Abstract
Population heterogeneity complicates the predictability of the outgrowth kinetics of individual spores. Flow cytometry sorting and monitoring of the germination and outgrowth of single dormant spores allowed the quantification of acid-induced spore population heterogeneity at pH 5.5 and in the presence of sorbic acid. This showed that germination efficiency was not a good predictor for heterogeneity in final outgrowth.
TEXT
Bacillus cereus is a food-spoiling and food-poisoning spore-forming organism that can be found in a large variety of foods and food ingredients (10). It has the ability to survive in harsh environments because it can form endospores that are resistant to heat, dehydration, and other physical and/or chemical stresses, and these spores may germinate and grow when conditions become more favorable. Thermal preservation methods that ensure complete inactivation of highly stress-resistant spores will negatively affect food quality, and the current trend toward milder preservation methods advocates application of mild food preservation factors, including mild heat treatments combined with addition of weak organic acids (11), to delay or inhibit germination and outgrowth of spores and cells.
We previously investigated the impact of sorbic acid (SA) on germination and outgrowth of B. cereus spores for a population as a whole (18), but heterogeneity in outgrowth between individual spores in the population was not quantified and will affect the germination and outgrowth profile. Furthermore, it is well documented that heat treatments can trigger activation of spores and accelerate germination (7) and thereby can influence outgrowth dynamics and possibly also heterogeneity. To date, the impact of heat treatment on germination and outgrowth kinetics has been described at the population level (for example, see references 8 and 16), but heterogeneity at the single-cell level has not been studied in combination with mild (acidic) stress factors to control the germination and outgrowth of B. cereus spores. Therefore, we assessed the impact of SA on germination and outgrowth kinetics at both the population level and single-cell level for spores that were heat shocked (HS) prior to initiation of germination. For that purpose, B. cereus ATCC 14579 spores were harvested from cultures grown in defined, minimal sporulation medium (4) and prepared as described previously (18) with the following modification: the Tween 80 concentrations were reduced from 0.1% to 0% in five daily washing steps. Pure spore crops devoid of vegetative cells and debris were stored in phosphate-buffered saline (PBS; pH 7) at 4°C for at least 2 weeks and not more than 8 weeks until use. Spore germination was assessed under either unstressed conditions (brain heart infusion [BHI] buffered with 100 mM PBS [bBHI]; pH 7) or under mild acid stress (bBHI at pH 5.5 or bBHI at pH 5.5 with supplementation with 0.75 mM undissociated sorbic acid [HSA]). Spore germination was monitored by the transition of phase-bright spores turning phase dark, which is observed as a drop in optical density (OD) in a spectrophotometer (VersaMax, Molecular Devices, United States) as described previously (5). Spore density was adjusted in all experiments to an optical density at 600 nm (OD600) of 0.8 to 0.9 to ensure that similar numbers of spores were used in all experiments (final concentration, ∼2 × 108 CFU/ml). Spores were either mildly heat shocked at 70°C for 10 min or not heat shocked by incubation at room temperature. After heat shock and prior to initiation of germination, spores were washed in PBS of the appropriate pH (pH 7 for the spores that were incubated at pH 7 and pH 5.5 for the spores germinated at pH 5.5 without and with addition of 0.75 mM HSA). Twenty microliters of spore solution was transferred to 250-μl wells in precooled microtiter plates, followed by addition of 180 μl of 1.1× concentrated bBHI at either pH 7 or pH 5.5 or at pH 5.5 with supplementation with 0.75 mM HSA. The OD600 was measured every 120 s for 8 h in a spectrophotometer (VersaMax; Molecular Devices, United States) that was prewarmed to 30°C. For each condition, two independent biological replicates were tested. Each replicate was repeated in eight wells (technical replicates), resulting in 16 data points per condition.
