Abstract
The aim of this investigation was to study the efficacy of the combined processes of UV light and mild temperatures for the inactivation of Salmonella enterica subsp. enterica and to explore the mechanism of inactivation. The doses to inactivate the 99.99% (4D) of the initial population ranged from 18.03 (Salmonella enterica serovar Typhimurium STCC 878) to 12.75 J ml−1 (Salmonella enterica serovar Enteritidis ATCC 13076). The pH and water activity of the treatment medium did not change the UV tolerance, but it decreased exponentially by increasing the absorption coefficient. An inactivating synergistic effect was observed by applying simultaneous UV light and heat treatment (UV-H). A less synergistic effect was observed by applying UV light first and heat subsequently. UV did not damage cell envelopes, but the number of injured cells was higher after a UV-H treatment than after heating. The synergistic effect observed by combining simultaneous UV and heat treatment opens the possibility to design combined treatments for pasteurization of liquid food with high UV absorptivity, such as fruit juices.
INTRODUCTION
Nowadays, UV light processing is one of the most promising alternatives to thermal food preservation treatments. The use of UV radiation has been suggested for disinfection of the surfaces of fresh products (50) and for pasteurization of liquids such as juices (12, 35), milk (10), liquid egg (9), soft drinks (19), beer (26), sugar syrup (45), and wines (13).
UV radiation covers part of the electromagnetic spectrum from 100 to 400 nm and is categorized as UV-A (320 to 400 nm), UV-B (280 to 320 nm), and UV-C (200 to 280 nm). UV-C is considered to be germicidal against most types of microorganisms since photons are absorbed by DNA at this specific wavelength (40). Photons interact with thymine and cystine nucleoside bases, causing the formation of cross-linked photoproducts, especially cyclobutyl pyrimidine dimers (CPD), which interrupt the transcription, translation, and replication of DNA and lead to cell death (43). UV-C light is lethal for most types of microorganisms, and their resistance varies significantly. In general, Gram-negative bacteria are more sensitive than Gram-positive bacteria, yeast, bacterial spores, molds, and viruses (27). UV resistance may vary with the species (27) and even with the strain (44). In addition, it is also known that microbial factors, such as the growth phase (2), can determinate microbial UV resistance.
Although a lot of investigations have extensively shown that UV light is effective in reducing microorganisms in food products, the industrial application is still limited because the depth of UV radiation in the surface of liquid food is very shallow (42). It is widely reported that the effectiveness of UV light inactivation rates decreases as the absorbance and the amount of suspended particles in the treatment medium increase (23, 33, 36). Thus, several strategies have been proposed to optimize the effect of UV radiation. On one hand, new UV reactors have been designed to ensure turbulent flow patterns so that all portions of a liquid food are exposed to UV light (24). On the other hand, UV light can be combined with other novel processing techniques or milder conventional preservation methods in a so-called “hurdle” approach to guarantee acceptable inactivation of pathogenic microorganisms (49). Some authors have suggested that a more efficient microbial reduction can be achieved by combining UV light with heating to temperatures that are lower than those used in pasteurization (18, 46).
Effective transfer of UV light technology to the industry requires a detailed knowledge of the resistance of different microbial species of interest in food safety, its inactivation kinetics, and the chemical and physical characteristics of the treatment medium involved in UV light efficacy. Most studies on UV-C microbial inactivation have been carried out with Escherichia coli because it is commonly used as a target pathogen (36) and because the serotype O157:H7 has been implicated in severe outbreaks of unpasteurized juice (48). In contrast, data on UV effectiveness against other food-borne pathogenic and food spoilage microorganisms are limited (25). Particularly, there are few data in the bibliography about the UV resistance of Salmonella. Salmonellosis is the second-most-often-reported food poisoning disease in Europe, accounting for 131,468 confirmed human cases in 2010 (about 21.5 persons per each 100,000) (11), and it has been implicated in food poisoning by the consumption of fruit juices (48). This is the reason that the U.S. FDA has identified this microorganism as a possible reference pathogen for UV pasteurization treatments (47).
The objective of this investigation was to compare the UV-C resistance of five strains of Salmonella enterica subsp. enterica and then study the influence of treatment medium characteristics (absorption coefficient, pH, and water activity [aw]) and microbiological factors (growth phase and UV damage and repair ability) with the most resistant strain. As a second step, we also studied the efficacy of the combined processes of UV light and middle temperatures, and we explored the mechanism of inactivation.
MATERIALS AND METHODS
Bacterial culture.
The strains of Salmonella enterica serovar Typhimurium STCC 878 and STCC 443, as well as Salmonella enterica serovar Senftenberg STCC 4384, were provided by the Spanish Type Culture Collection (STCC). The strains of Salmonella Senftenberg ATCC 43858 and Salmonella enteritidis serovar Enteritidis ATCC 13076 were provided by the American Type Culture Collection (ATCC). The bacterial cultures were kept frozen at −80°C in cryovials. Stationary-phase cultures were prepared by inoculating 10 ml of tryptone soy broth (Biolife, Milan, Italy) supplemented with 0.6% (wt/vol) yeast extract (Biolife) (TSBYE) with a loopful of growth from tryptone soy agar (Biolife) supplemented with 0.6% (wt/vol) yeast extract (TSAYE). The cultures were incubated at 35°C for 6 h in a shaking incubator. Fifty microliters of the cultures were inoculated into 50 ml of fresh TSBYE and incubated for 24 h under the same conditions, which resulted in stationary-phase cultures containing approximately 109 CFU ml−1.
UV treatments.
The UV equipment used in this investigation has been described previously (15). The whole system consisted of eight annular thin film flowthrough reactors connected in series with a sampling valve at the exit of each reactor. Each reactor consists of a low-pressure UV lamp (TUV 8WT5; Philips) with a nominal output power of 8 W (85% of energy emitted at a wavelength of 254 nm) enclosed by a quartz tube. The annular gap between the quartz tube and the outside glass sleeve, where liquid flowed, was 2.5 mm. The reactors were submerged in a tempered 90-liter water bath (selected temperature ±1.5°C) heated by the circulating water of a peripheral thermostatic bath (model Kattebad K12; Huber, Offenburg, Germany). Two thermocouples (model ZA 020-FS; Almeco, Bernburg, Germany), located at the input of the first reactor and at the outlet of the last reactor, allowed the treatment temperature to be controlled.
Treatment medium was added with the bacterial suspension to achieve 107 to 108 CFU ml−1 and pumped (8.5 liters h−1) through the heat exchanger to the reactors. Previous experiments demonstrated that bacterial loads lower than 5 × 108 CFU ml−1 did not affect the lethal efficacy of our equipment (data not shown). When the treatment conditions were stabilized, samples were withdrawn through the sampling valves at the outlet of each reactor and 0.1 ml or 1 ml was immediately pour plated in the recovery media. McIlvaine citrate-phosphate buffer (8) of different pHs (3.0, 4.0, 5.0, 6.0, and 7.0), water activity (0.94, 0.96, 0.98, and >0.99), and absorption coefficient (from 6.57 to 23.66 cm−1) were used as treatment media. Citrate-phosphate buffer of different water activities and absorption coefficients were prepared by adding different quantities of glycerol (Panreac, Barcelona, Spain) and tartrazine (Sigma-Aldrich, St. Louis, MO), respectively.
Analytical measurements.
Absorbance of media was measured at 254 nm using a Unicam UV500 spectrophotometer (Unicam Limited, Cambridge, United Kingdom). Sample solutions were diluted and evaluated using quartz cuvettes (Hellma, Müllheim, Germany) with path lengths of 1, 2, and 10 mm. The absorption coefficient of the sample solution was determined from the slope of the absorbance versus path length, correcting for the dilution factor. Turbidity was measured using a HI 83749 nephelometer (Hanna Instruments, Szeged, Hungary). The pH was adjusted using a Basic 20 pH meter (Crison Instruments, Barcelona, Spain). Water activity was measured at room temperature with a specially designed instrument (Water Activity System model CX-1; Decagon Devices, Pullman, WA). The mean illuminance (Klx) was measured with a FL A603 VL4 radiometer (Ahlborn, Holzkirchen, Germany).
Heat treatments.
