Abstract
Essential oils are marginally soluble in water, making it challenging to evenly disperse them in foods and resulting in an increased tendency to bind with food lipids and proteins, resulting in lowered antimicrobial efficacy. In the current study, free and nano-dispersed (ND) thymol were compared in terms of their antimicrobial efficacies against Escherichia coli O157:H7 ATCC 43889 and 43894 and Listeria monocytogenes strains Scott A and 101 in apple cider and 2% reduced-fat milk. Apple cider was adjusted to pHs 5.5 and 3.5, and antimicrobial tests were performed at 0.3-, 0.5-, 0.75-, and 1.0-g/liter thymol concentrations at 35, 32, 25, and 4°C. Overall, 0.5 and 1.0 g/liter thymol in nano-dispersion and along with free thymol were inhibitory and bactericidal, respectively, against bacterial strains under all treatment conditions. At pH 5.5, 0.5 g/liter ND thymol was bacteriostatic against L. monocytogenes and E. coli for up to 48 h. At pH 3.5, L. monocytogenes controls did not survive beyond 12 h but E. coli survived and was inhibited by 0.5 g/liter ND thymol after 12 and 48 h in apple cider. E. coli strains were significantly sensitive to 4°C and pH 3.5 (P < 0.05). When bacteria were tested in 2% reduced-fat milk at 35 or 32°C, ND and free thymol demonstrated inhibition at 4.5 g/liter. Thus, the current technology seems to be promising and novel, enabling thymol-containing nano-dispersions that are not only transparent but also effective against pathogens in food applications, especially in clear beverages.
INTRODUCTION
Considering the occurrence and severity of food-borne outbreaks caused by Escherichia coli O157:H7 and Listeria monocytogenes, improving methods to control these pathogens in foods is of primary concern. Existing preservation and control methods, along with effective sanitation, contribute to improved food safety. One of the control methods is the use of chemical antimicrobial compounds that are specific to foods. However, consumers are increasingly demanding minimally processed foods with “natural” flavors and few perceived “synthetic” additives (4). Unpasteurized apple cider and raw milk are examples of such products.
Typically considered a “low-risk” food due to its acidic pH of 4.0 (15, 30), apple cider has been associated with multiple food-borne illness outbreaks caused by E. coli O157:H7 and related E. coli strains from 1991 to 1998. There is concern regarding its ability to cause severe illness with low infectious doses (27). Investigations have revealed that some E. coli O157:H7 strains are acid tolerant or resistant (5, 23, 24, 26) and able to survive refrigeration temperatures for 1 to 2 weeks (31). Although L. monocytogenes has not been directly linked to apple cider outbreaks, potential cross-contamination of apple cider from environmental sources is possible. The widespread occurrence, relative heat resistance, and ability to grow under refrigerated/frozen conditions make L. monocytogenes an appropriate target organism for investigation of innovative preservative strategies (31).
The food industry is exploring new ways to develop effective natural antimicrobials to improve food safety and extend product shelf life. Plant essential oils (EO) are potential alternative natural antimicrobial ingredients. EO are classified as generally regarded as safe (GRAS) and are responsible for the aroma and flavor of many spices and herbs (2). Several reports confirm the antibacterial and antifungal properties of EO components, with the most effective ones coming from thyme (thymol), oregano (carvacrol), and clove (eugenol) (3).
Thymol, the major antimicrobial component from the aromatic plant thyme (Thymus vulgaris), is a hydrophobic, phenolic compound able to bind with bacterial proteins, which results in cell membrane disintegration and permeability, thus making it a potent, broad-spectrum antimicrobial (14). However, its hydrophobicity makes its application in foods challenging because of the difficulty of uniform dispersion in food matrices. Binding of thymol with hydrophobic constituents (lipids and proteins) of complex food systems, such as milk, reduces its antimicrobial effectiveness. Because of its limited water solubility, estimated to be 1 g/liter at 20°C (7), the undissolved thymol applied at a concentration above the solubility limit impacts visual quality and antimicrobial availability. Being a volatile EO component, thymol also has an intense spicy/medicinal aroma, particularly upon heating, thus adversely affecting sensory properties of foods upon incorporation in the free form or at high concentrations (8, 19, 28, 34).
Challenges in dealing with such hydrophobic compounds can be overcome by dissolving them in a solvent or solvent mixture with decreased polarity or dispersing them in emulsion droplets or biopolymer particles. For emulsion systems, oil droplets can be kinetically stabilized in the continuous aqueous phase by adopting appropriate emulsifiers and engineered interfaces. Milk proteins are among the best natural surfactants for food applications (29). Interfacial properties of milk proteins, particularly whey proteins, can be improved by conjugation with more hydrophilic oligosaccharides and polysaccharides (25). Compared to emulsions stabilized by proteins, the oligo- or polysaccharide moiety of conjugates on droplet surfaces provides steric hindrance against droplet aggregation and thereby improves emulsion stability. As such, conjugates of whey protein isolate (WPI) and maltodextrin (MD) have been applied to prepare emulsions of tomato volatiles (10) and conjugated linoleic acid (9) to minimize degradation and improve dispersibility in foods.
Encapsulation of lipophilic antimicrobials in nanoscale systems has several advantages. In aqueous dispersions, Brownian motion is the mechanism dominating the dynamics of nanoparticles, which prevents gravitational sedimentation or creaming of dispersed particles (35). Smaller particles scatter visible light less effectively and can eventually enable transparent dispersions at a sufficiently small dimension (35). The solubility of compounds with limited solubility increases when dispersed in a smaller structure (22), which, along with increased surface area for bacterial contact and even distribution in food matrices, improves the antimicrobial effectiveness (34). Proof of concept has been given by liposomal delivery systems containing carvacrol (28) and nanoscale surfactant micelles containing eugenol (16) when tested in growth media and milk. There is limited work using food biopolymers to deliver EO in foods.
