Abstract
The NF-κB pathway regulates innate immune responses to infection. NF-κB is activated after pathogen-associated molecular patterns are detected, leading to the induction of proinflammatory host responses. As a countermeasure, bacterial pathogens have evolved mechanisms to subvert NF-κB signaling. Enterotoxigenic Escherichia coli (ETEC) causes diarrheal disease and significant morbidity and mortality for humans in developing nations. The extent to which this important pathogen subverts innate immune responses by directly targeting the NF-κB pathway is an understudied topic. Here we report that ETEC secretes a heat-stable, proteinaceous factor that blocks NF-κB signaling normally induced by tumor necrosis factor (TNF), interleukin-1β, and flagellin. Pretreating intestinal epithelial cells with ETEC supernatant significantly blocked the degradation of the NF-κB inhibitor IκBα without affecting IκBα phosphorylation. Data from immunoprecipitation experiments suggest that the ETEC factor functions by preventing IκBα polyubiquitination. Inhibiting clathrin function blocked the activity of the secreted ETEC factor, suggesting that this yet-uncharacterized activity may utilize clathrin-dependent endocytosis to enter host cells. These data suggest that ETEC evades the host innate immune response by directly modulating NF-κB signaling.
INTRODUCTION
The NF-κB family plays a central role in controlling the expression of genes involved in proinflammatory immune responses (2). In unstimulated cells, NF-κB is maintained in the cytoplasm in an inactive state by the inhibitory NF-κB chaperone IκBα. Upon microbial infection or stimulation by cytokines such as tumor necrosis factor (TNF) and interleukin-1β (IL-1β), several receptor-mediated signaling pathways that result in the activation of the IκB kinase (IKK) complex are initiated (19). IKK phosphorylates IκBα on Ser32/36, leading to IκBα polyubiquitination by the β-TrCP E3 ubiquitin ligase. IκBα is then degraded by the 26S proteasome (23, 36), liberating NF-κB for nuclear translocation.
Bacterial pathogens have evolved different strategies to subvert host defense mechanisms to promote their survival and transmission. Intracellular microbial pathogens, such as Legionella pneumophila and Brucella abortus, prevent phagolysosome maturation and evade the host immune response by residing in host cells (28, 37). Extracellular pathogens, which are exposed to components of the host immune system, use virulence factors, such as NF-κB and mitogen-activated protein kinases (MAPKs) (27, 32, 41), that inhibit critical immune signaling pathways. For example, enterohemorrhagic and enteropathogenic Escherichia coli (EHEC and EPEC) deliver virulence factors that target different components of the NF-κB pathway by using a type three secretion system (T3SS) (17, 31, 35, 42, 44).
Enterotoxigenic E. coli (ETEC) is a significant source of human morbidity and mortality (13). In addition to causing diarrhea, the ETEC heat-labile enterotoxin (LT) plays multiple roles in modulating host cell function and bacterial adherence (21, 22). Soluble LT, as well as LT associated with outer membrane vesicles (OMVs), initiates host immune responses through distinct pathways (6). LT activates both NF-κB and MAPK pathways, and p38 MAPK activation is involved in LT-induced ETEC adherence (43). Patients infected with ETEC elicit a mild inflammatory response, as evidenced by the presence of fecal leukocytes (29) and fecal lactoferrin (18) in stool samples as well as elevated levels of fecal interleukin-1β (18).
Here we found that ETEC has evolved a means to subvert host innate immune responses by targeting the NF-κB pathway. We show that ETEC strain H10407 prevents NF-κB activation by disrupting the degradation-associated polyubiquitination of IκBα. An unidentified, secreted, heat-stable protein(s) is responsible for this phenotype after associating with the clathrin-dependent host endocytic machinery.
MATERIALS AND METHODS
Reagents.
Poly(ADP-ribose) polymerase (PARP) antibody was obtained from BD Biosciences. Extracellular signal-regulated kinase (ERK), p-ERK1/2, IκBα, p-IκBα, Jun N-terminal protein kinase (JNK), p-JNK, p38, and p-p38 antibodies, as well as TNF and IL-1β, were obtained from Cell Signaling. Tubulin and p65 antibodies, as well as brefeldin A (BFA), were obtained from Santa Cruz. Chlorpromazine, cycloheximide, filipin, lipopolysaccharide (LPS) (E. coli), methyl-β-cyclodextrin, MG132, phorbol 12-myristate 13-acetate (PMA), and clathrin heavy-chain (HC) antibody were obtained from Sigma.