Heat treatments were carried out in a specially designed resistometer (6). Briefly, this instrument consists of a 350-ml vessel provided with an electrical heater for thermostation, an agitation device to ensure inoculum distribution and temperature homogeneity, and ports for injecting the microbial suspension and for extraction of samples. Once the preset temperature had attained stability (temperature, ±0.05°C), 0.2 ml of an adequately diluted microbial cell suspension was inoculated into the corresponding treatment medium. After inoculation, 0.2-ml samples were collected at different heating times and immediately pour plated. Heat treatments were conducted at 50.0, 52.5, 55.0, 57.5, and 60.0°C, and samples were taken at the time of UV treatment (0.41, 0.90, 1.33, 1.76, 2.23, 2.66, 3.21, and 3.58 min).
Incubation of treated samples and survival counting.
The recovery medium was TSAYE. Where indicated, the maximum noninhibitory concentration (MNIC) of sodium chloride (TSAYE-SC) (Panreac, Barcelona, Spain) or bile salts (TSAYE-BS) (Biolife, Milán, Italy) was added to estimate the percentage of sublethally injured cells (28). The MNIC of sodium chloride (3% wt/vol) and bile salts (0.15% wt/vol) were chosen in previous experiments with nontreated cells (data not shown). The lack of tolerance to the presence of NaCl is attributed to damage to the functionality and/or integrity of the cytoplasmic membrane, whereas cells become sensitized and unable to grow on selective media containing bile salts if the outer membrane is damaged (28). Cells were also recovered in nonselective medium with 0.1% (wt/vol) of sodium pyruvate added (TSAYE-P) (Panreac, Barcelona, Spain), which removes peroxide and improves recovery of oxidative stressed cells (28). Samples recovered in the nonselective and sodium pyruvate-added media were incubated for 24 h at 35°C, and those recovered in selective media were incubated for 48 h at 35°C. TSAYE-P plates were incubated in anaerobiosis with a variable atmosphere incubator (MACS VA500; Don Whitley Scientific Limited, Shipley, United Kingdom). Previous experiments demonstrated that longer incubation times did not change the profile of survival curves. After incubation, CFU were counted with an improved Protos image analyzer automatic colony counter (Synoptics, Cambridge, United Kingdom) as described elsewhere (5). For photoreactivation, 20 μl of different dilutions of each sample were spread plated in TSAYE petri dishes under daylight fluorescent lamps of 13 W (T16 13W/827-EVG, G5; Osram, Munich, Germany) that emitted light in the 360- to 700-nm wavelength range. The distance between the surface of the test suspension and the lamp was 10 cm. The mean illuminance was 11.15 Klx, and the time of photoreactivation was 60 min at 20°C. Previously, it was demonstrated that longer incubation times and higher temperatures did not improve survival counts. In each experiment, a sample of UV-irradiated suspension was kept in the dark under the same conditions as a reference. After photoreactivation, the plates were incubated for 24 h at 35°C.
Dosage calculation, curve fitting, and statistical analysis.
Applied dose may be expressed as incident UV intensity (J cm−2), which is calculated with the irradiance in lamp surface and corrected for UV depth penetration using the Lambert-Beer law (37). In our study, the exposure dose was expressed in terms of the actual amount of energy transferred to the treatment media. The UV dose was calculated in J ml−1, using the total power output emitted by UV lamps and the experimentally calculated average residence time of our UV installation at 8.5 liters h−1 (15).
The survival curves of the UV treatments were obtained by plotting the logarithm of the survival fraction versus treatment doses, expressed in J ml−1. To fit the survival curves and calculate resistance parameters, the Geeraerd and Van Impe inactivation model-fitting tool (GInaFiT) was used (17). Because our survival curves did not show tails but rather shoulders, the log linear regression plus shoulder model (16) was used. This model describes the survival curves through two parameters: the shoulder length (SL) or dose before the exponential inactivation begins and the inactivation rate (kmax), defined as the slope of the exponential portion of the survival curve. For comparison purposes, GInaFiT also provides the parameter 4D, defined as the treatment dose necessary to inactivate 99.99% of the microbial population.
Statistical analyses.
A t test (P = 0.05) and analysis of variance (ANOVA) (P = 0.05), followed by Tukey's test, were carried out using GraphPad PRISM 4.1 software (GraphPad Software, Inc., San Diego, CA), and differences were considered significant for P values of ≤0.05. All microbial resistance determinations and analytical assays were performed at least three times on different working days. The error bars in the figures correspond to the mean standard deviation.
RESULTS
UV resistance of Salmonella enterica.
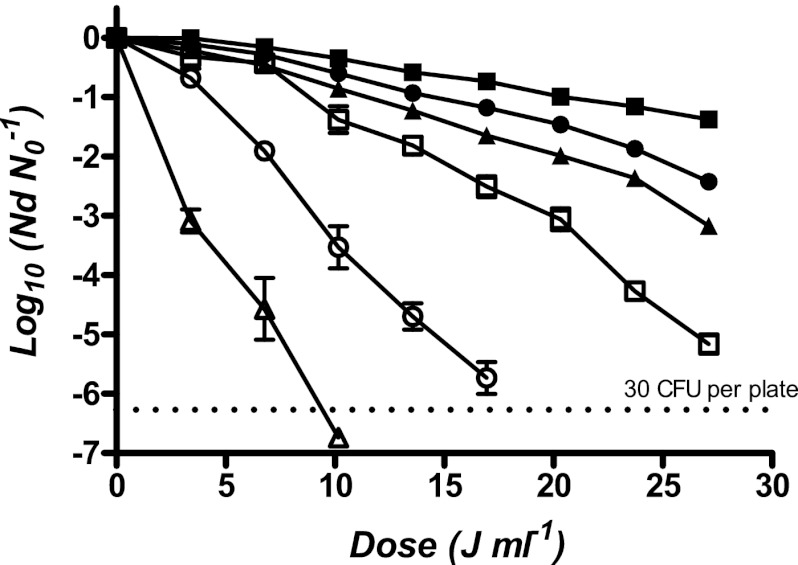
Figure 1 shows survival curves to UV light for the five strains investigated and suspended in McIlvaine buffer adjusted to a pH of 7.0 with 0.25 g liter−1 of tartrazine added (absorption coefficient of 10.81 cm−1). As observed in the figure, the log10 of survivors decreased with the delivered dose, but not linearly. Survival curves showed an initial lag phase followed by an exponential inactivation rate. Concave downward survival curves were fitted with the log linear regression plus shoulder model described by Geeraerd et al. (16). Table 1 shows the averages of the parameters calculated with Geeraerd et al.'s model under the reference conditions (pH 7; aw, >0.99; absorption coefficient, 10.81 cm−1) of the five strains recovered in different conditions. The table includes the coefficient of determination (R2) and the root mean square error (RMSE) values from the fitting, the rate of the log phase inactivation (kmax), the shoulder length (SL), and, for comparison purposes, the 4D parameter. The standard deviations of these values have also been included. UV resistance of different strains of Salmonella in the early stationary phase (24 h) varied widely: a treatment of 16.94 J ml−1 reached 3.78 ± 0.23, 5.12 ± 0.73, 4.69 ± 0.32, 5.08 ± 0.29, and 5.98 ± 0.55 log10 cycles of inactivation of Salmonella Typhimurium STCC 878 and STCC 443, Salmonella Senftenberg STCC 4384 and ATCC 43858, and Salmonella Enteritidis ATCC 13076, respectively (Fig. 1). As observed in Table 1, Salmonella Typhimurium STCC 878 was the most UV-resistant strain, showing the highest average dose to inactivate 99.99% of the initial population (18.03 ± 0.13 J ml−1), whereas Salmonella Enteritidis ATCC 13076 was the most sensitive (12.75 ± 0.44 J ml−1). Similar results were observed comparing the shoulder length of all strains: Salmonella Typhimurium STCC 878 presented an average shoulder length of 6.18 ± 0.30 J ml−1, and Salmonella Enteritidis ATCC 13076 presented an average shoulder length of 1.86 ± 1.40 J ml−1.
Fig 1.
