Abstract
Bacterial infections, including surgical site infections (SSI), are a common and serious complication of diabetes. Staphylococcus aureus, which is eliminated mainly by neutrophils, is a major cause of SSI in diabetic patients. However, the precise mechanisms by which diabetes predisposes to staphylococcal infection are not fully elucidated. The effect of insulin on this infection is also not well understood. We therefore investigated the effect of insulin treatment on SSI and neutrophil function in diabetic mice. S. aureus was inoculated into the abdominal muscle in diabetic db/db and high-fat-diet (HFD)-fed mice with or without insulin treatment. Although the diabetic db/db mice developed SSI, insulin treatment ameliorated the infection. db/db mice had neutrophil dysfunction, such as decreased phagocytosis, superoxide production, and killing activity of S. aureus; however, insulin treatment restored these functions. Ex vivo treatment (coincubation) of neutrophils with insulin and euglycemic control by phlorizin suggest that insulin may directly activate neutrophil phagocytic and bactericidal activity independently of its euglycemic effect. However, insulin may indirectly restore superoxide production by neutrophils through its euglycemic effect. HFD-fed mice with mild hyperglycemia also developed more severe SSI by S. aureus than control mice and had impaired neutrophil phagocytic and bactericidal activity, which was improved by insulin treatment. Unlike db/db mice, in HFD mice, superoxide production was increased in neutrophils and subsequently suppressed by insulin treatment. Glycemic control by insulin also normalized the neutrophil superoxide-producing capability in HFD mice. Thus, insulin may restore neutrophil phagocytosis and bactericidal activity, thereby ameliorating SSI.
INTRODUCTION
The number of patients with diabetes mellitus has increased greatly worldwide (8, 48). It is well known that diabetic patients are more prone to bacterial infections, including surgical site infections (SSI), than healthy individuals. Although many clinical reports have demonstrated that glycemic control reduces the risk of infections, the precise mechanisms by which diabetes predisposes to infections are not well understood (2, 21, 38). Control of bacterial infections has become more important for diabetic patients than in the past, because of the increase in diabetic patients and their susceptibility to infections. Foot infections following skin ulceration are also common causes of hospitalization for diabetic patients (6). These infectious complications seriously impair prognoses for diabetic patients (44).
Gram-positive bacteria cause more than half of cases of diabetes-related wound infections. Especially, Staphylococcus aureus is a major pathogen in these infections (44). Methicillin-resistant S. aureus (MRSA) also has become prevalent among both nosocomial and community-acquired infections in diabetic patients (44). Neutrophils play crucial roles in eliminating bacteria, including S. aureus, from hosts (22). Therefore, neutrophil dysfunction may be involved in the high susceptibility of diabetic patients to staphylococcal infection. However, results remain conflicting and equivocal with regard to neutrophil function in diabetic hosts (3, 15, 40). Although analyses of neutrophil function among diabetic patients may provide information to enable diabetic patients to more effectively overcome infectious complications, it has yet to be fully elucidated how diabetic hyperglycemia affects the neutrophil-mediated host defense and also how insulin treatment affects diabetes-related infections.
Diabetic db/db mice provide a monogenic model of obesity and type 2 diabetes (9). Insulin resistance is the earliest phenotypic change in db/db mice (10), and by approximately 8 to 12 weeks of age, these mice are severely obese, hyperglycemic, and insulin resistant (10). High-fat-diet (HFD)-induced obese and diabetic wild-type mice are also considered to be a model of type 2 diabetes (39). We herein investigated the effect of insulin treatment on SSI by S. aureus in diabetic db/db mice and HFD-fed wild-type mice, focusing on their neutrophil function.
MATERIALS AND METHODS
This study was conducted according to the guidelines of the Institutional Review Board for the Care of Animal Subjects at the National Defense Medical College, Japan.
Diabetic db/db mice and HFD-fed mice.
Male, 8-week-old diabetic db/db mice (C57BLKS/J lar-+Leprdb/+ Leprdb) and control lean nondiabetic mice (male, 8 weeks, C57BLKS/J lar-m+/m+) were purchased from Japan SLC (Shizuoka, Japan). Male, 4-week-old C57BL/6 mice were also purchased from Japan SLC and were fed a high fat diet (HFD) containing 60% fat (58Y1, Test Diet; PMI Nutrition International, Richmond, IN) or a control chow diet (CD) for 12 weeks. Twelve weeks after high-fat feeding, the animals' body weights increased to approximately 40 g and their blood glucose levels were approximately 12 to 14 mM. These mice were continuously fed the HFD during insulin treatment and postoperative observation. All mice were housed under controlled conditions with a 12-h light/dark cycle and given food (HFD or CD) and water ad libitum.
Insulin or phlorizin treatment.
Neutral protamine Hagedron (NPH) insulin (Humulin N; Eli Lilly, Indianapolis, IN) was subcutaneously (s.c.) injected into db/db mice and HFD-fed mice (16 weeks old) daily, morning and evening, for a week in doses individually adjusted to control blood glucose levels to below 8 mM. Blood glucose levels were measured routinely by a tail prick method by using a glucose monitoring device (FreeStyle, Nipro Co., Osaka, Japan). Each diabetic db/db mouse required approximately 10 to 50 IU/day of insulin to control blood glucose levels to <8 mM using a sliding scale of insulin. All db/db mice received almost the same doses of insulin for euglycemic control. Age-matched nontreated db/db mice and control mice (lar-m+/m+) similarly received s.c. injections with the vehicle (saline). Each HFD-fed wild-type mouse also required approximately 1 to 10 IU/day of insulin to control blood glucose levels. Age-matched, nontreated HFD and CD wild-type mice similarly received injections with vehicle (saline). To examine the effect of insulin treatment on the mice fed a control diet, the C57BL/6 mice (male, 8 weeks; Japan SLC) received 10 or 5 IU/day of insulin or vehicle (saline) for 3 days. Phlorizin (Sigma-Aldrich, Deisenhofen, Germany) directly decreases blood glucose independent of insulin-involved glucose metabolism, because phlorizin blocks glucose uptake/reabsorption through inhibition of the sodium-glucose symporters located in the proximal renal tubule and intestinal mucosa (13). Phlorizin dissolved in propylene glycol (Wako Pure Chemical Industries, Osaka, Japan) was also s.c. injected to db/db mice daily, morning and evening, for a week in doses individually adjusted to control blood glucose levels at approximately 11 mM. Each db/db mouse also required approximately 5 to 20 mg/day of phlorizin to control blood glucose levels. Age-matched db/db and control mice (lar-m+/m+) similarly received saline injections.