Bacterial strains and cell culture.
The bacterial strains used in this study are listed in Table 1. The intestinal epithelial cell lines HCT-8 and SW480, as well as the human monocyte-like cell line THP-1, were maintained at 37°C in 5% CO2 in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). THP-1 cells were cultured in the presence of 50 nM PMA for 48 h to induce their differentiation into macrophage-like cells before their use.
Table 1.
Bacterial strains used in this study
| Strain | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| ETEC H10407 | O78:H11 CFA/I LT+ ST+ (ST-H and ST-P) | 12 |
| ETEC H10407 ΔeltA | ΔeltA mutant of ETEC H10407 | 9 |
| ETEC H10407-P | Derivative of H10407 cured of the 92-kb pCS1 plasmid | 14 |
| ETEC B7A | O148:H28 CS6; LT+ ST+ (ST-H and ST-P) | 11 |
| ETEC E24377A | O139:H28 CS1+ CS3+ LT+ ST-H+ | 39 |
| ETEC 91.1626 | O6 ST-P+ LT+ | C. DebRoy |
| ETEC 91.1033 | O27 ST-P+ LT− | C. DebRoy |
| ETEC 3030-2 | K88ac LT+ STb+ | 15 |
| ETEC 1836-2 | K88ac+ EAST1+ | 45 |
| ETEC 2534-86 | O8:K87:NM:F4ac LT+ STb+ | 4 |
| ETEC B41 | Bovine isolate; O101:K99, F41 STa+ | 30 |
| E. coli G58-1 | O101:K28:NM | 3 |
| Shigella flexneri 2457T | Wild type; serotype 2a | 16 |
| S. flexneri 2457T(T3SS−)a | Virulence plasmid-cured 2457T derivative | 26 |
Mutant deficient in type III secretion system function.
ETEC culture supernatants.
ETEC strains were grown overnight without shaking at 37°C in LB broth, diluted 1:50 into serum- and antibiotic-free RPMI 1640 medium, and grown to an optical density at 600 nm (OD600) of 0.8. The bacterial culture was centrifuged (10,000 × g, 10 min), and the culture supernatant was filtered through a 0.22-μm filter and stored at 4°C. Where indicated, ETEC culture supernatants were preincubated (37°C, 1 h) with 10.0 μg/ml proteinase K, 10.0 μg/ml DNase I, 10.0 μg/ml RNase A, or 5.0 μg/ml polymyxin B. In some cases, culture supernatants were also heat inactivated (100°C, 20 min) and then cooled to room temperature before their incubation with HCT-8 cells. For size fractionation experiments, supernatants were passaged through an Amicon Ultra-15 centrifugal filter unit with an Ultracel-3 membrane.
Bacterial infection.
ETEC strains were grown overnight without shaking at 37°C in LB broth, diluted 1:50 into serum- and antibiotic-free RPMI 1640 medium, and grown to an OD600 of 0.8. Before infections, cell culture medium was replaced with serum- and antibiotic-free RPMI 1640 medium. HCT-8 cells were infected with ETEC strains at a multiplicity of infection of ∼50 for 2 h, after which the cells were washed with ice-cold phosphate-buffered saline (PBS) and then lysed for subsequent analyses.
RNA interference.
SW480 cells were grown to 50% confluence on 6-well plates and transfected with either 200 pmol of negative-control small interfering RNAs (siRNAs) or siRNAs directed against the clathrin heavy chain (HC) (Santa Cruz) using Lipofectamine 2000. The transfection mixture was replaced with complete growth medium, Dulbecco's modified Eagle medium (DMEM), after 5 h, and cells were further incubated for 68 h prior to the performance of other experiments. Knockdown efficiency was quantified using immunoblotting.
Immunoblotting.