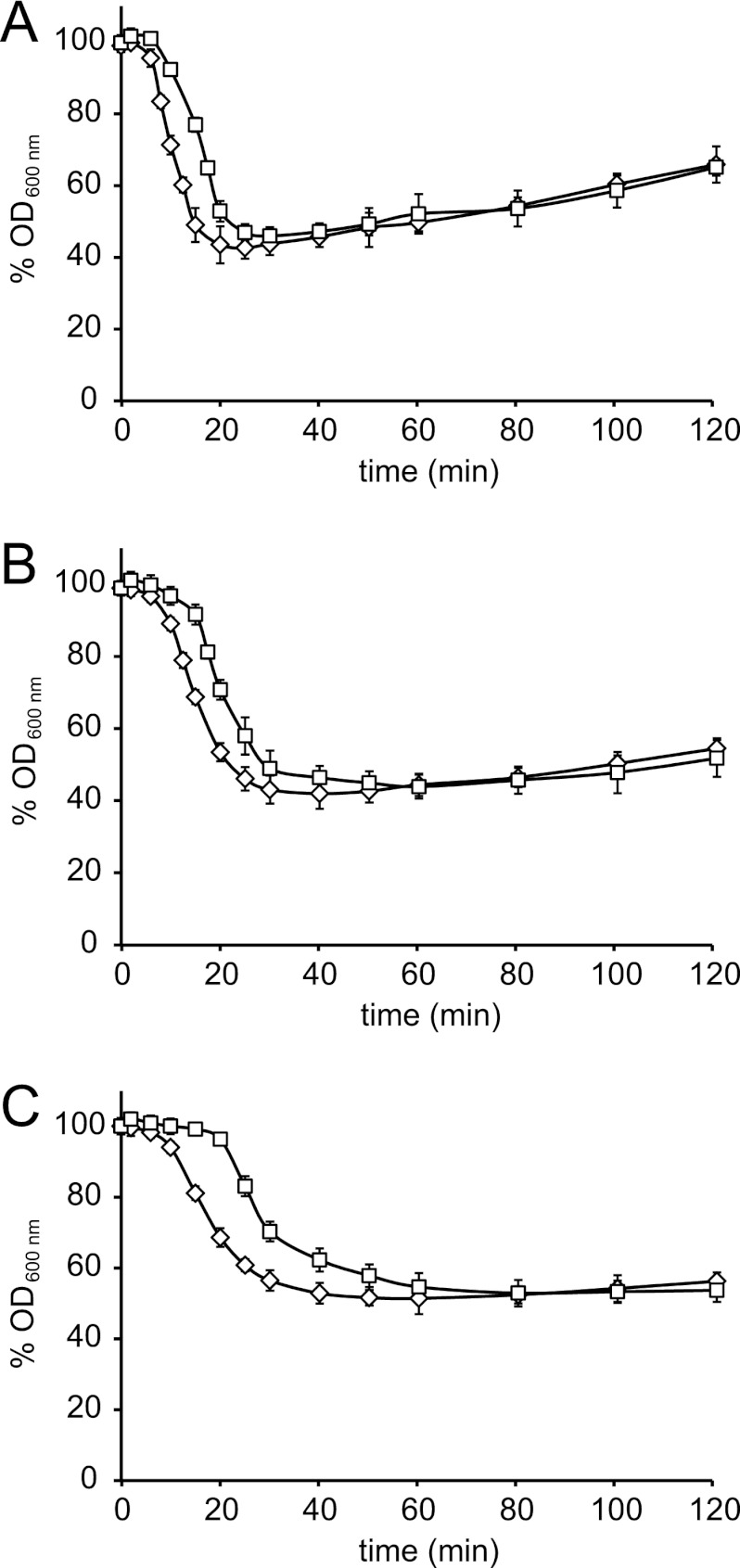
The optical density measurements showed that lowering the pH to 5.5 increased the lag time to initiation of germination (Fig. 1). The lag time increased further in the presence of 0.75 mM HSA at pH 5.5. The maximum drop in OD represented close to 100% germination at pH 7 and 5.5, as confirmed by plate counting of spores and cells (data not shown), whereas in the presence of 0.75 mM HSA, the germination efficiency was slightly lower and approximately 95%. Reduced germination efficiency by HSA may be caused by accumulation of sorbic acid, which is relatively hydrophobic, in the spore's inner membrane, and which may interfere with the signaling cascade in germinant-receptor-mediated germination (17). Heat shock reduced the time to reach a maximum OD drop at pH 7 and under both acidic conditions, underlining that the rather mild heat treatment applied in this study resulted in activation of spores and acceleration of germination. Acceleration of germination by heat treatment is a known phenomenon, and recently, it has been suggested that heat activation might increase the responsiveness of germination receptors and faster release of dipicolinic acid (DPA) (21). We now observed that this acceleration is still significant when combined with germination-delaying factors like the presence of sorbic acid.
