Abstract
The divalent cation Sr2+ induced repetitive transient spikes of the cytosolic Ca2+ activity [Ca2+]cy and parallel repetitive transient hyperpolarizations of the plasma membrane in the unicellular green alga Eremosphaera viridis. [Ca2+]cy measurements, membrane potential measurements, and cation analysis of the cells were used to elucidate the mechanism of Sr2+-induced [Ca2+]cy oscillations. Sr2+ was effectively and rapidly compartmentalized within the cell, probably into the vacuole. The [Ca2+]cy oscillations cause membrane potential oscillations, and not the reverse. The endoplasmic reticulum (ER) Ca2+-ATPase blockers 2,5-di-tert-butylhydroquinone and cyclopiazonic acid inhibited Sr2+-induced repetitive [Ca2+]cy spikes, whereas the compartmentalization of Sr2+ was not influenced. A repetitive Ca2+ release and Ca2+ re-uptake by the ER probably generated repetitive [Ca2+]cy spikes in E. viridis in the presence of Sr2+. The inhibitory effect of ruthenium red and ryanodine indicated that the Sr2+-induced Ca2+ release from the ER was mediated by a ryanodine/cyclic ADP-ribose type of Ca2+ channel. The blockage of Sr2+-induced repetitive [Ca2+]cy spikes by La3+ or Gd3+ indicated the necessity of a certain influx of divalent cations for sustained [Ca2+]cy oscillations. Based on these data we present a mathematical model that describes the baseline spiking [Ca2+]cy oscillations in E. viridis.
Transient elevations of the [Ca2+]cy play a central role in intracellular signal transduction in plant (Bush, 1995; Trewavas et al., 1996; Webb et al., 1996) and animal cells (Petersen et al., 1994; Bootman and Berridge, 1995). Brief transient [Ca2+]cy elevations with a rapid rising phase and a rapid falling phase are called [Ca2+]cy spikes, reflecting the shape of these [Ca2+]cy transients. Single [Ca2+]cy spikes can be induced by mechanical signals, cold shock, or elicitors in plant cells. A correlation between mechanical signal strength and amplitude of the resulting [Ca2+]cy spike has been shown (Knight et al., 1991, 1992), suggesting a role of [Ca2+]cy spikes in signal transduction in plant cells. In animal cells repetitive transient elevations of [Ca2+]cy, so-called [Ca2+]cy oscillations, are well established (Tsien and Tsien, 1990; Fewtrell, 1993; Petersen et al., 1994). For plant cells only a few reports about [Ca2+]cy oscillations exist. Phytohormone-induced [Ca2+]cy fluctuations reported earlier were strongly damped and ceased after a few repetitions (Felle, 1988; Schroeder and Hagiwara, 1990). Only recently stable [Ca2+]cy oscillations were observed in plant cells (Johnson et al., 1995; McAinsh et al., 1995; Ehrhardt et al., 1996; Bauer et al., 1997). In some cases these [Ca2+]cy oscillations display a baseline spiking pattern, which means that repetitive [Ca2+]cy spikes are separated by a constant [Ca2+]cy baseline (Ehrhardt et al., 1996; Bauer et al., 1997). There are indications for a physiological function of [Ca2+]cy oscillations in plant cells (Johnson et al., 1995; McAinsh et al., 1995; Ehrhardt et al., 1996). The mechanisms generating [Ca2+]cy oscillations in plant cells are unknown.
The unicellular green alga Eremosphaera viridis responds to various stimuli with single or repetitive [Ca2+]cy spikes (Bauer et al., 1997). For example, after a “light-off” stimulus a single [Ca2+]cy spike occurs, which is accompanied by a parallel transient hyperpolarization of the plasma membrane. The addition of caffeine or Sr2+ induces repetitive [Ca2+]cy spikes that are always accompanied by repetitive transient hyperpolarizations. This hyperpolarization of the plasma membrane is due to the opening of Ca2+-dependent K+ channels (Thaler et al., 1989; Förster, 1990), representing a qualitative indicator of [Ca2+]cy spikes (Bauer et al., 1997). In this study the long-lasting Sr2+-induced repetitive [Ca2+]cy spikes in E. viridis were used to elucidate the mechanism of [Ca2+]cy oscillations in a green plant cell. We developed a theoretical model that serves as a basis to discuss which intracellular Ca2+ stores might be involved in generating [Ca2+]cy spikes.
MATERIALS AND METHODS
Plant Material and Solutions
The coccal green alga Eremosphaera viridis de Bary (Algal Culture Collection Göttingen LB 228–1, Germany) was cultured and prepared for experiments as described by Köhler et al. (1983). Algal cells with diameters of at least 150 μm were used for measurements. Under standard conditions the external medium contained 0.1 mm KNO3, 0.1 mm MgCl2, 0.1 mm CaCl2, and 2 mm Mes adjusted to pH 5.6 by NaOH. For measurements at low external concentration of divalent cations, the medium contained 0.1 mm KNO3, 1 mm EGTA, and 2 mm Mes adjusted to pH 7.6 by NaOH. Under these conditions the total [Ca2+] was about 1.3 μm as measured by ICP-AES. Gd3+ and La3+ were added as chloride salts and Sr2+ was added as a chloride salt or as carbonate or gluconate. For high external [K+], KNO3 was used as well as the gluconate salt. TMB8 (Biomol, Hamburg, Germany) was used as 5 mm stock solution in water. Verapamil (Biomol) and DBHQ (Biomol) were used as 10 mm stock solutions in EtOH. CPA (Biomol) was used as 5 mm stock solution in DMSO. The resulting final concentrations of EtOH or DMSO in the external medium were ≤ 0.5% (v/v). Control experiments showed that both solvents at concentrations < 1% influenced neither the membrane potential nor cytosolic ion activities (see also Thaler et al., 1992; Sauer et al., 1993). The flow rate of the perfusion medium was adjusted to exchange the chamber volume in 1 min.
Measurement of [Ca2+]cy and Membrane Potential
The fluorescent Ca2+-sensitive dye fura-2-dextran (Mr = 10,000; Molecular Probes, Leiden, The Netherlands) was microinjected mechanically into the cytosol of the alga by a lab-made injection syringe as recently described (Plieth and Hansen, 1996). The concentration of the fluorescent dye in the cell was estimated by comparison of the fluorescence of the algal cell with the fluorescence of calibration capillaries (Plieth and Hansen, 1996). After microinjection the glass capillary was removed and a KCl-microelectrode filled with 1 m KCl was impaled to register the membrane potential in parallel with the Ca2+-dependent fura-2-dextran fluorescence. The ratiometric [Ca2+]cy measurement was performed according to the methods of Fenton and Crofts (1990) and [Ca2+]cy was determined by in vitro calibration (Grynkiewicz et al., 1985). A detailed description of membrane potential measurement and parallel [Ca2+]cy measurement including ratio imaging and in vitro calibration is given by Plieth and Hansen (1996). When recorded in parallel to ratiometric [Ca2+]cy measurements, computer-aided membrane potential measurements, discontinuously synchronized to [Ca2+]cy measurements (Plieth and Hansen, 1996), were performed. Membrane potential measurements (Axoclamp-2B, Axon Instruments, Foster City, CA) without parallel [Ca2+]cy measurements were continuously monitored on an oscilloscope, registered by an x/t-recorder, and scanned for final analysis and presentation.
Pressure Injection of Sr2+, Ca2+, Ruthenium Red, and Ryanodine
To increase directly the cytosolic Sr2+ activity, Sr2+ was microinjected into a single algal cell (Förster, 1990). A glass capillary containing 0.5 mm or 1 mm SrCl2 was connected via a polyethylene tube to a compressed-air cylinder and was impaled into the alga. For microinjection the turgor of the algal cell was decreased by 200 mm sorbitol in the external perfusion medium and a pressure of about 0.5 to 1.0 MPa was applied to the capillary, resulting in injection rates of 10 to 50 pL min−1. Internal concentrations were estimated from the volume of the cytoplasm and the rates of microinjection. The diameter of the spherical algae (≥ 150 μm) was measured for each experiment and the volume of the cytoplasm was calculated from the whole cell volume assuming 20% cytoplasm (Bethmann et al., 1995). The Sr2+ influx rates during microinjection ranged from 5 fmol min−1 (0.5 mm at 10 pL min−1) to 50 fmol min−1 (1 mm at 50 pL min−1). At 1 mm external SrCl2 an initial uptake rate of 4 μm min−1 corresponding to 15 fmol min−1 was calculated (see below) from cation uptake measurements shown in Figure 8. Ca2+, ruthenium red (Sigma-Aldrich, Deisenhofen, Germany), and ryanodine (Biomol) were microinjected in the same way by glass capillaries containing 0.1 mm CaCl2, 10 μm, 100 μm, or 1 mm ruthenium red, or 2 mm ryanodine in water. When Sr2+, Ca2+, ruthenium red, or ryanodine was microinjected, the membrane potential was measured continuously with an impaled microelectrode and was used as a Ca2+ indicator (Bauer et al., 1997). Since fura-2-dextran binds Sr2+, which results in a shift of the excitation spectrum hardly distinguishable from Ca2+ binding (Kwan and Putney, 1990), monitoring of [Ca2+]cy via fura-2-dextran fluorescence was not applicable for Sr2+ microinjection experiments.