Survival curves of Salmonella Typhimurium STCC 878 (■) and STCC 443 (●), Salmonella Senftenberg STCC 4384 (▲) and ATCC 43858 (○), and Salmonella Enteritidis ATCC 13076 (△) in McIlvaine buffer adjusted to a pH of 7.0, with an absorption coefficient of 10.81 cm−1. Stationary-phase cultures were prepared by inoculating 50 ml of fresh TSBYE and incubated for 24 h at 35°C, which resulted in stationary-phase cultures containing approximately 109 CFU ml−1. The UV equipment consisted of eight annular thin-film flowthrough reactors connected in series with a sampling valve at the exit of each reactor. The annular gap, where liquid flowed, was 2.5 mm. The recovery medium was TSAYE. Plates were incubated for 24 h at 35°C before survival counts.
Table 1.
UV resistance parameters (SL, kmax, and 4D) obtained from the fitting of Geeraerd et al.'s model to the survival curves of different Salmonella strains at different growth phases and recovered in different mediaa
| Strain | Growth phase (h) | Recovery medium | SL (J ml−1) | kmax (ml J−1) | 4D (J ml−1) | R2 | RMSE |
|---|---|---|---|---|---|---|---|
| Salmonella Typhimurium STCC 878 | Stationary (24) | TSAYE | 6.18 (0.30)aA | 0.77 (0.05)aA | 18.03 (0.13)aA | 0.994 | 0.209 |
| Stationary (24) | TSAYE-SC | 6.09 (0.49) | 0.82 (0.03) | 17.37 (0.96) | 0.997 | 0.151 | |
| Stationary (24) | TSAYE-BS | 5.02 (0.66)* | 0.74 (0.02) | 17.62 (0.57) | 0.997 | 0.160 | |
| Stationary (24) | TSAYE-P | 6.86 (0.52) | 0.82 (0.02) | 18.16 (0.47) | 0.999 | 0.197 | |
| Stationary (24) | TSAYE plus visible light | 6.73 (0.16)* | 0.83 (0.24) | 18.95 (0.49)* | 0.989 | 0.245 | |
| Exponential (5) | TSAYE | 4.21 (1.16)B | 0.87 (0.09)A | 14.87 (0.57)B | 0.963 | 0.474 | |
| Exponential (5) | TSAYE-SC | 5.36 (0.82) | 0.76 (0.09) | 13.52 (0.59) | 0.981 | 0.365 | |
| Exponential (5) | TSAYE-BS | 4.41 (1.58) | 0.74 (0.05) | 13.99 (0.66) | 0.998 | 0.540 | |
| Exponential (5) | TSAYE-P | 5.60 (1.18) | 0.83 (0.34) | 13.04 (0.38)* | 0.984 | 0.264 | |
| Stationary (72) | TSAYE | 6.71 (1.01)A | 0.82 (0.02)A | 18,98 (1.24)A | 0.975 | 0.427 | |
| Stationary (72) | TSAYE-SC | 6.30 (1.13) | 0.79 (0.05) | 18.16 (0.55) | 0.990 | 0.286 | |
| Stationary (72) | TSAYE-BS | 7.10 (1.51) | 0.79 (0.07) | 18.70 (0.25) | 0.981 | 0.419 | |
| Stationary (72) | TSAYE-P | 6.90 (1.18) | 0.71 (0.05) | 19.06 (1.19) | 0.989 | 0.319 | |
| Salmonella Typhimurium STCC 443 | Stationary (24) | TSAYE | 5.67 (0.60)b | 1.01 (0.13)b | 14.94 (0.86)b | 0.989 | 0.335 |
| Stationary (24) | TSAYE-SC | 5.80 (1.43) | 1.06 (0.12) | 14.64 (0.36) | 0.978 | 0.485 | |
| Stationary (24) | TSAYE-BS | 4.16 (1.14) | 1.12 (0.08) | 14.94 (0.26) | 0.991 | 0.310 | |
| Stationary (24) | TSAYE-P | 6.02 (0.88) | 0.86 (0.05)* | 14.43 (0.23) | 0.991 | 0.316 | |
| Salmonella Senftenberg STCC 4384 | Stationary (24) | TSAYE | 4.42 (0.54)c | 0.84 (0.10)b | 15.57 (0.36)c | 0.992 | 0.264 |
| Stationary (24) | TSAYE-SC | 4.78 (2.87) | 0.81 (0.11) | 16.26 (0.63) | 0.973 | 0.386 | |
| Stationary (24) | TSAYE-BS | 4.52 (0.92) | 0.85 (0.04) | 15.42 (0.21) | 0.994 | 0.453 | |
| Stationary (24) | TSAYE-P | 5.54 (1.02) | 0.88 (0.05) | 16.13 (0.23) | 0.991 | 0.299 | |
| Salmonella Senftenberg ATCC 43858 | Stationary (24) | TSAYE | 4.69 (0.41)c | 0.89 (0.03)b | 15.23 (0.51)b | 0.992 | 0.278 |
| Stationary (24) | TSAYE-SC | 4.78 (1.31) | 1.02 (0.09) | 14.82 (0.33) | 0.984 | 0.418 | |
| Stationary (24) | TSAYE-BS | 5.12 (0.86) | 0.91 (0.05) | 15.42 (0.20) | 0.994 | 0.261 | |
| Stationary (24) | TSAYE-P | 4.53 (1.78) | 0.80 (0.06) | 15.18 (0.41) | 0.983 | 0.461 | |
| Salmonella Enteritidis ATCC 13076 | Stationary (24) | TSAYE | 1.86 (1.40)d | 0.87 (0.10)c | 12.75 (0.44)d | 0.990 | 0.310 |
| Stationary (24) | TSAYE-SC | 1.20 (1.11) | 0.92 (0.05) | 11.38 (0.31)* | 0.992 | 0.313 | |
| Stationary (24) | TSAYE-BS | 2.98 (1.53) | 0.93 (0.08) | 13.01 (0.39) | 0.984 | 0.413 | |
| Stationary (24) | TSAYE-P | 2.85 (0.99) | 0.95 (0.05) | 12.60 (0.26) | 0.993 | 0.275 |
Lowercase letters a, b, c, and d indicate significant differences (P ≤ 0.05) among mean values of each strain at stationary phase (24 h) recovered in TSAYE. Uppercase letters A and B indicate significant differences (P ≤ 0.05) among growth phases of Salmonella Typhimurium STCC 878 recovered in TSAYE. An asterisk indicates a significant difference (P ≤ 0.05) among mean values of different recovery media compared with counts in TSAYE. Values in parentheses represent the standard deviations (SD) of the means.
The effect of the growth phase on UV susceptibility was also studied with the most resistant strain. Table 1 includes resistance parameters of Salmonella Typhimurium STCC 878 in mid-log (5 h), early stationary (24 h), and late stationary growth phases (72 h). The dose necessary to inactivate 99.99% of the population in the exponential growth phase was 14.87 ± 0.57 J ml−1, whereas 18.03 ± 0.13 J ml−1 was needed for stationary-growth-phase cells. The highest UV susceptibility of mid-log-phase cells can be attributed to the reduction of SL (from 6.18 ± 0.30 to 4.21 ± 1.16 J ml−1), while no significant differences were found between kmax (P > 0.05). In contrast, the UV resistance of stationary-phase cells did not change, even when increasing incubation time up to 72 h.
No significant differences (P > 0.05) were found between survivor counts in nonselective and selective media (Table 1). This indicated that UV light treatments did not damage the functionality and integrity of cell envelopes in any of the strains and growth phases investigated. Counts obtained in the nonselective medium with sodium pyruvate indicated that there was no oxidative damage. However, the photoreactivation step before incubation improved the UV survival of the most resistant strain. The 4D value of Salmonella Typhimurium STCC 878 significantly increased from 18.03 ± 0.13 to 18.95 ± 0.49 J ml−1 due to an SL increase (Table 1).
UV inactivation of Salmonella Typhimurium STCC 878 in different treatment media.
To study the effect of physicochemical properties of the treatment medium on UV lethality, Salmonella Typhimurium STCC 878 was irradiated in citrate-phosphate buffer with different pHs (3.0 to 7.0) and water activities (>0.99 to 0.96), with an absorption coefficient adjusted to 10.81 cm−1. The calculated kmax, SL, and 4D values are summarized in Table 2. The pH and water activity did not show a significant effect (P > 0.05) on the inactivation by UV light. Neither interaction between both variables was found.
Table 2.