Fig 1.
Impact of heat treatment and subsequent acid stress exposure on germination and initial outgrowth kinetics. Prior to germination, dormant spores were heat shocked for 10 min at 70°C (diamonds) or not heat-shocked (squares), after which germination and outgrowth were monitored at 30°C in buffered BHI at pH 7 (A), pH 5.5 (B), and pH 5.5 with supplementation with 0.75 mM undissociated sorbic acid (C). Error bars represent standard deviations of the repetitions (n = 16).
Germination curves as shown in Fig. 1 represent the summation of behavior of individual spores within a population, and mild preservation stresses have been shown to induce heterogeneity in growth potential. Both low temperature and salt stress exposure have been reported to induce heterogeneity for exponentially growing B. cereus cells (2, 3), but data on stress-induced heterogeneity for dormant spores are not available. Heterogeneity for germination and outgrowth of spores complicates the predictability of spore behavior in response to preservation treatments like acidification. Single-cell performance analysis provides more accurate insight into population heterogeneity and is necessary to better understand and quantify the heterogeneity in germination and outgrowth potential in an acidic environment. Therefore, growth curves initiating from single dormant spores were obtained using optical density measurements. Dormant B. cereus spores were sorted with a Beckman Coulter EPICS Elite flow cytometer with 488-nm excitation from a 15-mW argon laser into sterile 96-well microtiter plates (flat-bottom; Greiner Bio-One, the Netherlands). Dormant spores were distinguished from germinated spores by using the fluorescent reporter dye SYTO9 (Invitrogen, the Netherlands) as described previously (18), with the exception that a final concentration of 2 μM dye was used. Plates were closed, subsequently sealed with Parafilm M laboratory film, and stored at −20°C until use (pilot experiments with BHI at pH 7 showed that long-term storage at −20°C did not affect the germination properties of single spores). Before use, each plate was thawed at room temperature for 10 min, and in the experiments with heat treatment, plates were incubated for 10 min at 70°C. After thawing and/or heat treatment, the plates were kept on ice while 200-μl aliquots of bBHI at either pH 7 or 5.5 or at pH 5.5 with supplementation with 0.75 mM HSA were added to each well. The plates were incubated at 30°C in a spectrophotometer (VersaMax; Molecular Devices, United States) for up to 72 h, and during this period, the optical density was measured every 10 min at a wavelength of 600 nm.
