev. 90:1377–1406 [Google Scholar]
- 11. Calvert SE, Karlin RE. 1991. Relationships between sulphur, organic carbon, and iron in the modern sediments of the Black Sea. Geochim. Cosmochim. Acta 55:2483–2490 [Google Scholar]
- 12. Canfield DE, Farquhar J, Zerkle AL. 2010. High isotope fractionations during sulfate reduction in a low-sulfate euxinic ocean analog. Geology 38:415–418 [Google Scholar]
- 13. Canfield DE, Raiswell R. 1999. The evolution of the sulfur cycle. Am. J. Sci. 299:679–723 [Google Scholar]
- 14. Chambers LA, Trudinger PA. 1979. Microbiological fractionation of stable sulfur isotopes: a review and critique. Geomicrobiol. J. 1:249–293 [Google Scholar]
- 15. Chambers LA, Trudinger PA, Smith JW, Burns MS. 1975. Fractionation of sulfur isotopes by continuous cultures of Desulfovibrio desulfuricans. Can. J. Microbiol. 21:1602–1607 [DOI] [PubMed] [Google Scholar]
- 16. Cline JD. 1969. Spectrophotometric determination of hydrogen sulfide in natural water. Limnol. Oceanogr. 14:454–458 [Google Scholar]
- 17. Cotter PA, Darie S, Gunsalus RP. 1992. The effect of iron limitation on expression of the aerobic and anaerobic electron transport pathway genes in Escherichia coli. FEMS Microbiol. Lett. 79:227–232 [DOI] [PubMed] [Google Scholar]
- 18. Davison W. 1991. The solubility of iron sulphides in synthetic and natural water at ambient temperature. Aquat. Sci. 53:309–329 [Google Scholar]
- 19. Del Don C, Hanselmann KW, Peduzzi R, Bachofen R. 2001. The meromictic alpine Lake Cadagno: orographical and biochemical description. Aquat. Sci. 63:70–90 [Google Scholar]
- 20. Detmers J, Bruchert V, Habicht K, Kuever J. 2001. Diversity of sulfur isotope fractionations by sulfate reducing prokaryotes. Appl. Environ. Microbiol. 67:888–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dilworth MJ. 1966. Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochim. Biophys. Acta 127:285–294 [DOI] [PubMed] [Google Scholar]
- 22. Dwyer DF, Weeg-Aerssens E, Shelton DR, Tiedje JM. 1988. Bioenergetic conditions of butyrate metabolism by a syntrophic, anaerobic bacterium in coculture with hydrogen-oxidizing methanogenic and sulfidogenic bacteria. Appl. Environ. Microbiol. 54:1354–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farquhar J, et al. 2003. Multiple sulphur isotopic interpretations of biosynthetic pathways: implications for biological signatures in the sulphur isotope record. Geobiology 1:27–36 [Google Scholar]
- 24. Fulweiler RW, Nixon SW, Buckley BA, Granger SL. 2007. Reversal of the net dinitrogen gas flux in coastal marine sediments. Nature 448:180–182 [DOI] [PubMed] [Google Scholar]
- 25. Gavel OY, et al. 1998. ATP sulfurylases from sulfate-reducing bacteria of the genus Desulfovibrio. A novel metalloprotein containing cobalt and zinc. Biochemistry 37:16225–16232 [DOI] [PubMed] [Google Scholar]
- 26. Giblin AE, Howarth RW. 1984. Porewater evidence for a dynamic sedimentary iron cycle in salt marshes. Limnol. Oceanogr. 29:47–63 [Google Scholar]
- 27. Habicht KS, Gabe M, Thamdrup B, Berg P, Canfield DE. 2002. Calibration of sulfate levels in the Archean ocean. Science 298:2372–2374 [DOI] [PubMed] [Google Scholar]
- 28. Habicht KS, Salling L, Thamdrup B, Canfield DE. 2005. Effect of low sulfate concentrations on lactate oxidation and isotope fractionation during sulfate reduction by Archaeoglobus fulgidus strain Z. Appl. Environ. Microbiol. 71:3770–3777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Halm H, et al. 2009. Co-occurrence of denitrification and nitrogen fixation in a meromictic lake, Lake Cadagno (Switzerland). Environ. Microbiol. 11:1945–1958 [DOI] [PubMed] [Google Scholar]
- 30. Handley KH, Boothman C, Mills RA, Pancost RD, Lloyd JR. 2010. Functional diversity of bacteria in a ferruginous hydrothermal sediment. ISME J. 4:1193–1205 [DOI] [PubMed] [Google Scholar]