SSI and measurement of neutrophil count.
After anesthetization of mice using an intraperitoneal injection of pentobarbital (50 mg/kg; Abbott Laboratories, Abbott Park, IL), a 5-mm midline skin incision to expose the abdominal muscle was made. Thereafter, to develop a surgical site infection (SSI), 1 × 106 CFU of S. aureus (S. aureus 209P, ATCC 6538P) dissolved in 20 μl of PBS was inoculated into the subfascial layer of the abdominal muscle using a syringe with a 29-gauge needle (Terumo, Tokyo, Japan) under a microscope. The incisions were carefully closed using 5-0 silk. Seven days after bacterial inoculation, the lesions of SSI were evaluated. Blood samples were obtained from the retro-orbital plexus in mice after surgery and bacterial inoculation to measure neutrophil counts using a hematology analyzer PEC-170 (Beckman Coulter, Inc., Miami, FL).
Isolation of neutrophils.
As previously described (22, 23), a blood sample was drawn into a heparinized syringe from the abdominal inferior vena cava under lethal pentobarbital anesthesia. Leukocytes were isolated by dextran sedimentation. Thereafter, neutrophils were separated from mononuclear cells by centrifugation using Pancoll for mouse (PAN Biotech GmbH, Aidenbach, Germany) followed by hypotonic lysis of erythrocytes. The resultant cells contained nearly 90% neutrophils, as assessed by microscopy with Wright-Giemsa stain.
Ex vivo treatment (coincubation) of neutrophils with insulin.
Neutrophils (2.5 × 106/ml) were obtained from nontreated db/db and control mice (without surgery) and were incubated with 10 mIU/ml of insulin or vehicle (saline) in 10% fetal bovine serum (FBS)-RPMI 1640 medium for 2 h in 5% CO2 at 37°C. To determine the in vitro insulin concentration, we used as a reference the serum insulin levels in the db/db mice that resulted in appropriate glycemic control by insulin (30). Thereafter, the following functional analyses of neutrophils were performed.
Determination of bactericidal activity of neutrophils against S. aureus.
Neutrophils (5 × 105/200 μl of medium without antibiotic) were cultured with 5 × 103 CFU of S. aureus for 6 h in 5% CO2 at 37°C, as previously described (22). Thereafter, culture medium was serially diluted 10-fold with phosphate-buffered saline (PBS), placed using a spiral platter on brain heart infusion agar plates, and incubated at 37°C for 24 h. The number of viable bacteria was then counted according to the observed colonies on the agar plates.
Determination of microsphere phagocytosis by neutrophils.
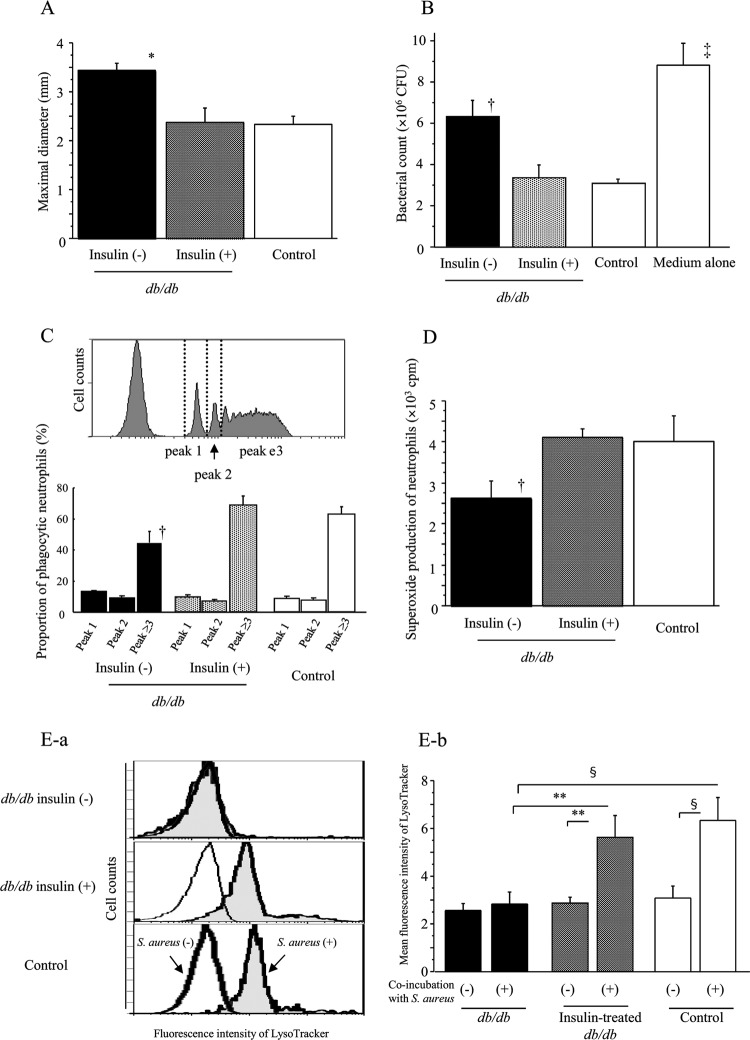
Neutrophils were incubated with Fluoresbrite YG (FITC) carboxylate microspheres (75-nm diameter; Polysciences Europe, Eppelheim, Germany; here called FITC microspheres; 1 × 108/ml) in 500 μl medium for 20 min in 5% CO2 at 37°C. After staining neutrophils with phycoerythrin (PE)-conjugated anti-mouse Gr-1 monoclonal antibody (MAb) (eBioscience, San Diego, CA), phagocytosis of FITC microspheres by Gr-1+ neutrophils was analyzed using FC500 instruments (Beckman Coulter Inc.) as previously described (22). FITC fluorescent intensity is dependent on the number of ingested microspheres. Peaks on the histogram in Fig. 3C correspond to neutrophils that contain no ingested microspheres or one (peak 1), two (peak 2), or more (peak ≥3) microspheres (from left to right in figure, respectively).
Fig 3.