The objective of the present study was to evaluate the efficacy of thymol dispersed in WPI-MD nanocapsules in inhibition of E. coli O157:H7 and L. monocytogenes in model food systems, including apple cider and 2% reduced-fat milk, under different pH and temperature conditions.
MATERIALS AND METHODS
Materials.
Thymol (99%) was obtained from Acros Organics (Thermo Fisher Scientific, Morris Plains, NJ). WPI was a gift from Hilmar Cheese Company (Hilmar, CA). MD180, with an average dextrose equivalent of 18, was a product of Grain Processing Corporation (Muscatine, IA). Tryptic soy broth (TSB), peptone, and agar (chemical grade) were purchased from Becton, Dickinson and Company (Sparks, MD). Other chemicals, such as hexane and methanol, were obtained from Fisher Scientific (Pittsburgh, PA). Apple cider was a product of M.H. Zeigler and Sons, LLC (Lansdale, PA). Ultra-high-temperature (UHT) pasteurized organic milk with 2% fat was purchased from Private Selection, a brand of the Kroger company (Cincinnati, OH).
Preparation and characterization of nano-dispersion.
An emulsion-evaporation process was used to encapsulate thymol (32). An oil phase (with 20% [wt/vol] thymol in hexane) was emulsified at 10% (vol/vol) into an aqueous phase with 11.1% (wt/vol) conjugates, followed by spray drying. Conjugates were previously prepared by spray drying a solution containing WPI and MD180 at a mass ratio of 1:2, followed by dry heating the spray-dried powder at 90°C for 2 h for conjugation (Maillard reaction). All spray-drying experiments were performed using a model B-290 mini spray dryer (BÜCHI Labortechnik AG, Flawil, Switzerland) at an inlet temperature of 150°C, a feed rate of 0.11 ml/s, 600 kPa compressed air pressure, a 35-m3/h airflow rate, and a recorded outlet temperature of 80 to 90°C. The spray-dried capsules were collected and stored in a freezer at −18°C.
The amount of thymol in spray-dried powder was quantified using a high-performance liquid chromatography (HPLC) method detailed previously (32). To prepare nano-dispersions for characterization of physical properties or the antimicrobial tests described below, spray-dried powder was hydrated for 14 h at 5% (wt/wt) or to a specific concentration (% [wt/vol]) of thymol in deionized water at room temperature. The dispersions containing 5% (wt/vol) spray-dried powder were adjusted to pHs 3.0, 5.0, and 7.0 using 1 N HCl or 1 N NaOH for measurement of absorbance at 600 nm using a UV-vis spectrophotometer (Biomate 5; Thermo Electron Corporation, Woburn, MA) and particle size distribution using a Delsa Nano-Zeta potential and submicron particle size analyzer (Beckman Coulter, Inc., Brea, CA). The particle size distribution was used to calculate volume-length mean particle diameter (d4,3) using equation 1, where ni is the number of particles corresponding to diameter di:
| (1) |
Culture preparation.
Stock cultures of E. coli O157:H7 ATCC 43889 and ATCC 43894 and L. monocytogenes strains Scott A and 101 were obtained from the Department of Food Science and Technology at the University of Tennessee, Knoxville. All cultures were grown in TSB and stored at −20°C in glycerol as stocks, and 100-μl samples were inoculated in 50 ml TSB and incubated for 24 h at 35 or 32°C to obtain working cultures.
Antimicrobial susceptibility test in model food systems.
Inhibition of E. coli and L. monocytogenes was studied in apple cider adjusted to pHs 3.5 and 5.5 and milk adjusted to pH 6.8 using time-kill assays (12). Enumeration was done using pour plating to determine viable count as CFU/ml. Growth media with and without bacterial culture were used as positive and negative controls, respectively, in all treatments.
Statistical analysis.
Values presented were data from four replicate experiments pooled to calculate the statistical mean and standard error of the mean for each treatment. The mixed-model analysis of variance (ANOVA) (P < 0.05) test was used to analyze data, using SAS 9.2 (SAS Institute, NC).
RESULTS AND DISCUSSION
Nano-dispersion properties.
The nano-dispersions with 5% (wt/vol) spray-dried powder contained 5.3 g/liter thymol, well above the solubility of 1 g/liter at 20°C (7), and were transparent at room temperature (21°C) and under refrigeration conditions. The d4,3 values at pHs 3.0, 5.0, and 7.0 were 67, 100, and 58 nm, respectively, and absorbance values of dispersions were below 0.5 at 600 nm (Table 1). It is well established that conjugation with carbohydrates can improve emulsifying properties of proteins (25). This occurs because the protein molecules anchor the oil droplets during emulsion formation, while the hydrophilic carbohydrate moieties position themselves into the aqueous phase, which forms a steric stabilizing layer that prevents oil droplets from coalescing (1, 13). The emulsion was turbid before spray drying, and evaporation of hexane created hollow, micrometer-sized capsules (32) that reformed to nanometer-sized particles (Table 1) upon hydration. Glycation of whey protein with MD effectively prevented aggregation, enabling transparent dispersions, particularly at pH 5.0, where unconjugated whey proteins would aggregate easily because of the close-to-zero net charge at this acidity near the isoelectric points of major whey proteins β-lactoglobulin, α-lactalbumin, and bovine serum albumin (6).
Table 1.
