Abstract
Among 1,341 blood samples from rodents that were trapped in Southeast Asia between 2008 and 2010, we found a prevalence of Bartonella infection ranging from 9.6 to 11.9%. Bartonella species identified (143 isolates) included B. elizabethae, B. coopersplainsensis, B. phoceensis, B. queenslandensis, B. rattimassiliensis, B. tribocorum, and three new putative Bartonella species.
TEXT
Bartonella species are intracellular parasites of erythrocytes and endothelial cells of various mammalian host species, such as humans and wild and domestic animals, including rodents (5, 7, 14). Bartonella infections in rodents have been reported worldwide (2, 4, 8, 10, 11, 16). The objectives of this study were to investigate the prevalence and to evaluate the genetic diversity of Bartonella infections from a large series of blood samples from rodents and shrews that were trapped from two sites in Cambodia, two sites in Lao PDR, and three sites in Thailand (Fig. 1). Pictures, habitat descriptions, and the coordinates of the trap lines are available at the CERoPath project website (www.ceropath.org).
Fig 1.
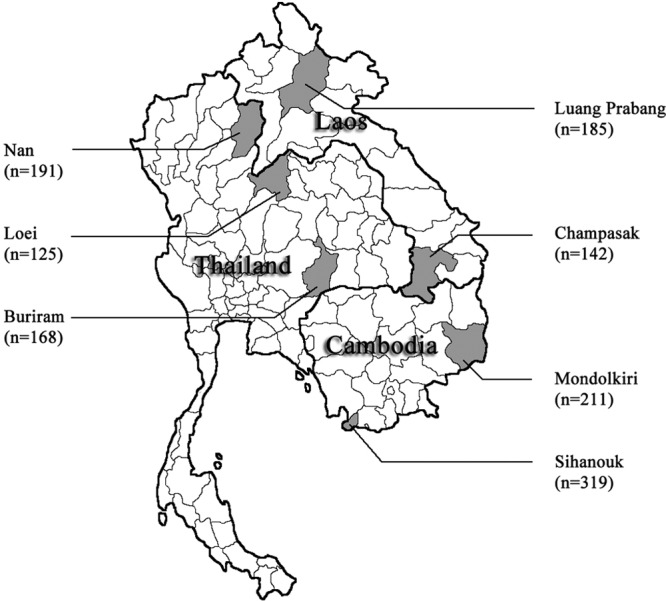
Geographical distribution of the trapped rodents and the study areas consist of the sampling sites in two provinces of Cambodia (Mondolkiri and Sihanouk), two provinces of Lao PDR (Luang Prabang and Champasuk), and three provinces of Thailand (Nan, Loei, and Buriram). (Template map adapted from Google Earth [U.S. Department of State Geographer, 2012, Mapabc.com; Data SIO, NOAA, U.S. Navy, NGA, GEBCO, 2012, Tele Atlas].)
Blood samples were collected by cardiocentesis and stored at −80°C before shipment on dry ice to the URMITE CNRS-IRD UMR laboratory in Marseille, France. Overall, DNA from blood samples (n = 1,341) was extracted and screened using real-time PCR that targeted the 16S-23S rRNA intergenic spacer region (ITS), as previously described (12). Blood samples were also tested by culture method on Columbia agar supplemented with 5% sheep blood and incubated at 37°C in 5% CO2 for up to 4 weeks. A single colony for each positive sample was picked, its DNA was extracted, and the species was identified by using standard PCR amplification and a sequencing analysis targeting two housekeeping genes (gltA and rpoB) and the ITS fragment (9, 13). The sequence data were aligned and compared with the sequences of the Bartonella reference strains, which were retrieved from GenBank (see Table S1 in the supplemental material) and analyzed using Clustal W. Phylogenetic analyses were conducted on concatenated gltA and rpoB sequences by using the MEGA 5 software and maximum parsimony method. The prevalence of Bartonella infections in the three countries were analyze using R statistical software.