UV resistance parameters (SL, kmax, and 4D) obtained from the fitting of Geeraerd et al.'s model to the survival curves of Salmonella Typhimurium STCC 878 in McIlvaine buffer of different pHs and water activities, with an absorption coefficient of 10.81 cm−1a
| pH | aw | SL (J ml−1) | kmax (ml J−1) | 4D (J ml−1) | R2 | RMSE |
|---|---|---|---|---|---|---|
| 3.0 | 0.99 | 7.02 (0.45) | 0.80 (0.03) | 19.06 (0.63) | 0.983 | 0.353 |
| 4.0 | 0.99 | 6.11 (0.85) | 0.73 (0.11) | 18.88 (1.33) | 0.990 | 0.257 |
| 5.0 | 0.99 | 6.28 (1.05) | 0.68 (0.20) | 19.42 (1.34) | 0.990 | 0.264 |
| 6.0 | 0.99 | 6.67 (0.21) | 0.68 (0.06) | 16.45 (0.60) | 0.993 | 0.223 |
| 7.0 | 0.99 | 6.18 (0.20) | 0.77 (0.03) | 18.03 (0.32) | 0.994 | 0.209 |
| 7.0 | 0.98 | 6.52 (0.50) | 0.75 (0.04) | 19.06 (0.19) | 0.997 | 0.430 |
| 7.0 | 0.96 | 6.42 (0.06) | 0.77 (0.07) | 19.61 (1.91) | 0.996 | 0.151 |
| 7.0 | 0.94 | 6.74 (0.19) | 0.73 (0.06) | 19.34 (1.72) | 0.993 | 0.222 |
| 3.0 | 0.94 | 5.92 (0.70) | 0.78 (0.02) | 17.89 (0.73) | 0.996 | 0.189 |
Values in parentheses represent the SD of the means.
The effect of the absorptivity of the media on the inactivation of Salmonella Typhimurium STCC 878 was studied by adjusting the absorbance of the buffer while other parameters were held constant (pH, 7.0; aw, >0.99; turbidity, 5.92 nephelometric turbidity units [NTU]). Table 3 shows UV resistance parameters obtained in McIlvaine buffer, with an absorption coefficient ranging from 6.57 to 23.66 cm−1. Results demonstrated that increasing the absorptivity resulted in a higher 4D value (Table 3). An exponential relationship was found between the inactivation rates (kmax) and the absorption coefficient (α) (Fig. 2). The regression line that defined this relationship (log10 kmax, −0.0526α + 0.338; R2, 0.956) allowed us to conclude that the inactivation rate decreased 10 times by increasing the absorption coefficient by 18.98 cm−1.
Table 3.
UV resistance parameters (SL, kmax, and 4D) obtained from the fitting of Geeraerd et al.'s model to the survival curves of Salmonella Typhimurium STCC 878 in McIlvaine buffer, with different absorption coefficientsa
| Absorption coefficient (cm−1) | SL (J ml−1) | kmax (ml J−1) | 4D (J ml−1) | R2 | RMSE |
|---|---|---|---|---|---|
| 6.57 | 1.73 (1.07) | 1.39 (0.06) | 11.11 (0.65) | 0.996 | 0.199 |
| 8.76 | 4.45 (0.81) | 0.89 (0.01) | 15.10 (0.76) | 0.991 | 0.197 |
| 10,81 | 6.18 (0.20) | 0.77 (0.03) | 18.03 (0.32) | 0.994 | 0.209 |
| 12.80 | 7.44 (0.64) | 0.56 (0.09) | 22.41 (0.78) | 0.991 | 0.294 |
| 14.50 | 7.95 (0.93) | 0.48 (0.04) | 25.52 (1.94) | 0.993 | 0.153 |
| 16.21 | 8.48 (0.32) | 0.26 (0.01) | 0.994 | 0.059 | |
| 18.78 | 9.04 (0.91) | 0.23 (0.04) | 0.990 | 0.065 | |
| 20.44 | 8.86 (0.58) | 0.19 (0.01) | 0.977 | 0.093 | |
| 23.66 | 11.31 (1.51) | 0.16 (0.02) | 0.980 | 0.070 |
Values in parentheses represent the SD of the means.
Fig 2.
Relationship between treatment medium absorptivity (α) and UV inactivation rate (log10 kmax) of stationary-phase cells of Salmonella Typhimurium STCC 878. Treatments were carried out in citrate-phosphate buffer of pH 7.0. Buffers of different absorption coefficients were prepared by adding different quantities of tartrazine. The TSAYE recovery plates were incubated for 24 h at 35°C. The relationship (log10 kmax, −0.0526α + 0.338; R2, 0.956) allowed us to conclude that the inactivation rate decreased 10 times by increasing the absorption coefficient by 18.98 cm−1.
Inactivation of Salmonella Typhimurium STCC 878 by UV light at different temperatures.
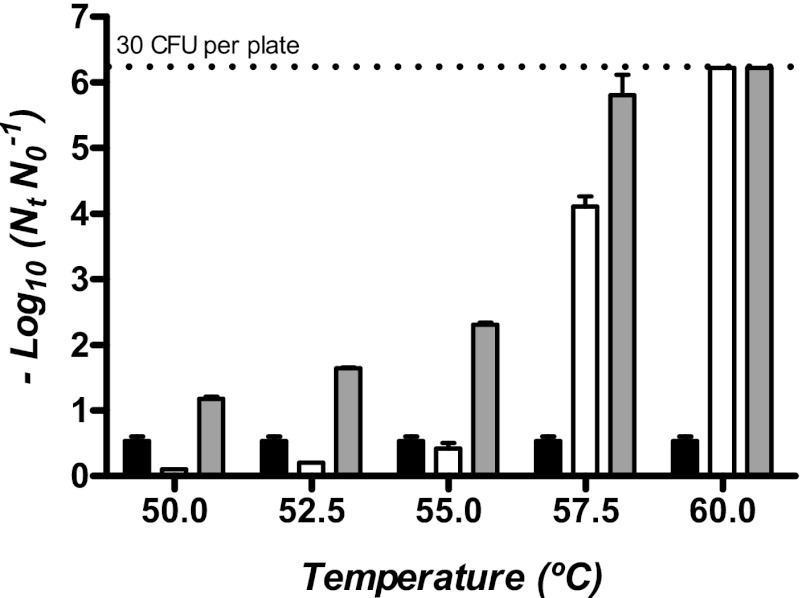
The effects of the combined processes of UV light and mild temperatures were studied in McIlvaine buffer adjusted to a pH of 7.0, with an absorption coefficient of 23.66 cm−1. In this medium, at room temperature, the treatment at the maximum power (27.10 J ml−1) caused only a small decrease in the number of survivors at room temperature (inactivation of 1.12 ± 0.19 log10 cycles) (Fig. 3). Figure 3 shows the survival curves of Salmonella Typhimurium STCC 878 to UV light at different temperatures in the same treatment medium. The simultaneous combination of both technologies (UV-H treatment) significantly increased the inactivation rate with temperature: a UV dose of 16.94 J ml−1 at 50.0, 52.5, 55.0, 57.5, and 60.0°C reduced the surviving population of 1.18 ± 0.06, 1.64 ± 0.13, 2.44 ± 0.28, 5.71 ± 0.60, and more than 6.22 log10 cycles, respectively, while UV light treatment at room temperature hardly achieved 0.64 ± 0.08 log10 cycles. The UV resistance parameters that were calculated by fitting survival curves with Geeraerd et al.'s model have been summarized in Table 4.
Fig 3.
Survival curves to UV treatments at different temperatures of stationary-phase cells of Salmonella Typhimurium STCC 878. Treatments were carried out in McIlvaine buffer adjusted to a pH of 7.0, with an absorption coefficient of 23.66 cm−1 at 25.0°C (■), 50.0°C (●), 52.5°C (▲), 55°C (□), 57.5°C (○), and 60.0°C (△). The UV reactors were submerged in a tempered 90-liter water bath (temperature, ±1.5°C) heated by the circulating water of a peripheral thermostatic bath. Two thermocouples, located at the input of the first reactor and at the outlet of the last reactor, allowed the treatment temperature to be controlled. The figure demonstrates that the shoulder length decreases and the inactivation rate increases with temperature.
Table 4.
