Abstract
Studying the interaction of dendritic cells (DCs) with bacteria controlled by T-cell-mediated immune responses may reveal novel adjuvants for the induction of cellular immunity. Murine studies and the observation that nocardias infect predominantly immunosuppressed patients have suggested that these bacteria may possess an adjuvant potential. Moreover, adjuvants on the basis of the nocardial cell wall have been applied in clinical studies. Since the handling of adjuvants by DCs may determine the type of immune responses induced by a vaccine, the present study aimed at investigating the interaction of immature human monocyte-derived DCs with live or inactivated Nocardia farcinica in vitro and determining the cellular phenotypic changes as well as alterations in characteristic functions, such as phagocytosis, induction of T-cell proliferation, and cytokine secretion. Human DCs ingested N. farcinica and eradicated the bacterium intracellularly. DCs exposed to inactivated N. farcinica were activated, i.e., they developed a mature phenotype, downregulated their phagocytic capacity, and stimulated allogeneic T cells in mixed leukocyte reactions. Soluble factors were not involved in this process. To elucidate the potential adjuvant effect of N. farcinica on the induction of T-cell-mediated immune responses, we characterized the cytokines produced by nocardia-exposed DCs and detected substantial amounts of tumor necrosis factor alpha (TNF-α) and interleukin-12 p40 (IL-12p40). However, nocardia-treated DCs secreted only small amounts of IL-12p70, which were significantly smaller than the amounts of IL-23. Thus, N. farcinica activates DCs, but adjuvants based on this bacterium may have only a limited capacity to induce Th1 immune responses.
INTRODUCTION
Mycobacterial antigens are potent adjuvants for the induction of cellular immune responses. The toxicity of their components, however, has been a major obstacle to their clinical availability. Nocardias are Gram-positive and partially acid-fast bacilli with a close relationship to mycobacteria (49). In contrast to some mycobacterial species, which can cause severe infections in immunocompetent subjects (e.g., tuberculosis, leprosy, and Buruli ulcer), nocardias affect mainly immunosuppressed patients, particularly those suffering from cellular immune dysfunctions (13). Because nocardias are widespread and commonly found in the environment, this cannot be explained by limited exposure but may rather reflect the induction of protective immune responses against nocardias in the immunocompetent host. Murine studies underline the role of cellular immunity for protection against nocardias. Nude mice are more susceptible to Nocardia brasiliensis infection, and immunity to N. asteroides infection has been transferred with primed splenic T lymphocytes (8, 9, 16, 21). In early N. brasiliensis infection of BALB/c mice, serum levels of interleukin-4 (IL-4), IL-6, IL-10, and particularly gamma interferon (IFN-γ) are elevated, followed by substantial lymphocyte proliferation in the spleen and lymph nodes (47). In addition, CXC chemokine receptor 2-mediated chemotaxis of neutrophils has been shown to be essential in pulmonary N. asteroides infection (43), while humoral immunity may be less critical for immunity in murine nocardiosis (10).
Intravesical immunotherapy with live Mycobacterium bovis bacillus Calmette-Guérin (BCG) has been introduced clinically for the treatment and prevention of relapses of superficial bladder cancer (3). Side effects are rare but potentially serious (38). In clinical studies, a commercially available preparation of the cell wall skeleton of Nocardia rubra that induces tumor necrosis factor alpha (TNF-α) and IL-1 secretion in human monocytes in vitro and activates murine macrophages in vivo following intraperitoneal injection (29, 42) was less effective than BCG regarding numbers of immune cells attracted to the bladder and cytokine induction in patients with superficial bladder cancer (15). Although this may have been due to different preparations of antigen, i.e., the application of viable bacilli versus the bacterial cell wall, a better understanding of the cellular and molecular mechanisms underlying the efficacy of adjuvants based on preparations of nocardias could potentially lead to the development of more effective and safer therapeutics.
Dendritic cells (DCs) are key cells in the induction of cellular immune responses and are therefore of interest in vaccine research (39). DCs reside in an immature state in peripheral tissues and recognize pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors (PRRs). These include Toll-like receptors (TLRs), C-type lectins, and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) (55). Mature DCs are found in secondary lymphoid tissues, where their high expression of major histocompatibility complex (MHC) and costimulatory molecules as well as secretion of cytokines enable them to potently activate naive T lymphocytes and thereby induce antigen-specific immune responses (5). IL-12 is a key cytokine in T-lymphocyte activation and differentiation, and individuals with inherited IL-12 deficiency appear to have a greater risk of acquiring nocardial infections (44). IL-12 consists in its biologically active form, IL-12p70, of a light chain (IL-12p35) and a heavy chain (IL-12p40) and favors the differentiation of Th1 cells, although its production may not be indispensable for the induction of Th1 responses (53). The stimulation of antigen-presenting cells through only one PRR, e.g., TLR or NLR, is usually not sufficient for the production of IL-12p70, and a second stimulus, either through a different PRR or derived by another signal, such as CD40 ligand (CD40L) or IFN-γ, is required (54). IL-12p40 (together with a p19 subunit) is also part of IL-23, which drives the development of T helper cells that secrete IL-17 (Th17 cells) (1).
While several studies have focused on the effects of mycobacteria on DCs (4, 27, 32), it is not known how human DCs interact with nocardias. Studies on this interaction, however, could elucidate how nocardial antigens can be used more efficiently as adjuvants for the induction of effective cellular immune responses. Our study therefore aimed at analyzing the effects of nocardias on immature human monocyte-derived DCs regarding phenotypic alterations and functional consequences, including the secretion of cytokines involved in Th1 or Th17 induction, as well as IL-10, a key anti-inflammatory cytokine. We chose N. farcinica for our studies because its genome has been determined completely and thereby may allow the future identification of candidate genes involved in the induction of protective immune responses (30). Here we show that nocardias are readily taken up by human DCs in vitro, which leads to the upregulation of typical maturation markers, an increased potential to stimulate T cells, and cytokine secretion. Notably, N. farcinica-activated DCs produce only negligible amounts of IL-12p70.
MATERIALS AND METHODS
Bacteria.