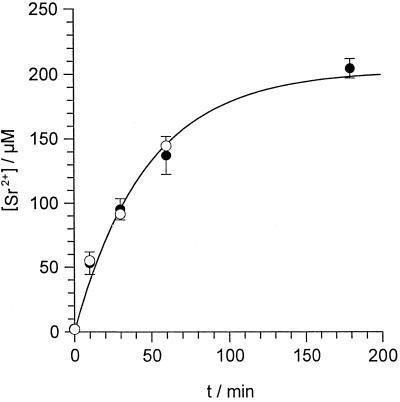
Figure 8.
Sr2+ uptake into E. viridis. Algal cells were incubated in the standard medium plus 1 mm SrCl2 either containing no ER Ca2+-ATPase inhibitor (control, •) or containing additionally 10 μm DBHQ or CPA (○). After different incubation times (t/min) cells were separated from the medium and the [Sr2+] of the cell sap ([Sr2+]/μm) was determined. Data points of the control (•) are given as means ± se of three to six measurements; data points measured in the presence of ER Ca2+-ATPase inhibitors (○) are means of three measurements. Data points were described by Equation 1. With an initial [Sr2+] of [Sr2+]0 = 2.1 μm, a fit to 19 separate data points for the control (•) yielded an equilibrium [Sr2+] of [Sr2+]EQ = 204 ± 18 μm, and a rate constant of a = 0.021 ± 0.004 min−1 (fit indicated by the solid line). When the data points measured in the presence of ER Ca2+-ATPase inhibitors (○) were included in the fit, parameters did not significantly change ([Sr2+]EQ = 202 ± 18 μm, a = 0.022 ± 0.004 min−1).
Measurement of Cellular Cation Concentrations
The cation content of total cells (cell sap plus cell wall) and of cell sap of E. viridis was determined by ICP-AES analysis as described by Bethmann et al. (1995). One-milliliter probes of a dense algal suspension were taken and incubated in 500 mL of standard medium without any addition or containing 1 mm SrCl2, 100 or 250 μm GdCl3, 100 μm LaCl3, 1 mm SrCl2 plus 10 μm DBHQ, or 1 mm SrCl2 plus 10 μm CPA. Samples of total cells were dried overnight at 80°C and analyzed. Samples for cell sap were heated to 100°C for 30 min and centrifuged at 4750g for 30 min to remove cell walls. The supernatant representing the cell sap was analyzed.
Data Analysis and Mathematical Modeling
All results in the text are given as mean ± sd. The nonlinear regression analysis of the data in Figure 8 was done with Grafit (Erithacus Software, London, UK) based on the Marquardt algorithm. A stability analysis of Equation 2 was performed by calculating the eigenvalues (Stucki and Somogyi, 1994) for different parameter sets (Mathcad, MathSoft, Cambridge, MA). For some parameter sets yielding stable oscillations (positive eigenvalues) Equation 2 was solved numerically (Mathematica, Wolfram Research, Champaign, IL).
RESULTS
[Ca2+]cy and membrane potential of E. viridis were measured simultaneously in the same algal cell. The free-running membrane potential (E/mV) under standard conditions (in 0.1 mm KNO3, MgCl2, CaCl2, and 2 mm Mes adjusted to pH 5.6 by NaOH) was −84 ± 20 mV (n = 332), and the steady-state [Ca2+]cy was 163 ± 42 nm (n = 50) based on in vitro calibrations.
The Effect of Sr2+ on [Ca2+]cy and Membrane Potential
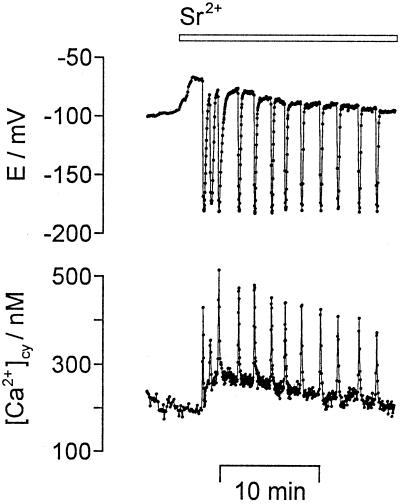
The effect of Sr2+ on [Ca2+]cy and on membrane potential in E. viridis is shown in Figure 1. In 95% of the experiments (n = 27) the addition of 1 mm Sr2+ to the external medium induced repetitive [Ca2+]cy spikes that were always accompanied by parallel, repetitive, transient hyperpolarizations of the plasma membrane. The repetitive transient changes of [Ca2+]cy and of membrane potential continued for more than 2 h under continuous perfusion of 1 mm SrCl2. When Sr2+ was removed from the external medium the repetitive changes continued in 54% of all measurements with a decreasing frequency; 1 mm SrCO3 or 1 mm Sr2+ gluconate had the same effect as 1 mm SrCl2. In the presence of Sr2+ a systrophe (a chloroplast translocation to the center of the cell) was frequently observed.
Figure 1.
Sr2+-induced repetitive [Ca2+]cy spikes and repetitive transient hyperpolarizations. The [Ca2+]cy/nM (bottom) and the membrane potential (E/mV, top) were recorded simultaneously in a single algal cell. The addition of 1 mm SrCl2 to the external medium (bar on top gives the perfusion protocol) induced repetitive [Ca2+]cy spikes and parallel repetitive transient hyperpolarizations of the plasma membrane. Sampling frequency was 1/3 s−1.
The discrete [Ca2+]cy spikes in E. viridis had a duration of 24 ± 8 s (n = 28) with a rapid rising phase and a rapid falling phase, and were separated by intervals with a constant baseline of [Ca2+]cy. The [Ca2+]cy spikes had an amplitude of about 365 ± 71 nm (n = 36). Amplitudes of up to 900 nm were observed (see Fig. 4). The baseline value of [Ca2+]cy during Sr2+-induced oscillations was 168 ± 43 nm (n = 27). This did not differ significantly from the steady-state value of 163 ± 42 nm in the absence of SrCl2 mentioned above. The duration of a single transient hyperpolarization of the plasma membrane was 35 ± 9 s with an amplitude of −181 ± 7 mV (n = 68). The frequency of Sr2+-induced [Ca2+]cy oscillations ranged from up to 0.8 min−1 (see Fig. 1) to 0.2 min−1 (see Fig. 5) and less. A quantitative analysis showed that lower frequencies (< 0.3 min−1) were correlated with cytosolic fura-2-dextran concentrations above 10 μm (2 μm referring to the whole cell).
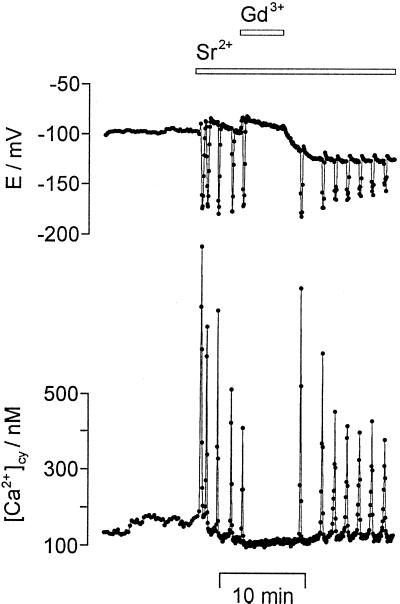
Figure 4.
Gd3+ inhibited Sr2+-induced repetitive [Ca2+]cy spikes and repetitive transient hyperpolarizations. Repetitive [Ca2+]cy spikes (bottom) and repetitive transient hyperpolarizations (E/mV, top) were induced by 1 mm SrCl2. The additional perfusion of 1 mm GdCl3 for 5 min (bars on top) reversibly inhibited the repetitive [Ca2+]cy and potential changes. Sampling frequency was 1/1.5 s−1.
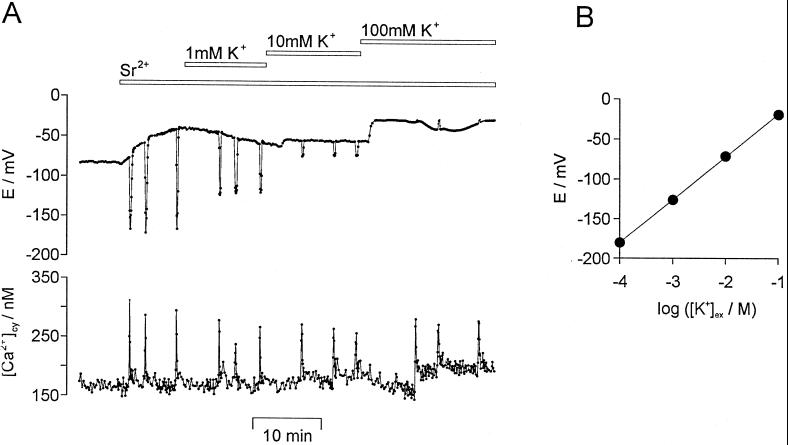
Figure 5.
The effect of different external [K+] on Sr2+-induced repetitive [Ca2+]cy spikes and repetitive transient hyperpolarizations. A, Repetitive [Ca2+]cy spikes (bottom) and repetitive transient hyperpolarizations (E/mV, top) were induced by the addition of 1 mm SrCl2 to the medium. The external [K+] was increased from 0.1 mm KCl (standard medium) to 1 mm, to 10 mm, and finally to 100 mm (bars on top). Sampling frequency was 1/3 s−1. B, The amplitude of the transient hyperpolarizations (E/mV) was plotted against the logarithm of the external [K+]: log ([K+]ex/M). A linear regression analysis (solid line, r = 0.999) yielded a slope of 53.8 ± 0.4 mV for each 10-fold increase in [K+]. Data points are given as means ± se (n = 15) with se being smaller than the symbol size.