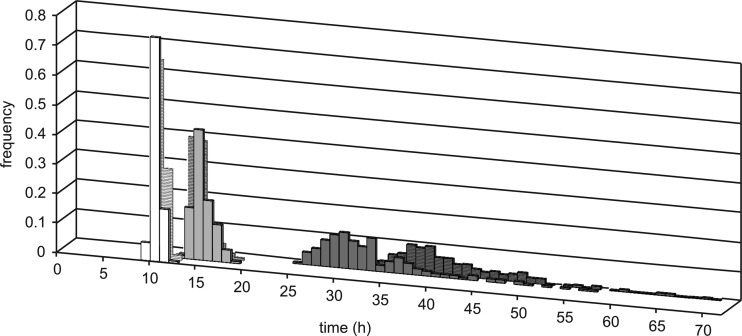
A lower pH of 5.5 resulted in more heterogeneity in germination and outgrowth compared to the optimal growth condition, and this heterogeneity was even larger in the presence of 0.75 mM HSA (Fig. 2A, B, and C). For the latter stress condition, no growth was detected in approximately 40% of the wells within the time frame of the experiment, underlining that it is conceivable that the observed heterogeneity is underestimated. Heat shock did not decrease the percentage of wells in which no growth was detected and only seemed to have an impact on heterogeneity when spores were exposed to 0.75 mM HSA at pH 5.5 (Fig. 2F). To assess the observed heterogeneity in more detail, the first time point at which each well reached the OD600 value of 0.2 (which equals 2 times the background signal) was calculated and defined as the time to detection. These times to detection were used to visualize the heterogeneity in spore germination and outgrowth (Fig. 3). The distributions became more spread and skewed to the right under more severe stress. Lowering the pH to 5.5 and addition of HSA at pH 5.5 increased both the means and the associated standard deviations of the time-to-detection distributions and also increased the coefficient of variation (Table 1), showing that, relatively, variability had been increased with increasing stress conditions. To test the significance of these observations, the data were normalized with the mean per data set using the formula normalized value = value/mean, and the variances were compared using Levene's test. Levene's test confirmed that germination and outgrowth at pH 5.5 and at pH 5.5 plus 0.75 mM HSA significantly increased the variability compared to optimal conditions (P < 0.05). In addition, heat treatment of spores indeed only reduced the variability when spores were subsequently exposed to 0.75 mM HSA at pH 5.5. This reduced heterogeneity in germination and outgrowth kinetics, introduced by a relatively mild heat pretreatment of spores, is in line with previous findings in other sporeformers where mild heat treatment of spores made the distributions smaller in Bacillus subtilis (9), whereas more severe lethal heat treatment widened the distribution not only for B. subtilis (9), but also for Clostridium botulinum (14). We demonstrated now for B. cereus that mild heat pretreatment only affected the heterogeneity in outgrowth when spores were exposed to rather stressful conditions, like 0.75 mM HSA at pH 5.5. This suggested that mild heat-induced acceleration of germination at pH 7 and 5.5 (Fig. 1) did not significantly affect heterogeneity in outgrowth performance (Fig. 2 and 3). Additionally, although a rather high germination efficiency was observed in the presence of 0.75 mM HSA—the maximum OD drop corresponded with approximately 95% of germinated spores—the heterogeneity in outgrowth was substantial (Fig. 3) and might even be underestimated as approximately 40% of the wells did not reach the turbidity detection threshold. These observations underlined that germination efficiency did not seem to be a good predictor for heterogeneity in final outgrowth. The observed heterogeneity is the sum of heterogeneity during germination, the first doubling phases, and further outgrowth and is likely to result from multiple sources (6, 15). For C. botulinum, it has been shown that the first spore that germinated was not the first to develop into a vegetative cell, also under stress conditions, highlighting the lack of correlation between germination and outgrowth times (12–14, 19). The most important source of variability in outgrowth might depend on the relative magnitude of the contributions of the different phases in outgrowth. The positively skewed distributions we observed for B. cereus spore development under acid stress conditions were similar to the shapes of distribution previously reported for C. botulinum spore germination and outgrowth under stressful conditions (1, 13). This implies that when mean outgrowth times are used in risk assessment, the number of spores that have shorter development times will be underestimated, resulting in a “fail-dangerous” scenario. Heterogeneity in germination and outgrowth complicates their predictability, and recent publications suggest that the levels of germination receptors might vary significantly between spores in a population (20, 21) and might contribute to heterogeneity in performance. We demonstrated that mild acid preservation will increase the heterogeneity in outgrowth kinetics of dormant spores. The next step will be to correlate germination and outgrowth kinetics to spore physiology at the single-cell level, which will provide valuable mechanistic understanding of sources of spore germination and outgrowth heterogeneity. Quantitative information and mechanistic knowledge on heterogeneity in germination and outgrowth will give relevant insights to optimize application of mild preservation factors to control and balance food quality and food safety.