N. farcinica ATCC 3308 was stored frozen at −80°C in Cryobank storage tubes (Mast Diagnostica, Reinfeld, Germany) until transfer to chocolate agar plates for recovery, when desired. After 3 to 5 days of incubation in 5% CO2 at 37°C, 2 colonies were suspended in 10 ml RPMI 1640 supplemented with 2 mM l-glutamine, 10 mM HEPES (all from Gibco, Invitrogen, Karlsruhe, Germany), 50 μM 2-mercaptoethanol (Sigma, Taufkirchen, Germany), and 10% fetal calf serum (Biochrom, Berlin, Germany) without antibiotics and then incubated for 2 days under the same conditions. The culture medium was then differentially centrifuged to remove filaments of nocardias and retrieve coccoid forms of the bacteria as previously described (20). Briefly, bacterial suspensions were centrifuged at 60 × g for 7 min, and the supernatant was carefully transferred to another tube and centrifuged at 900 × g for 15 min. The resulting pellet was then resuspended in medium to obtain a just visible opacification (McFarland standard of 1) and serially diluted to adjust for various multiplicities of infection (MOI) (e.g., for an MOI of 0.1, we used 1 × 104 CFU N. farcinica plus 1 × 105 DCs). To determine the MOI, 100-μl serial dilutions of each bacterial suspension were plated on chocolate agar plates. After incubation for 3 to 5 days as described above, colonies were counted and the resulting MOIs were calculated. Since N. farcinica is not readily inactivated by heat, bacteria were inactivated by incubation in medium containing 5 μg/ml amikacin (ICN Biomedicals, Eschwege, Germany) and penicillin (1,000 U/ml)-streptomycin (1,000 μg/ml) (both from Gibco) for 24 h. Complete inactivation of the bacteria was documented by an absence of growth after inoculation of the suspension in brain heart infusion broth (Oxoid, Wedel, Germany), incubation for up to 7 days, and a final subculture attempt on chocolate agar. The stimulatory effects induced by inactivated bacteria on DCs approximately equaled those observed with live bacteria at an MOI of 1.
Generation of human DCs and isolation of human T cells.
Buffy coats (German Red Cross, Berlin, Germany) or peripheral blood from healthy volunteers were used to isolate peripheral blood mononuclear cells (PBMCs). Monocyte-derived DCs were generated from magnetically separated CD14+ monocytes (Miltenyi Biotec, Bergisch-Gladbach, Germany) as previously described (33). Briefly, monocytes were cultured in medium as described above, with supplementation of the medium with 1,000 U/ml human recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF) (sargramostim; Leukine, Berlex, Richmond, CA) and 100 U/ml human rIL-4 (R&D Systems, Wiesbaden-Nordenstadt, Germany), at 3 × 106 cells/well in 6-well cell culture dishes (Nunc, Roskilde, Denmark) for 6 days, and fresh medium and cytokines were added every other day. Penicillin (100 U/ml)-streptomycin (100 μg/ml) was added in experiments without viable bacteria (see below). The endocytic functions of DCs were studied by using 10 μg/ml dextran-fluorescein isothiocyanate (dextran-FITC) (Molecular Probes/Invitrogen) as described previously (33, 40).
Human T cells were enriched from CD14-depleted PBMCs by HLA-DR-negative magnetic bead selection (33). A total of 1 × 108 cells/ml were incubated with FITC-conjugated monoclonal antibodies (MAbs; 1:50 dilution) against human HLA-DR (BD Pharmingen, Heidelberg, Germany), the MAbs were washed out, and cells were incubated with anti-FITC antibody-conjugated magnetic beads (Miltenyi Biotec). HLA-DR-negative cells were harvested, washed twice, and used as responder cells in allogeneic mixed leukocyte reactions (MLRs). The purity of the cells was monitored by fluorescence-activated cell sorter (FACS) analysis and was ≥90% in all experiments.
Incubation of human DCs with nocardias.
At day 6, DCs were harvested and transferred to 96-well round-bottom plates at 105 cells/well in 100 μl culture supernatant free of antibiotics. One-hundred-microliter bacterial suspensions at various MOIs were added, and the cells were incubated. After 30 min, amikacin and penicillin-streptomycin were added at final concentrations of 5 μg/ml, 1,000 U/ml, and 1,000 μg/ml, respectively, in 100 μl medium.
Since we observed Nocardia-induced cell death in initial experiments, apoptosis of infected cells was determined by a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay as described previously (28). Briefly, cells were washed twice with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde-PBS (wt/vol) for 30 min at room temperature. Cells were washed, permeabilized in 0.1% Triton X-100 (Sigma) in 0.1% sodium citrate, washed again, and incubated in the TUNEL reaction or control mixture (Roche Molecular Biochemicals, Mannheim, Germany) for 60 min at 37°C and 5% CO2. After washing, the cells were analyzed by FACS.
CFU were determined by plating 50-μl aliquots of culture supernatants or serial dilutions of the supernatants onto chocolate agar plates and counting the CFU after 5 days of incubation at 37°C with 5% CO2. To determine the intracellular survival of nocardias at days 2, 4, and 10, extracellular bacteria were treated for 2 h with antibiotics as described above. After extensive washing, the cells were lysed by adding distilled water at 37°C for 30 min. Lysis was confirmed by trypan blue staining. Lysates were then plated in serial dilutions onto chocolate agar plates, and colonies were counted after 5 days of incubation at 37°C with 5% CO2.
Suspensions of inactivated nocardias were added to the cultures at a ratio of 1:10 and incubated in the presence of penicillin (100 U/ml) and streptomycin (100 μg/ml). Controls consisted of DCs stimulated with (i) human rIL-6, rTNF, rIL-1β (all at 10 ng/ml; R&D Systems) and 10 μM prostaglandin E2 (PGE2; Sigma), which reliably induce DC maturation (35), or (ii) lipopolysaccharide (LPS) (derived from Escherichia coli; Sigma), and (iii) DCs were kept in an immature state in medium.
Flow cytometric analyses.
The phenotype of DCs was monitored by flow cytometry with phycoerythrin (PE)- or FITC-labeled anti-human MAbs to the surface molecules HLA-DR, CD14, CD25, CD80, CD86 (all from BD Pharmingen), and CD83 (Caltag Laboratories, Hamburg, Germany) or with the appropriate isotype controls, as previously described (33). For detection of intracellular antigens, cells were fixed for 30 min in 4% paraformaldehyde-PBS (wt/vol), permeabilized by washing twice in 0.5% saponin, and subsequently stained with a polyclonal anti-Mycobacterium bovis BCG antiserum (pAbBCG) with known cross-reactivity to nocardias (56) for 60 min in 0.5% saponin, followed by incubation with a secondary FITC-conjugated goat anti-rabbit IgG MAb (Jackson) in 0.5% saponin for another 60 min. The purity of T cells was also monitored by FACS analysis (PE-conjugated MAbs against CD3, CD14, and CD20; all from BD Pharmingen). All cell populations were fixed with 10% formaldehyde-PBS (vol/vol) before analysis on a FACSCalibur flow cytometer with CellQuestPro software (BD Pharmingen). Mean fluorescence intensities (MFIs) were calculated and are given as medians with 25th and 75th percentiles.
Allogeneic mixed leukocyte reaction assays.