Mammalian cell pellets were resuspended in ice-cold lysis buffer A (10.0 mM HEPES, 1.5 mM MgCl2, 10.0 mM KCl, 0.5 mM dithiothreitol [DTT], 0.05% NP-40) supplemented with protease and phosphatase inhibitor cocktails (Thermo Scientific) and incubated on ice for 30 min. Lysates were centrifuged (17,000 × g, 4°C, 10 min), and the supernatant was retained as the cytosolic fraction. The pellet was washed with lysis buffer A and resuspended in buffer B (5.0 mM HEPES, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 26% glycerol, 300 mM NaCl). This resuspended nuclear fraction was passed through a 25-gauge needle to shear genomic DNA and then incubated on ice for 30 min with intermittent vortexing. The supernatant, which contains nuclear proteins, was collected by centrifugation. Protein concentrations were determined using the bicinchoninic acid (BCA) protein assay kit (Pierce). Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with Odyssey blocking buffer (LI-COR) at room temperature for 1 h, followed by incubation with primary antibodies overnight at 4°C. Membranes were washed three times in PBS plus 0.1% Tween and incubated with secondary antibodies for 2 h at room temperature. Immunoblots were developed using the Odyssey infrared imaging system. Poly(ADP-ribose) polymerase (PARP) and tubulin abundance were used to normalize protein loading for nuclear and cytoplasmic fractions, respectively.
Immunoprecipitation.
IκBα immunoprecipitation was performed using an IκBα (L35A5) mouse monoclonal antibody (MAb)-Sepharose bead conjugate. HCT-8 cells were lysed with ice-cold lysis buffer A (10.0 mM HEPES, 1.5 mM MgCl2, 10.0 mM KCl, 0.5 mM DTT, 0.05% NP-40) and centrifuged. The supernatant was incubated with anti-IκBα antibody-conjugated beads overnight at 4°C. After centrifugation, the beads were washed three times with buffer A and then resuspended in SDS-PAGE loading buffer for analysis.
Real-time quantitative reverse transcription-PCR (RT-PCR).
cDNA from HCT-8 cells was prepared by using a SYBR green cells-to-CT kit by following the manufacturer's instructions (Ambion). Real-time PCR was performed in a SYBR green PCR master mix (Applied Biosystems) with detection in a Fast 7500 system (Applied Biosystems) and with the following primers: for GAPDH, 5′-AC2AG2TG2TCTC2TCTGACT2C and 5′-GTG2TCGT2GAG3CA2TG; for TNF, 5′-TGCTC2TCAC3ACAC2AT and 5′-G2AG2T2GAC2T2G2TCTG2TA. Relative transcription levels were calculated using the threshold cycle (ΔΔCT) method.
Luciferase assays.
HCT-8 cells were cotransfected at a ratio of 10:1 (2.0 μg total DNA) with a firefly luciferase construct driven by a consensus κB site, together with the Renilla luciferase plasmid (Promega). Approximately 48 h after transfection, cells were treated with ETEC supernatants for 2 h and then stimulated with 20 ng/ml TNF, 10 ng/ml IL-1β, or 500 ng/ml flagellin for 30 min. Firefly and Renilla luciferase levels were measured using a dual luciferase reporter system kit (Promega) according to the manufacturer's instructions.
Statistics.
Throughout the manuscript, data are presented as means and standard errors of the means (SEM) (n ≥ 3), with asterisks used to indicate statistically significant differences (P < 0.05; Student's t test), as specified in the figure legends.
RESULTS
ETEC supernatant inhibits IκBα degradation.