Absorbance and mean particle diametersa of thymol-containing nano-dispersions
| pH | Absorbance at 600 nm | Mean particle diam d4,3 (nm) |
|---|---|---|
| 3.0 | 0.230 ± 0.03 | 67 ± 1 |
| 5.0 | 0.453 ± 0.20 | 100 ± 1 |
| 7.0 | 0.247 ± 0.02 | 58 ± 1 |
Values are means ± standard errors of means from four measurements, two measurements from each of two replicates.
Antimicrobial activity of free and ND thymol in apple cider.
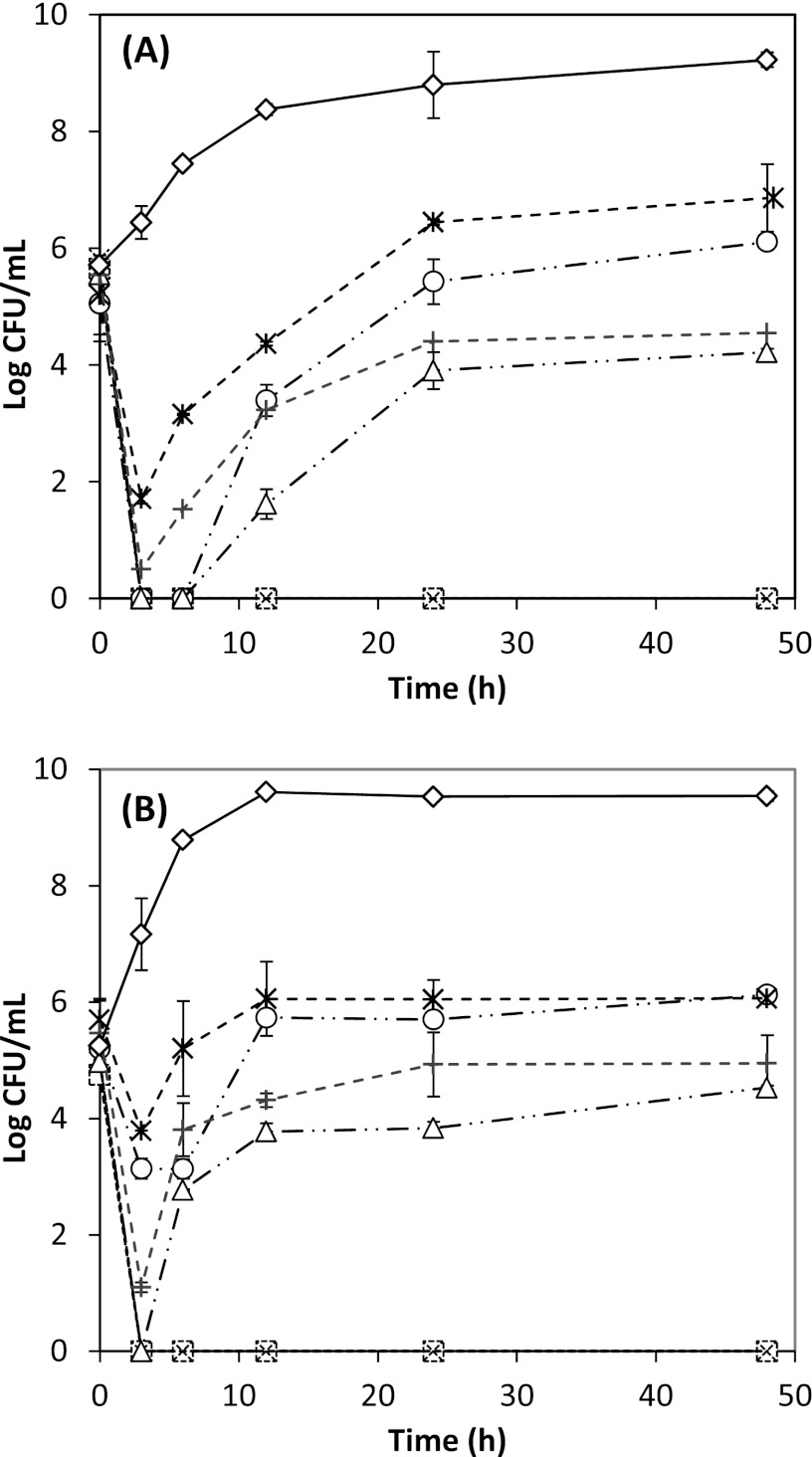
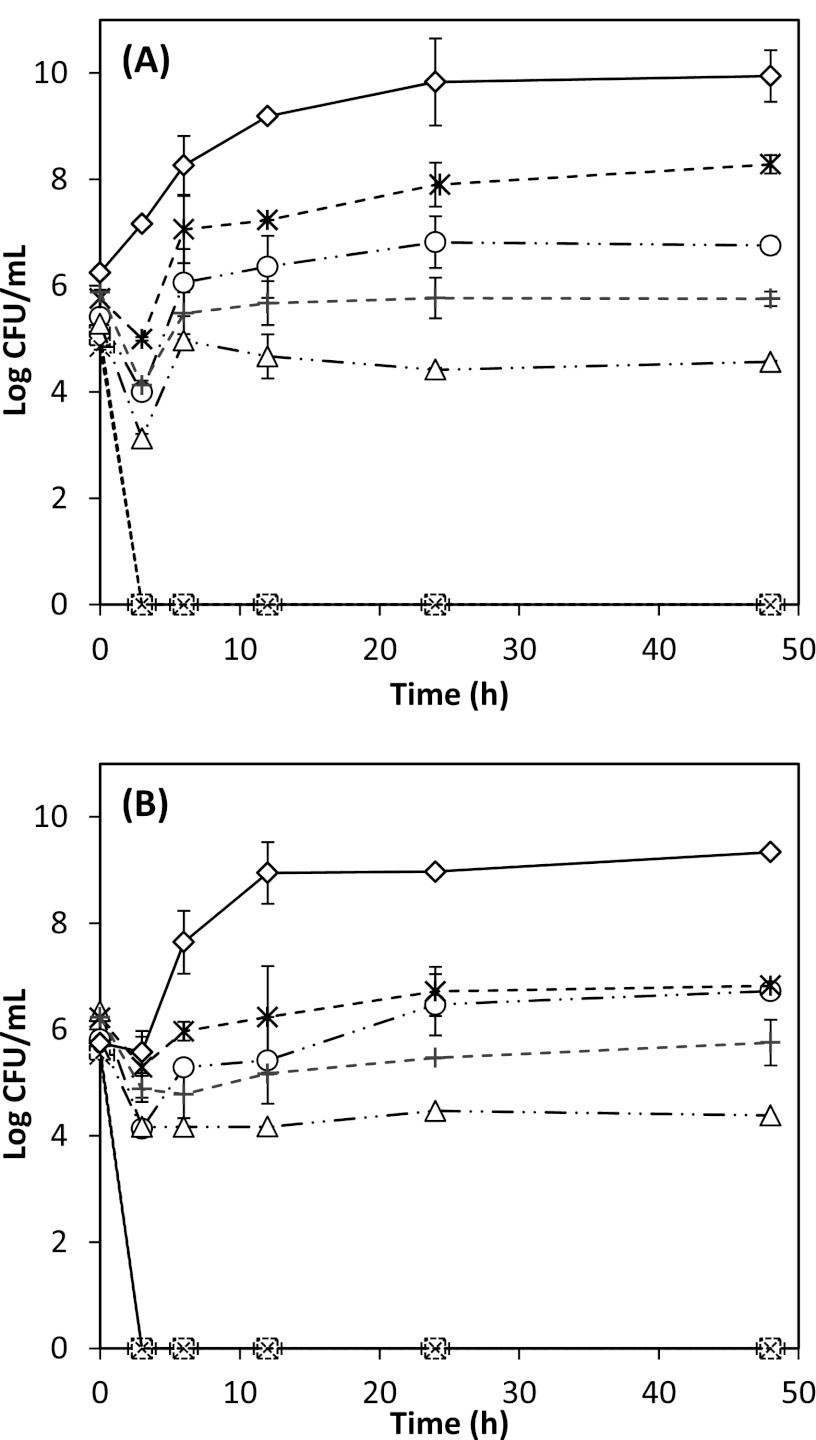
The antimicrobial efficacies of 0.3 to 1.0 g/liter free and nano-dispersed (ND) thymol in apple cider adjusted to pH 5.5 at optimum incubation temperatures for E. coli ATCC 43889 and ATCC 43894 and L. monocytogenes strains Scott A and 101 are presented in Fig. 1 and 2. At pH 5.5, 1.0 g/liter thymol resulted in complete bacterial inhibition, while 0.5 g/liter thymol was bacteriostatic at 35°C for E. coli and 32°C for L. monocytogenes.
Fig 1.
Antimicrobial activities of free and nano-dispersed (ND) thymol used at overall thymol concentrations of 0.3, 0.5, and 1.0 g/liter in apple cider adjusted to pH 5.5, against Escherichia coli O157:H7 ATCC 43889 (A) and 43894 (B) at 35°C. Error bars represent standard deviations of the means from four measurements, two from each of two replicates. ND thymol:  , 0.3 g/liter; ○, 0.5 g/liter; □, 1 g/liter. Free thymol: +, 0.3 g/liter; △, 0.5 g/liter; ×, 1 g/liter. Control: ♢, bacterial culture with no thymol.