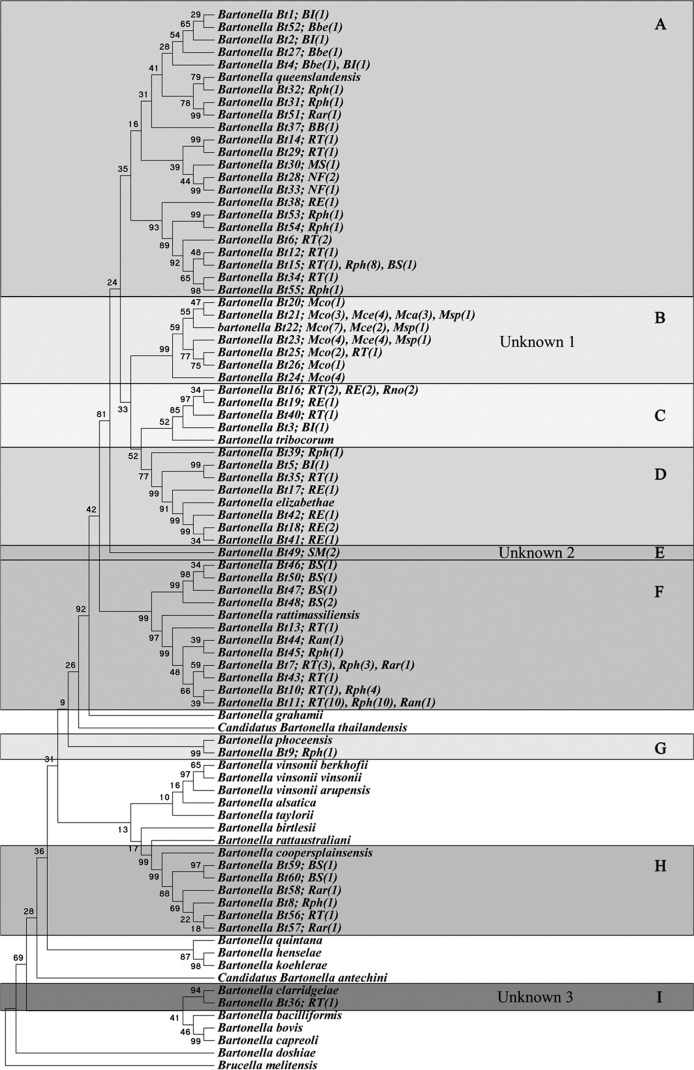
Overall, of the trapped animals, 19 species of rodents and 2 species of shrews were identified. Bartonella spp. were isolated from 143 of the 1,341 (10.7%) blood samples. The highest prevalence of Bartonella spp. was found in Lao PDR (11.9%), followed by Thailand (11%) and Cambodia (9.6%) (Table 1). However, these differences were not statistically significant (chi-square test, all P values were >0.05). The prevalence of Bartonella infection varied among host species, from 1% for Maxomys surifer to 75% for Mus spp. (Table 1). Among the positive animals, species of the genus Rattus displayed the highest prevalence of Bartonella infection, followed by rodents of the genera Mus and Berylmys. Of the total 143 isolates from 140 animal blood samples, 3 samples contained multiple Bartonella species. Phylogenetic analyses revealed that the concatenated gltA and rpoB gene sequences could be classified into 9 clusters (Fig. 2, clusters A to I). The phylogenetic tree suggests that 6 clusters (A, C, D, F, G, and H) are closely related to known Bartonella species, including B. queenslandensis (n = 33), B. rattimassiliensis (n = 42), B. tribocorum (n = 11), B. elizabethae (n = 7), B. coopersplainsensis (n = 6), and B. phoceensis (n = 1). Conversely, three clusters (B, E, and I) are associated with the three new putative species (Fig. 2, unknowns 1, 2, and 3). The GenBank accession numbers for the nucleotide sequences of these new species are reported below in the text; accession numbers for identified species can be found in Table S1 of the supplemental material.