Fig 2.
Heterogeneity in outgrowth initiated from single dormant spores. Before initiation of germination, spores were not heat shocked (A, B, and C) or were heat shocked for 10 min at 70°C (D, E, and F), after which germination and outgrowth were monitored at 30°C in buffered BHI at pH 7 (A and D), pH 5.5 (B and E), and pH 5.5 with supplementation with 0.75 mM undissociated sorbic acid (C and F). The figures are representative examples of growth curves generated in 96-well microtiter plates.
Fig 3.
Frequency distribution of times to detection (OD600, 0.2) initiated from single dormant spores that were not heat shocked (dashed bars) or were heat shocked for 10 min at 70°C (undashed bars) and incubated at 30°C in buffered BHI at pH 7 (white bars), pH 5.5 (light gray bars), and pH 5.5 with supplementation with 0.75 mM undissociated sorbic acid (dark gray bars).
Table 1.
Effects of heat treatment and subsequent acid stress exposure on heterogeneity in outgrowth
| Condition(s) | No. of wellsa | Time to detection (h)b |
||
|---|---|---|---|---|
| Mean | SD | Coefficient of variation | ||
| pH 7 | 509 | 9.9 | 0.45 | 0.045 |
| pH 7, HS | 331 | 9.7 | 0.44 | 0.045 |
| pH 5.5 | 563 | 13.5 | 1.06 | 0.079 |
| pH 5.5, HS | 305 | 14.0 | 1.11 | 0.079 |
| pH 5.5, 0.75 mM HSA | 406 | 39.3 | 7.85 | 0.199 |
| pH 5.5, 0.75 mM HSA, HS | 191 | 31.5 | 5.91 | 0.188 |
Number of monitored wells that reached the time to detection.
Times to detection (OD600, 0.2) were generated for single dormant spores that were not heat shocked or were heat shocked for 10 min at 70°C (HS) and incubated in buffered BHI at pH 7 or 5.5 or at pH 5.5 with supplementation with 0.75 mM undissociated sorbic acid (HSA).
ACKNOWLEDGMENT
Nathalie van Egmond is gratefully acknowledged for assistance with the flow cytometer experiments.
Footnotes
Published ahead of print 21 September 2012
REFERENCES
- 1. Billon CMP, McKirgan CJ, McClure PJ, Adair C. 1997. The effect of temperature on the germination of single spores of Clostridium botulinum 62A. J. Appl. Microbiol. 82:48–56 [DOI] [PubMed] [Google Scholar]
- 2. den Besten HMW, Garcia D, Moezelaar R, Zwietering MH, Abee T. 2010. Direct-imaging-based quantification of Bacillus cereus ATCC 14579 population heterogeneity at a low incubation temperature. Appl. Environ. Microbiol. 76:927–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. den Besten HMW, et al. 2007. Quantitative analysis of population heterogeneity of the adaptive salt stress response and growth capacity of Bacillus cereus ATCC 14579. Appl. Environ. Microbiol. 73:4797–4804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Vries YP, Hornstra LM, de Vos WM, Abee T. 2004. Growth and sporulation of Bacillus cereus ATCC 14579 under defined conditions: temporal expression of genes for key sigma factors. Appl. Environ. Microbiol. 70:2514–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hornstra LM, de Vries YP, de Vos WM, Abee T, Wells-Bennik MHJ. 2005. gerR, a novel ger operon involved in l-alanine- and inosine-initiated germination of Bacillus cereus ATCC 14579. Appl. Environ. Microbiol. 71:774–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hornstra LM, Ter Beek A, Smelt JP, Kallemeijn WW, Brul S. 2009. On the origin of heterogeneity in (preservation) resistance of Bacillus spores: input for a ‘systems’ analysis approach of bacterial spore outgrowth. Int. J. Food Microbiol. 134:9–15 [DOI] [PubMed] [Google Scholar]