To investigate the dose dependency, the effect of different external [SrCl2] on the latency period and on the frequency of repetitive transient hyperpolarizations of the plasma membrane was determined (Table I). These experiments were performed as membrane potential measurements only, to avoid a frequency decrease by fura-2-dextran. The addition of 0.01 mm SrCl2 had no effect on the membrane potential of E. viridis. The addition of increasing [SrCl2] caused increasing depolarizations (Table I, ΔE). After a latency period, τ, which decreased at larger [SrCl2], repetitive transient hyperpolarizations were induced in 69, 95, and 97% of the measurements at 0.1, 1, and 10 mm SrCl2, respectively (Table I). The frequency, ν, increased, whereas the amplitude of the repetitive transient hyperpolarizations decreased at increasing [SrCl2] (Table I). A comparable decrease of amplitudes was observed at increasing external [Mg2+] for transient hyperpolarizations induced by darkening (Sauer et al., 1994). Routinely, we used 1 mm Sr2+ because this concentration was sufficient to induce long-lasting repetitive [Ca2+]cy spikes with a high probability and frequency.
Table I.
The effect of different external Sr2+ concentrations ([SrCl2]/mm) on the membrane potential
| [SrCl2]/mm | ΔEa | Pb | τc | νd | Amplitudee | n |
|---|---|---|---|---|---|---|
| mV | % | min | min−1 | mV | ||
| 0.01 | 0 | 0 | – | – | – | 4 |
| 0.1 | 7 ± 2 | 69 | 4.2 ± 1.5 | 0.2 ± 0.05 | −191 ± 4 | 23 |
| 1 | 19 ± 4 | 95 | 0.5 ± 0.3 | 0.5 ± 0.2 | −181 ± 7 | 273 |
| 10 | 38 ± 10 | 97 | 0.3 ± 0.4 | 0.5 ± 0.2 | −167 ± 4 | 35 |
Probability to induce repetitive transient hyperpolarizations.
Lag phases after addition of Sr2+ before repetitive transient hyperpolarizations started.
Frequency of repetitive transient hyperpolarizations.
Maximum amplitude of the hyperpolarizations.
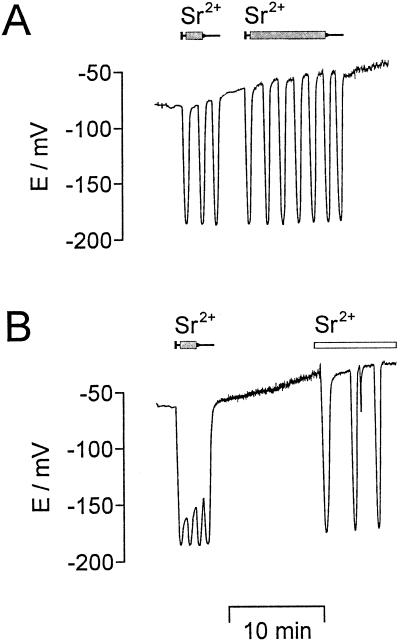
To test the effect of a direct increase in cytosolic [Sr2+], SrCl2 was microinjected into the cytoplasm of the algal cell. The microinjection of SrCl2 (n = 15) always resulted in hyperpolarizations (Fig. 2). At lower Sr2+ injection rates (Fig. 2A) repetitive transient hyperpolarizations were induced with a frequency comparable to those observed at 1 mm external SrCl2 (compare Figs. 1 and 2A). At higher Sr2+ injection rates (Fig. 2B) a massive hyperpolarization of the plasma membrane occurred. The frequency of the transient hyperpolarizations increased to more than 1 min−1, resulting in a “fusion” of the repetitive potential spikes to give rise to a fluctuating permanent hyperpolarization.
Figure 2.
Microinjection of Sr2+ induced hyperpolarizations. A, With 0.5 mm SrCl2 inside the injection pipette and small injection rates (about 10 pL/min) repetitive transient hyperpolarizations were induced as long as pressure was applied (small syringes on top indicate the duration of pressure application). B, SrCl2 (1 mm) inside the injection pipette and larger injection rates (about 50 pL/min) resulted in a nearly permanent hyperpolarization as long as pressure was applied (duration indicated by the small syringe above). When a Sr2+ injection was followed by the external perfusion of 1 mm SrCl2 (duration indicated by white bar), repetitive transient hyperpolarizations were observed. During long-lasting microinjection experiments, a continuous depolarization of the plasma membrane was frequently observed. The membrane potential was registered continuously.
The effect of different concentrations of CaCl2 or MgCl2 in the external medium on repetitive transient hyperpolarizations induced by 0.1 or 1 mm external SrCl2 was tested. Neither the probability to induce hyperpolarizations nor their duration or frequency were influenced by the external concentration of Ca2+ (or Mg2+) in the range from about 1 μm Ca2+ (1 mm EGTA, pH 7.6; n = 10) up to 1 mm Ca2+ (n = 8) or 10 mm Mg2+ (n = 7).
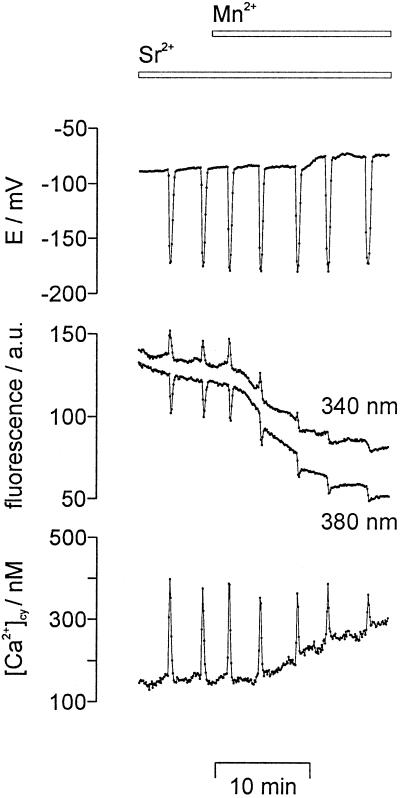
Mn2+ was used to study the influx of divalent cations during Sr2+-induced repetitive [Ca2+]cy spikes (Fig. 3). Mn2+ is known to bind to fura-2 with about a 40-fold higher affinity than Ca2+, and it quenches the fluorescence of the dye at all excitation wavelengths (Kwan and Putney, 1990). The addition of 0.1 mm MnCl2 to the external medium resulted in a continuous decrease of the fluorescence intensity at both excitation wavelengths (340 and 380 nm, Fig. 3, middle). This quenching of the fluorescence indicates a continuous Mn2+ influx into the cytoplasm of the cell. It resulted in an apparent increase of [Ca2+]cy (Fig. 3, bottom), whereas membrane potential was hardly influenced (Fig. 3, top).
Figure 3.
Mn2+ quenched the fura-2-dextran fluorescence intensity. Repetitive [Ca2+]cy spikes (bottom) and repetitive transient hyperpolarizations of the plasma membrane (E/mV, top) were induced by 1 mm SrCl2 in the external medium. The additional perfusion of 0.1 mm MnCl2 (bars on top) decreased the fluorescence intensity emitted by fura-2-dextran (middle traces, given in arbitrary units, a.u.) at both excitation wavelengths, 340 nm (shifted upward by 25 a.u. for clarity) and 380 nm. Sampling frequency was 1/6 s−1.
For a further characterization of the permeability of the plasma membrane for divalent cations, the plant plasma membrane Ca2+ channel blockers Gd3+ and La3+ (Huang et al., 1994; Marshall et al., 1994; Rengel, 1994; Piñeros and Tester, 1995) were used. Figure 4 shows that 1 mm GdCl3 in the external medium reversibly blocked repetitive [Ca2+]cy spikes in parallel to repetitive transient hyperpolarizations of the plasma membrane. This was observed in all measurements in which 1 mm GdCl3 or LaCl3 was used (n = 7). Investigating the concentration dependence revealed the following behavior. The addition of 100 μm GdCl3 or LaCl3 to the external medium had no effect on repetitive changes of [Ca2+]cy and membrane potential induced by 1 mm SrCl2 (n = 14). At concentrations of GdCl3 or LaCl3 of 200 μm, repetitive transient hyperpolarizations of the plasma membrane were influenced in all measurements. In 2 out of 10 measurements the hyperpolarizations were reversibly blocked, in 8 measurements the frequency was reduced by up to 70% as compared with the frequency in the absence of GdCl3 or LaCl3. Repetitive transient hyperpolarizations induced by 0.1 mm SrCl2 were already reversibly blocked at 100 μm GdCl3 or LaCl3 (n = 5). This indicates that Sr2+ and La3+ or Gd3+ competitively interacted with the same plasma membrane Ca2+ channel. The transient hyperpolarization observed after a “light-off” stimulus was not affected by 100 μm LaCl3 (n = 12) or GdCl3 (n = 10). This shows that these trivalent cations do not block the plasma membrane K+ channel, which gives rise to the transient hyperpolarization.
Besides La3+ and Gd3+, the effect of the Ca2+ channel blocker verapamil was investigated. Verapamil (50 μm) reversibly inhibited repetitive transient hyperpolarizations induced by 1 mm SrCl2 in 50% of the measurements (n = 8).