UV resistance parameters (SL, kmax, and 4D) obtained from the fitting of Geeraerd et al.'s model to the survival curves of Salmonella Typhimurium STCC 878 in McIlvaine buffer, with an absorption coefficient of 23.66 cm−1 at different temperaturesa
| Temp (°C) | SL (min) | kmax (min−1) | 4D (min) | R2 | RMSE |
|---|---|---|---|---|---|
| 25.0 | 1.40 (0.10) | 1.22 (0.09) | 8.97 (0.99) | 0.993 | 0.025 |
| 50.0 | 1.20 (0.23) | 2.15 (0.55) | 6.08 (0.32) | 0.993 | 0.077 |
| 52.5 | 0.59 (0.21) | 2.25 (0.16) | 4.69 (0.13) | 0.989 | 0.131 |
| 55.0 | 0.44 (0.11) | 2.60 (0.09) | 3.34 (0.71) | 0.997 | 0.083 |
| 57.5 | 0.19 (0.02) | 6.71 (0.45) | 1.58 (0.10) | 0.994 | 0.249 |
| 60.0 | 0 | 10.44 (1.07) | 0.69 (0.12) | 0.988 | 0.392 |
Values in parentheses represent the SD of the means.
To estimate the contribution of heat to the lethal effect of the combined process, the thermal resistance of Salmonella Typhimurium STCC 878 was determined at the same treatment conditions and temperatures. Figure 4 shows the log10 cycles of inactivation reached by UV treatments of 16.94 J ml−1 (2.23 min) at 25°C, by thermal treatments for 2.23 min at different temperatures, and by the combined UV-H process at the same temperatures for the same time. As shown, the thermal inactivation at 50.0, 52.5, 55.0, 57.5, and 60.0°C for 2.23 min was 0.10, 0.19, 0.42 ± 0.15, 4.11 ± 0.26, and more than 6.22 log10 cycles, respectively. The sum of the heat and UV inactivation was lower than that reached by the simultaneous application of both technologies, and the existence of a synergistic effect was deduced. The log10 cycles of the synergistic effect after 2.23 min of treatment were calculated by subtracting UV and heat inactivation cycles from those obtained during UV-H treatment at different temperatures: 0.44, 0.88, 1.38, and 0.96 log10 cycles of synergism at 50.0, 52.5, 55.0, and 57.5°C were obtained.
Fig 4.
Inactivation of stationary-phase cells of Salmonella Typhimurium STCC 878 by UV light (black bars) (19.94 J ml−1/2.23 min), heat at different temperatures (white bars) (2.23 min), and the combined process (gray bars) (19.94 J ml−1/2.23 min) in McIlvaine buffer adjusted to a pH of 7.0, with an absorption coefficient of 23.66 cm−1. The sum of the heat and UV inactivation was lower than that reached by the simultaneous application of both technologies. The log10 cycles of the synergistic effect, calculated by subtracting UV and heat inactivation cycles from those obtained during UV-H treatment, were 0.44, 0.88, 1.38, and 0.96 log10 cycles at 50.0, 52.5, 55.0, and 57.5°C, respectively.
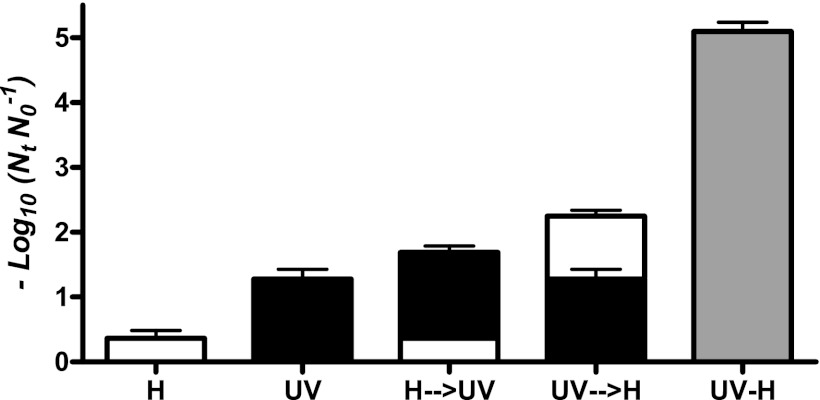
To explore the mechanism of the synergistic effect of the combined treatment, we applied both technologies sequentially in two possible orders. For this purpose, a temperature of 55.0°C was chosen since heat treatment showed a minimal lethal effect compared to that achieved by the simultaneous combination of heat and UV light. A heat treatment of 55.0°C for 3.58 min was followed by a UV treatment at the highest intensity applicable in our equipment (27.10 J ml−1); and, vice versa, a UV treatment of the same intensity followed by a thermal treatment at 55.0°C for 3.58 min was carried out. Figure 5 summarizes our results. The sequential application of heat followed by the UV treatment showed an additive effect: the total microbial inactivation of the combined treatment of heat and UV light (1.69 ± 0.30 log10 cycles) was not significantly different (P > 0.05) from the sum (1.59 ± 0.21 log10 cycles) of heat inactivation (0.47 ± 0.20 log10 cycles) and UV light at room temperature inactivation (1.12 ± 0.19 log10 cycles). The combination of UV light followed by heat treatment showed a greater lethal effect (2.34 ± 0.31 log10 cycles). However, the synergism was much lower than that observed when both technologies were applied simultaneously (5.09 ± 0.28 log10 cycles).
Fig 5.
Inactivation of Salmonella Typhimurium STCC 878 by UV light (UV; black bars) (19.94 J ml−1/2.23 min), heat treatment (H; white bars) (55.0°C for 2.23 min), and combined treatment of both technologies simultaneously (UV-H; gray bars) and sequentially in both orders: UV followed by heat treatment (UV→H), and heat followed by UV treatment (H→UV). The H→UV treatment showed an additive effect (1.69 ± 0.30 log10 cycles) that was not significantly different (P > 0.05) from the sum of heat (0.47 ± 0.20 log10 cycles) and UV inactivation (1.12 ± 0.19 log10 cycles). The UV→H treatment showed a greater lethal effect (2.34 ± 0.31 log10 cycles) but lower than that observed when both technologies were applied simultaneously (5.09 ± 0.28 log10 cycles).
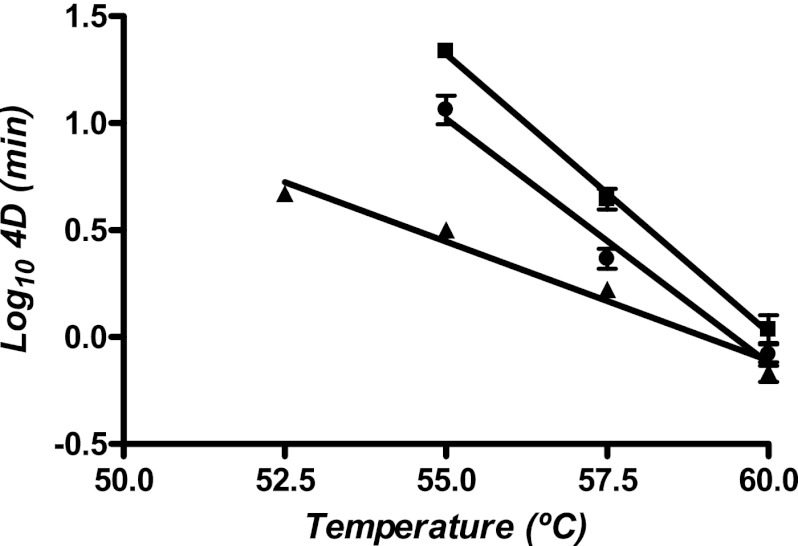
To thoroughly investigate the effect of temperature on the two combined processes that showed a synergistic effect, heat treatment preceded by UV light and UV-H treatment, survival curves of the combined processes at different temperatures were obtained. The resistance parameters were calculated fitting survival curves with the Geeraerd et al.'s model, and results are summarized in Fig. 6. The 4D values to microbial inactivation by heat were also included in the figure for comparison. As observed in the figure, heat inactivation of Salmonella Typhimurium STCC 878 showed a thermodependence similar to published data (29), with a z value (°C increase to decrease 4D value 10 times) of 3.89°C. The sequential application of UV light and heat treatment showed a nonsignificant different z value (4.37°C). The lethal effect of the simultaneous application of UV light and heat showed lower thermodependence: the 4D value would decrease 10 times by increasing the temperature by 9.01°C.
Fig 6.