T cells were prepared from CD14-negative PBMCs by magnetic depletion of HLA-DR+ cells, and the cells were ≥95% CD3+. T cells were stained with 0.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) before coincubation of 2 × 105 T cells with graded numbers of stimulated or unstimulated DCs in flat-bottom 96-well culture plates (Nunc). T cells alone (2 × 105/well) served as controls. Six days later, cells were harvested and stained with CD3-peridinin chlorophyll protein (CD3-PerCP) and CD4-allophycocyanin (CD4-APC) (both from BD BioSciences), and the CFSE dilution of CD4+ T cells was analyzed on a FACSCalibur flow cytometer using CellQuestPro software (both from BD BioSciences).
Analysis of cytokine secretion by DCs.
Forty-eight-hour cell-free supernatants of DC cultures were harvested and stored at −80°C until analysis by sandwich enzyme-linked immunosorbent assays (ELISAs) for IL-12p40, IL-12p70, IL-10 (all from U-CyTech, Utrecht, The Netherlands), or IL-23 (eBioscience).
Analysis of p38 MAPK phosphorylation.
p38 mitogen-activated protein kinase (MAPK) phosphorylation was determined as described before (33). Briefly, 1.5 × 106 immature human DCs were incubated with inactivated N. farcinica at 37°C for 15, 30, and 60 min, and cells kept in medium alone were used as controls. Cells were washed twice, resuspended in 100 μl cell lysis buffer containing protease and phosphatase inhibitors (Sigma), and incubated for 60 min on ice. Cell debris was removed by centrifugation, and supernatants were harvested and stored at −80°C. Supernatants were mixed with 2× SDS sample buffer (Bio-Rad, Hercules, CA), boiled for 5 min, subjected to 10% SDS-PAGE, and transferred to Trans-Blot nitrocellulose membranes (Bio-Rad). Membranes were blocked with blocking buffer (5% nonfat dry milk in Tris-buffered saline) for 1 h at room temperature and incubated overnight at 4°C with MAbs to phospho-p38 or total p38 MAPK (Cell Signaling Technology, Beverly, MA). Bands were visualized with horseradish peroxidase-conjugated MAbs by use of an enhanced chemiluminescence (ECL) system (Cell Signaling Technology).
Statistics.
Data were analyzed statistically using the nonparametric Mann-Whitney test. When more than two conditions were compared, the nonparametric Friedman test followed by Dunn's multiple-comparison test as a posttest was used. Differences were considered statistically significant for P values of <0.05.
RESULTS
Human immature DCs ingest and eradicate nocardias in vitro.
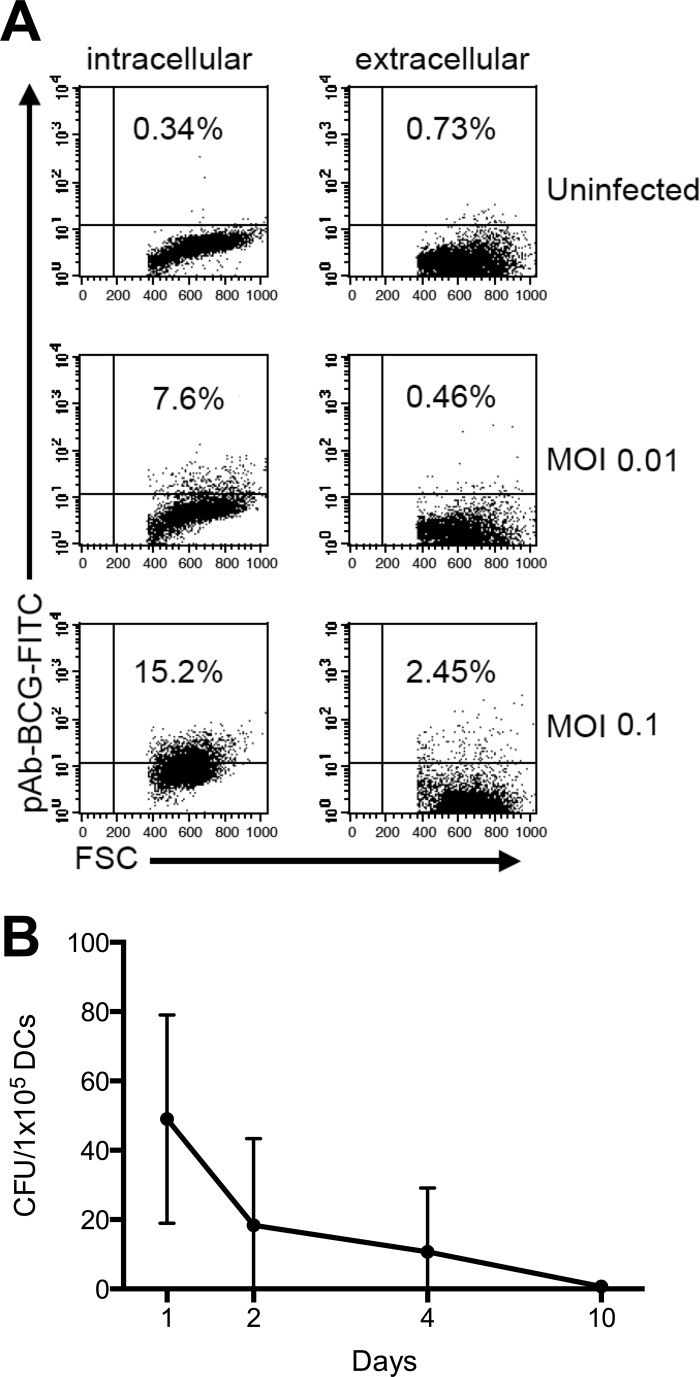
We first examined the interaction of DCs with viable nocardias at low MOIs (0.01 and 0.1). After 48 h of incubation, extracellular and intracellular bacteria could be detected by light microscopy (data not shown). Intracellular flow cytometric analysis using pAbBCG, which cross-reacts with nocardias (56), confirmed the intracellular location of the bacteria. Following intracellular staining, we observed an MOI-dependent increase of positive cells in infected cultures (Fig. 1A), whereas extracellular staining did not reveal a similar increase in the number of positive cells. Uninfected DCs stained negative both intra- and extracellularly (Fig. 1A).
Fig 1.
N. farcinica infects DCs in vitro but does not persist over prolonged periods. (A) DCs were incubated in medium alone (uninfected) or with N. farcinica at an MOI of 0.01 or 0.1, as described in Materials and Methods. Cells were then fixed, permeabilized with saponin (intracellular) or not permeabilized (extracellular), and incubated with cross-reacting rabbit pAbBCG followed by a secondary, FITC-conjugated monoclonal anti-rabbit IgG. The staining was then visualized by flow cytometry. Data from one representative of seven independent experiments are shown. (B) The presence of live intracellular bacteria after 2, 4, or 10 days in culture was assessed by lysing DCs. To inactivate possible extracellular bacteria, a bactericidal dose of antibiotics was added to the medium before washing and lysing of the cells, serial dilutions of the lysates were plated onto chocolate agar plates, and CFU were determined after 2 days of incubation. Data are mean numbers of CFU/105 DCs ± standard deviations (SD) for three independent experiments.