To test the relationship between the transient hyperpolarization of the plasma membrane and the [Ca2+]cy spike, the external [K+] ([K+]ex/mm) was changed during Sr2+-induced repetitive [Ca2+]cy spikes. As shown in Figure 5A, an increase of [K+]ex from 0.1 to 1 to 10 and finally to 100 mm decreased the amplitude of the transient hyperpolarizations. In contrast to this, the amplitude of the [Ca2+]cy spikes (321 ± 19 nm, n = 17) was not influenced by [K+]ex even at 100 mm. Figure 5B shows that a 10-fold increase in [K+]ex resulted in a decrease of the amplitude of transient hyperpolarizations of about 54 mV, following the Nernst potential for K+. The steady-state membrane potential became less negative at [K+]ex > 1 mm (n = 8). Depending on the steady-state membrane potential, the transient potential changes in the presence of 100 mm K+ resulted in small transient hyperpolarizations or small transient depolarizations. Even [Ca2+]cy spikes accompanied by a transient depolarization of the plasma membrane did not significantly differ from those observed under standard conditions (0.1 mm KNO3), as shown in Figure 5A. K+ gluconate had the same effect as KCl.
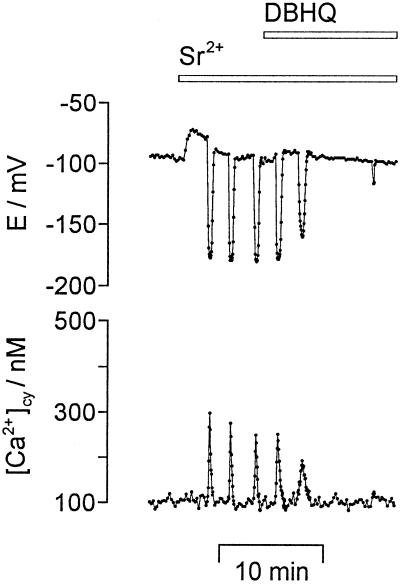
To study the involvement of internal Ca2+ stores, the effect of the ER Ca2+-ATPase blockers DBHQ and CPA (Inesi and Sagara, 1994) on Sr2+-induced repetitive [Ca2+]cy spikes was investigated. As shown in Figure 6, repetitive [Ca2+]cy spikes and repetitive transient hyperpolarizations were inhibited by 10 μm DBHQ after 2.9 ± 1.2 min (n = 8). The inhibitory effect was reversible when DBHQ was washed out for more than 20 min (n = 7). After a 5-min preperfusion of 10 μm DBHQ, the addition of Sr2+ (n = 8) induced one or a few repetitive transient hyperpolarizations with reduced amplitudes, but never sustained oscillations (not shown). Longer preperfusions inhibited Sr2+-induced transient hyperpolarizations. CPA at a concentration of 10 μm had the same effects on Sr2+-induced repetitive transient hyperpolarizations as 10 μm DBHQ (n = 5). The baseline [Ca2+]cy level was not increased in the presence of DBHQ (Fig. 6), showing that a cytosolic Ca2+ homeostasis was still achieved by active Ca2+ transport systems that are not sensitive to DBHQ. A transient hyperpolarization induced by pressure injection of Ca2+ was not affected by 10 μm DBHQ, indicating that DBHQ did not act on the Ca2+-dependent plasma membrane K+ channel.
Figure 6.
The ER Ca2+-ATPase inhibitor DBHQ blocked Sr2+-induced repetitive [Ca2+]cy spikes and repetitive transient hyperpolarizations. Repetitive [Ca2+]cy spikes (bottom) and repetitive transient hyperpolarizations (E/mV, top) induced by 1 mm SrCl2 were inhibited after the addition of 10 μm DBHQ to the external medium (bars on top). Sampling frequency was 1/3 s−1.
TMB8, an antagonist of InsP3-induced Ca2+ release from intracellular stores in animal (Zhang and Melvin, 1993) and plant cells (Schumaker and Sze, 1987; Förster, 1990), did not block Sr2+-induced repetitive [Ca2+]cy spikes and transient hyperpolarizations at concentrations up to 200 μm (n = 28).
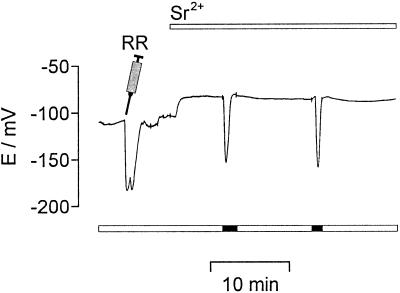
The effect of ruthenium red (Ma, 1993) and ryanodine (Smith et al., 1988), known antagonists of the ryanodine/cADPR Ca2+ release channel, were investigated. Both were microinjected into the cytoplasm of algal cells either before 1 mm SrCl2 was added or during Sr2+-induced repetitive transient hyperpolarizations. At cytosolic ruthenium red concentrations below 10 μm the amplitudes of the transient hyperpolarizations were decreased and they were completely inhibited at ruthenium red concentrations above 10 μm (n = 21) (Fig. 7). At cytosolic ryanodine concentrations below 100 μm, amplitudes and frequency of transient hyperpolarizations were decreased. At cytosolic ryanodine concentrations above 100 μm, Sr2+-induced repetitive transient hyperpolarizations were completely blocked (n = 6). The transient hyperpolarization observed after a “light-off” stimulus was influenced neither by ruthenium red (Fig. 7) nor by ryanodine at concentrations as high as several hundred micrometers.
Figure 7.
The ryanodine/cADPR Ca2+ release channel antagonist ruthenium red (RR) blocked Sr2+-induced hyperpolarizations. With 1 mm inside the pipette ruthenium red was microinjected (indicated by the syringe), resulting in a cytosolic concentration of about 100 μm. The microinjection itself caused a transient hyperpolarization. Afterward the addition of 1 mm SrCl2 to the external medium failed to induce any hyperpolarization, whereas darkening (light protocol given by the bars below) still induced a transient hyperpolarization. The membrane potential was recorded continuously.
Measurements of Cation Uptake
To investigate whether Sr2+ enters E. viridis, algal cells were incubated in the standard medium containing additionally 1 mm SrCl2, and the [Sr2+] ([Sr2+]/μm) of the total cells (cell sap plus cell wall) and the cell sap were determined after different incubation times. As shown in Figure 8, the [Sr2+] of the cell sap increased, reaching a stable value within 2 to 3 h. In the presence of 10 μm DBHQ or CPA, Sr2+ uptake into E. viridis was not significantly influenced (Fig. 8). The average (n = 14) [Sr2+] of the total cells was 24 μm larger compared with the concentration in the cell sap, regardless of the incubation time. This reflects a constant amount of Sr2+, which was bound to the cell wall. The time course of the increase of the [Sr2+] in the cell sap was mathematically described under the assumption that the Sr2+ influx was compensated by a Sr2+ export that linearly increases with internal [Sr2+]: [Sr2+] starts at an initial value [Sr2+]0 at t = 0 and reaches an equilibrium concentration [Sr2+]EQ at t → ∞ according to
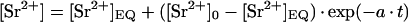 |
1 |
where a is the rate constant in min−1. The initial [Sr2+] of the cell sap was determined by ICP-AES analysis before Sr2+ was added to the external medium as [Sr2+]0 = 2.1 ± 0.75 μm (n = 5). On the basis of Equation 1, a nonlinear regression analysis of the data points summarized in Figure 8 yielded an equilibrium [Sr2+] of the cell sap of [Sr2+]EQ = 200 μm and a rate constant of a = 0.02 min−1. From these data, using the first derivation of Equation 1, an initial Sr2+ influx at t = 0 of 4.0 μm min−1 was calculated that corresponds to a current of about 24 pA for a spherical algal cell with a diameter of 150 μm (34 μA cm−2).
Besides Sr2+, the concentrations of La3+ and Gd3+ were measured in the total cells and in the cell sap of E. viridis after 60 min of incubation at 100 μm LaCl3 or up to 250 μm GdCl3 in the external medium, respectively. The [La3+] in the total cells was about 1.0 mm, whereas the [La3+] in the cell sap was below the detection limit of about 0.8 μm. Similar values were obtained for Gd3+. A concentration of about 0.5 mm Gd3+ was measured for total cells whereas the concentration in the cell sap remained below the detection limit of about 1.0 μm. This indicates that La3+ and Gd3+ were not taken up into E. viridis to a considerable amount. It also demonstrated that a proper separation of the cell wall from the cell sap was achieved. In the presence of 250 μm GdCl3, Sr2+ uptake after 60 min was decreased from about 145 to 59 μm.
DISCUSSION
There are two fundamental questions about [Ca2+]cy oscillations: How do they arise? And what is their physiological function? The above measurements provide access to the understanding of the mechanism of [Ca2+]cy oscillations in a green plant cell. In this context Sr2+ was used as a tool to induce long-lasting repetitive [Ca2+]cy spikes. In animal cells Sr2+ is frequently used to induce Ca2+ release from intracellular Ca2+ stores (Mironov and Juri, 1990; Grégoire et al., 1993) and Sr2+ was shown to induce repetitive Ca2+ spikes (Bos-Mikich et al., 1995). Regarding the physiological response to increasing [Ca2+]cy in E. viridis, it should be mentioned that [Ca2+]cy oscillations are accompanied by a systrophe, which is a chloroplast translocation to the center of the cell (Schönknecht et al., 1998). This systrophe is observed as a reaction to excess light and is also induced in E. viridis by blue light in the presence of external Ca2+ (Weidinger and Ruppel, 1985).