Relationship between treatment temperature and the time to inactivate 99.99% of the initial population (4D value) of Salmonella Typhimurium STCC 878 by heat (■), by heat after a UV treatment of 27.10 J ml−1 (●), and by both technologies applied simultaneously (▲). 4D values were calculated fitting survival curves with the Geeraerd and Van Impe inactivation model-fitting tool (GInaFiT). Survival curves to thermal treatments were obtained with a mixing method (thermoresistometer TR-SC).
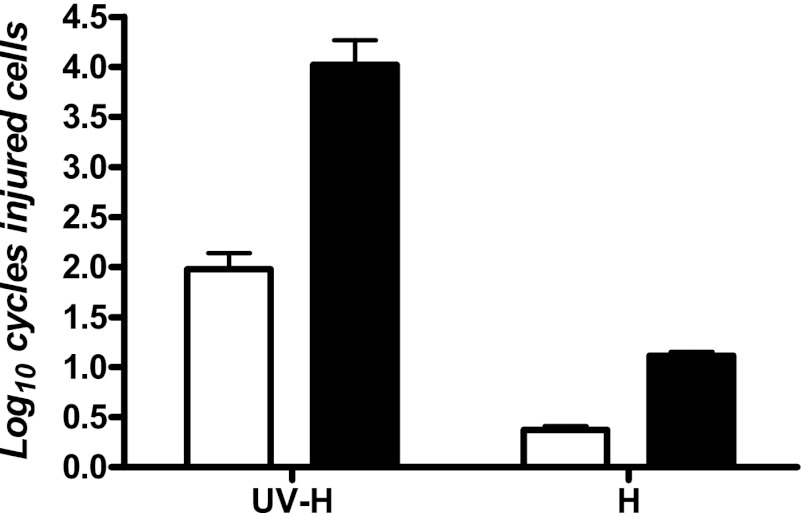
In a further step, survival curves to UV-H, UV, and heat treatments were obtained by recovering cells in nonselective and selective media. The log10 cycles of damaged cells were calculated by subtracting the log10 number of survivors in the nonselective media from the log10 number of survivors in the selective media. Results of these experiments are summarized in Fig. 7. A heat treatment at 55.0°C for 2.23 min reached 0.37 ± 0.07 log10 cycles of damaged cells in the plasmatic membrane and 1.17 ± 0.07 log10 cycles in the external membrane. UV treatments at 25.0°C did not affect cell envelopes (Table 1). After UV-H treatment at the same temperature for the same time, 2 and 4 log10 cycles of damaged cells were found in cytoplasmic and outer membranes, respectively.
Fig 7.
Injured stationary-phase cells of Salmonella Typhimurium STCC 878 treated by heat (55.0°C for 2.23 min) and the combined UV-H process (55.0°C + 19.94 J ml−1 for 2.23 min). The log10 cycles of injured cells were calculated by subtracting the log10 number of survivors in the nonselective media (TSAYE) from the log10 number of survivors in the selective media. Selective media were as follows: TSAYE with 3% (wt/vol) sodium chloride added (white bars) to detect cytoplasmic membrane damage and TSAYE with 0.15% (wt/vol) bile salts added (black bars) to detect outer membrane damage. Survivors recovered in the nonselective media were incubated for 24 h at 35°C, and those recovered in selective media were incubated for 48 h at 35°C.
DISCUSSION
Some authors have observed that UV microbial inactivation followed first-order kinetics (20), but it is not unusual to find deviation from the linearity, such as shoulders, tails, or both (40). All UV survival curves obtained in this investigation showed initial shoulders followed by an exponential order of death (Fig. 1). Therefore, they were fitted with the log linear regression plus shoulder model of Geeraerd et al. (16). Initial shoulder phase is attributed to the action of DNA repair mechanisms (40). UV light acts by causing mutated bases that compromise cell functionality. However, bacteria have developed DNA repair mechanisms to restore DNA structure and functionality. This phenomenon is reflected in the shape of the inactivation curves. After the initial shoulder, the maximum DNA repair capability is surpassed, additional UV exposure would be lethal for microorganisms, and survivors exponentially decline (27).
A wide variation to UV light susceptibility was observed among the five tested Salmonella strains. The 4D values ranged from 18.03 ± 0.13 J ml−1 for Salmonella Typhimurium STCC 878 to 12.75 ± 0.44 J ml−1 for the Salmonella Enteritidis strain. It has been demonstrated that Salmonella Senftenberg is much more resistant to heat (29) than Salmonella Typhimurium; however, the resistances of these two bacteria to ultrasound (US) (30) and pulsed electric fields (PEF) (1) are very similar. There are few data in the bibliography about the UV resistance variability among Salmonella strains. Gabriel et al. (14) reported that the tested Salmonella Enteritidis strain was less resistant than Salmonella Typhimurium in phosphate-buffered saline (PBS) buffer. In our investigation, differences in UV resistance were mainly due to different lengths of the shoulders (Table 1), which have been related with a higher DNA repair ability (15). We have also observed a longer shoulder of survival curves of Salmonella after a photoreparation step (Table 1). Therefore, the high UV resistance of Salmonella Typhimurium STCC 878 was probably due to a more efficient DNA repair system. This is an interesting aspect that deserves further investigation.
By comparing results from Table 1 to those obtained with other species in the same experimental conditions, we can conclude that the most UV-resistant strain of Salmonella (4D, 18.03 ± 0.13 J ml−1) was less sensitive to UV light than the most UV-resistant of five E. coli strains (4D, 16.60 ± 0.48 J ml−1) (15). Kim et al. (22) also observed that Salmonella Typhimurium was more resistant than E. coli O157:H7, but Yaun et al. (50) reported that UV resistance of Salmonella and E. coli O157:H7 did not differ, and Gabriel et al. (14) demonstrated that Salmonella Typhimurium was less resistant than E. coli O157:H7 and Listeria monocytogenes. Disagreements in the literature may be due to the wide variation of UV resistance between strains, as we have observed in Salmonella strains (Table 1).
It is well known that stationary-phase cells of several bacterial species show enhanced UV-C resistance (2, 4). Accordingly, Salmonella Typhimurium STCC 878 in mid-log phase was more UV susceptible than cells in the stationary phase, showing a shorter shoulder. Child et al. (4) observed that cells of an RpoS mutant of Salmonella Typhimurium were much more sensitive to UV light than the corresponding wild type, suggesting that RpoS gene expression is implicated in the UV resistance. Since shoulder length is related to damage and repair mechanisms (40), our results indicate that these mechanisms are regulated, at least in part, by the global stress response induced by the RpoS regulon.
It is well established that UV light inactivates microorganisms by damaging their nucleic acid, thereby preventing microorganisms from replicating. However, it has also been suggested that photons can interact with components of cell envelopes (32) and favor the oxidation of unsaturated fatty acid residues of lipids and phospholipids (23). In our study, no significant differences (P > 0.05) in the proportion of either envelope-damaged cells or oxidative-damaged cells were found using selective growth media. In contrast, the exposure of survivors to visible light, before their incubation at 35°C, increased the recovery of Salmonella Typhimurium STCC 878 (Table 1). From our results, it is noteworthy that survival curves after photoreparation showed longer shoulder length, whereas kmax values barely changed. This evidences that shoulders are related to DNA repair mechanisms.
It is well known that the physicochemical characteristics of the treatment media may change the bactericidal efficacy of most food preservation technologies. For Salmonella, pH and water activity of the treatment medium are the most influential factors (1, 29). Our results demonstrated that acidification from pH 7.0 to 3.0 did not influence the UV resistance of Salmonella Typhimurium STCC 878 (Table 2). These results are in agreement with published data regarding other bacterial species (23, 33, 37). Data in the bibliography about the effect of water activity on the lethal effect of UV light are scarce. Our results demonstrated that water activity, in the range of 0.94 to >0.99, did not change the UV resistance of Salmonella Typhimurium (Table 2). It has been hypothesized that lowering the water activity of the treatment medium led to water loss within the cells, which increased DNA condensation and increased UV light resistance (41). The protective effect of sucrose to most inactivating technologies was demonstrated to be more pronounced than that of glycerol (7, 31). To avoid misinterpretations, we studied the effect of water activity in the same media added with sucrose (aw values from 0.94 to >0.99) and reached the same conclusion as with glycerol (data not shown). In regards to UV technology, the absorption coefficient is the most influential environmental factor. As was shown by our results, UV efficacy exponentially decreased with the absorption coefficient in the range of 6.57 to 23.66 cm−1 (Fig. 2 and Table 3).