, 0.3 g/liter; ○, 0.5 g/liter; □, 1 g/liter. Free thymol: +, 0.3 g/liter; △, 0.5 g/liter; ×, 1 g/liter. Control: ♢, bacterial culture with no thymol.
Fig 2.
Antimicrobial activities of free and nano-dispersed (ND) thymol used at overall thymol concentrations of 0.3, 0.5, and 1.0 g/liter in apple cider adjusted to pH 5.5, against Listeria monocytogenes strains Scott A (A) and 101 (B) at 32°C. Error bars represent standard deviations of the means from four measurements, two from each of two replicates. ND thymol:  , 0.3 g/liter; ○, 0.5 g/liter; □, 1 g/liter. Free thymol: +, 0.3 g/liter; △, 0.5 g/liter; ×, 1 g/liter. Control: ♢, bacterial culture with no thymol.
, 0.3 g/liter; ○, 0.5 g/liter; □, 1 g/liter. Free thymol: +, 0.3 g/liter; △, 0.5 g/liter; ×, 1 g/liter. Control: ♢, bacterial culture with no thymol.
At 0.3 g/liter thymol, L. monocytogenes strains differed in their susceptibility (P < 0.05) (Fig. 1 and 2), while E. coli strains demonstrated similar extents of inactivation (P > 0.05). Free thymol was generally more effective than ND thymol. However, at 4°C, insoluble particles were observed based on visual inspection when free thymol was suspended in water above 0.5 g/liter (data not shown). This visual defect, while microbiologically more effective, may impact consumer acceptance, especially for transparent beverages. In contrast, transparent nano-dispersions were observed at all tested thymol concentrations, demonstrating promise in clear beverage applications. Thus, from this point forward, results will be discussed for ND thymol only.