To further investigate the fate of nocardias ingested by immature DCs, we determined the numbers of intracellular bacteria after lysis of the cells with distilled water at different time points postinfection (MOI 0.1). We observed a decline in the number of intracellular bacteria that could be isolated from infected DC cultures, and 10 days after infection, no or only minimal numbers of nocardias could be grown from cell lysates (Fig. 1B). Therefore, while human immature DCs ingest N. farcinica in vitro, the cells probably do not provide a niche for bacterial survival, as the number of intracellular bacteria declines over time.
We also detected a dose-dependent cell loss in N. farcinica-infected DCs compared to uninfected DCs, and changes in size (forward scatter [FSC]) and granularity (side scatter [SSC]) of the infected cell population were observed by FACS analysis (data not shown). These changes are characteristic of apoptotic cell death, and large numbers of TUNEL-positive cells could be detected in nocardia-treated cells at MOIs of ≥1 (data not shown).
Phenotypic activation of N. farcinica-treated human DCs.
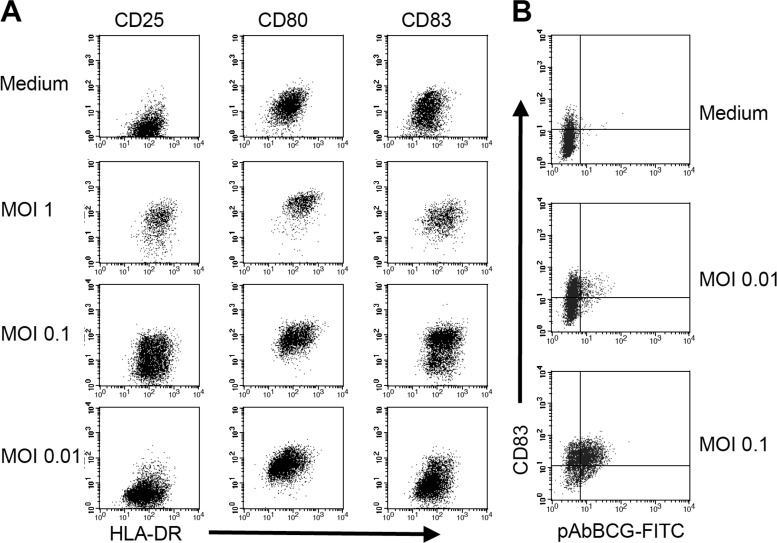
To examine the effects of nocardias on immature human DCs, we determined the phenotype of nocardia-exposed DCs 2 days after infection with N. farcinica at various MOIs. Following exposure to N. farcinica at an MOI of 1, many cells died of apoptosis, but the remaining DCs displayed a mature phenotype, i.e., upregulation of MHC class II molecules and costimulatory molecules (CD80 and CD86) and expression of CD25 and CD83 (Fig. 2A). Cells exposed to nocardias at an MOI of 0.1 still expressed more HLA-DR, CD25, and CD83 than control cells incubated in medium only (Fig. 2A; Table 1), while incubation at an MOI of 0.01 resulted in only marginal and nonsignificant upregulation of activation markers.
Fig 2.
N. farcinica induces DC maturation. (A) MOI-dependent upregulation of markers for DC activation upon exposure to various MOIs of N. farcinica. Cells were incubated in medium alone or in the presence of N. farcinica at an MOI of 1, 0.1, or 0.01. Thirty minutes after the addition of bacteria, cultures were treated with antibiotics, harvested after 48 h, and stained with the indicated MAbs. (B) After incubation with medium alone or N. farcinica at an MOI of 0.01 or 0.1, cells were harvested and stained intracellularly for the presence of nocardias as described in the legend to Fig. 1. After extensive washing, anti-CD83 MAb was added. Data from one representative of four independent experiments are shown.
Table 1.
Expression of DC activation markers by immature human DCs incubated in the presence or absence of viable N. farcinica at different MOIs
| Treatment | Expression of surface molecule (median MFI [25th, 75th percentile])a |
||
|---|---|---|---|
| HLA-DR | CD25 | CD83 | |
| N. farcinica at MOI of 0.1 | 155.4 (86.7, 211.9)* | 39.9 (19.0, 60.6)** | 18.1 (9.9, 50.0)** |
| N. farcinica at MOI of 0.01 | 91.6 (51.4, 142.2) | 3.5 (1.3, 23.3) | 8.6 (3.5, 32.1) |
| Medium | 61.1 (37.4, 105.3) | 0.7 (0.02, 11.3) | 2.9 (2.1, 30.8) |
Data are based on MFIs from five independent experiments (MFIs of isotype controls were subtracted). *, P < 0.05; **, P < 0.01 (Friedman test and Dunn's multiple-comparison test).
Since the experiments with MOIs of <1 indicated that uninfected DCs might also become activated following exposure to nocardias, we next investigated the ratio of activated DCs within nocardia-positive and -negative cells by double staining with antibodies against the DC activation marker CD83 and with pAbBCG. We observed an MOI-dependent increase of nocardia-positive cells; however, mature cells were observed in both nocardia-positive and nocardia-negative DC populations (Fig. 2B), suggesting that either solid bacterial components or cytokines secreted by infected cells (or both), rather than the ingestion of the bacterium itself, may activate N. farcinica-exposed immature DCs.
Nocardia-induced DC activation is due to direct interaction of cells with bacteria and not to soluble mediators.
We next elucidated whether soluble factors produced by the bacteria, the infected DCs, or both were responsible for the observed bystander activation of uninfected cells. When cell-free, sterile-filtered supernatants from DC cultures infected at MOIs previously shown to be effective at activating DCs were transferred to immature DCs, no upregulation of surface markers for DC maturation was detected 48 h after the transfer of supernatants (data not shown).
Since the presence of bacteria was essential for the previously observed stimulatory effects of nocardias on DCs, we next inactivated N. farcinica by antibiotic treatment prior to its addition to the cell cultures. Notably, inactivated nocardias also provided a potent stimulus for the activation of DCs, as shown by a significant upregulation of CD25, CD83, and CD86 on DCs incubated with inactivated nocardias for 48 h (Table 2). Similarly, there was a strong trend for an increased HLA-DR upregulation (P = 0.05) by DCs exposed to inactivated nocardias. Thus, at the MOIs investigated, insoluble bacterial antigens that are also provided by inactivated bacteria most likely induced DC activation.
Table 2.