Sr2+ Uptake and Compartmentalization
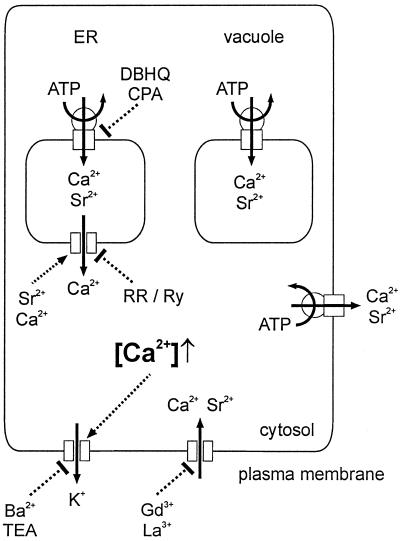
Sr2+ was rapidly taken up into E. viridis, as shown in Figure 8. It is known that binding of Sr2+ to fura-2-dextran results in a fluorescence excitation spectrum very similar to the spectrum caused by Ca2+, with a 30-fold lower affinity of fura-2-dextran for Sr2+ compared with Ca2+ (Kwan and Putney, 1990). The baseline value of [Ca2+]cy during Sr2+-induced oscillations did not differ from the steady-state [Ca2+]cy level in the absence of Sr2+ (see Figs. 1, 4, 5A, and 6). Therefore, the free cytosolic Sr2+ activity did not exceed 1 to 2 μm. On the other hand, [Sr2+] of up to 200 μm were measured in the cell sap. This shows that Sr2+ was effectively compartmentalized into internal organelles, which is comparable to animal cells (Kwan and Putney, 1990; Mironov and Juri, 1990). As documented in Figure 2, besides being effective, the intracellular compartmentalization of Sr2+ was also rapid; immediately after stopping Sr2+ microinjection membrane potential oscillations ceased. The only organelle that can contribute to the measured intracellular steady-state concentration of 200 μm Sr2+ (Fig. 8) is the vacuole. All other organelles have such a small volume compared with the total volume of the cell (1.8 nL), that an accumulation of this amount of Sr2+ (360 fmol) in another compartment is unlikely. A vacuolar [Sr2+] of 250 μm (80% of the cell volume) is comparable to the vacuolar [Ca2+] of about 400 μm in E. viridis (Bethmann et al., 1995). The plasma membrane Ca2+ channel blocker Gd3+ (Marshall et al., 1994; Rengel, 1994; Piñeros and Tester, 1995) considerably decreased Sr2+ uptake. Sr2+ uptake and compartmentalization were not affected by the ER Ca2+-ATPase blockers CPA or DBHQ (Fig. 8), indicating that these inhibitors influenced neither the Sr2+ influx and efflux across the plasma membrane (compare Eq. 1) nor Sr2+ uptake into the vacuole (summarized in Fig. 10).
Figure 10.
A schematic model for Sr2+-induced [Ca2+]cy spikes in E. viridis. Sr2+ enters the cell via Ca2+ channels in the plasma membrane that are competitively blocked by Gd3+ or La3+. Most of the Sr2+ taken up into the cell is compartmentalized into the vacuole. At steady state, the same amount of Sr2+ (and Ca2+) that enters the cell is transported out of the cell by plasma membrane Ca2+-ATPases. The Ca2+-ATPases that pump Sr2+ (and Ca2+) into the vacuole or out of the cell are not blocked by DBHQ or CPA. Inside the cell Sr2+ induces a Ca2+-release from the ER. The ER Ca2+ channel is inhibited by either ruthenium red (RR) or ryanodine (Ry), pointing to a ryanodine/cADPR-like Ca2+ channel. The rapid increase in [Ca2+]cy is compensated for by Ca2+-ATPases of internal Ca2+ stores and the plasma membrane. The ER is refilled by Ca2+-ATPases that are blocked by DBHQ or CPA. The [Ca2+]cy spike causes the opening of plasma membrane K+ channels, resulting in a transient hyperpolarization. This K+ channel is known to be blocked by Ba2+ or TEA (Köhler et al., 1983; Thaler et al., 1989).
Sr2+-Induced Repetitive [Ca2+]cy Spikes in E. viridis Show a Baseline Spiking Pattern
[Ca2+]cy oscillations in animal and plant cells display a variety of patterns. Besides more irregular repetitive [Ca2+]cy changes, sinusoidal [Ca2+]cy oscillations are observed, which differ from baseline spiking [Ca2+]cy oscillations displaying discrete [Ca2+]cy spikes separated by a baseline of [Ca2+]cy (Fewtrell, 1993). In animal cells sinusoidal [Ca2+]cy oscillations and baseline spiking [Ca2+]cy oscillations are mechanistically different. Whereas for sinusoidal [Ca2+]cy oscillations the agonist dose increases the amplitude but not the frequency, for baseline spiking [Ca2+]cy oscillations the frequency is determined by the agonist dose, whereas the amplitude is independent from the agonist dose, and the latency period before the first [Ca2+]cy spike is inversely related to the agonist dose (Thomas et al., 1996). Furthermore, Ca2+ signals with a baseline spiking pattern continue for a long period of stimulation. The agonist dose dependency of Sr2+-induced baseline spiking oscillations in E. viridis is the same. The frequency increased with increasing external [Sr2+] (Table I), as well as with increasing microinjected [Sr2+] (Fig. 2). The latency period decreased with increasing external [Sr2+] (Table I). Moreover, Sr2+-induced repetitive [Ca2+]cy spikes in E. viridis lasted a very long time. Not only the pattern but also the dose dependency of the repetitive [Ca2+]cy spikes observed in E. viridis was comparable to animal cells. This is the first indication to our knowledge that similar patterns of [Ca2+]cy oscillations in plant and animal cells may be based on a similar mechanism.
Changes in [Ca2+]cy are involved in many different signal transduction processes ( Trewavas et al., 1996; Webb et al., 1996), which raises the question of how a stimulus specificity is achieved. [Ca2+]cy oscillations may encode information about the stimulus by the frequency, amplitude, or duration (Clapham, 1995; Berridge, 1997). In animal cells there is considerable evidence that frequency encoding does contribute to a stimulus-specific reaction: [Ca2+]cy oscillations have been shown to depend on the type or strength of a stimulus (Petersen et al., 1994; Thomas et al., 1996), and biochemical mechanisms are documented that are able to decode [Ca2+]cy oscillations (Hajnóczky et al., 1995; De Koninck and Schulman, 1998). Agonist-induced frequency modulation has not been shown in plants, but the results presented here suggest that the relevant mechanisms for frequency encoding exist in E. viridis.
In Commelina communis guard cells an increase to 100 μm of the external [Ca2+] induces baseline spiking [Ca2+]cy oscillations, however, a further increase to 1 mm results in asymmetric and irregular Ca2+-induced [Ca2+]cy elevations (McAinsh et al., 1995). In E. viridis the external concentration of Ca2+ or Mg2+ had no effect on Sr2+-induced oscillations.
Membrane Potential Oscillations Are Caused by [Ca2+]cy Oscillations
The measurements presented here (Figs. 1 and 3–6) demonstrate the close correlation between [Ca2+]cy spikes and transient hyperpolarizations. This correlation raises the question of whether the [Ca2+]cy spikes cause transient hyperpolarizations or whether the transient hyperpolarizations cause Ca2+ spikes. For animal cells both possibilities are well documented. On the one hand, autonomous [Ca2+]cy oscillations driven by Ca2+ release from internal stores may cause membrane potential oscillations due to the opening of Ca2+-dependent plasma membrane ion channels (Lee and Earm, 1994; Wojnowski et al., 1994; D'Andrea and Thorn, 1996). On the other hand, autonomous plasma membrane oscillations may cause [Ca2+]cy oscillations due to Ca2+ influx via voltage-dependent Ca2+ channels (Li et al., 1995b; Larsson et al., 1996). Within the time resolution of our measurements (1.5–6 s) [Ca2+]cy normally increased at the same time as the membrane potential started to hyperpolarize. The experiments illustrated in Figure 5 show that the plasma membrane potential as changed by external [K+] does not exert any significant effect on Sr2+-induced [Ca2+]cy spikes. This is opposite to what is expected for [Ca2+]cy spikes caused by an influx of Ca2+ across the plasma membrane. Such [Ca2+]cy spikes vary with the membrane potential, but this was not observed (Fig. 5). Even when the transient hyperpolarization switched to a depolarization, the amplitude and time course of the [Ca2+]cy spikes were not influenced. This clearly shows that the [Ca2+]cy spikes caused the membrane potential changes rather than the other way around. The close correlation between [Ca2+]cy spikes and transient hyperpolarizations in E. viridis (Figs. 1 and 3–6; Bauer et al., 1997) is due to the Ca2+-dependent opening of plasma membrane K+ channels (Fig. 5B).