Effect of insulin treatment on the maximal diameter of SSI at 7 days after surgery in db/db mice (A). Effect of insulin treatment on neutrophil bactericidal activity against S. aureus (B), microsphere-phagocytic activity (C), PMA-induced superoxide production (D), and LysoTracker response to S. aureus (E) in db/db mice just before surgery. (B to E) Neutrophils were obtained from the db/db or control mice after insulin/sham treatment for 1 week (without surgery) to examine their function. (C) The percentage of each peak is shown for the three mouse groups in the lower panel. Fluorescence intensity of LysoTracker in neutrophils with or without S. aureus coincubation for 20 min is shown (E-a; data shown are representative in each group), and mean fluorescence intensity of LysoTracker in neutrophils is shown in the right panel (E-b). Data are means ± SE from 10 mice in each group: *, P < 0.01; †, P < 0.05 versus other groups; ‡, P < 0.01 versus insulin + db/db and control, P < 0.05 versus insulin − db/db; §, P < 0.01; **, P < 0.05.
Determination of superoxide production by neutrophils.
Superoxide production of neutrophils was determined by 2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo[1,2-alpha]pyrazin-3-one (MCLA)-dependent chemiluminescence as described previously (23, 27). A cuvette containing neutrophils (5 × 105) and MCLA (2 μM) in 200 μl of Hank's balanced salt solution was placed in a luminometer (Gene Light GL-200; Microtec Co., Chiba, Japan). Thereafter, 4 μg of phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) was added to the cuvette to determine the MCLA-dependent chemiluminescence intensity induced by PMA. After adding PMA, superoxide dismutase (final concentration, 0.5 μM; Sigma-Aldrich) was then added to assess the role of superoxide anion in the MCLA-dependent chemiluminescence emission from neutrophils.
Determination of fluorescence intensity of LysoTracker in neutrophils stimulated with S. aureus.
Neutrophils (5 × 105/200 μl medium) were incubated with or without 5 × 103 CFU of S. aureus for 5 min in 5% CO2 at 37°C. Thereafter, neutrophils were further incubated with 100 nM LysoTracker Red DND-99 (Invitrogen Life Technologies, Carlsbad, CA) for 15 min followed by staining with FITC-conjugated anti-mouse Gr-1 MAb (eBioscience) for 10 min at 4°C. Fluorescence intensity of LysoTracker in Gr-1+ neutrophils was measured using FC500 instruments (Beckman Coulter Inc.), as previously described (31). LysoTracker freely permeates the cell membrane and labels and tracks acidic organelles in live cells, and its staining indicates phagosome acidification and maturation (31, 41).
Pathological examination.
Specimens of SSI were obtained from the sites of surgery and bacterial inoculation at 7 days after surgery. Sections were prepared from formalin-fixed, paraffin-embedded tissue samples stained with routine procedures by hematoxylin and eosin.
Statistical analysis.
Data are presented as mean values ± standard errors (SE). Statistical analyses were performed using the StatView 4.02J software package (Abacus Concepts, Berkeley, CA). Statistical evaluations were compared using standard one- or two-way analysis of variance followed by the Fisher's protected least significant difference test. P values of <0.05 were considered to indicate a significant difference.
RESULTS
Blood glucose levels after surgery in insulin-treated db/db mice.
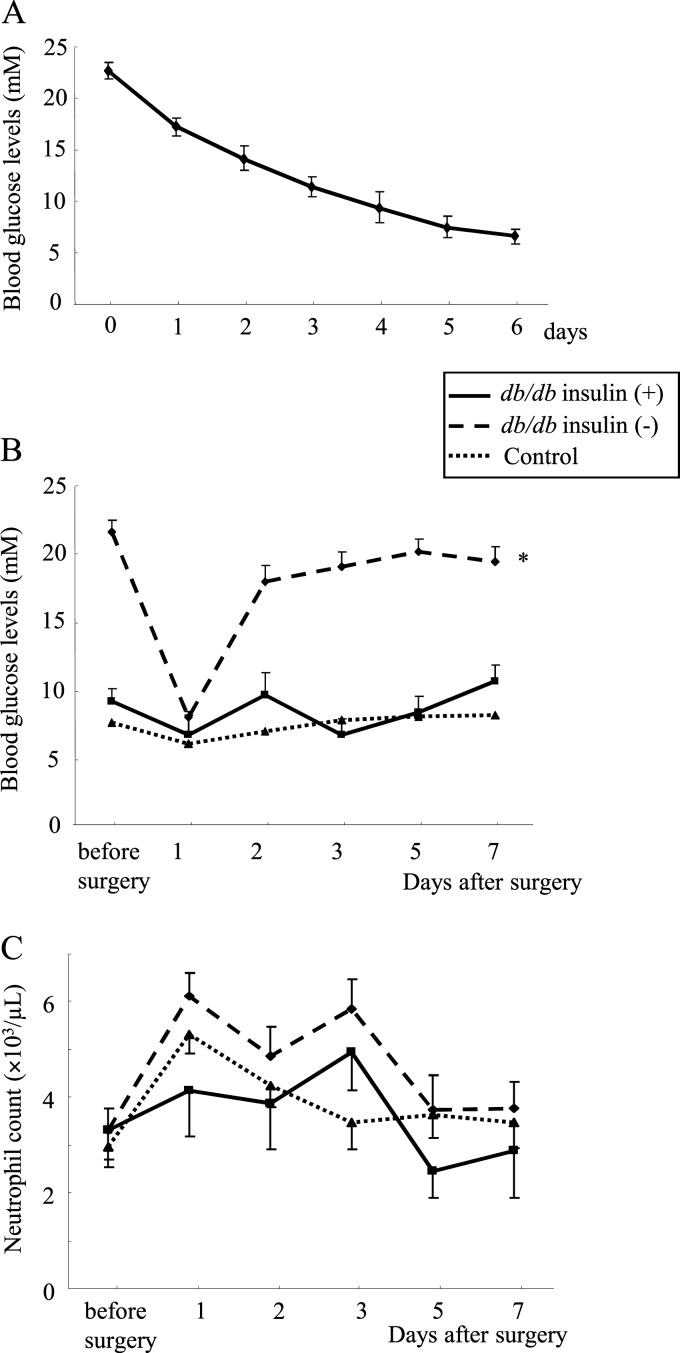
Insulin treatment of db/db mice for 1 week decreased their blood glucose levels to less than 7 mM (Fig. 1A) without changes in body weight (before treatment, 37 ± 1; after, 39 ± 1 g). Thereafter, db/db mice with and without insulin treatment and control mice underwent surgery and bacterial inoculation. One day after surgery, the blood glucose levels markedly decreased in nontreated db/db mice but the next day rapidly returned to a hyperglycemic state (>17 mM) (Fig. 1B). In contrast, insulin-treated db/db mice as well as control mice kept their glucose levels at approximately 6 to 12 mM after surgery (Fig. 1B). Body weight did not change between the period before and after surgery in any of the three groups, although both insulin-treated and nontreated db/db mice were markedly heavier than control mice (db/db mice, 40 ± 1 g; insulin-treated db/db mice, 37 ± 1 g; control, 19 ± 0.2 g, at 7 days after surgery; P < 0.01). No significant differences in neutrophil counts were statistically observed among the three mouse groups (Fig. 1C).