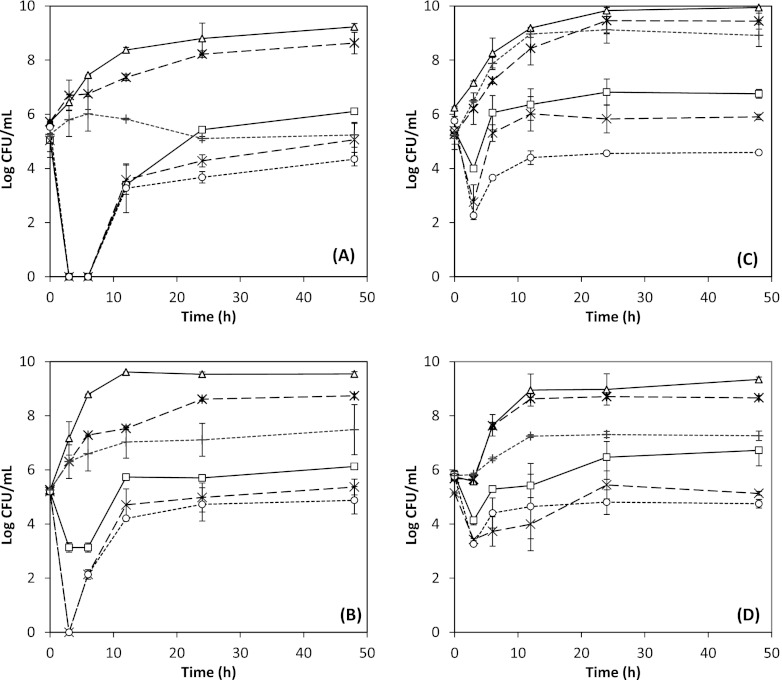
The antimicrobial activities of 0.5 g/liter ND thymol against E. coli O157:H7 strains ATCC 43889 and ATCC 43894 and L. monocytogenes strains Scott A and 101 in apple cider adjusted to pH 5.5 were further studied at 25 and 4°C (Fig. 3). As at the optimal growth temperature (Fig. 1 and 2), the initial reduction of bacterial populations was followed by a recovery during incubation at 25 and 4°C, to a level that was lower at a lower incubation temperature (Fig. 3). The two strains of both bacteria also showed different susceptibility to ND thymol (P < 0.05). Although differences were observed for two bacteria (more effective against E. coli O157:H7 than L. monocytogenes) and their two strains initially, the ND thymol did not show differences against the two bacteria and their two strains after 48 h. The recovery ability of bacteria after treatment by EO was reported previously (17) for oregano oil, which contains carvacrol, an isomer of thymol, as its main EO component. Potential mechanisms for this response include temporal adaptation to sublethal doses (11) and/or protection by food constituents allowing recovery from injury (18).
Fig 3.
Antimicrobial activities of nano-dispersed (ND) thymol used at an overall thymol concentration of 0.5 g/liter in apple cider adjusted to pH 5.5, against Escherichia coli O157:H7 ATCC 43889 (A) and ATCC 43894 (B) and Listeria monocytogenes strains Scott A (C) and 101 (D). Error bars represent standard deviations of the means from four measurements, two from each of two replicates. ND thymol: ○, 4°C; ×, 25°C; □, 35 or 32°C. Controls: bacterial culture with no thymol at 4°C (+), 25°C ( ), and 35°C for E. coli or 32°C for L. monocytogenes (△). The optimal growth temperature treatments in Fig. 1 and 2 are plotted here for comparison.
), and 35°C for E. coli or 32°C for L. monocytogenes (△). The optimal growth temperature treatments in Fig. 1 and 2 are plotted here for comparison.
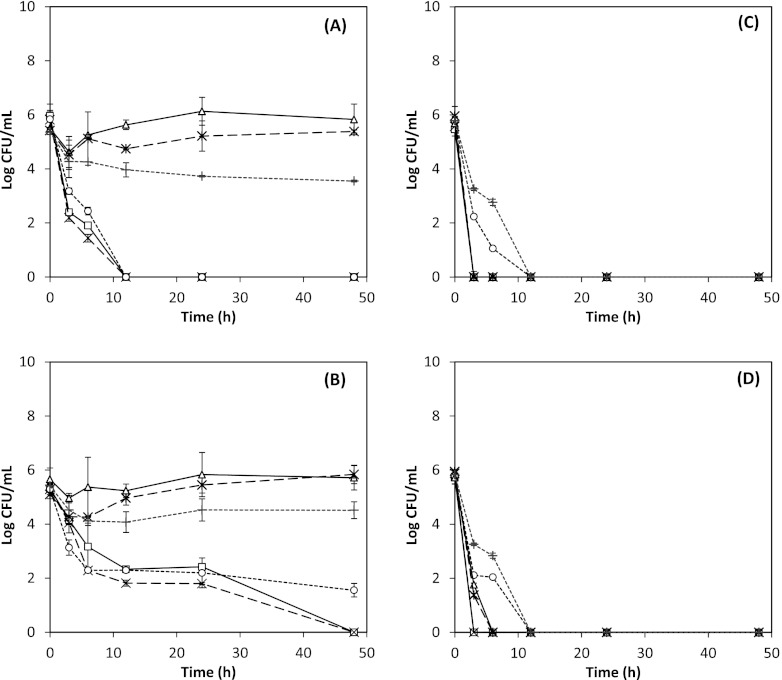
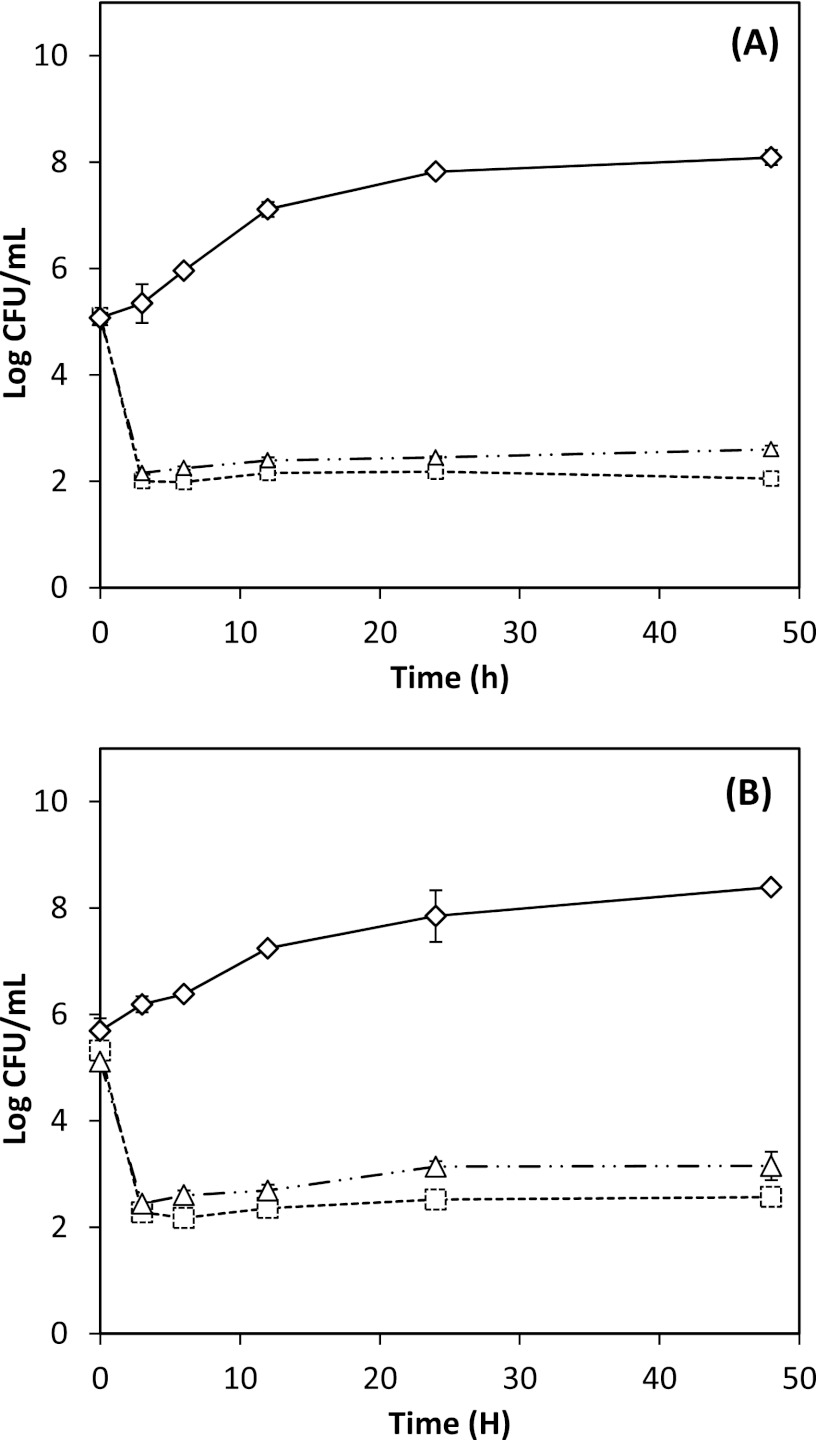