The Role of Ca2+ Release and Re-Uptake by Internal Stores
Most models describing the mechanism of [Ca2+]cy oscillations in animal cells are based on a repetitive Ca2+ release and re-uptake by intracellular Ca2+ stores (Tsien and Tsien, 1990; Fewtrell, 1993; Petersen et al., 1994). We used DBHQ (Fig. 6) and CPA to demonstrate the role of Ca2+ release and re-uptake by intracellular Ca2+ stores for [Ca2+]cy oscillations in E. viridis. CPA and DBHQ are well-established inhibitors of ER Ca2+-ATPases in animal cells (Inesi and Sagara, 1994). In plant cells DBHQ and CPA recently have been shown to act specifically on ER Ca2+-ATPases as well (Logan and Venis, 1995; Hwang et al., 1997; Liang et al., 1997). Both inhibitors are lipophilic and readily enter the cell (Busch and Sievers, 1993; Du et al., 1994; Trebacz et al., 1996), which explains the long duration needed for a wash out with E. viridis. In line with a specific action on ER Ca2+-ATPases, CPA or DBHQ influenced neither the transport of divalent cations across the plasma membrane and the tonoplast (Fig. 8) nor the steady-state [Ca2+]cy (Fig. 6). Blocking ER Ca2+-ATPases by CPA or DBHQ prevents the re-uptake of Ca2+ into the ER, resulting in a rapid emptying of the Ca2+ store that drives [Ca2+]cy oscillations. Thus, a Sr2+-induced Ca2+ release is no longer possible, i.e. [Ca2+]cy oscillations come to an end.
The Role of Ca2+ Fluxes across the Plasma Membrane
Since plasma membrane Ca2+-ATPases transport Ca2+ out of the cell, especially during [Ca2+]cy spikes, a certain Ca2+ influx is necessary to prevent a depletion in Ca2+. In animal cells Ca2+ fluxes across the plasma membrane have been shown to be substantial for sustained [Ca2+]cy oscillations (Tsien and Tsien, 1990; Fewtrell, 1993; Petersen et al., 1994). The quenching of the fura-2-dextran fluorescence by externally added Mn2+ (Kwan and Putney, 1990) (Fig. 3) indicated that the plasma membrane of E. viridis had a significant permeability for Mn2+, which is known to permeate plant plasma membrane Ca2+ channels (Piñeros and Tester, 1995).
During transient hyperpolarizations Mn2+ quenching significantly increased (Fig. 3). This could be caused by the increasing electrical driving force for Mn2+ influx and does not necessarily point to a voltage-dependent conductance increase. An increasing Mn2+ quenching indicates an increasing Ca2+ influx, suggesting that a component of the [Ca2+]cy spikes might arise directly via Ca2+ influx during the transient hyperpolarization. However, neither the external [Ca2+] nor the membrane potential (see Fig. 5) have a significant influence on [Ca2+]cy spike amplitudes. Therefore, a Ca2+ influx during transient hyperpolarizations does not seem to contribute significantly to [Ca2+]cy spike amplitudes.
The application of the plant plasma membrane Ca2+ channel blockers La3+ or Gd3+ (Huang et al., 1994; Marshall et al., 1994; Rengel, 1994; Piñeros and Tester, 1995) reversibly inhibited Sr2+-induced repetitive [Ca2+]cy spikes and transient hyperpolarizations in E. viridis (Fig. 4). The cation uptake measurements showed that neither La3+ nor Gd3+ reached micromolar intracellular concentrations, which were reported to block ER (Klüsener et al., 1995) or tonoplast Ca2+ channels (Johannes et al., 1992; Pantoja et al., 1992). Therefore, La3+ and Gd3+ are very likely to act on plasma membrane Ca2+ channels. Accordingly, the dark-induced transient hyperpolarization that is caused by a single [Ca2+]cy spike in E. viridis (Bauer et al., 1997), probably due to Ca2+ release from the chloroplast (Schönknecht et al., 1998), was not affected by these plasma membrane Ca2+ channel blockers. As the results in Figure 5 show, [Ca2+]cy spikes in E. viridis are not caused by membrane potential-driven Ca2+ influx. However, the inhibitory effect of La3+ or Gd3+ indicates that, comparable to animal cells, a certain Ca2+ influx across the plasma membrane is necessary for sustained [Ca2+]cy oscillations. Since La3+ or Gd3+ also block Sr2+ uptake, and Sr2+ is rapidly compartmentalized at the same time, the effect of the trivalent cations may alternatively be explained by a decrease of the cytosolic Sr2+ activity, resulting in cessation of oscillations. However, caffeine-induced repetitive [Ca2+]cy spikes in E. viridis have recently been shown to be reversibly inhibited by La3+ or Gd3+ as well (Bauer et al., 1997), corroborating the view that a certain Ca2+ influx is essential for sustained [Ca2+]cy oscillations.
A Mathematical Model for Sr2+-Induced Repetitive [Ca2+]cy Spikes
On the basis of the results mentioned above, it becomes possible to describe the repetitive [Ca2+]cy spikes in E. viridis theoretically by adapting a mathematical model proposed by Stucki and Somoggyi (1994).
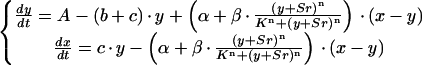 |
2 |
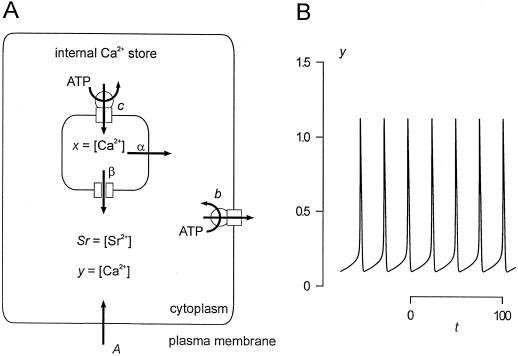
The two differential equations describe the changes of the Ca2+ activities in the cytosol and in the store due to the repetitive release and re-uptake of Ca2+ by internal stores plus Ca2+ fluxes across the plasma membrane. This is illustrated in Figure 9A. y = [Ca2+]cy is increased by a steady influx A across the plasma membrane. Ca2+-ATPases pump cytosolic Ca2+ into internal stores (c) or across the plasma membrane (b) with a rate proportional to y. The fluxes out of the store depend on the difference between the Ca2+ activities in the store and in the cytosol (x–y). There is a linear leak (α), and a Ca2+ release channel (β), which is assumed to be modulated in a cooperative manner (K, n) by cytosolic Ca2+ (y) and Sr2+ (Sr in Eq. 2) activities. The change in Ca2+ activity inside the store x is determined by the balance of the flux into the store (c · y) and the fluxes out of the store already mentioned. The assumption of a Sr2+-induced Ca2+ release is a feature additionally introduced to the original mathematical model of Stucki and Somogyi (1994). This Sr2+ dependence is established for different animal cells (Mironov and Juri, 1990; Grégoire et al., 1993). Figure 9B demonstrates that this theoretical approach is sufficient to model [Ca2+]cy oscillations with a baseline spiking pattern. With the parameters chosen for Figure 9B there are no oscillations in the absence of Sr2+ (Sr = 0) in the cytoplasm. Only in the presence of a certain amount of Sr2+ is Ca2+ released by the Ca2+ release channel from the intracellular Ca2+ store, initiating sustained [Ca2+]cy oscillations.
Figure 9.
A theoretical model of the repetitive [Ca2+]cy spikes in E. viridis. A, Schematic model of the Ca2+ fluxes involved in generating repetitive [Ca2+]cy spikes in E. viridis. The [Ca2+]cy, y, and Sr2+ activity, Sr, cause a Ca2+/Sr2+-induced Ca2+ release (β) from an internal Ca2+ store. The Ca2+ store is refilled by a Ca2+-ATPase (c), and there is a continuous Ca2+ efflux due to a “leak” (α). A plasma membrane Ca2+-ATPase removes Ca2+ from the cytoplasm (b), and there is a Ca2+ influx from the external medium (A). B, Temporal response of the system depicted in A and described by Equation 2. The [Ca2+]cy, y, is plotted as a function of time, t, for: A = 0.2; b = 1; c = 2; α = 0.05; β = 15.7; K = 1; Sr = 0.1; n = 4; x(t=0) = 2.8; y(t=0) = 0.2. For the sake of simplicity no units are given and the axes are given in arbitrary units.
The theoretical model described by Equation 2 and depicted in Figure 9 is a minimal model that does not consider buffer capacities, the influx and compartmentalization of Sr2+, or the volumes of different compartments. However, it contains all the elements discussed above (see Fig. 10): An intracellular Ca2+ store that is filled by a DBHQ or CPA-sensitive Ca2+-ATPase, a continuous Ca2+ efflux from the Ca2+ store, which slowly depletes the store when the Ca2+-ATPase is blocked, and a Ca2+ influx pathway across the plasma membrane that is blocked by Gd3+ or La3+.