Fig 1.
(A) Blood glucose levels in insulin-treated db/db mice. Diabetic db/db mice (n = 10) were treated with insulin for 1 week, and their blood glucose levels were monitored. Changes in blood glucose levels (B) and neutrophil counts (C) after surgery and bacterial inoculation. Data are means ± SE from 10 mice in each group: *, P < 0.01 versus others.
Improvement of SSI by insulin treatment in db/db mice.
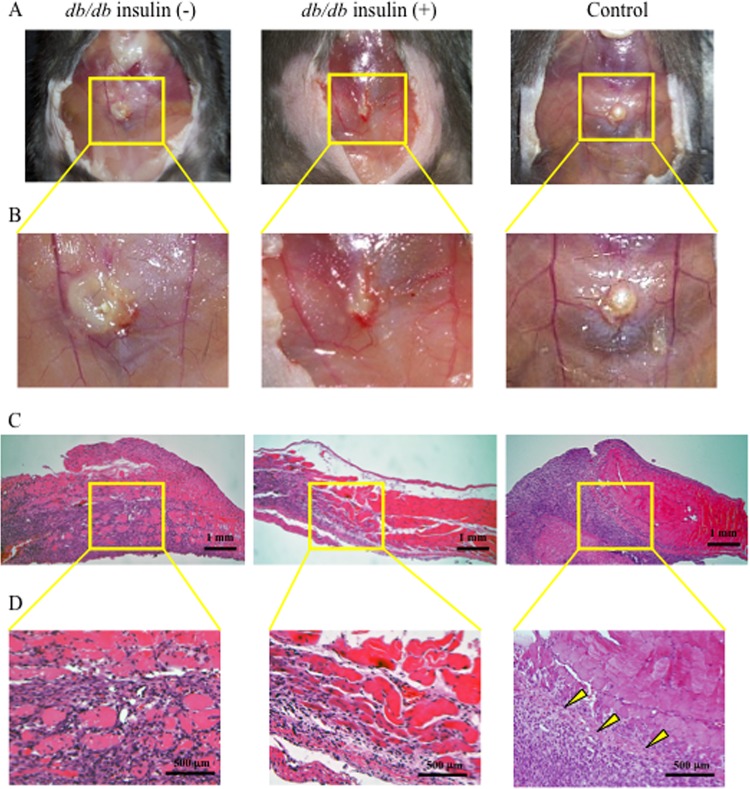
Although db/db mice had significantly larger SSI 7 days after surgery than control mice, insulin treatment substantially suppressed development of SSI in db/db mice (Fig. 2A and B and Fig. 3A). In control mice, there was a proliferation of fibroblasts in the border of the infectious lesion, suggesting the recovery phase of tissue injury/inflammation (as indicated by arrowheads in Fig. 2D), while in nontreated db/db mice, there was diffuse infiltration of neutrophils in the border of the lesions, suggesting vigorous expansion of SSI (Fig. 2C and D). However, such marked infiltration of neutrophils was not observed in the insulin-treated db/db mice (Fig. 2C and D). Proliferation of fibroblasts in the border of the lesions was not found in the db/db mice even by insulin treatment (Fig. 2C and D).
Fig 2.
Macroscopic (A, B) and microscopic (C, D) findings of the SSI at day 7 after surgery in nontreated db/db, insulin-treated db/db, and control mice. The mouse skin was widely opened to examine the SSI. Panels B and D are magnified images of the square areas in panels A and C in each group, respectively. Representative data are shown for each group.
Improvement of neutrophil dysfunction by insulin treatment in db/db mice.
We examined neutrophil function in insulin-treated and nontreated db/db mice and control mice (without surgery). Although S. aureus-killing activity of neutrophils from db/db mice was remarkably impaired compared to that of control mice, insulin treatment significantly improved bactericidal activity of neutrophils in db/db mice (Fig. 3B). In diabetic db/db mice, the proportion of neutrophils having potent phagocytic activity was decreased (Fig. 3C, peak ≥3), but insulin treatment markedly restored this activity (Fig. 3C). PMA-induced superoxide production by neutrophils was decreased in db/db mice; however, insulin treatment significantly improved production (Fig. 3D). Although no significant differences in fluorescence intensity of LysoTracker in neutrophils, which indicates phagolysosome maturation (31, 41), were observed among the three mouse groups under a nonstimulative condition, in insulin-treated db/db mice as well as control mice, there were significant increases in the LysoTracker-intensity of neutrophils by stimulation with S. aureus, while no such increase was shown in nontreated db/db mice (Fig. 3E-a and -b). We also examined the effect of insulin treatment on neutrophil function in control diet-fed wild-type mice. When the mice received 10 IU/day of insulin for 3 days, they showed severe hypoglycemia (<1.2 mM), and half of them (5/10) died within 3 days. Neutrophils from the survived mice showed a significantly lower phagocytic activity than that of the vehicle (saline)-treated mice (microsphere phagocytosis; peak ≥3; with insulin treatment, 22% ± 3%, versus without insulin, 56% ± 2%; P < 0.01) and did not show a significant superoxide production by PMA stimulation (data not shown), suggesting an impairment in neutrophil function. Treatment with 5 IU/day of insulin for 3 days also rendered the mice mildly hypoglycemic (approximately 2.5 to 3 mM), but no mice died. Their neutrophils did not show significant functional changes, such as phagocytic activity, compared to those from vehicle-treated mice (data not shown).
Direct effects of insulin on neutrophil function in db/db mice.