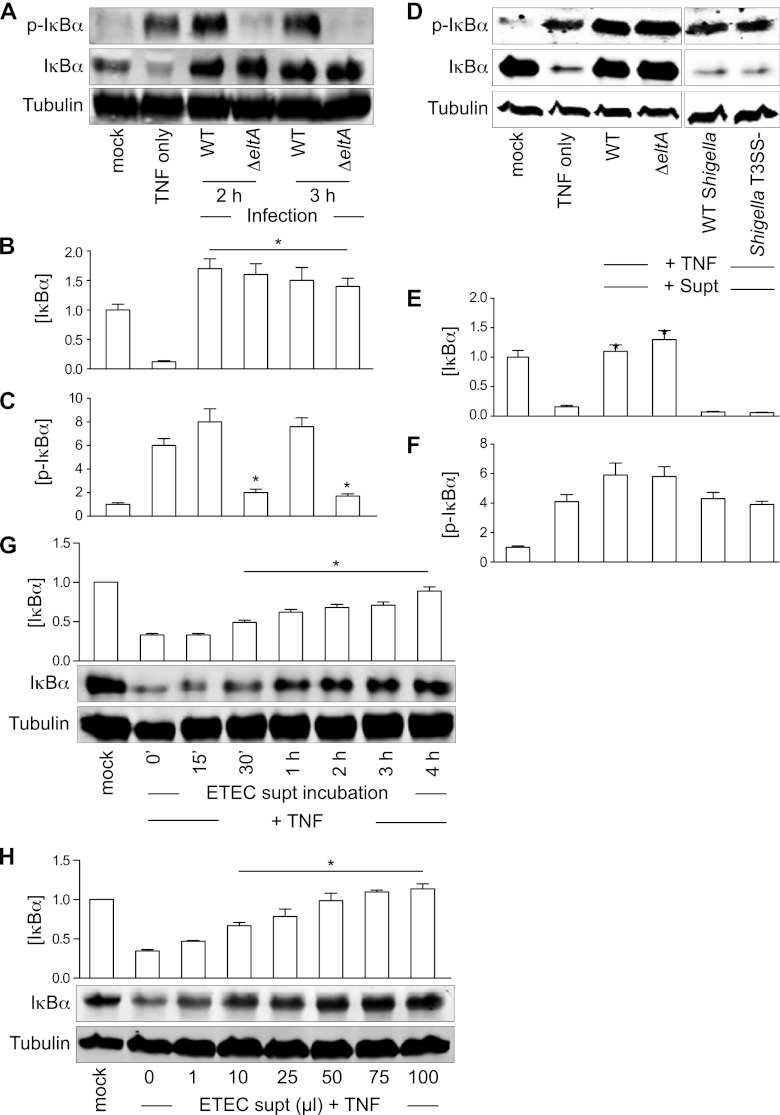
Many pathogens have evolved strategies to prevent NF-κB pathway activation. To determine whether ETEC can also modulate this pathway, we infected HCT-8 intestinal epithelial cells with the commonly studied human ETEC strain H10407 (12). We also infected cells with an H10407 mutant lacking the enzymatic subunit of the heat-labile enterotoxin LT (ΔeltA), which exhibits an attenuated ability to induce NF-κB activation (43). Although infection of HCT-8 cells with wild-type (WT) ETEC induced significant IκBα phosphorylation, the subsequent degradation of IκBα was blocked (Fig. 1A to C). The positive control, TNF treatment, resulted in the expected phosphorylation and subsequent degradation of IκBα (Fig. 1A to C).
Fig 1.
ETEC supernatants protect IκBα from TNF-induced degradation. (A) Immunoblotting of total and phospho(Ser32/36)-IκBα after TNF stimulation or ETEC (WT or ΔeltA mutant) infection. Tubulin immunoblotting was used to normalize protein abundance for quantification. (B and C) Quantification of IκBα and phospho(Ser32/36)-IκBα abundance from data shown in panel A. Asterisks indicate protein abundance significantly different from that in the TNF-only lane. (D) Immunoblotting of total and phospho-IκBα after incubating HCT-8 cells with ETEC or Shigella supernatants (supt) and then stimulating the cells with TNF. (E and F) Quantification of IκBα and phospho(Ser32/36)-IκBα abundance from data shown in panel D. Asterisks indicate protein abundance significantly different from that in the TNF-only lane. (G) IκBα protection as a function of incubation time with the ETEC supernatant. Asterisks indicate protein abundance significantly different from that in the TNF-only lane. (H) IκBα protection as a function of ETEC supernatant volume. Asterisks indicate protein abundance significantly different from that in the TNF-only lane.