- 31. Harder W, Dijkhuizen L. 1983. Physiological responses to nutrient limitation. Annu. Rev. Microbiol. 37:1–23 [DOI] [PubMed] [Google Scholar]
- 32. Harrison AG, Thode HG. 1958. Mechanism of the bacterial reduction of sulphate from isotope fractionation studies. Trans. Faraday Soc. 54:84–92 [Google Scholar]
- 33. Herbert RB, Jr, Benner SG, Pratt AR, Blowes DW. 1998. Surface chemistry and morphology of poorly crystalline iron sulfides precipitated in media containing sulfate-reducing bacteria. Chem. Geol. 144:87–97 [Google Scholar]
- 34. Hetzel A, Böttcher ME, Wortmann UG, Brumsack H-J. 2009. Paleo-redox conditions during OAE2 reflected in Demerara Rise sediment geochemistry (ODP Leg 207). Palaeogeogr. Palaeoclimatol. Palaeoecol. 273:302–328 [Google Scholar]
- 35. Hines ME, Bazylinski DA, Tugel JB, Lyons WB. 1991. Anaerobic microbial biogeochemistry in sediments from two basins in the Gulf of Maine: evidence for iron and manganese reduction. Estuar. Coast. Shelf Sci. 32:313–324 [Google Scholar]
- 36. Holmes RM, Aminot A, Kerouel R, Hooker BA, Peterson BJ. 1999. A simple and precise method for measuring ammonium in marine and fresh water ecosystems. Can. J. Fish. Aquat. Sci. 56:1801–1808 [Google Scholar]
- 37. Hubbard JAM, Lewandowska KB, Hughes MN, Poole RK. 1986. Effects of iron-limitation of Escherichia coli on growth, the respiratory chains and gallium uptake. Arch. Microbiol. 146:80–86 [DOI] [PubMed] [Google Scholar]
- 38. Johnston WA, Huang W, de Voss JJ, Hayes MA, Gillam EM. 2008. Quantitative whole-cell cytochrome P450 measurement suitable for high-throughput application. J. Biomol. Screen. 13:135–141 [DOI] [PubMed] [Google Scholar]
- 39. Jones GA, Pickard MD. 1980. Effect of titanium (III) citrate as reducing agent on growth of rumen bacteria. Appl. Environ. Microbiol. 39:1144–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaplan IR, Rittenberg SC. 1964. Microbiological fractionation of sulphur isotopes. J. Gen. Microbiol. 34:195–212 [DOI] [PubMed] [Google Scholar]
- 41. Keller KL, Wall JD. 2011. Genetics and molecular biology of the electron flow for sulfate respiration in Desulfovibrio. Front. Microbiol. 2:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. King GM, Klug MJ, Wiegert RG, Chalmers AG. 1982. Relation of soil water movement and sulfide concentration to Spartina alterniflora production in a Georgia salt marsh. Science 218:61–63 [DOI] [PubMed] [Google Scholar]
- 43. Kleikemper J, et al. 2002. Activity and diversity of sulfate-reducing bacteria in a petroleum hydrocarbon-contaminated aquifer. Appl. Environ. Microbiol. 68:1516–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kremer DR, Nienhuis-Kuiper HE, Timmer CJ, Hansen TA. 1989. Catabolism of malate and related dicarboxylic acids in various Desulfovibrio strains and the involvement of an oxygen-labile NADPH dehydrogenase. Arch. Microbiol. 151:34–39 [Google Scholar]
- 45. Lamperia J, Pereira AS, Moura JJG. 1994. Adenylylsulfate reductase from sulfate-reducing bacteria. Methods Enzymol. 243:241–260 [Google Scholar]
- 46. Lewis BL, Landing WM. 1991. The biogeochemistry of manganese and iron in the Black Sea. Deep Sea Res. 38:S773–S803 [Google Scholar]
- 47. Meyer KM, Kump LR. 2008. Oceanic euxinia in Earth history: causes and consequences. Annu. Rev. Earth Planet. Sci. 36:251–288 [Google Scholar]
- 48. Moreau JW, Zierenberg RA, Banfield JF. 2010. Diversity of dissimilatory sulfate reductase genes (dsrAB) in a salt marsh impacted by long-term acid mine drainage. Appl. Environ. Microbiol. 76:4819–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Odom JM, Peck HD., Jr 1981. Hydrogen cycling as a general mechanism for energy coupling in the sulfate reducing bacteria, Desulfovibrio sp. FEMS Microbiol. Lett. 12:47–50 [Google Scholar]