- 7. Keynan A, Evenchik Z. 1969. Activation, p 359–396 In Gould GW, Hurst A. (ed), The bacterial spore. Academic Press, New York, NY [Google Scholar]
- 8. Løvdal IS, Hovda MB, Granum PE, Rosnes JT. 2011. Promoting Bacillus cereus spore germination for subsequent inactivation by mild heat treatment. J. Food Prot. 74:2079–2089 [DOI] [PubMed] [Google Scholar]
- 9. Smelt JPPM, Bos AP, Kort R, Brul S. 2008. Modelling the effect of sub(lethal) heat treatment of Bacillus subtilis spores on germination rate and outgrowth to exponentially growing vegetative cells. Int. J. Food Microbiol. 128:34–40 [DOI] [PubMed] [Google Scholar]
- 10. Stenfors Arnesen LP, Fagerlund A, Granum PE. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32:579–606 [DOI] [PubMed] [Google Scholar]
- 11. Stratford M, Eklund T. 2003. Organic acids and esters, p 48–84 In Russell NJ, Gould GW. (ed), Food preservatives, 2nd ed Kluwer Academic/Plenum Publishers, New York, NY [Google Scholar]
- 12. Stringer SC, Webb MD, George SM, Pin C, Peck MW. 2005. Heterogeneity of times required for germination and outgrowth from single spores of nonproteolytic Clostridium botulinum. Appl. Environ. Microbiol. 71:4998–5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stringer SC, Webb MD, Peck MW. 2009. Contrasting effects of heat treatment and incubation temperature on germination and outgrowth of individual spores of nonproteolytic Clostridium botulinum bacteria. Appl. Environ. Microbiol. 75:2712–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stringer SC, Webb MD, Peck MW. 2011. Lag time variability in individual spores of Clostridium botulinum. Food Microbiol. 28:228–235 [DOI] [PubMed] [Google Scholar]
- 15. Ter Beek A, et al. 2011. Models of the behaviour of (thermally stressed) microbial spores in foods: tools to study mechanisms of damage and repair. Food Microbiol. 28:678–684 [DOI] [PubMed] [Google Scholar]
- 16. Van der Voort M, García D, Moezelaar R, Abee T. 2010. Germinant receptor diversity and germination responses of four strains of the Bacillus cereus group. Int. J. Food Microbiol. 139:108–115 [DOI] [PubMed] [Google Scholar]
- 17. Van Melis CCJ, Nierop Groot MN, Abee T. 2011. Impact of sorbic acid on germinant receptor-dependent and -independent germination pathways in Bacillus cereus. Appl. Environ. Microbiol. 77:2552–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Melis CCJ, Nierop Groot MN, Tempelaars MH, Moezelaar R, Abee T. 2011. Characterization of germination and outgrowth of sorbic acid-stressed Bacillus cereus ATCC 14579 spores: phenotype and transcriptome analysis. Food Microbiol. 28:275–283 [DOI] [PubMed] [Google Scholar]
- 19. Webb MD, Pin C, Peck MW, Stringer SC. 2007. Historical and contemporary NaCl concentrations affect the duration and distribution of lag times from individual spores of nonproteolytic Clostridium botulinum. Appl. Environ. Microbiol. 73:2118–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yi X, Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J. Bacteriol. 192:3424–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang P, Garner W, Yi X, Yu J, Li Y-Q, Setlow P. 2010. Factors affecting variability in time between addition of nutrient germinants and rapid dipicolinic acid release during germination of spores of Bacillus species. J. Bacteriol. 192:3608–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]