Figure 4 presents the antimicrobial activity of 0.5 g/liter ND thymol against E. coli O157:H7 and L. monocytogenes at different temperatures in apple cider adjusted to pH 3.5. At pH 3.5, L. monocytogenes controls did not grow, but the bacteria survived and were detectable up to 3.0 log CFU/ml at 6 h and reduced to undetectable levels at 12 h, with no statistical difference observed between both strains (P > 0.05). Similar results were reported by Baskaran and others (4), who applied trans-cinnamaldehyde in apple cider and observed rapid inactivation of L. monocytogenes at pH 3.5, even without the antimicrobial. For E. coli O157:H7, both strains demonstrated survival (acid tolerance), to an extent more noticeable at a higher temperature, with significantly lower counts at 4°C (P < 0.05). The strain ATCC 43894 was more resistant than ATCC 43889 (Fig. 4A versus B). The present findings are in agreement with studies in which TSB was employed (15, 30), reporting that E. coli O157:H7 strains ATCC 43889 and ATCC 43894 were both acid tolerant and that the tolerance may have been induced when the growth medium became slightly acidic (from pH 7.4 to pH 6.0) upon prior overnight incubation of cultures. The results are also in agreement with a report that temperature does not affect the acid tolerance of E. coli O157:H7 ATCC 43894 at pHs 2.0 to 4.0 (30). Bactericidal activity of EO against E. coli has been shown to increase with incubation temperature when tested at 4, 21, and 37°C in apple juice (15). However, the testing times were 5, 60, and 120 min, which may have been too short to capture the recovery or stress response of pathogens under changed environmental conditions. A noteworthy finding in their study was that E. coli strains were viable at refrigerated temperatures for up to 2 h and that carvacrol, cinnamaldehyde, and thymol retained their antimicrobial properties. Thus, continued inactivation of pathogens by these natural antimicrobials in apple cider (Fig. 4) is promising in developing intervention strategies, even during refrigerated storage.
Fig 4.