Table 1.
Prevalence of Bartonella infection in rodents and shrews from three countries in southeastern Asia (Cambodia, Lao PDR, and Thailand) between 2008 and 2010
| Species (total no. trapped) | No. of animals trapped per country |
No. (% of total trapped animals) with Bartonella infection by country |
Total no. infected (% of total trapped animals) | ||||
|---|---|---|---|---|---|---|---|
| Cambodia | Lao PDR | Thailand | Cambodia | Lao PDR | Thailand | ||
| Bandicota indica (74) | 3 | 6 | 65 | 0 | 0 | 5 (7.7) | 5 (6.8) |
| Bandicota savilei (112) | 74 | 14 | 24 | 7 (9.5) | 1 (7.1) | 0 | 8 (7.1) |
| Bandicota sp. (1) | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Berylmys berdmorei (31) | 11 | 5 | 15 | 1 (9.1) | 1 (20) | 2 (13.3) | 4 (12.9) |
| Berylmys bowersi (5) | 0 | 2 | 3 | 0 | 0 | 0 | 0 |
| Cannomys badius (1) | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Crocidura sp. (1) | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Leopoldamys edwardsi (3) | 2 | 1 | 0 | 0 | 0 | 0 | 0 |
| Maxomys surifer (101) | 92 | 3 | 6 | 1 (1.1) | 0 | 0 | 1 (1) |
| Mus caroli (49) | 0 | 23 | 26 | 0 | 1 (4.8) | 2 (8) | 3 (6.1) |
| Mus cervicolor (48) | 0 | 0 | 48 | 0 | 0 | 10 (20.8) | 10 (20.8) |
| Mus cookii (90) | 0 | 55 | 35 | 0 | 17 (30.) | 6 (17.1) | 23 (25.6) |
| Mus sp. (4) | 0 | 0 | 4 | 0 | 0 | 3 (75) | 3 (75) |
| Niviventer fulvescens (19) | 10 | 1 | 8 | 1 (10) | 0 | 1 (12.5) | 2 (10.5) |
| Rattus andamanensis (5) | 0 | 5 | 0 | 0 | 2 (40) | 0 | 2 (40) |
| Rattus argentiventer (44) | 42 | 0 | 2 | 4 (9.5) | 0 | 0 | 4 (9.1) |
| Rattus exulans (354) | 115 | 97 | 142 | 4 (3.5) | 0 | 6 (4.2) | 10 (2.8) |
| Rattus losea (47) | 0 | 8 | 39 | 0 | 0 | 0 | 0 |
| Rattus nitidus (13) | 0 | 13 | 0 | 0 | 0 | 0 | 0 |
| Rattus norvegicus (24) | 24 | 0 | 0 | 2 (8.3) | 0 | 0 | 2 (8.3) |
| Rattus tanezumi (116) | 0 | 78 | 38 | 0 | 17 (21.8) | 12 (31.6) | 29 (25) |
| R. tanezumi clade R3 (159) | 117 | 13 | 29 | 29 (24.8) | 0 | 6 (20.7) | 35 (22) |
| Rhizomys pruinosus (1) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Suncus murinus (39) | 39 | 0 | 0 | 2 (5.1) | 0 | 0 | 2 (5.1) |
| Total | 530 | 327 | 484 | 51 (9.6) | 39 (11.9) | 53 (11) | 143 |
| All samples | 1,341 | 143 (10.7) | |||||
Fig 2.
By using the maximum parsimony method, we concatenated a phylogenetic tree of the partial gltA and rpoB sequences of the Bartonella isolates from this study and compared them to validated Bartonella species. The 60 different sequences were classified into 9 clusters (clusters A to I). Abbreivations for species of the infected hosts: Bbe, B. berdmorei; BI, B. indica; BS, B. savilei; Bsp, Bandicota sp.; Mca, M. caroli; Mce, M. cervicolor; Mco, M. cookii; Msp, Mus sp.; MS, M. surifer; NF, N. fulvescens; Rar, R. argentiventer; Ran, R. andamanensis; RE, R. exulans; Rno, R. norvegicus; Rph, R. tanezumi clade R3; RT, R. tanezumi; SM, S. murinus.