The difficulty of UV light treatment in achieving a 5 log10 reduction due to the low penetration capacity of UV photons on liquid foods has prompted several authors to develop hurdle strategies combining UV light with other novel processing techniques or milder conventional preservation methods (3, 49). The combination of heat at sublethal temperatures with other nonthermal technologies such as high hydrostatic pressure (HHP), US, and PEF at low intensity results in an equivalent or even higher degree of microbial inactivation (38, 39). However, data in literature about the combination of UV light and mild heat treatment are scarce. Our research focused on the effect of the combined processes of UV light and mild temperatures applied both simultaneously (UV-H treatment) and sequentially. UV lethality increased with the temperature ranging from 50°C to 60°C (Fig. 3).
Comparing inactivation of the simultaneous UV-H combined treatment with thermal inactivation at the same temperature and with UV light at room temperature, a synergistic lethal effect was deduced for both technologies (Fig. 4). This synergistic effect increased with the temperature ranging from 50.0°C to 55.0°C, a range at which thermal inactivation was negligible. Meanwhile, at 57.5°C, the synergistic lethal effect of the combined treatment decreased, and at 60.0°C, it disappeared. This can be also deduced from the relationship between inactivation parameters and temperature for both heat and UV-H treatments (Fig. 6). The UV death time curve of the UV-H process was less thermodependent than the thermal death time curve (z = 3.89°C); and both curves tended to join at 60.0°C. These behaviors reveal that the synergistic interaction between heat and UV light is only effective in a specific temperature range.
The sequence of the treatments seemed to be important to maximize the lethal effect of the combined process, which is likely to be related with the mechanism of action. A synergistic effect was observed after UV light treatment followed by a heat treatment at 55.0°C, but lower than that observed with both technologies applied simultaneously. Unlike the UV-H treatments, the death curve to the sequential application of UV light and heat showed a similar thermodependence (z = 4.37°C) than heat treatment alone, which means that the magnitude of the synergistic effect hardly changed with temperature. On the contrary, an additive effect was observed in the reverse order of treatment. Overall our observations lead to an interesting conclusion: the mechanism through which the combination of UV light and heat acted synergistically was different when the technologies were applied simultaneously than when they were applied successively.
The inactivation mechanisms of the combination of UV light and heat are not well understood and it could be explained by two hypotheses that are not exclusive. On the one hand, it might be attributed to a reduction in cellular capacity to repair damage (34). However, we observed no UV sensitization after a heat treatment of 3.58 min at 55.0°C, while in the UV-H treatment a synergistic lethal effect was detected for the same time and temperature of treatment. On the other hand, synergism might be related to additional lethal damage that arises from the interaction of sublesions induced by both agents.
To clarify the mechanism of action of the observed synergistic lethal effect, sublethal damage in cell envelopes of Salmonella Typhimurium was studied. It is well known than heat treatment induces damage in bacterial cell envelopes, and the results in Table 1 demonstrated that UV treatments at 25.0°C did not injure these structures. However, our results indicated that the number of envelope-injured cells was higher after a UV-H treatment than after heating, and this difference was more notorious in external membrane damage than in cytoplasmic membrane damage. This indicates that the mechanism of the synergistic effect of UV light and heat is related to a sensitization of cell envelopes or the cell's inability to repair these structures. The enhanced inactivation at higher temperatures might be due to the phase transition of the phospholipid molecules, within the cell membrane, from gel to liquid crystalline (21). This is an interesting question from both a microbial physiology and food technology point of view, and specific investigations to clarify the mechanisms of inactivation of the synergistic effect should be designed.
From our results, we reached some interesting conclusions. The UV resistance of different strains of Salmonella may differ widely. The entrance in the stationary growth phase increased the UV resistance of Salmonella Typhimurium STCC 878. Survivors to UV-C treatments showed neither oxidative damage nor injury in cell envelopes, but the incubation of survivors after photoreactivation below visible light increased the number of survivors. The pH and water activity of the treatment medium did not change the UV tolerance, but the lethal effect of the treatments decreased exponentially by increasing the absorption coefficient. An additive lethal effect was observed when applying a heat treatment followed by a UV light. A synergistic lethal effect was observed after UV light treatment followed by a heat treatment, but the effect was lower than that observed when both technologies were applied simultaneously. Unlike the UV-H treatments, the death curve to the sequential application of UV light and heat showed a similar thermodependence to heat treatment, which means that the magnitude of the synergistic effect hardly changed with temperature. The number of envelope-injured cells was higher after a UV-H treatment than after heating, and this difference was more notorious in the outer membrane than in the cytoplasmic membrane. This indicates that the mechanism of the synergistic effect of UV light and heat was related to a sensitization of cell envelopes or the cell's inability to repair these structures.
ACKNOWLEDGMENTS
This study has been carried out with financial support from the Ministerio de Ciencia e Innovación, EU-FEDER (CIT020000-2009-40) and the Departamento de Ciencia, Tecnología y Universidad del Gobierno de Aragón. E. Gayán and M. J. Serrano gratefully acknowledge the financial support for her doctoral studies provided by the Ministerio de Educación y Ciencia.
Footnotes
Published ahead of print 21 September 2012
REFERENCES
- 1. Álvarez I, Mañas P, Sala FJ, Condón S. 2003. Inactivation of Salmonella enterica serovar Enteritidis by ultrasonic waves under pressure at different water activities. Appl. Environ. Microbiol. 69:668–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bucheli-Witschel M, Bassin C, Egli T. 2010. UV-C inactivation in Escherichia coli is affected by growth conditions preceding irradiation, in particular by the specific growth rate. J. Appl. Microbiol. 109:1733–1744 [DOI] [PubMed] [Google Scholar]
- 3. Char CD, Mitilinaki E, Guerrero SN, Alzamora SM. 2010. Use of high-intensity ultrasound and UV-C light to inactivate some microorganisms in fruit juices. Food Bioprocess Technol. 3:797–803 [Google Scholar]
- 4. Child M, Strike P, Pickup R, Edwards C. 2002. Salmonella Typhimurium displays cyclical patterns of sensitivity to UV-C killing during prolonged incubation in the stationary phase of growth. FEMS Microbiol. Lett. 213:81–85 [DOI] [PubMed] [Google Scholar]
- 5. Condón S, Oria R, Sala FJ. 1987. Heat resistance of microorganisms: an improved method for survival counting. J. Microbiol. Methods 7:37–44 [Google Scholar]
- 6. Condón S, Arrizubieta MJ, Sala FJ. 1993. Microbial heat resistance determinations by the multipoint system with the thermoresistometer TR-SC. Improvement of this methodology. J. Microbiol. Methods 18:357–366 [Google Scholar]
- 7. Coroller L, Leguerinel I, Mafart P. 2001. Effect of water activities of heating and recovery media on apparent heat resistance of Bacillus cereus spores. Appl. Environ. Microbiol. 67:317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dawson RMC, Elliot DC, Elliot WH, Jones KM. 1974. pH, buffers and physiological media. Clarendon Press, Oxford, United Kingdom [Google Scholar]