Antimicrobial activities of nano-dispersed thymol used at an overall thymol concentration of 0.5 g/liter in apple cider adjusted to pH 3.5, against Escherichia coli O157:H7 ATCC 43889 (A) and ATCC 43894 (B) and Listeria monocytogenes strains Scott A (C) and 101 (D). Error bars represent standard deviations of the means from four measurements, two from each of two replicates. ND thymol: ○, 4°C; ×, 25°C; □, 35 or 32°C. Controls: bacterial culture with no thymol at 4°C (+), 25°C ( ), and 35°C for E. coli or 32°C for L. monocytogenes (△).
), and 35°C for E. coli or 32°C for L. monocytogenes (△).
Antimicrobial efficacy of thymol in milk.
Figure 5 shows similar antimicrobial activities of 4.5 g/liter free and ND thymol against E. coli O157:H7 ATCC 43889 and L. monocytogenes strain Scott A. Compared to that needed in apple cider (Fig. 1 and 2), the concentration needed for inhibition of bacteria in milk was 9 times higher. The requirement for higher concentrations of antimicrobials in complex foods than in simple foods (e.g., apple cider) or microbiological growth media can be explained by loss of activity due to interactions of phenolic compounds with food components, including lipids, proteins, and salts (20, 21). It has been previously reported that up to 100-fold-greater concentrations of EO are needed in food systems than are found in studies based on growth media (33). At high concentrations, EO and EO components may adversely affect sensory properties and lower consumer acceptance of foods. While the ND thymol did not improve antimicrobial activity, it does provide a unique advantage of being able to deliver poorly water-soluble thymol at concentrations well beyond its solubility limit, ca. 1.0 g/liter at 20°C (7), in the form of dispersions, thus expanding its applications. We are currently conducting more comprehensive investigations to study the impacts of EO level and type, food matrices, and conjugate structures on the antimicrobial properties of ND EO.
Fig 5.
Antimicrobial activities of free (△) and nano-dispersed (□) thymol used at 4.5 g/liter in 2% reduced-fat milk against Escherichia coli O157:H7 ATCC 43889 at 35°C (A) and Listeria monocytogenes strain Scott A at 32°C (B), respectively. Error bars represent standard deviations of the means from four measurements, two from each of two replicates. Control: (♢) bacterial culture with no thymol at pH 6.8 and 35°C for E. coli and 32°C for L. monocytogenes.
Conclusions.
The efficacy of thymol against E. coli O157:H7 and L. monocytogenes was affected by pH and food matrix. Both ND thymol and free thymol showed antimicrobial effectiveness in both model foods. In apple cider, E. coli was significantly more sensitive to a treatment combination of 4°C and pH 3.5, while L. monocytogenes did not survive at such a low pH. The application of thymol at 0.5 g/liter was effective in inhibiting both bacteria at pH 3.5, regardless of temperature. The thymol concentration needed to inhibit both pathogens showed a 9-fold increase from ca. 0.5 g/liter in apple cider at pH 5.5 to 4.5 g/liter in 2% reduced-fat milk at pH 6.8. Although impacts on organoleptic properties were not investigated in this study, the nano-encapsulated thymol system is able to deliver a higher concentration of antimicrobial without separating into distinct phases and thereby impacting visual appearance of food products. This study thus demonstrates a strategy to uniformly distribute lipophilic antimicrobials in food systems to enhance microbial safety.
ACKNOWLEDGMENTS
This work was supported by the University of Tennessee and the USDA National Institute of Food and Agriculture under project number TEN02010-03476.
Footnotes
Published ahead of print 28 September 2012
REFERENCES
- 1. Akhtar M, Dickinson E. 2007. Whey protein-maltodextrin conjugates as emulsifying agents: an alternative to gum arabic. Food Hydrocoll. 21:607–616 [Google Scholar]
- 2. Arfa AB, Chrakabandhu Y, Preziosi-Belloy L, Chalier P, Gontard N. 2007. Coating papers with soy protein isolates as inclusion matrix of carvacrol. Food Res. Int. 40:22–32 [Google Scholar]
- 3. Baranauskiene R, Venskutonis PR, Dewettinck K, Verhé R. 2006. Properties of oregano (Origanum vulgare L.), citronella (Cymbopogon nardus G.) and marjoram (Majorana hortensis L.) flavors encapsulated into milk protein-based matrices. Food Res. Int. 39:413–425 [Google Scholar]
- 4. Baskaran SA, Amalaradjou MAR, Hoagland T, Venkitanarayanan K. 2010. Inactivation of Escherichia coli O157:H7 in apple juice and apple cider by trans-cinnamaldehyde. Int. J. Food Microbiol. 141:126–129 [DOI] [PubMed] [Google Scholar]
- 5. Benjamin MM, Datta AR. 1995. Acid tolerance of enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 61:1669–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bryant CM, McClements DJ. 1998. Molecular basis of protein functionality with special consideration of cold-set gels derived from heat-denatured whey. Trends Food Sci. Technol. 9:143–151 [Google Scholar]
- 7. Budavari S. 1989. The Merck index - encyclopedia of chemicals. Merck and Co., Inc., Rahway, NJ [Google Scholar]
- 8. Burt S. 2004. Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 94:223–253 [DOI] [PubMed] [Google Scholar]
- 9. Choi KO, Ryu J, Kwak HS, KO S. 2010. Spray-dried conjugated linoleic acid encapsulated with Maillard reaction products of whey proteins and maltodextrin. Food Sci. Biotechnol. 19:957–965 [Google Scholar]