Neutrophils were also obtained from nontreated db/db mice and control mice (without surgery) to coincubate with or without insulin. Coincubation with insulin significantly restored S. aureus-killing activities in vitro of neutrophils obtained from db/db mice, suggesting that insulin directly stimulates neutrophils to restore bactericidal activity, although insulin did not further augment neutrophil bactericidal activity in the control mice (Table 1). Insulin also directly augmented phagocytic activity of neutrophils in not only db/db mice but also in control mice, suggesting a direct enhancing effect of insulin on neutrophil phagocytosis (Table 1). Coincubation with insulin tended to increase superoxide production by neutrophils in db/db mice; however, the difference was not significant (Table 1). Nevertheless, coincubation with insulin significantly restored the LysoTracker reaction of neutrophils to bacterial stimulation (Table 1), suggesting the functional augmentation of phagolysosomes of neutrophils.
Table 1.
Effect of in vitro insulin coincubation on neutrophil functionsa
| Characteristic | Result |
||||
|---|---|---|---|---|---|
| db/db | Control | Medium alone | |||
| Coincubation with insulin | − | + | − | + | |
| Bacterial count after 6 h coculture (×106 CFU) | 6.9 ± 1.2† | 4.3 ± 0.8 | 4.6 ± 0.5 | 4.4 ± 0.7 | 9.3 ± 1.2§ |
| Proportion of phagocytic neutrophils (peak ≥ 3) (%) | 44 ± 2* | 53 ± 3 | 58 ± 1 | 71 ± 2* | |
| Superoxide production of neutrophils (PMA stimulation; ×103 cpm) | 2.3 ± 0.6‡ | 2.5 ± 0.5‡ | 4.2 ± 0.5 | 4.1 ± 0.6 | |
| Mean fluorescence intensity of LysoTracker (S. aureus stimulation) | 2.9 ± 0.2† | 5.6 ± 1.3 | 5.8 ± 0.8 | 5.7 ± 0.9 | |
Neutrophils obtained from the nontreated db/db and control mice (without surgery) were incubated with (+) or without (−) 10 mIU/ml of insulin or vehicle (saline) for 2 h in 5% CO2 at 37°C. Thereafter, neutrophil functions were examined. Mean flow intensity of LysoTracker in neutrophils coincubated with S. aureus for 20 min are shown. All data are pooled from three or four individual experiments with five mice in each group. Data are means ± SE.
, P < 0.01;
, P < 0.05 versus other groups;
, P < 0.05 versus controls with and without insulin;
, P < 0.01 versus db/db with insulin, with controls, with and without insulin, P < 0.05 versus db/db without insulin.
Effects of phlorizin on neutrophil function in db/db mice.
Phlorizin treatment markedly decreased blood glucose levels in db/db mice (before treatment, 23 ± 1 mM; after, 12 ± 1 mM; P < 0.01) without significant changes in their body weight (before treatment, 37 ± 1 g; after, 36 ± 2 g). The antihyperglycemic effect of phlorizin is independent of insulin-involved glucose metabolism (13). In contrast to insulin treatment, phlorizin treatment improved neither bactericidal nor phagocytic activity of neutrophils in db/db mice, although superoxide production and LysoTracker intensity were significantly improved (Table 2).
Table 2.
Effect of phlorizin treatment on neutrophil functionsa
| Characteristic | Value |
|||
|---|---|---|---|---|
|
db/db |
Control | Medium alone | ||
| Phlorizin (minus) | Phlorizin (+) | |||
| Bacterial count after 6 h coculture (×106 CFU) | 6.2 ± 0.3 | 5.7 ± 0.3 | 3.5 ± 0.5* | 9.8 ± 1.5* |
| Proportion of phagocytic neutrophils (peak ≥ 3) (%) | 39 ± 6 | 35 ± 5 | 55 ± 5† | |
| Superoxide production of neutrophils (PMA stimulation; ×103 cpm) | 2.7 ± 0.3‡ | 3.6 ± 0.3 | 4.2 ± 0.3 | |
| Mean fluorescence intensity of LysoTracker (S. aureus stimulation) | 2.8 ± 0.2† | 4.6 ± 0.4 | 5.2 ± 0.8 | |
Neutrophils were obtained from the phlorizin-treated and nontreated db/db mice and control mice (without surgery). Mean flow intensity of LysoTracker in neutrophils coincubated with S. aureus for 20 min are shown. Data are means ± SE from 10 mice in each group.
, P < 0.01;
, P < 0.05 versus other groups;
, P < 0.01 versus control, P < 0.05 versus phlorizin-treated db/db.
Blood glucose levels after insulin treatment in HFD-fed wild-type mice.
Although HFD-fed wild-type mice had substantially higher glucose levels than chow-diet (CD)-fed wild-type mice, insulin treatment decreased their glucose levels (before treatment, 13 ± 1 mM; after, 6 ± 1 mM; P < 0.01) without a change in body weight (before/after, 40 ± 1 g). After surgery, blood glucose levels in insulin-treated HFD-fed wild-type mice were maintained at approximately 5 to 6 mM, while in nontreated HFD-fed wild-type mice and CD-fed wild-type mice, the blood glucose levels were approximately 9 to 10 mM and 5 to 7 mM, respectively. No changes in body weight between before and after surgery were observed in any group, although both insulin-treated and nontreated HFD-fed mice were heavier than the CD mice (both insulin-treated and nontreated HFD-fed mice, 40 ± 1 g, versus CD-fed mice, 29 ± 0.3 g, P < 0.01, at 7 days after surgery). No significant changes in neutrophil counts were observed before and after surgery in any group (data not shown).
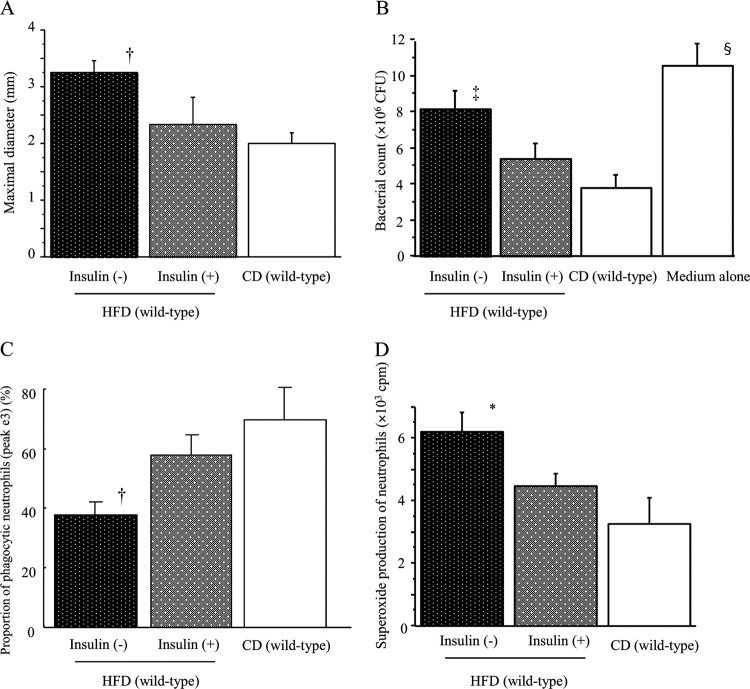
Improvement of SSI and neutrophil dysfunction by insulin treatment in HFD-fed wild-type mice.