DC activation markers expressed by DCs incubated in the presence or absence of inactivated N. farcinica
| Treatment | Expression of surface molecule (median MFI [25th, 75th percentile])a |
|||
|---|---|---|---|---|
| HLA-DR | CD25 | CD83 | CD86 | |
| Inactivated N. farcinica | 194.5 (136.7, 585.1) | 7.9* (2.3, 28.2) | 36.1* (23.9, 41.6) | 314.0* (264.1, 475.5) |
| Medium | 97.8 (58.3, 305.4) | 0 (0, 0) | 2.8 (1.5, 4.6) | 12.0 (5.8, 28.0) |
Data are based on MFIs from nine independent experiments (MFIs of isotype controls were subtracted). *, P ≤ 0.01 compared to DCs kept in medium (Mann-Whitney test).
N. farcinica-activated DCs downregulate their phagocytic activity but possess an increased potential to stimulate CD4+ T-cell proliferation.
Activation of immature DCs leads to several functional alterations of the cells, including downregulation of their phagocytic capacity (23, 48). We therefore tested the effects of inactivated nocardias on the phagocytosis of dextran and observed a significant impairment of this process in DCs incubated previously in the presence of inactivated N. farcinica compared with immature DCs (P = 0.008) (Fig. 3).
Fig 3.
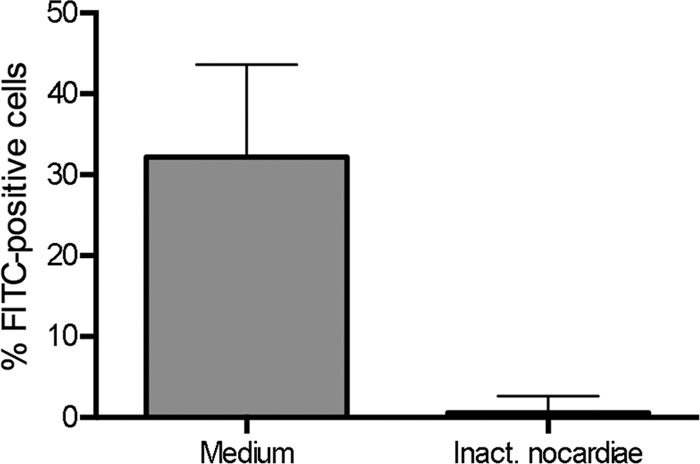
Reduced phagocytosis of dextran by DCs after exposure to nocardias. Nonstimulated DCs (medium) or DCs stimulated by inactivated nocardias (as described in Materials and Methods) were incubated with 20 μg/ml dextran-FITC for 30 min at 37°C, or at 4°C to detect nonspecific binding. After 30 min, the cells were washed, and the percentage of FITC-positive cells was determined by flow cytometry. Results (after subtraction of fluorescence data obtained at 4°C) from five independent experiments are shown as medians plus interquartile ranges.
Activation of DCs is also reflected by an increased capacity of the cells to stimulate the proliferation of T lymphocytes. We incubated DCs with inactivated nocardias, harvested the cells 48 h later, and plated them in graded numbers with allogeneic T cells in MLR assays, while cells kept in medium were used as controls. In correlation with the phenotypic changes of the cells registered before, we observed an increased potential of the nocardia-activated DCs to stimulate the proliferation of CD4+ T cells (P < 0.01 for DC/T-cell ratios of 1:40 to 1:1,080 and P < 0.05 for a DC/T-cell ratio of 1:3,240) (Fig. 4). Therefore, the previously observed phenotypic maturation of DCs induced by nocardias corresponds to an impaired phagocytic capacity and to an increased capability of the cells to stimulate the proliferation of CD4+ T cells.
Fig 4.
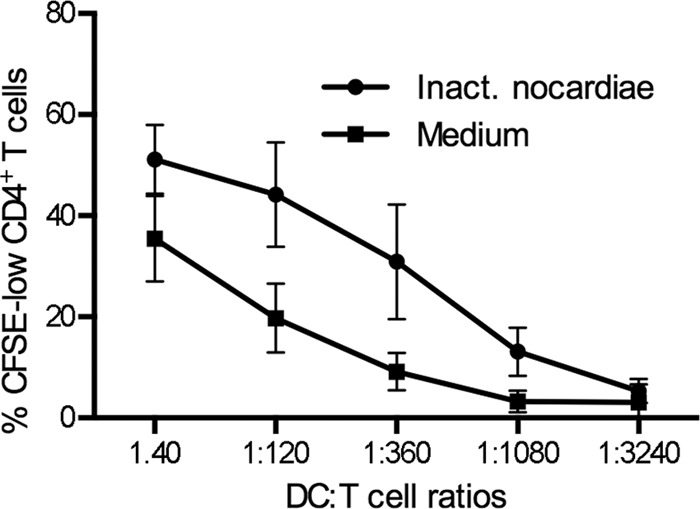
DCs matured by inactivated nocardias potently activate allogeneic T-cell proliferation. DCs were incubated for 48 h with medium or inactivated nocardias (1:10). T cells were isolated from PBMCs by consecutive magnetic depletion of CD14+ monocytes and remaining HLA-DR+ cells and then were stained with CFSE. T cells were incubated with DCs in serial dilutions, as indicated. After 7 days, cells were harvested and T-cell proliferation was assessed as the percentage of CFSElow cells, with gating on live CD3+ CD4+ cells. Data from eight independent experiments are shown as means ± SD.
Nocardia-activated DCs secrete IL-12/23p40 and IL-10.