The Nature of the Ca2+ Release
Most [Ca2+]cy oscillations investigated in nonexcitable animal cells turned out to be caused by Ca2+ release via the InsP3-activated Ca2+ channel (Berridge, 1993; Fewtrell, 1993; Li et al., 1995a). TMB8, which was shown to inhibit InsP3-induced Ca2+ release in E. viridis (Förster, 1990), had no effect on Sr2+-induced [Ca2+]cy oscillations, indicating that InsP3-activated Ca2+ channels are not involved. In animal cells, besides caffeine, Sr2+ is known to induce Ca2+ release from internal stores via the ryanodine/cADPR Ca2+ channel (Meissner, 1994; Lee et al., 1995). In E. viridis caffeine and Sr2+ alike induce repetitive [Ca2+]cy spikes and transient hyperpolarizations (Bauer et al., 1997). Ruthenium red (Ma, 1993) and ryanodine (Smith et al., 1988) are known to be specific for the ryanodine/cADPR Ca2+ channel, not interacting with the InsP3-activated Ca2+ channel (Ehrlich et al., 1994). Ruthenium red and ryanodine were recently shown to block Ca2+ release in plant cells as well (Allen et al., 1995; Muir and Sanders, 1996). Both inhibitors when microinjected into E. viridis blocked Sr2+-induced repetitive transient hyperpolarizations (Fig. 7). Neither ruthenium red (Fig. 7) nor ryanodine affected the transient hyperpolarization observed after darkening, indicating that both inhibitors specifically blocked Sr2+-induced Ca2+ release and not the [Ca2+]cy spike induced by darkening (Bauer et al., 1997). Most likely, in E. viridis Sr2+ induced a Ca2+ release from intracellular Ca2+ stores by activating a type of ryanodine/cADPR Ca2+ channel (Fig. 10).
Probably this ryanodine/cADPR Ca2+ channel is located in the ER (Fig. 10). This is evident from the effect of CPA or DBHQ, which specifically inhibit ER Ca2+-ATPases in animal (Inesi and Sagara, 1994) as well as in plant cells (Logan and Venis, 1995). As discussed above, in E. viridis CPA or DBHQ influenced neither the transport of divalent cations across the plasma membrane and the tonoplast (Fig. 8) nor the steady-state [Ca2+]cy (Fig. 6). Moreover, a preperfusion of DBHQ or CPA for more than 5 min completely blocked Sr2+-induced oscillations, indicating a rather small volume or Ca2+ content of the affected Ca2+ store. In E. viridis as in animal cells (Kass et al., 1989; Demaurex et al., 1992), there is probably a continuous Ca2+ efflux from the store and when the compensating Ca2+ uptake by Ca2+-ATPases is blocked, this results in a Ca2+ store depletion even in the absence of Sr2+-induced [Ca2+]cy oscillations. This depletion within a few minutes excludes the involvement of the vacuole, since the vacuole is a huge store of free Ca2+ in E. viridis (Bethmann et al., 1995). Little is known about Ca2+ release channels of the plant ER. Klüsener et al. (1995) isolated and reconstituted a voltage-dependent Ca2+ channel from the ER of a higher plant mechanoreceptor organ that was not affected by InsP3 or ryanodine. In E. viridis as in other plant cells, the vacuole is the largest internal Ca2+ store and probably plays a key role in ion homeostasis and compartmentalization (see above). However, the [Ca2+]cy oscillations described here are driven by a rather small internal Ca2+ store, probably the ER, and not the vacuole (Fig. 10). In good agreement, Plieth et al. (1998) recently demonstrated that the elevation in [Ca2+]cy during action potentials in Chara sp. is neither caused by Ca2+ influx across the plasma membrane nor by Ca2+ release from the vacuole. A Ca2+ release from internal stores different from the vacuole gives rise to elevated [Ca2+]cy (Plieth et al., 1998).
The Sr2+-induced [Ca2+]cy oscillations in the unicellular green alga E. viridis show a dose-dependent frequency increase (Table I). This suggests that in E. viridis, comparable to animal cells, [Ca2+]cy oscillations might encode information about external stimuli by their frequency, mediating stimulus-specific reactions by Ca2+-dependent signal transduction processes. It is likely that Sr2+-induced repetitive [Ca2+]cy spikes are initialized by a Sr2+-induced Ca2+ release from the ER via a type of ryanodine/cADPR Ca2+ release channel. Our current working model of the different transmembrane Ca2+ fluxes and compartments involved in Sr2+-induced [Ca2+]cy oscillations in E. viridis is summarized in Figure 10.
ACKNOWLEDGMENTS
We thank Prof. Sattelmacher, Kiel, Germany, for his generous support and cooperation, and Mrs. F. Reisberg for analyses by ICP-AES.
Abbreviations:
- [Ca2+]cy
cytosolic Ca2+ activity
- cADPR
cyclic ADP-Rib
- CPA
cyclopiazonic acid
- DBHQ
2,5-di-tert-butylhydroquinone
- ICP-AES
induction coupled plasma-atomic emission spectroscopy
- InsP3
inositol 1,4,5-trisphosphate
- TMB8
3,4,5-trimethoxybenzoic acid 8-diethylaminooctyl ester
Footnotes
This work was financially supported by the Deutsche Forschungsgemeinschaft within the SFB 176 (TP B11) and a travel grant to O.P., and by a grant from the University of Würzburg to C.S.B.
LITERATURE CITED
- Allen GJ, Muir SR, Sanders D. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science. 1995;268:735–737. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- Bauer CS, Plieth C, Hansen U-P, Sattelmacher B, Simonis W, Schönknecht G. Repetitive Ca2+ spikes in a unicellular green alga. FEBS Lett. 1997;405:390–393. doi: 10.1016/s0014-5793(97)00231-7. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The AM and FM of calcium signalling. Nature. 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- Bethmann B, Thaler M, Simonis W, Schönknecht G. Electrochemical potential gradients of H+, K+, Ca2+, and Cl− across the tonoplast of the green alga Eremosphaera viridis. Plant Physiol. 1995;109:1317–1326. doi: 10.1104/pp.109.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Berridge MJ. The elemental principles of calcium signaling. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Bos-Mikich A, Swann K, Whittingham DG. Calcium oscillations and protein synthesis inhibition synergistically activate mouse oocytes. Mol Reprod Dev. 1995;41:84–90. doi: 10.1002/mrd.1080410113. [DOI] [PubMed] [Google Scholar]
- Busch MB, Sievers A. Membrane traffic from the endoplasmic reticulum to the Golgi apparatus is disturbed by an inhibitor of the Ca2+-ATPase in the ER. Protoplasma. 1993;177:23–31. [Google Scholar]
- Bush DS. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- D'Andrea P, Thorn P. Ca2+ signalling in rat chromaffin cells: interplay between Ca2+ release from intracellular stores and membrane potential. Cell Calcium. 1996;19:113–123. doi: 10.1016/s0143-4160(96)90080-9. [DOI] [PubMed] [Google Scholar]
- De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- Demaurex N, Lew DP, Krause K-H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J Biol Chem. 1992;267:2318–2324. [PubMed] [Google Scholar]
- Du GG, Ashley CC, Lea TJ. Effects of thapsigargin and cyclopiazonic acid on the sarcoplasmic reticulum Ca2+ pump of skinned fibres from frog skeletal muscle. Pflügers Arch. 1994;429:169–175. doi: 10.1007/BF00374309. [DOI] [PubMed] [Google Scholar]
- Ehrhardt DW, Wais R, Long SR. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca2+-release channels. Trends Pharmacol Sci. 1994;15:145–149. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Felle H. Auxin causes oscillations of cytosolic free calcium and pH in Zea mays coleoptiles. Planta. 1988;174:495–499. doi: 10.1007/BF00634478. [DOI] [PubMed] [Google Scholar]
- Fenton JM, Crofts AR. Computer aided fluorescence imaging of photosynthetic systems: application of video imaging to the study of fluorescence induction of green plants and photosynthetic bacteria. Photosynth Res. 1990;26:59–66. doi: 10.1007/BF00048977. [DOI] [PubMed] [Google Scholar]
- Fewtrell C. Ca2+ oscillations in non-excitable cells. Annu Rev Physiol. 1993;55:427–454. doi: 10.1146/annurev.ph.55.030193.002235. [DOI] [PubMed] [Google Scholar]
- Förster B. Injected inositol 1,4,5-trisphosphate activates Ca2+-sensitive K+ channels in the plasmalemma of Eremosphaera viridis. FEBS Lett. 1990;269:197–201. doi: 10.1016/0014-5793(90)81153-f. [DOI] [PubMed] [Google Scholar]
- Grégoire G, Loirand G, Pacaud P. Ca2+ and Sr2+ entry induced Ca2+ release from the intracellular Ca2+ store in smooth muscle cells of rat portal vein. J Physiol. 1993;474:483–500. doi: 10.1113/jphysiol.1993.sp019957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescent properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Huang JW, Grunes DL, Kochian LV. Voltage-dependent Ca2+ influx into right-side-out plasma membrane vesicles isolated from wheat roots: characterization of a putative Ca2+ channel. Proc Natl Acad Sci USA. 1994;91:3473–3477. doi: 10.1073/pnas.91.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Ratterman DM, Sze H. Distinction between endoplasmic reticulum-type and plasma membrane-type Ca2+ pumps. Partial purification of a 120-kilodalton Ca2+-ATPase from endomembranes. Plant Physiol. 1997;113:535–548. doi: 10.1104/pp.113.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G, Sagara Y. Specific inhibitors of intracellular Ca2+ transport ATPases. J Membr Biol. 1994;141:1–6. doi: 10.1007/BF00232868. [DOI] [PubMed] [Google Scholar]