HFD-fed wild-type mice with and without insulin treatment and CD-fed wild-type mice underwent surgery and S. aureus inoculation. Although HFD mice developed significantly larger SSI 7 days after surgery than the CD mice, insulin treatment markedly decreased the size of the SSI in HFD mice (Fig. 4A). Next, we examined neutrophil function in these mice. Bactericidal activity and phagocytic activity of neutrophils were remarkably decreased in HFD wild-type mice, and insulin treatment significantly restored those activities (Fig. 4B and C). In contrast, unlike findings for db/db mice, neutrophils in HFD wild-type mice had a potent superoxide-producing capability in comparison with CD wild-type mice (Fig. 4D). Interestingly, insulin treatment significantly suppressed the enhanced superoxide production by neutrophils in HFD-fed mice (Fig. 4D). However, no significant differences in the LysoTracker response of neutrophils to S. aureus were observed among the three mouse groups (data not shown).
Fig 4.
Effect of insulin treatment on the maximal diameter of SSI at day 7 after surgery in HFD-fed wild-type mice (A). Effect of insulin treatment on neutrophil bactericidal activity against S. aureus (B), microsphere-phagocytic activity (C), and PMA-induced superoxide production (D) in HFD-fed wild-type mice. (B to D) Neutrophils were obtained from the HFD-fed or control mice after insulin/sham treatment for 1 week (without surgery) to examine their function. Data are means ± SE from 10 mice in each group: *, P < 0.01 versus other groups; †, P < 0.01 versus CD, P < 0.05 versus insulin + HFD; ‡, P < 0.01 versus CD, P < 0.05 versus insulin + HFD and medium alone; §, P < 0.01 versus insulin + HFD and CD, P < 0.05 versus insulin − HFD.
DISCUSSION
Impaired neutrophil bactericidal activity is crucial in the defense against S. aureus infection by diabetic mice, because S. aureus is eliminated mainly by neutrophils (22). Once neutrophils encounter bacteria, they are activated to promptly phagocytose them into phagosomes, where they are digested by reactive oxygen species (ROS), i.e., superoxide anions, and proteolytic enzymes (16). Thus, phagocytosis and superoxide production are important for neutrophils to kill bacteria. Phagolysosome maturation, which we assessed by the LysoTracker reaction, also participates in bacterial digestion by neutrophils. These neutrophil functions require ATP-involved energy, which is produced mainly by the metabolism of glucose to lactate (5). Since neutrophils from diabetic hosts represent impaired glucose metabolism (14), the reduced energy of neutrophils in diabetic hosts may render them functionally refractory. Because db/db mice have a recessive, autosomal mutation in the leptin receptor (9, 10), we could not completely deny the possibility that leptin receptor deficiency may have a role in neutrophil dysfunction in db/db mice (46). Park et al. demonstrated that db/db mice are susceptible to foot and ankle infection by S. aureus due to neutrophil dysfunction (34). Our present results showed the potent effect of insulin in improving neutrophil dysfunction and ameliorating staphylococcal infection in db/db mice.
Ex vivo treatment (coincubation) of neutrophils with insulin and euglycemic control by phlorizin suggest that insulin treatment renders diabetic mice resistant to infections independent of its antihyperglycemic effect, although insulin-induced severe hypoglycemia in normal mice may seriously damage neutrophil function. Clinical reports have demonstrated that glycemic control decreased perioperative infections (2, 21, 38). These beneficial effects of glycemic control on infections may be caused by the direct effect of insulin, because most diabetic/hyperglycemic patients are treated with insulin. In contrast, insulin treatment for db/db mice may indirectly, probably through its antihyperglycemic effect, restore the superoxide-producing capability of neutrophils. Hypertonic glucose reportedly exerted a dose-dependent inhibitory effect on superoxide production by rat neutrophils (37). Activation of NADP (NADPH) oxidase is closely related to superoxide production by neutrophils (16, 47). In the presence of excessive glucose levels, the hexokinase pathway (glucose is converted to glucose-6-phosphatase) is saturated and glucose is converted to sorbitol using the polyol pathway in neutrophils. Activation of the polyol pathway decreases the availability of NADPH, leading to reductions of NADPH oxidase activity and superoxide production (43).
Activated neutrophils release superoxide anions not only into phagosomes but also into the extracellular surroundings (16). We measured phagosomal and extracellular superoxide together by a luminometer. Hydrogen peroxide that is formed from dismutation of the superoxide radical is bactericidal only at high concentrations (e.g., in phagosomes), but extracellularly generated superoxide does not kill bacteria and presumably accounts for the destructive capacity of neutrophils (19). To be effective in combating bacteria, superoxide should be produced near the targets in phagosomes. LysoTracker staining may be involved in superoxide production in phagolysosomes of activated neutrophils (4). On one hand, insulin may directly augment neutrophil phagocytosis, resulting in an increase in microbe-ingesting phagolysosomes and augmentation of the LysoTracker reaction. On the other hand, euglycemia by phlorizin may augment not only extracellular but also phagosomal superoxide production in neutrophils, which may also possibly augment the LysoTracker reaction. Because phlorizin did not augment bactericidal activity of neutrophils, phagocytosis, which is the first step in the bactericidal process, may be crucial for killing of bacteria.