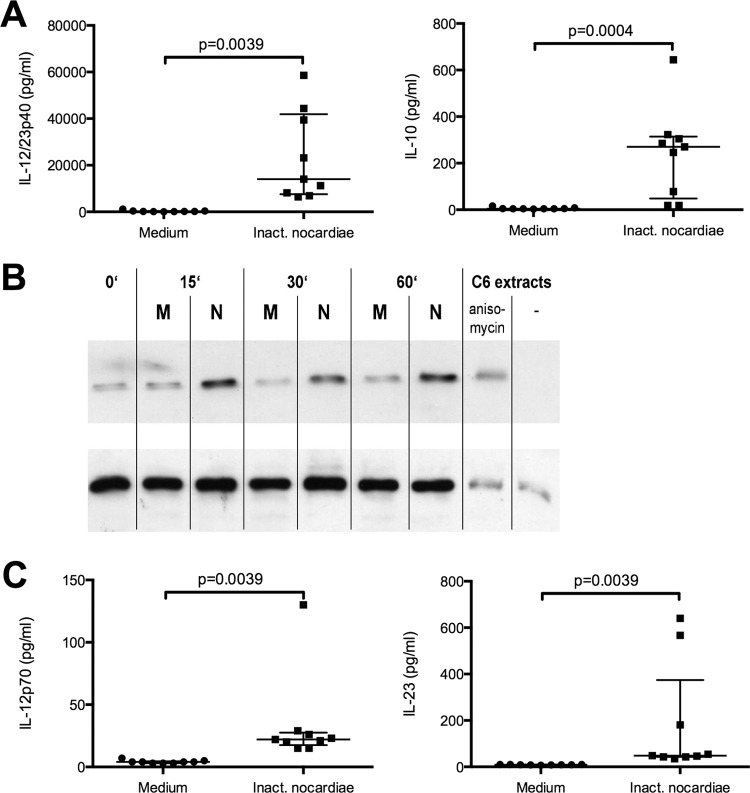
Activated DCs secrete cytokines and chemokines that are involved in T-cell activation and differentiation. To analyze the production of proinflammatory IL-12/23p40 versus anti-inflammatory IL-10 by N. farcinica-activated human DCs and to compare it to that induced by a different bacterial stimulus, we incubated immature DCs with either inactivated nocardias or LPS for 48 h and determined the concentrations of IL-12/23p40 and IL-10. Cells kept in medium alone served as negative controls. Nocardia-treated DCs secreted considerable amounts of IL-12/23p40 and IL-10, while nonstimulated cells produced only negligible amounts of both cytokines (Fig. 5A). Both nocardias and LPS induced substantially more IL-12/23p40 than IL-10 (50-fold more for N. farcinica and 70-fold more for LPS). Notably, LPS induced around 7-fold more IL-12/23p40 and 5-fold more IL-10 than inactivated N. farcinica (data not shown).
Fig 5.
DCs incubated with inactivated N. farcinica secrete IL-12/IL-23p40, which correlates with p38 MAPK phosphorylation, as well as IL-10, IL-23, and a little IL-12p70. For cytokine analysis, DCs were incubated for 48 h with medium or inactivated nocardias (1:10). Supernatants were harvested after 48 h and analyzed by ELISA for the presence of IL-12/IL-23p40 and IL-10 (A) or IL-12p70 and IL-23 (C). Medians and interquartile ranges are given for nine independent experiments. (B) For analysis of p38 MAPK phosphorylation, DCs were incubated without (medium [M]) or with (N) inactivated N. farcinica for the indicated periods. Cells were then harvested and lysed. Phosphorylated (upper row) and total (lower row) p38 MAPK forms were determined by Western blot analysis. Data are representative of three independent experiments.
IL-12/23p40 secretion by N. farcinica-activated DCs involves the p38 MAPK pathway.
The IL-12/23p40 production of human DCs induced by CD154 or TNF-α is signaled via the p38 MAPK phosphorylation pathway (57). We were therefore interested in whether this pathway is also relevant to the Nocardia-induced IL-12/23p40 production of human DCs observed before. Stimulation of immature DCs with inactivated N. farcinica was followed by a sharp increase in phosphorylation of p38 MAPK, which was still detectable after 60 min. In contrast, no increased p38 MAPK phosphorylation was detected in unstimulated immature DCs (Fig. 5B). Thus, similar to the effects of noninfectious agents inducing IL-12/23p40 via the p38 MAPK pathway, the same signal transduction pathway is involved following the interaction of human DCs with N. farcinica.
Nocardia-activated DCs produce only a little IL-12p70.
To further investigate whether IL-12/23p40 was partially produced in the form of IL-12p70, IL-23, or both, we analyzed the concentrations of these cytokines by use of ELISAs detecting the specific subunits, i.e., p35 for IL-12p70 and p19 in the case of IL-23. While N. farcinica induced only negligible amounts of IL-12p70, significantly more IL-23 (P = 0.0027) was produced by nocardia-treated DCs (Fig. 5C). Notably, LPS-treated DCs produced comparable amounts of IL-23 (median, 95 pg/ml; 25th percentile, 61.5 pg/ml; 75th percentile, 197 pg/ml) (P = 0.3526) as nocardia-treated DCs but 12-fold more IL-12p70 (median, 264 pg/ml; 25th percentile, 199 pg/ml; 75th percentile, 620 pg/ml) (P = 0.0004) than DCs stimulated by N. farcinica. Therefore, inactivated N. farcinica is only a weak inducer of the Th1-promoting cytokine IL-12p70 in human DCs.
DISCUSSION
Human DCs ingested N. farcinica and were able to kill the bacterium intracellularly. While the enhanced resistance of N. asteroides against intracellular killing involves nocardial superoxide dismutase (7, 11), the lack of intracellular survival of N. farcinica in DCs may reflect the reduced virulence of this species compared with N. asteroides. One possible mechanism could involve lysosomal acid phosphatase, which has been shown to correlate with intracellular degradation of N. farcinica by murine macrophages (12). At MOIs of ≥1, N. farcinica induced apoptosis of human DCs, and similar data have been reported for the effect of N. asteroides on human epithelial cells (6).
Viable or inactivated N. farcinica activated human DCs, and DCs might therefore also be involved in the induction of antinocardial cellular immune responses in vivo. DC activation did not involve soluble cellular and/or bacterial factors, as supernatants from infected cell cultures did not transfer DC-activating capacity to uninfected cells. The nocardial cell wall contains proteins, lipids, glycolipids, lipoproteins, glycolipoproteins, and peptidoglycan, which is characteristic of the family Mycobacteriaceae, and these structures could be involved in DC activation, which was readily induced by inactivated N. farcinica. Early studies have indicated that proteins, but not lipid antigens or carbohydrates, are the immunodominant structures of nocardias capable of inducing protective immunity in a murine model of N. asteroides infection (24–26). Interestingly, Trevino-Villarreal et al. (52) reported recently that delipidated N. brasiliensis induced more expression of MHC-II, CD40, and CD80 in murine bone marrow-derived DCs. At the same time, the cells produced significantly less transforming growth factor beta (TGF-β). If this is also associated with altered IL-12 secretion and these data can be translated to human cells, delipidated Nocardia spp. might provide a more immunostimulatory adjuvant than the bacteria tested in our system.
DCs might become activated through the interaction of nocardial antigens with the extracellular receptor TLR2, which is expressed by monocyte-derived human DCs (31) and has previously been shown to also recognize antigenic structures derived from the cell wall of N. coeliaca (51). Increased TLR2 expression has also been observed in inflammatory lesions of murine mycetoma caused by N. brasiliensis (41). TLR2 is known to bind a large variety of ligands from Gram-positive and Gram-negative bacteria, but also from parasites and fungi (2). Nocardial lipoproteins/lipopeptides and peptidoglycan might constitute putative TLR2 ligands, and for the closely related mycobacteria, lipomannans have also been shown to be recognized through TLR2 (45). Notably, some mycobacterial antigens can be recognized by TLR4 (34), which is also expressed by monocyte-derived DCs (31). This might be different for nocardias, however, since the TNF-α secretion of macrophages from TLR4 knockout mice was unimpaired upon incubation with nocardial cell wall preparations derived from N. coeliaca (51). Additionally, TLR9 has been shown to be involved in the induction of Th1 immune responses to Mycobacterium tuberculosis, in cooperation with TLR2 (4), and likewise, TLR9 is required for induction of IFN-γ by Propionibacterium acnes (36). TLR9, however, is not expressed by human myeloid DCs (31).