- 50. O'Leary MH. 1989. Multiple isotope effects on enzyme-catalyzed reactions. Annu. Rev. Biochem. 58:377–401 [DOI] [PubMed] [Google Scholar]
- 51. Ono S, Keller NS, Rouxel O, Alt J. 2012. Sulfur-33 cosnstraints on the origin of secondary pyrite in altered oceanic basement. Geochim. Cosmochim. Acta 87:323–340 [Google Scholar]
- 52. Ono S, Wing B, Rumble D, Farquhar J. 2006. High precision analysis of all four stable isotope of sulfur (32S, 33S, 34S and 36S) at nanomole levels using a laser fluorination isotope-ratio-monitoring gas chromatography-mass spectrometry. Chem. Geol. 225:30–39 [Google Scholar]
- 53. Pagni M, Beffa T, Isch C, Aragno M. 1992. Linear growth and poly (β-hydroxybutyrate) synthesis in response to pulse-wise addition of the growth-limiting substrate to steady-state heterotrophic continuous cultures of Aquaspirillum autotrophicum. J. Gen. Microbiol. 138:429–436 [DOI] [PubMed] [Google Scholar]
- 54. Pereira IAC, et al. 2011. A comparative genomic analysis of energy metabolism in sulfate reducing bacteria and archaea. Front. Microbiol. 2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Postgate JR. 1956. Iron and synthesis of cytochrome c3. J. Gen. Microbiol. 15:186–193 [DOI] [PubMed] [Google Scholar]
- 56. Postgate JR. 1982. The fundamental of nitrogen fixation. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 57. Postgate JR, Campbell LL. 1966. Classification of Desulfovibrio species, the non-sporulating sulfate-reducing bacteria. Bacteriol. Rev. 30:732–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rabus R, Hansen TA, Widdel F. 2006. Dissimilatory sulfate- and sulfur-reducing prokaryotes, p 659–786 In Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackbrandt E. (ed), The prokaryotes. Springer-Verlag, New York, NY [Google Scholar]
- 59. Rees CE. 1973. A steady-state model for sulphur isotope fractionation in bacterial reduction processes. Geochim. Cosmochim. Acta 37:1141–1162 [Google Scholar]
- 60. Rickard D, Luther GW., III 2007. Chemistry of iron sulfides. Chem. Rev. 107:514–562 [DOI] [PubMed] [Google Scholar]
- 61. Rudnicki MD, Elderfield H, Spiro B. 2001. Fractionation of sulfur isotopes during bacterial sulfate reduction in deep ocean sediments at elevated temperatures. Geochim. Cosmochim. Acta 65:777–789 [Google Scholar]
- 62. Sim MS, Ono S, Donovan K, Templer SP, Bosak T. 2011. Effect of electron donors on the fractionation of sulfur isotopes by a marine Desulfovibrio sp. Geochim. Cosmochim. Acta 75:4244–4259 [Google Scholar]
- 63. Sim MS, Bosak T, Ono S. 2011. Large sulfur isotope fractionation does not require disproportionation. Science 333:74–77 [DOI] [PubMed] [Google Scholar]
- 64. Stams FJM, Veenhuis M, Weenk GH, Hanssen TA. 1983. Occurrence of polyglucose as a storage polymer in Desulfovibrio species and Desulfobulbus propionicus. Arch. Microbiol. 136:54–59 [Google Scholar]
- 65. Sweeny RE, Kaplan IR. 1980. Stable isotope composition of dissolved sulfate and hydrogen sulfide in the Black Sea. Mar. Chem. 9:145–152 [Google Scholar]
- 66. Thode HG, Monster J, Dunford HB. 1961. Sulphur isotope geochemistry. Geochim. Cosmochim. Acta 25:158–174 [Google Scholar]
- 67. Wang G, Spivack AJ, D'Hondt S. 2010. Gibbs energies of reaction and microbial mutualism in anaerobic deep subseafloor sediments of ODP site 1226. Geochim. Cosmochim. Acta 74:3938–3947 [Google Scholar]
- 68. Widdel F, Kohring GW, Mayer F. 1983. Studies on dissimilatory sulfate-reducing bacteria that decomposed fatty acids. III. Characterization of the filamentous gliding Desulfonema limocola gen. nov. sp. nov. and Desulfonema magnum sp. nov. Arch. Microbiol. 134:286–294 [Google Scholar]
- 69. Wright RT, Coffin RB. 1984. Measuring microzooplankton grazing on planktonic marine bacteria by its impact on bacterial production. Microb. Ecol. 10:137–149 [DOI] [PubMed] [Google Scholar]