Although hyperglycemia-induced ROS production from β-cell mitochondria is known to contribute to the development of insulin resistance and pancreatic β-cell failure (25, 35), conflicting results have been reported about ROS production by neutrophils in diabetes. Some reports have demonstrated that ROS generation by neutrophils are impaired/decreased in diabetic hosts, resulting in attenuating host defenses (12, 32). Other reports have demonstrated that hyperglycemia caused by diabetes increases ROS generation by neutrophils, resulting in oxidative stress (3, 26, 42). The db/db mice used in the current study had substantially higher blood glucose levels than the HFD-fed wild-type mice (23 ± 1 versus 13 ± 1 mM, P < 0.01). Diabetic db/db mice seem to have a more severe diabetic state than HFD mice.
Type 1 diabetes results from destruction of pancreatic β-cells, leading to absolute insulin deficiency, while type 2 diabetes results from insulin resistance caused by obesity, leading to relative insulin deficiency (1). Systemic inflammation, although of low grade, is a feature of the insulin-resistant states related to obesity and type 2 diabetes (11). Patients with type 2 diabetes have elevated levels of proinflammatory cytokines, such as TNF in skeletal muscle (36), adipose tissue (17), and sera (28). Proinflammatory cytokines, including TNF, augment superoxide production by neutrophils (45). This hyperproductivity of superoxide by neutrophils was also observed in the HFD mice but not in the db/db mice. From this aspect, HFD mice may be suitable as a model of type 2 diabetes, in which hyperglycemia develops gradually and in the earlier stages is often not severe enough for the patients to be aware of the clinical symptoms of diabetes (1).
It was shown that p47phox is a key protein in the assembly of NADPH oxidase and that the translocation of cytosolic p47phox to plasma membrane is important for ROS generation (33, 47). Although severe hyperglycemia may inhibit neutrophil superoxide production (37), mild hyperglycemia mechanistically stimulates the translocation of p47phox (29, 33), leading to priming neutrophils to produce superoxide. HFD-fed mice reportedly upregulate p47phox expression in endothelial cells (7), so it could be speculated that their neutrophils also may upregulate p47phox-mediated superoxide production. In contrast, db/db mice of the C57BL/6J strain reportedly showed no significant expression of p47phox in neutrophils (18). Leptin receptor genes and the p47phox gene are independent of each other, because the former mutated genes are mapped on chromosome 4 in db/db mice (24), while the latter gene is mapped on chromosome 5 (20). Thus, the difference in diabetic states between db/db and HFD mice may affect superoxide-producing capability of neutrophils, although their bactericidal/phagocytic activities were similarly impaired in both mice.
Interestingly, glycemic control by insulin may normalize either excessive or suppressive superoxide-producing capability of neutrophils in HFD-fed or db/db mice, respectively. Generally, insufficient superoxide production by neutrophils is harmful for hosts because it reduces bactericidal activity while excessive superoxide production by neutrophils is also injurious to hosts because of its tissue destructive properties. Although normalization of blood glucose appeared to be beneficial to both groups of diabetic mice, further studies are required to reveal the precise mechanisms of insulin-induced pleiotropic effects on superoxide production by neutrophils.
ACKNOWLEDGMENTS
None of the authors has a conflict of interests.
The present study was supported in part by a grant-in-aid for a Special Research Program (host defense against internal and external factors) from the Ministry of Defense to M.K.
Footnotes
Published ahead of print 1 October 2012
REFERENCES
- 1. American Diabetes Association 2010. Diagnosis and classification of diabetes mellitus. Diabetes Care 33(Suppl 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ata A, Lee J, Bestle SL, Desemone J, Stain SC. 2010. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch. Surg. 145:858–864 [DOI] [PubMed] [Google Scholar]
- 3. Ayilavarapu S, et al. 2010. Diabetes-induced oxidative stress is mediated by Ca2+-independent phospholipase A2 in neutrophils. J. Immunol. 184:1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bassoe CF, et al. 2003. Investigations of phagosomes, mitochondria, and acidic granules in human neutrophils using fluorescent probes. Cytometry B Clin. Cytom. 51:21–29 [DOI] [PubMed] [Google Scholar]
- 5. Borregaard N, Herlin T. 1982. Energy metabolism of human neutrophils during phagocytosis. J. Clin. Invest. 70:550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boulton AJ, Kirsner RS, Vileikyte L. 2004. Clinical practice. Neuropathic diabetic foot ulcers. N. Engl. J. Med. 351:48–55 [DOI] [PubMed] [Google Scholar]
- 7. Chen JX, Stinnett A. 2008. Critical role of the NADPH oxidase subunit p47phox on vascular TLR expression and neointimal lesion formation in high-fat diet-induced obesity. Lab. Invest. 88:1316–1328 [DOI] [PubMed] [Google Scholar]
- 8. Cheung BM, et al. 2009. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am. J. Med. 122:443–453 [DOI] [PubMed] [Google Scholar]
- 9. Coleman DL. 1982. Diabetes-obesity syndromes in mice. Diabetes 31:1–6 [DOI] [PubMed] [Google Scholar]
- 10. Coleman DL, Hummel KP. 1974. Hyperinsulinemia in pre-weaning diabetes (db) mice. Diabetologia 10(Suppl):607–610 [DOI] [PubMed] [Google Scholar]
- 11. Dandona P, Aljada A, Bandyopadhyay A. 2004. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 25:4–7 [DOI] [PubMed] [Google Scholar]
- 12. Daoud AK, Tayyar MA, Fouda IM, Harfeil NA. 2009. Effects of diabetes mellitus versus in vitro hyperglycemia on select immune cell functions. J. Immunotoxicol. 6:36–41 [DOI] [PubMed] [Google Scholar]
- 13. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. 2005. Phlorizin: a review. Diabetes Metab. Res. Rev. 21:31–38 [DOI] [PubMed] [Google Scholar]
- 14. Esmann V. 1983. The polymorphonuclear leukocyte in diabetes mellitus. J. Clin. Chem. Clin. Biochem. 21:561–567 [PubMed] [Google Scholar]
- 15. Fejfarova V, et al. 2006. Effect of acute hyperglycemia and/or hyperinsulinemia on polymorphonuclear functions in healthy subjects. Metabolism 55:811–818 [DOI] [PubMed] [Google Scholar]