Peptidoglycans from Gram-positive bacteria can also be recognized by the intracellular PRR NOD2 (46), and Mycobacterium paratuberculosis antigens stimulate NOD2-transfected HEK cells (19). Likewise, NOD2 recognizes N-glycolyl muramyl dipeptide derived from N. asteroides and mycobacteria (14). Since human myeloid DCs can be stimulated in vitro through NOD2 (22), this receptor might be involved in the recognition of nocardial peptidoglycans in our system.
DCs incubated with N. farcinica produced only negligible amounts of IL-12p70, but this is a key feature of cells stimulated only through TLR2 (37). This can be overcome in vitro and in vivo through various mechanisms, e.g., through the synergistic effects of distinct TLR ligands (54), but this might not occur in nocardial infection, as discussed before. In the absence of an additional TLR ligand, other molecules can also support the induction of IL-12p70 production. The ligation of CD40 may cooperate with TLR signaling in the induction of enhanced IL-12p70 secretion (17, 50). Likewise, IFN-γ can drive the production of IL-12p70 (54), and interestingly, neutrophils, which have a critical role in murine nocardial pneumonia (43) and also accumulate after injection of the N. rubra cell wall (42), have been shown to produce IFN-γ during murine pulmonary infection with N. asteroides (18). Thus, the limited secretion of IL-12p70 by isolated DCs stimulated with nocardial antigens in vitro may not reflect the in vivo situation, where other molecules might contribute to the induction of protective immunity.
In conclusion, N. farcinica activates human DCs, and TLR2 may be involved in this process. Compared to other DC-activating stimuli, such as LPS, nocardial antigens induce only a weak IL-12p70 secretion by isolated DCs. Further studies are warranted to identify soluble or membrane-bound molecules that may cooperate with TLR2 in the induction of antinocardial immune responses. However, if these responses are also characterized by a reduced Th1 activity in vivo, adjuvants on the basis of N. farcinica may be less suited for the induction of strong Th1 responses.
ACKNOWLEDGMENTS
This study was supported by the H. W. & J. Hector Foundation and by the Charité-Universitätsmedizin Berlin.
Footnotes
Published ahead of print 17 September 2012
REFERENCES
- 1. Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910–1914 [DOI] [PubMed] [Google Scholar]
- 2. Akira S, Takeda K. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511 [DOI] [PubMed] [Google Scholar]
- 3. Alexandroff AB, Jackson AM, O'Donnell MA, James K. 1999. BCG immunotherapy of bladder cancer: 20 years on. Lancet 353:1689–1694 [DOI] [PubMed] [Google Scholar]
- 4. Bafica A, et al. 2005. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J. Exp. Med. 202:1715–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392:245–252 [DOI] [PubMed] [Google Scholar]
- 6. Barry DP, Beaman BL. 2007. Nocardia asteroides strain GUH-2 induces proteasome inhibition and apoptotic death of cultured cells. Res. Microbiol. 158:86–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beaman BL, Beaman L. 1994. Nocardia species: host-parasite relationships. Clin. Microbiol. Rev. 7:213–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beaman BL, Gershwin ME, Maslan S. 1978. Infectious agents in immunodeficient murine models: pathogenicity of Nocardia asteroides in congenitally athymic (nude) and hereditarily asplenic (Dh/+) mice. Infect. Immun. 20:381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beaman BL, Goldstein E, Gershwin ME, Maslan S, Lippert W. 1978. Lung response to congenitally athymic (nude), heterozygous, and Swiss Webster mice to aerogenic and intranasal infection by Nocardia asteroides. Infect. Immun. 22:867–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beaman BL, Maslan S. 1977. Effect of cyclophosphamide on experimental Nocardia asteroides infection in mice. Infect. Immun. 16:995–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beaman L, Beaman BL. 1990. Monoclonal antibodies demonstrate that superoxide dismutase contributes to protection of Nocardia asteroides within the intact host. Infect. Immun. 58:3122–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Black CM, Beaman BL, Donovan RM, Goldstein E. 1985. Intracellular acid phosphatase content and ability of different macrophage populations to kill Nocardia asteroides. Infect. Immun. 47:375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ., Jr 2006. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin. Microbiol. Rev. 19:259–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coulombe F, et al. 2009. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J. Exp. Med. 206:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Boer EC, De Reijke TM, Vos PC, Kurth KH, Schamhart DH. 2000. Immunostimulation in the urinary bladder by local application of Nocardia rubra cell-wall skeletons (Rubratin) and bacillus Calmette-Guerin as therapy for superficial bladder cancer: a comparative study. Clin. Infect. Dis. 31(Suppl 3):S109–S114 [DOI] [PubMed] [Google Scholar]
- 16. Deem RL, Beaman BL, Gershwin ME. 1982. Adoptive transfer of immunity to Nocardia asteroides in nude mice. Infect. Immun. 38:914–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards AD, et al. 2002. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J. Immunol. 169:3652–3660 [DOI] [PubMed] [Google Scholar]
- 18. Ellis TN, Beaman BL. 2002. Murine polymorphonuclear neutrophils produce interferon-gamma in response to pulmonary infection with Nocardia asteroides. J. Leukoc. Biol. 72:373–381 [PubMed] [Google Scholar]
- 19. Ferwerda G, et al. 2007. Mycobacterium paratuberculosis is recognized by Toll-like receptors and NOD2. J. Leukoc. Biol. 82:1011–1018 [DOI] [PubMed] [Google Scholar]
- 20. Filice GA, Fischer JE. 1986. Lack of synergy between phagocytes and antimicrobials against Nocardia asteroides. J. Antimicrob. Chemother. 17:353–360 [DOI] [PubMed] [Google Scholar]
- 21. Folb PI, Timme A, Horowitz A. 1977. Nocardia infections in congenitally athymic (nude) mice and in other inbred mouse strains. Infect. Immun. 18:459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fritz JH, et al. 2005. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur. J. Immunol. 35:2459–2470 [DOI] [PubMed] [Google Scholar]
- 23. Garrett WS, et al. 2000. Developmental control of endocytosis in dendritic cells by Cdc42. Cell 102:325–334 [DOI] [PubMed] [Google Scholar]