- 10. Christiansen KF, et al. 2011. Flavor release of the tomato flavor enhancer, 2-isobutylthiazole, from whey protein stabilized model dressings. Food Sci. Technol. Int. 17:143–154 [DOI] [PubMed] [Google Scholar]
- 11. Davidson PM, Harrison MA. 2002. Resistance and adaptation to food antimicrobials, sanitizers, and other process controls. Food Technol. 56:69–78 [Google Scholar]
- 12. Davidson PM, Parish ME. 1989. Methods for testing the efficacy of food antimicrobials. Food Technol. 43:148–155 [Google Scholar]
- 13. Donsì F, Annunziata M, Vincensi M, Ferrari G. 2012. Design of nanoemulsion-based delivery systems of natural antimicrobials: effect of the emulsifier. J. Biotechnol. 159:342–350 [DOI] [PubMed] [Google Scholar]
- 14. Falcone PM, Mastromatteo M, Del Nobile MA, Corbo MR, Sinigaglia M. 2007. Evaluating in vitro antimicrobial activity of thymol toward hygiene-indicating and pathogenic bacteria. J. Food Prot. 70:425–431 [DOI] [PubMed] [Google Scholar]
- 15. Friedman M, Henika PR, Levin CE, Mandrell RE. 2004. Antibacterial activities of plant essential oils and their components against Escherichia coli O157:H7 and Salmonella enterica in apple juice. J. Agric. Food Chem. 52:6042–6048 [DOI] [PubMed] [Google Scholar]
- 16. Gaysinsky S, Davidson PM, Bruce B, Weiss J. 2005. Growth inhibition of Escherichia coli O157:H7 and Listeria monocytogenes by carvacrol and eugenol encapsulated in surfactant micelles. J. Food Prot. 68:2559–2566 [DOI] [PubMed] [Google Scholar]
- 17. Ghalfi H, Benkerroum N, Doguiet DDK, Bensaid M, Thonart P. 2007. Effectiveness of cell-adsorbed bacteriocin produced by Lactobacillus curvatus CWBI-B28 and selected essential oils to control Listeria monocytogenes in pork meat during cold storage. Lett. Appl. Microbiol. 44:268–273 [DOI] [PubMed] [Google Scholar]
- 18. Gill AO, Holley RA. 2006. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 108:1–9 [DOI] [PubMed] [Google Scholar]
- 19. Guarda A, Rubilar JF, Miltz J, Galotto MJ. 2011. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 146:144–150 [DOI] [PubMed] [Google Scholar]
- 20. Gutierrez J, Barry-Ryan C, Bourke P. 2009. Antimicrobial activity of plant essential oils using food model media: efficacy, synergistic potential and interactions with food components. Food Microbiol. 26:142–150 [DOI] [PubMed] [Google Scholar]
- 21. Gutierrez J, Barry-Ryan C, Bourke P. 2008. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 124:91–97 [DOI] [PubMed] [Google Scholar]
- 22. Hiemenz PC, Rajagopalan R. 1997. Principles of colloid and surface chemistry, 3rd ed Marcel Dekker, New York, NY [Google Scholar]
- 23. Hsin-Yi C, Chou CC. 2001. Acid adaptation and temperature effect on the survival of E. coli O157:H7 in acidic fruit juice and lactic fermented milk product. Int. J. Food Microbiol. 70:189–195 [DOI] [PubMed] [Google Scholar]
- 24. Jordan KN, Oxford L, O'Byrne CP. 1999. Survival of low-pH stress by Escherichia coli O157:H7: correlation between alterations in the cell envelope and increased acid tolerance. Appl. Environ. Microbiol. 65:3048–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kato A. 2002. Industrial applications of Maillard-type protein-polysaccharide conjugates. Food Sci. Technol. Res. 8:193–199 [Google Scholar]
- 26. Leyer G, Wang L, Johnson E. 1995. Acid adaptation of Escherichia coli O157:H7 increases survival in acidic foods. Appl. Environ. Microbiol. 61:3752–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin J, et al. 1996. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 62:3094–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liolios CC, Gortzi O, Lalas S, Tsaknis J, Chinou I. 2009. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 112:77–83 [Google Scholar]
- 29. Ly MH, et al. 2008. Interactions between bacterial surfaces and milk proteins, impact on food emulsions stability. Food Hydrocoll. 22:742–751 [Google Scholar]
- 30. Miller L, Kaspar C. 1994. Escherichia coli O157:H7 acid tolerance and survival in apple cider. J. Food Prot. 57:460–464 [DOI] [PubMed] [Google Scholar]
- 31. Roering AM, et al. 1999. Comparative survival of Salmonella typhimurium DT 104, Listeria monocytogenes, and Escherichia coli O157:H7 in preservative-free apple cider and simulated gastric fluid. Int. J. Food Microbiol. 46:263–269 [DOI] [PubMed] [Google Scholar]
- 32. Shah B, Ikeda S, Davidson PM, Zhong Q. 2012. Nanodispersing thymol in whey protein isolate-maltodextrin conjugate capsules produced using the emulsion–evaporation technique. J. Food Eng. 113:79–86 [Google Scholar]
- 33. Solomakos N, Govaris A, Koidis P, Botsoglou N. 2008. The antimicrobial effect of thyme essential oil, nisin and their combination against Escherichia coli O157:H7 in minced beef during refrigerated storage. Meat Sci. 80:159–166 [DOI] [PubMed] [Google Scholar]
- 34. Weiss J, Gaysinsky S, Davidson PM, McClements DJ. 2009. Nanostructured encapsulation systems: food antimicrobials, p 425–479 In Barbosa-Canovas G, et al. (ed), Global issues in food science and technology. Academic Press, San Diego, CA [Google Scholar]
- 35. Zhong Q, Shah B. 2012. Engineering nanostructures in foods and beverages for improved sensory and nutritional quality, p 179–207 In Huang Q. (ed), Nanotechnology in the food, beverage and nutraceutical industries. Woodhead Publishing Ltd., Cambridge, United Kingdom [Google Scholar]