- 16. Hampton MB, Kettle AJ, Winterbourn CC. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007–3017 [PubMed] [Google Scholar]
- 17. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. 1995. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 95:2409–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang CK, Zhan L, Hannigan MO, Ai Y, Leto TL. 2000. P47(phox)-deficient NADPH oxidase defect in neutrophils of diabetic mouse strains, C57BL/6J-m db/db and db/+. J. Leukoc. Biol. 67:210–215 [DOI] [PubMed] [Google Scholar]
- 19. Hyslop PA, et al. 1995. Hydrogen peroxide as a potent bacteriostatic antibiotic: implications for host defense. Free Radic. Biol. Med. 19:31–37 [DOI] [PubMed] [Google Scholar]
- 20. Jackson SH, et al. 1994. Cloning and functional expression of the mouse homologue of p47phox. Immunogenetics 39:272–275 [DOI] [PubMed] [Google Scholar]
- 21. King JT, Jr, Goulet JL, Perkal MF, Rosenthal RA. 2011. Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann. Surg. 253:158–165 [DOI] [PubMed] [Google Scholar]
- 22. Kinoshita M, et al. 2011. Enhancement of neutrophil function by interleukin-18 therapy protects burn-injured mice from methicillin-resistant Staphylococcus aureus. Infect. Immun. 79:2670–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kinoshita M, et al. 2005. Opposite effects of enhanced tumor necrosis factor-alpha production from Kupffer cells by gadolinium chloride on liver injury/mortality in endotoxemia of normal and partially hepatectomized mice. Shock 23:65–72 [DOI] [PubMed] [Google Scholar]
- 24. Leibel RL, Chung WK, Chua SC., Jr 1997. The molecular genetics of rodent single gene obesities. J. Biol. Chem. 272:31937–31940 [DOI] [PubMed] [Google Scholar]
- 25. Ma ZA, Zhao Z, Turk J. 2012. Mitochondrial dysfunction and beta-cell failure in type 2 diabetes mellitus. Exp. Diabetes Res. 2012:703538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marin DP, Bolin AP, Macedo Rde C, Sampaio SC, Otton R. 2011. ROS production in neutrophils from alloxan-induced diabetic rats treated in vivo with astaxanthin. Int. Immunopharmacol. 11:103–109 [DOI] [PubMed] [Google Scholar]
- 27. Masuda Y, Kinoshita M, Ono S, Tsujimoto H, Mochizuki H. 2006. Diverse enhancement of superoxide production from Kupffer cells and neutrophils after burn injury or septicemia. J. Clin. Biochem. Nutr. 38:25–32 [Google Scholar]
- 28. Mishima Y, et al. 2001. Relationship between serum tumor necrosis factor-alpha and insulin resistance in obese men with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 52:119–123 [DOI] [PubMed] [Google Scholar]
- 29. Mohanty P, et al. 2000. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J. Clin. Endocrinol. Metab. 85:2970–2973 [DOI] [PubMed] [Google Scholar]
- 30. Nagel JM, et al. 2010. Insulin glargine and NPH insulin increase to a similar degree epithelial cell proliferation and aberrant crypt foci formation in colons of diabetic mice. Horm. Cancer 1:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakashima M, et al. 2012. Pivotal advance: characterization of mouse liver phagocytic B cells in innate immunity. J. Leukoc. Biol. 91:537–546 [DOI] [PubMed] [Google Scholar]
- 32. Nielson CP, Hindson DA. 1989. Inhibition of polymorphonuclear leukocyte respiratory burst by elevated glucose concentrations in vitro. Diabetes 38:1031–1035 [DOI] [PubMed] [Google Scholar]
- 33. Omori K, et al. 2008. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J. Leukoc. Biol. 84:292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park S, Rich J, Hanses F, Lee JC. 2009. Defects in innate immunity predispose C57BL/6J-Leprdb/Leprdb mice to infection by Staphylococcus aureus. Infect. Immun. 77:1008–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Poitout V, Robertson RP. 2008. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr. Rev. 29:351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. 1996. The expression of TNF alpha by human muscle. Relationship to insulin resistance. J. Clin. Invest. 97:1111–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sato N, et al. 1993. Hypertonic glucose inhibits the production of oxygen-derived free radicals by rat neutrophils. Life Sci. 52:1481–1486 [DOI] [PubMed] [Google Scholar]
- 38. Sehgal R, et al. 2011. Risk factors for surgical site infections after colorectal resection in diabetic patients. J. Am. Coll. Surg. 212:29–34 [DOI] [PubMed] [Google Scholar]
- 39. Seitz O, et al. 2010. Wound healing in mice with high-fat diet- or ob gene-induced diabetes-obesity syndromes: a comparative study. Exp. Diabetes Res. 2010:476969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stegenga ME, et al. 2008. Effect of acute hyperglycaemia and/or hyperinsulinaemia on proinflammatory gene expression, cytokine production and neutrophil function in humans. Diabet. Med. 25:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Via LE, et al. 1998. Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 111(Pt 7):897–905 [DOI] [PubMed] [Google Scholar]
- 42. Walrand S, Guillet C, Boirie Y, Vasson MP. 2004. In vivo evidences that insulin regulates human polymorphonuclear neutrophil functions. J. Leukoc. Biol. 76:1104–1110 [DOI] [PubMed] [Google Scholar]
- 43. Wilson RM, Tomlinson DR, Reeves WG. 1987. Neutrophil sorbitol production impairs oxidative killing in diabetes. Diabet. Med. 4:37–40 [DOI] [PubMed] [Google Scholar]
- 44. Yates C, et al. 2009. Wound chronicity, inpatient care, and chronic kidney disease predispose to MRSA infection in diabetic foot ulcers. Diabetes Care 32:1907–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yoshikawa T, et al. 1992. Augmentative effects of tumor necrosis factor-alpha (human, natural type) on polymorphonuclear leukocyte-derived superoxide generation induced by various stimulants. Int. J. Immunopharmacol. 14:1391–1398 [DOI] [PubMed] [Google Scholar]
- 46. Zarkesh-Esfahani H, et al. 2004. Leptin indirectly activates human neutrophils via induction of TNF-alpha. J. Immunol. 172:1809–1814 [DOI] [PubMed] [Google Scholar]
- 47. Zhan Y, He D, Newburger PE, Zhou GW. 2004. p47(phox) PX domain of NADPH oxidase targets cell membrane via moesin-mediated association with the actin cytoskeleton. J. Cell Biochem. 92:795–809 [DOI] [PubMed] [Google Scholar]
- 48. Zimmet P, Alberti KG, Shaw J. 2001. Global and societal implications of the diabetes epidemic. Nature 414:782–787 [DOI] [PubMed] [Google Scholar]