- 24. Gupta R, Pancholi V, Vinayak VK, Khuller GK. 1985. Immune responses to the protein, carbohydrate and lipid antigens of Nocardia asteroides in experimental nocardiosis in mice. J. Med. Microbiol. 20:255–261 [DOI] [PubMed] [Google Scholar]
- 25. Gupta R, Pancholi V, Vinayak VK, Khuller GK. 1985. Immunological responses to protein, carbohydrate and lipid fractions of Nocardia asteroides in mice. J. Med. Microbiol. 20:263–274 [DOI] [PubMed] [Google Scholar]
- 26. Gupta R, Pancholi V, Vinayak VK, Khuller GK. 1985. Protective immunity to systemic nocardiosis in mice immunized with cell extract antigens of Nocardia asteroides. Med. Microbiol. Immunol. 174:157–166 [DOI] [PubMed] [Google Scholar]
- 27. Hanekom WA, et al. 2003. Mycobacterium tuberculosis inhibits maturation of human monocyte-derived dendritic cells in vitro. J. Infect. Dis. 188:257–266 [DOI] [PubMed] [Google Scholar]
- 28. Ignatius R, et al. 2000. Canarypox virus-induced maturation of dendritic cells is mediated by apoptotic cell death and tumor necrosis factor alpha secretion. J. Virol. 74:11329–11338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Inamura N, Sone S, Ogawa T, Nishio M, Ogura T. 1992. Human blood monocyte activation by Nocardia rubra cell wall skeleton for productions of interleukin 1 and tumor necrosis factor-alpha. Biotherapy 4:155–163 [DOI] [PubMed] [Google Scholar]
- 30. Ishikawa J, et al. 2004. The complete genomic sequence of Nocardia farcinica IFM 10152. Proc. Natl. Acad. Sci. U. S. A. 101:14925–14930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iwasaki A, Medzhitov R. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995 [DOI] [PubMed] [Google Scholar]
- 32. Jang S, Uematsu S, Akira S, Salgame P. 2004. IL-6 and IL-10 induction from dendritic cells in response to Mycobacterium tuberculosis is predominantly dependent on TLR2-mediated recognition. J. Immunol. 173:3392–3397 [DOI] [PubMed] [Google Scholar]
- 33. Jasny E, et al. 2008. IL-12-impaired and IL-12-secreting dendritic cells produce IL-23 upon CD154 restimulation. J. Immunol. 180:6629–6639 [DOI] [PubMed] [Google Scholar]
- 34. Jo EK, Yang CS, Choi CH, Harding CV. 2007. Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell. Microbiol. 9:1087–1098 [DOI] [PubMed] [Google Scholar]
- 35. Jonuleit H, et al. 1997. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur. J. Immunol. 27:3135–3142 [DOI] [PubMed] [Google Scholar]
- 36. Kalis C, et al. 2005. Requirement for TLR9 in the immunomodulatory activity of Propionibacterium acnes. J. Immunol. 174:4295–4300 [DOI] [PubMed] [Google Scholar]
- 37. Kapsenberg ML. 2003. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3:984–993 [DOI] [PubMed] [Google Scholar]
- 38. Lamm DL, et al. 1992. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J. Urol. 147:596–600 [DOI] [PubMed] [Google Scholar]
- 39. Mazzoni A, Segal DM. 2004. Controlling the Toll road to dendritic cell polarization. J. Leukoc. Biol. 75:721–730 [DOI] [PubMed] [Google Scholar]
- 40. Mehlhop E, et al. 2002. Enhanced in vitro stimulation of rhesus macaque dendritic cells for activation of SIV-specific T cell responses. J. Immunol. Methods 260:219–234 [DOI] [PubMed] [Google Scholar]
- 41. Millan-Chiu BE, Hernandez-Hernandez F, Perez-Torres A, Mendez-Tovar LJ, Lopez-Martinez R. 2011. In situ TLR2 and TLR4 expression in a murine model of mycetoma caused by Nocardia brasiliensis. FEMS Immunol. Med. Microbiol. 61:278–287 [DOI] [PubMed] [Google Scholar]
- 42. Mine Y, et al. 1986. In vivo activation of functional properties in mouse peritoneal macrophages by Nocardia rubra cell wall skeleton. Arzneimittelforschung 36:1651–1655 [PubMed] [Google Scholar]
- 43. Moore TA, et al. 2000. Bacterial clearance and survival are dependent on CXC chemokine receptor-2 ligands in a murine model of pulmonary Nocardia asteroides infection. J. Immunol. 164:908–915 [DOI] [PubMed] [Google Scholar]
- 44. Picard C, et al. 2002. Inherited interleukin-12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds. Am. J. Hum. Genet. 70:336–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Quesniaux VJ, et al. 2004. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J. Immunol. 172:4425–4434 [DOI] [PubMed] [Google Scholar]
- 46. Royet J, Dziarski R. 2007. Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nat. Rev. Microbiol. 5:264–277 [DOI] [PubMed] [Google Scholar]
- 47. Salinas-Carmona MC, Torres-Lopez E, Ramos AI, Licon-Trillo A, Gonzalez-Spencer D. 1999. Immune response to Nocardia brasiliensis antigens in an experimental model of actinomycetoma in BALB/c mice. Infect. Immun. 67:2428–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sallusto F, Cella M, Danieli C, Lanzavecchia A. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saubolle MA, Sussland D. 2003. Nocardiosis: review of clinical and laboratory experience. J. Clin. Microbiol. 41:4497–4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schulz O, et al. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity 13:453–462 [DOI] [PubMed] [Google Scholar]
- 51. Takeuchi O, et al. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443–451 [DOI] [PubMed] [Google Scholar]
- 52. Trevino-Villarreal JH, Vera-Cabrera L, Valero-Guillen PL, Salinas-Carmona MC. 2012. Nocardia brasiliensis cell wall lipids modulate macrophage and dendritic responses favoring development of experimental actinomycetoma in BALB/c mice. Infect. Immun. 80:3587–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133–146 [DOI] [PubMed] [Google Scholar]
- 54. Trinchieri G, Sher A. 2007. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 7:179–190 [DOI] [PubMed] [Google Scholar]
- 55. Ueno H, et al. 2007. Dendritic cell subsets in health and disease. Immunol. Rev. 219:118–142 [DOI] [PubMed] [Google Scholar]
- 56. Ulrichs T, et al. 2005. Modified immunohistological staining allows detection of Ziehl-Neelsen-negative Mycobacterium tuberculosis organisms and their precise localization in human tissue. J. Pathol. 205:633–640 [DOI] [PubMed] [Google Scholar]
- 57. Yu Q, Kovacs C, Yue FY, Ostrowski MA. 2004. The role of the p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, and phosphoinositide-3-OH kinase signal transduction pathways in CD40 ligand-induced dendritic cell activation and expansion of virus-specific CD8+ T cell memory responses. J. Immunol. 172:6047–6056 [DOI] [PubMed] [Google Scholar]