The presence of Bartonella infections in rodents has previously been reported in southeastern Asia, including Thailand, Lao PDR, and Indonesia (1, 3, 6, 15, 17). However, this study is the first report on Bartonella infections in rodents and shrews from Cambodia. Previously, Angelakis et al. (in 2008), Bai et al. (in 2009), Castle et al. (in 2004), and Saisongkorh et al. (in 2009) reported that B. berdmorei, B. indica, B. savilei, Cannomys badius, Mus cervicolor, M. surifer, Rattus species R. argentiventer, R. exulans, R. losea, R. norvegicus, and R. tanezumi collected in Lao PDR and Thailand were infected with Bartonella spp. (1, 3, 6, 15, 17); these findings were in agreement with our study results. Interestingly, we detected new infected rodent species in several localities, including Niviventer fulvescens and Rattus andamanensis, which were mainly trapped in forest areas, and Mus cookii, which was mainly trapped in agricultural areas.
The overall mean prevalence of Bartonella infection for the three countries was about 11%: Lao PDR, 11.9%; Thailand, 11%; Cambodia, 9.6%. In the present study, the prevalence of Bartonella in Lao PDR was lower than the prevalence reported by Angelakis et al. (2008). Those authors determined a prevalence of 25.5% for rodents trapped in agricultural lands and urban areas, which are different habitats from those in our study. In Thailand, our prevalence of Bartonella infection was similar to that reported by Castle et al. (6) and Saisongkorh et al. (15) (8.7% and 8.5%, respectively) for rodents trapped in the northern and northeastern parts of Thailand (the same region observed in our study); however, our reported prevalence of Bartonella infection was lower than that reported by Bai et al. (41.1%) (3) for rodents trapped in all regions of Thailand. The prevalence of Bartonella infection in shrews has only been reported in southeastern Asia, but a similar prevalence for S. murinus in Indonesia was reported by Winoto et al. (17). Interestingly, we found three putative new species in the three countries, based on the criteria of La Scola et al. (9). However, to eventually confirm whether all three unknown species are new species, further genetic identification by genome sequencing will be required and is ongoing.
In summary, this is the first report on Bartonella species isolated from a large number of rodents and shrews from areas near the Mekong river (Cambodia, Lao PDR, and Thailand) and the first report showing the important species diversity of rodents and shrews in these areas that were infected. The zoonotic Bartonella elizabethae was also found in this study. Rodents and shrews live in a wide range of habitats that are frequented by humans, which warrants further investigations on the transmission ecology of Bartonella in order to improve bartonellosis prevention. Since Bartonella species may be transmitted to humans by ticks, future surveys of Bartonella distributions within ticks in these areas are warranted.
Nucleotide sequence accession numbers.
The partial gltA and rpoB sequences of these new species (unknowns 1, 2, and 3) have been deposited in GenBank under accession numbers JX087928 to JX087945.
Supplementary Material
ACKNOWLEDGMENTS
We thank the CERoPath Project team (drivers, students, and staff) for sample collection, with special thanks to Hul Vibol, Kim Aun, Kim Blasdell, and Kone in Cambodia and Kittipong Chaisiri in Thailand. Special thanks are also due to Philippe Buchy (Pasteur Institute of Cambodia) and Bouneuang Douangboupha (NAFRI of Lao PDR). We also thank Annick Bernard and Linda Hadjadj for their technical assistance during the study.
This work was supported by the ANR 07 BDIV 012 CERoPath Project (Community Ecology of Rodents and Their Pathogens in Southeast Asia, France; www.ceropath.org), Infectipôle Sud and Center of Excellence on Agricultural Biotechnology, the Science and Technology Postgraduate Education and Research Development Office, the Office of Higher Education Commission, Ministry of Education. Part of this research was funded by the Center of Advanced Studies for Agriculture and Food, Institute for Advanced Studies, Kasetsart University.