- Johannes E, Brosnan JM, Sanders D. Parallel pathways for intracellular Ca2+ release from the vacuole of higher plants. Plant J. 1992;2:97–102. [Google Scholar]
- Johnson CH, Knight MR, Kondo T, Masson P, Sedbrook J, Haley A, Trewavas A. Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science. 1995;269:1863–1865. doi: 10.1126/science.7569925. [DOI] [PubMed] [Google Scholar]
- Kass GEN, Duddy SK, Moore GA, Orrenius S. 2,5-Di(tert-butyl)-1,4-benzohydroquinone rapidly elevates cytosolic Ca2+ concentration by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. J Biol Chem. 1989;264:15192–15198. [PubMed] [Google Scholar]
- Klüsener B, Boheim G, Liss H, Engelberth J, Weiler EW. Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO J. 1995;14:2708–2714. doi: 10.1002/j.1460-2075.1995.tb07271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effect of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Knight MR, Smith SM, Trewavas AJ. Wind-induced plant motion immediately increases cytosolic calcium. Proc Natl Acad Sci USA. 1992;89:4967–4971. doi: 10.1073/pnas.89.11.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler K, Geisweid H-J, Simonis W, Urbach W. Changes in membrane potential and resistance caused by transient increase of potassium conductance in the unicellular alga Eremosphaera viridis. Planta. 1983;159:165–171. doi: 10.1007/BF00392988. [DOI] [PubMed] [Google Scholar]
- Kwan C-Y, Putney JW. Uptake and intracellular sequestration of divalent cations in resting and methacholine-stimulated mouse lacrimal acinar cells: dissociation by Sr2+ and Ba2+ of agonist-stimulated divalent cation entry from the refilling of the agonist-sensitive intracellular pool. J Biol Chem. 1990;265:678–684. [PubMed] [Google Scholar]
- Larsson O, Kindmark H, Bränström R, Fredholm B, Berggren PO. Oscillations in KATP channel activity promote oscillations in cytoplasmic free Ca2+ concentration in the pancreatic β cell. Proc Natl Acad Sci USA. 1996;93:5161–5165. doi: 10.1073/pnas.93.10.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Aarhus R, Graeff RM. Sensitization of calcium-induced calcium release by cyclic ADP-ribose and calmodulin. J Biol Chem. 1995;270:9060–9066. doi: 10.1074/jbc.270.16.9060. [DOI] [PubMed] [Google Scholar]
- Lee SH, Earm YE. Caffeine induces periodic oscillations of Ca2+-activated K+ current in pulmonary arterial smooth muscle cells. Pflügers Arch. 1994;426:189–198. doi: 10.1007/BF00374771. [DOI] [PubMed] [Google Scholar]
- Li YX, Keizer J, Stojilkovic SS, Rinzel J. Ca2+ excitability of the ER membrane: an explanation for IP3-induced Ca2+ oscillations. Am J Physiol Cell Physiol. 1995a;38:C1079–C1092. doi: 10.1152/ajpcell.1995.269.5.C1079. [DOI] [PubMed] [Google Scholar]
- Li YX, Rinzel J, Vergara L, Stojilkovic SS. Spontaneous electrical and calcium oscillations in unstimulated pituitary gonadotrophs. Biophys J. 1995b;69:785–795. doi: 10.1016/S0006-3495(95)79952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Cunningham KW, Harper JF, Sze H. ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1997;94:8579–8584. doi: 10.1073/pnas.94.16.8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DC, Venis MA. Characterisation and immunological identification of a calmodulin-stimulated Ca2+-ATPase from maize shoots. J Plant Physiol. 1995;145:702–710. [Google Scholar]
- Ma J. Block by ruthenium red of the ryanodine-activated calcium release channel of skeletal muscle. J Gen Physiol. 1993;102:1031–1056. doi: 10.1085/jgp.102.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J, Corzo A, Leigh RA, Sanders D. Membrane potential-dependent calcium transport in right-side-out plasma membrane vesicles from Zea mays L. roots. Plant J. 1994;5:683–694. [Google Scholar]
- McAinsh MR, Webb AAR, Taylor JE, Hetherington AM. Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell. 1995;7:1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu Rev Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Juri MU. Sr and Ba transients in isolated snail neurones studied with fura-2: the recovery from depolarization induced load and modulation of Ca release from intracellular stores. Neurosci Lett. 1990;112:184–189. doi: 10.1016/0304-3940(90)90200-s. [DOI] [PubMed] [Google Scholar]
- Muir SR, Sanders D. Pharmacology of Ca2+ release from red beet microsomes suggests the presence of ryanodine receptor homologs in higher plants. FEBS Lett. 1996;395:39–42. doi: 10.1016/0014-5793(96)01000-9. [DOI] [PubMed] [Google Scholar]
- Pantoja O, Gelli A, Blumwald E. Voltage-dependent calcium channels in plant vacuoles. Science. 1992;255:1567–1570. doi: 10.1126/science.255.5051.1567. [DOI] [PubMed] [Google Scholar]
- Petersen OH, Petersen CCH, Kasai H. Calcium and hormone action. Annu Rev Physiol. 1994;56:297–319. doi: 10.1146/annurev.ph.56.030194.001501. [DOI] [PubMed] [Google Scholar]
- Piñeros M, Tester M. Characterization of a voltage-dependent Ca2+-selective channel from wheat roots. Planta. 1995;195:478–488. [Google Scholar]
- Plieth C, Hansen U-P. Methodological aspects of pressure loading of fura-2 into Characean cells. J Exp Bot. 1996;47:1601–1612. [Google Scholar]
- Plieth C, Sattelmacher B, Hansen U-P, Thiel G. The action potential in Chara: Ca2+ release from internal stores visualized by Mn2+-induced quenching of fura-dextran. Plant J. 1998;13:167–175. [Google Scholar]
- Rengel Z. Effects of Al3+, rare earth elements, and other metals on net 45Ca2+ uptake by Amaranthus protoplasts. J Plant Physiol. 1994;143:47–51. [Google Scholar]
- Sauer G, Simonis W, Schönknecht G. An inwardly rectifying cation current across the plasma membrane of the green alga Eremosphaera viridis. Plant Cell Physiol. 1993;34:1275–1282. [Google Scholar]
- Sauer G, Simonis W, Schönknecht G. Divalent cation and anion currents are activated during the dark-induced transient hyperpolarization of the plasma membrane of the green alga Eremosphaera viridis. J Exp Bot. 1994;45:1403–1412. [Google Scholar]
- Schönknecht G, Bauer CS, Simonis W. Light-dependent signal transduction and transient changes in cytosolic Ca2+ in a unicellular green alga. J Exp Bot. 1998;49:1–11. [Google Scholar]
- Schroeder JI, Hagiwara S. Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proc Natl Acad Sci USA. 1990;87:9305–9309. doi: 10.1073/pnas.87.23.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker KS, Sze H. Inositol 1,4,5-trisphosphate releases Ca2+ from vacuolar membrane vesicles of oat roots. J Biol Chem. 1987;262:3944–3946. [PubMed] [Google Scholar]
- Smith JS, Imagawa T, Ma J, Fill M, Campbell KP, Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988;92:1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucki JW, Somogyi R. A dialogue on Ca2+ oscillations: an attempt to understand the essentials of mechanisms leading to hormone-induced intracellular Ca2+ oscillations in various kinds of cells on a theoretical level. Biochim Biophys Acta. 1994;1183:453–472. doi: 10.1016/0005-2728(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Thaler M, Simonis W, Schönknecht G. Light-dependent changes of the cytoplasmic H+ and Cl− activity in the green alga Eremosphaera viridis. Plant Physiol. 1992;99:103–110. doi: 10.1104/pp.99.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler M, Steigner W, Förster B, Köhler K, Simonis W, Urbach W. Calcium activation of potassium channels in the plasmalemma of Eremosphaera viridis. J Exp Bot. 1989;40:1195–1203. [Google Scholar]
- Thomas AP, Bird GSJ, Hajnóczky G, Robb-Gaspers LD, Putney JW. Spatial and temporal aspects of cellular calcium signaling. FASEB J. 1996;10:1505–1517. [PubMed] [Google Scholar]
- Trebacz K, Busch MB, Hejnowicz Z, Sievers A. Cyclopiazonic acid disturbs the regulation of cytosolic calcium when repetitive action potentials are evoked in Dionaea traps. Planta. 1996;198:623–626. doi: 10.1007/BF00262650. [DOI] [PubMed] [Google Scholar]
- Trewavas A, Read N, Campbell AK, Knight M. Transduction of Ca2+ signals in plant cells and compartmentalization of the Ca2+ signal. Biochem Soc Trans. 1996;24:971–974. doi: 10.1042/bst0240971. [DOI] [PubMed] [Google Scholar]
- Tsien RW, Tsien RY. Calcium channels, stores and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Webb AAR, McAinsh MR, Taylor JE, Hetherington AM. Calcium ions as intracellular second messengers in higher plants. Adv Bot Res. 1996;22:45–96. [Google Scholar]
- Weidinger M, Ruppel HG. Ca2+-requirement for a blue-light-induced chloroplast translocation in Eremosphaera viridis. Protoplasma. 1985;124:184–187. [Google Scholar]
- Wojnowski L, Schwab A, Hoyland J, Mason WT, Sibernagl S, Oberleithner H. Cytoplasmic Ca2+ determines the rate of Ca2+ entry into Mardin-Darby canine kidney-focus (MDCK-f) cells. Pflügers Arch. 1994;426:95–100. doi: 10.1007/BF00374676. [DOI] [PubMed] [Google Scholar]
- Zhang GH, Melvin JE. Inhibitors of the intracellular Ca2+ release mechanism prevent muscarinic-induced Ca2+ influx in rat sublingual mucous acini. FEBS Lett. 1993;327:1–6. doi: 10.1016/0014-5793(93)81026-v. [DOI] [PubMed] [Google Scholar]