- 9. de Souza PM, Fernández A. 2011. Effects of UV-C on physicochemical quality attributes and Salmonella Enteritidis inactivation in liquid egg products. Food Control 22:1385–1392 [Google Scholar]
- 10. Donaghy J, et al. 2009. Inactivation of Mycobacterium avium ssp paratuberculosis in milk by UV treatment. Lett. Appl. Microbiol. 49:217–221 [DOI] [PubMed] [Google Scholar]
- 11. European Food Safety Authority 2012. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2010. EFSA J. 10:2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Franz CMAP, Specht I, Cho G, Graef V, Stahl MR. 2009. UV-C-inactivation of microorganisms in naturally cloudy apple juice using novel inactivation equipment based on Dean vortex technology. Food Control 20:1103–1107 [Google Scholar]
- 13. Fredericks IN, Du Toit M, Krügel M. 2011. Efficacy of ultraviolet radiation as an alternative technology to inactivate microorganisms in grape juices and wines. Food Microbiol. 28:510–517 [DOI] [PubMed] [Google Scholar]
- 14. Gabriel AA, Nakano H. 2009. Inactivation of Salmonella, E. coli and Listeria monocytogenes in phosphate-buffered saline and apple juice by ultraviolet and heat treatments. Food Control 20:443–446 [Google Scholar]
- 15. Gayán E, Monfort S, Álvarez I, Condón S. 2011. UV-C inactivation of Escherichia coli at different temperatures. Innov. Food Sci. Emerg. 12:531–541 [Google Scholar]
- 16. Geeraerd AH, Herremans CH, Van Impe JF. 2000. Structural model requirements to describe microbial inactivation during a mild heat treatment. Int. J. Food Microbiol. 59:185–209 [DOI] [PubMed] [Google Scholar]
- 17. Geeraerd AH, Valdramidis VP, Van Impe JF. 2005. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Microbiol. 102:95–105 [DOI] [PubMed] [Google Scholar]
- 18. Geveke DJ. 2008. UV inactivation of E. coli in liquid egg white. Food Bioprocess Technol. 1:201–206 [Google Scholar]
- 19. Gibbs C. 2000. UV disinfection. Soft Drinks Int. 1:32–34 [Google Scholar]
- 20. Guerrero-Beltrán JA, Barbosa-Cánovas GV. 2004. Advantages and limitations on processing foods by UV light. Food. Sci. Technol. Int. 10:137–147 [Google Scholar]
- 21. Jayaram S, Castle GSP, Margaritis A. 1992. Kinetics of sterilization of Lactobacillus brevis cells by the application of high-voltage pulses. Biotechnol. Bioeng. 40:1412–1420 [DOI] [PubMed] [Google Scholar]
- 22. Kim T, Silva JL, Chen TC. 2002. Effects of UV irradiation on selected pathogens in peptone water and on stainless steel and chicken meat. J. Food Prot. 65:1142–1145 [DOI] [PubMed] [Google Scholar]
- 23. Koutchma T, Keller S, Chirtel S, Parisi B. 2004. Ultraviolet disinfection of juice products in laminar and turbulent flow reactors. Innov. Food Sci. Emerg. 5:179–189 [Google Scholar]
- 24. Koutchma T, Paris B, Patazca E. 2007. Validation of UV coiled tube reactor for fresh juices. J. Environ. Eng. Sci. 6:319–328 [Google Scholar]
- 25. Koutchma T, Forney LJ, Moraru CL. 2009. Ultraviolet light in food technology. CRC Press, Boca Raton, FL [Google Scholar]
- 26. Le G, et al. 2010. UV inactivation of microorganisms in beer by a novel thin-film apparatus. Food Control 21:1312–1317 [Google Scholar]
- 27. López-Malo A, Palau E. 2005. Ultraviolet light and food preservation, p 464–484 In Barbosa-Cánovas GV, Tapia MS, Cano MP. (ed), Novel food processing technologies. CRC Press, Madrid, Spain [Google Scholar]
- 28. Mackey BM. 2000. Injured bacteria, p 315–341 In Lund M, Baird-Parker TC, Gould GW. (ed), The microbiological safety and quality of food. Aspen Publishers Inc., Gaithersburg, MD [Google Scholar]
- 29. Mañas P, Pagán R, Raso J, Condón S. 2003. Predicting thermal inactivation in media of different pH of Salmonella grown at different temperatures. Int. J. Food Microbiol. 87:45–53 [DOI] [PubMed] [Google Scholar]
- 30. Mañas P, Pagan R, Raso J, Sala FJ, Condón S. 2000. Inactivation of Salmonella enteritidis, Salmonella Typhimurium, and Salmonella Senftenberg by ultrasonic waves under pressure. J. Food Prot. 63:451–456 [DOI] [PubMed] [Google Scholar]
- 31. Molina-Hoppner A, Doster W, Vogel RF, Ganzle MG. 2004. Protective effect of sucrose and sodium chloride for Lactococcus lactis during sublethal and lethal high-pressure treatments. Appl. Environ. Microbiol. 70:2013–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Montgomery JM. 1985. Water treatment: principles and design. John Wiley, New York, NY [Google Scholar]
- 33. Murakami EG, Jackson L, Madsen K, Schickedanz B. 2006. Factors affecting the ultraviolet inactivation of Escherichia coli K12 in apple juice and a model system. J. Food Process. Eng. 29:53–71 [Google Scholar]
- 34. Okuda A. 1974. Effects of mild heating on recovery from ultraviolet inactivation in Escherichia coli B/r. II. Removal of heat-induced effects by sobsequent incubation. J. Radiat. Res. 15:140–143 [Google Scholar]
- 35. Oteiza JM, Giannuzzi L, Zaritzky N. 2010. Ultraviolet treatment of orange juice to inactivate E. coli O157:H7 as affected by native microflora. Food Bioprocess Tech. 3:603–614 [Google Scholar]
- 36. Oteiza JM, Peltzer M, Gannuzzi L, Zaritzky N. 2005. Antimicrobial efficacy of UV radiation on Escherichia coli O157:H7 (EDL 933) in fruit juices of different absorptivities. J. Food Prot. 68:49–58 [DOI] [PubMed] [Google Scholar]
- 37. Quintero-Ramos A, Churey JJ, Hartman P, Barnard J, Worobo RW. 2004. Modeling of Escherichia coli inactivation by UV irradiation at different pH values in apple cider. J. Food Prot. 67:1153–1156 [DOI] [PubMed] [Google Scholar]
- 38. Rajkovic A, Smigic N, Devlieghere F. 2010. Contemporary strategies in combating microbial contamination in food chain. Int. J. Food Microbiol. 141:S29–S42 [DOI] [PubMed] [Google Scholar]
- 39. Raso J, Barbosa-Cánovas GV. 2003. Nonthermal preservation of foods using combined processing techniques. Crit. Rev. Food. Sci. Nutr. 43:265–285 [DOI] [PubMed] [Google Scholar]
- 40. Sastry SK, Datta K, Worobo RW. 2000. Ultraviolet light. J. Food Saf. 65:90–92 [Google Scholar]
- 41. Setlow P. 1995. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu. Rev. Microbiol. 49:29–54 [DOI] [PubMed] [Google Scholar]
- 42. Shama G. 1992. Ultraviolet-irradiation apparatus for disinfecting liquids of high ultraviolet absorptivities. Lett. Appl. Microbiol. 15:69–72 [Google Scholar]
- 43. Sizer CE, Balasubramaniam VM. 1999. New intervention processes for minimally processed juices. Food Technol. 53:64–67 [Google Scholar]
- 44. Sommer R, Lhotsky M, Haider T, Cabaj A. 2000. UV inactivation, liquid-holding recovery, and photoreactivation of Escherichia coli O157 and other pathogenic Escherichia coli strains in water. J. Food Prot. 63:1015–1020 [DOI] [PubMed] [Google Scholar]
- 45. Stother B. 1999. UV disinfection in liquid sugar manufacture. Int. Sugar J. 101:361–363 [Google Scholar]
- 46. Ukuku DO, Geveke DJ. 2010. A combined treatment of UV-light and radio frequency electric field for the inactivation of Escherichia coli K-12 in apple juice. Int. J. Food Microbiol. 138:50–55 [DOI] [PubMed] [Google Scholar]
- 47. U.S. Food and Drug Administration 2001. Hazard Analysis and Critical Control Point (HACCP); procedures for the safe and sanitary processing and importing of juice, final rule. Federal Register 66:6137–6202 U.S. Food and Drug Administration, Washington, DC [Google Scholar]
- 48. Vojdani JD, Beuchat LR, Taure RV. 2008. Juice-associated outbreaks of human illness in the United States, 1995 through 2005. J. Food Prot. 71:356–364 [DOI] [PubMed] [Google Scholar]
- 49. Walkling-Ribeiro M, et al. 2008. Reduction of Staphylococcus aureus and quality changes in apple juice processed by ultraviolet irradiation, pre-heating and pulsed electric fields. J. Food Eng. 89:267–273 [Google Scholar]
- 50. Yaun BR, Sumner SS, Eifert JD, Marcy JE. 2004. Inhibition of pathogens on fresh produce by ultraviolet energy. Int. J. Food Microbiol. 90:1–8 [DOI] [PubMed] [Google Scholar]