Footnotes
Published ahead of print 14 September 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Angelakis E, et al. 2009. Molecular detection of Bartonella species in rodents from the Lao PDR. Clin. Microbiol. Infect. 15(Suppl 2):95–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bai HM, Yang FL, Yang H, Zhang Q. 2005. Study on Bartonella species in rodents in western Yunnan, China. Zhonghua Liu Xing Bing Xue Za Zhi 26:868–870 (In Chinese.) [PubMed] [Google Scholar]
- 3. Bai Y, Kosoy MY, Lerdthusnee K, Peruski LF, Richardson JH. 2009. Prevalence and genetic heterogeneity of Bartonella strains cultured from rodents from 17 provinces in Thailand. Am. J. Trop. Med. Hyg. 81:811–816 [DOI] [PubMed] [Google Scholar]
- 4. Birtles RJ, et al. 2001. Longitudinal monitoring of the dynamics of infections due to Bartonella species in UK woodland rodents. Epidemiol. Infect. 126:323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breitschwerdt EB, Kordick DL. 2000. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin. Microbiol. Rev. 13:428–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castle KT, et al. 2004. Prevalence and diversity of Bartonella in rodents of northern Thailand: a comparison with Bartonella in rodents from southern China. Am. J. Trop. Med. Hyg. 70:429–433 [PubMed] [Google Scholar]
- 7. Dehio C. 2004. Molecular and cellular basis of Bartonella pathogenesis. Annu. Rev. Microbiol. 58:365–390 [DOI] [PubMed] [Google Scholar]
- 8. Gundi VA, et al. 2004. Isolation of Bartonella rattimassiliensis sp. nov. and Bartonella phoceensis sp. nov. from European Rattus norvegicus. J. Clin. Microbiol. 42:3816–3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. La Scola B, Zeaiter Z, Khamis A, Raoult D. 2003. Gene-sequence-based criteria for species definition in bacteriology: the Bartonella paradigm. Trends Microbiol. 11:318–321 [DOI] [PubMed] [Google Scholar]
- 10. Liu Q, et al. 2010. Detection of Bartonella species in small mammals from Zhejiang Province, China. J. Wildl. Dis. 46:179–185 [DOI] [PubMed] [Google Scholar]
- 11. Pretorius AM, Beati L, Birtles RJ. 2004. Diversity of bartonellae associated with small mammals inhabiting Free State Province, South Africa. Int. J. Syst. Evol. Microbiol. 54:1959–1967 [DOI] [PubMed] [Google Scholar]
- 12. Raoult D, et al. 2006. First isolation of Bartonella alsatica from a valve of a patient with endocarditis. J. Clin. Microbiol. 44:278–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roux V, Raoult D. 1995. Inter- and intraspecies identification of Bartonella (Rochalimaea) species. J. Clin. Microbiol. 33:1573–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saisongkorh W, Rolain JM, Suputtamongkol Y, Raoult D. 2009. Emerging Bartonella in humans and animals in Asia and Australia. J. Med. Assoc. Thai. 92:707–731 [PubMed] [Google Scholar]
- 15. Saisongkorh W, Wootta W, Sawanpanyalert P, Raoult D, Rolain JM. 2009. “Candidatus Bartonella thailandensis”: a new genotype of Bartonella identified from rodents. Vet. Microbiol. 139:197–201 [DOI] [PubMed] [Google Scholar]
- 16. Tea A, Alexiou-Daniel S, Papoutsi A, Papa A, Antoniadis A. 2004. Bartonella species isolated from rodents, Greece. Emerg. Infect. Dis. 10:963–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winoto IL, et al. 2005. Bartonella species in rodents and shrews in the greater Jakarta area. Southeast Asian J. Trop. Med. Public Health 36:1523–1529 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.