We speculated that ETEC H10407 secretes a virulence factor that blocks IκBα degradation. To test this idea, we incubated HCT-8 cells with cell-free ETEC supernatants for 2 h and then stimulated the cells with TNF. For comparison with an enteric pathogen that induces significant host inflammation, we also used WT Shigella flexneri and an S. flexneri mutant deficient in type III secretion system (T3SS) function (24). Pretreating HCT-8 cells with ETEC H10407 supernatants (both the WT and the ΔeltA mutant) completely blocked TNF-induced IκBα degradation but not IκBα phosphorylation (Fig. 1D to F). In contrast, pretreating HCT-8 cells with S. flexneri supernatants did not block IκBα degradation (Fig. 1D to F). IκBα abundance after TNF treatment increased as a function of both the time (Fig. 1G) and the volume of the ETEC supernatant (Fig. 1H) incubated with HCT8 cells.
ETEC secreted factor has heat-stable, protein-like qualities and is expressed by multiple human ETEC strains.
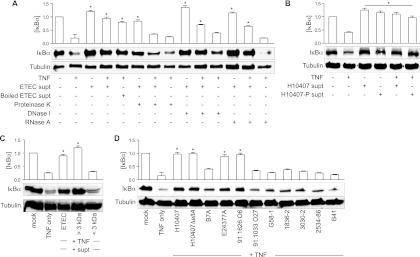
To determine the biological characteristics of the ETEC secreted factor (ESF), we treated ETEC supernatants with proteinase K, DNase I, or RNase A and then tested these treated supernatants for their ability to inhibit TNF-induced IκBα degradation. Proteinase K treatment completely abrogated the ability of ESF to block TNF-induced IκBα degradation. In contrast, DNase I or RNase A had no impact on ESF activity, suggesting that the secreted factor is a protein (Fig. 2A). We also incubated ETEC supernatants at 100°C for 20 min and then retested their activity. Boiled ETEC supernatant still retained an inhibitory activity against TNF-induced IκBα degradation, suggesting that the active protein is heat stable (Fig. 2A).
Fig 2.
ESF has heat-stable, proteinaceous qualities. (A) IκBα immunoblotting after treating cells with TNF or with ETEC supernatants first incubated with either proteinase K, boiling, DNase I, or RNase A. (B) IκBα immunoblotting after treating cells with TNF and supernatants derived from ETEC H01047 possessing or lacking the major virulence plasmid (H10407-P). (C) IκBα immunoblotting after treating cells with ETEC supernatant subjected to size fractionation (3-kDa cutoff). (D) IκBα immunoblotting after treating cells with TNF and supernatants derived from the indicated human, porcine, and bovine ETEC strains. For all quantitative data in this figure, asterisks indicate IκBα abundance significantly different from that in the TNF-only lane.
We tested whether a strain of ETEC cured of the large pCS1 virulence plasmid (H10407-P) retained the inhibitory activity. This CFA/I-encoding plasmid also encodes the heat-stable toxin H (ST-H/ST-1b) and other recently identified ETEC adhesins (14). We found that H10407-P supernatants still blocked TNF-induced IκBα degradation (Fig. 2B), suggesting that ESF is not encoded on this virulence plasmid. By fractionating the ETEC supernatant by size exclusion, using Amicon filtration devices with a 3-kDa cutoff, we determined that ESF is larger than 3 kDa (Fig. 2C), suggesting that the activity is unlikely to be attributable to the small ETEC enterotoxins (34).
To determine whether the secreted protein is conserved among other ETEC strains, we prepared culture supernatants from different ETEC isolates and tested their activity. Supernatants from the sequenced human ETEC isolate E24377A (39) as well as a relatively uncharacterized human isolate (91.1626; C. DebRoy, personal communication) also inhibited TNF-induced IκBα degradation (Fig. 2D). In contrast, the human ETEC isolate 91.1033 and ETEC strains of porcine (1836-2, 3030-2, 2534-86) and bovine (B41) tropism did not block TNF-induced IκBα degradation (Fig. 2D). Another sequenced human ETEC isolate, B7A (11), had no significant impact on TNF-induced IκBα degradation. We failed to detect the inhibitory activity in both the laboratory E. coli strain BL21 (not shown) and a porcine commensal E. coli isolate (G58-1) (3) (Fig. 2D). Thus, some strains of ETEC of significance to human health appear to encode the ability to inhibit TNF-induced IκBα degradation while others do not.
ESF inhibits NF-κB nuclear translocation and transcriptional activity.
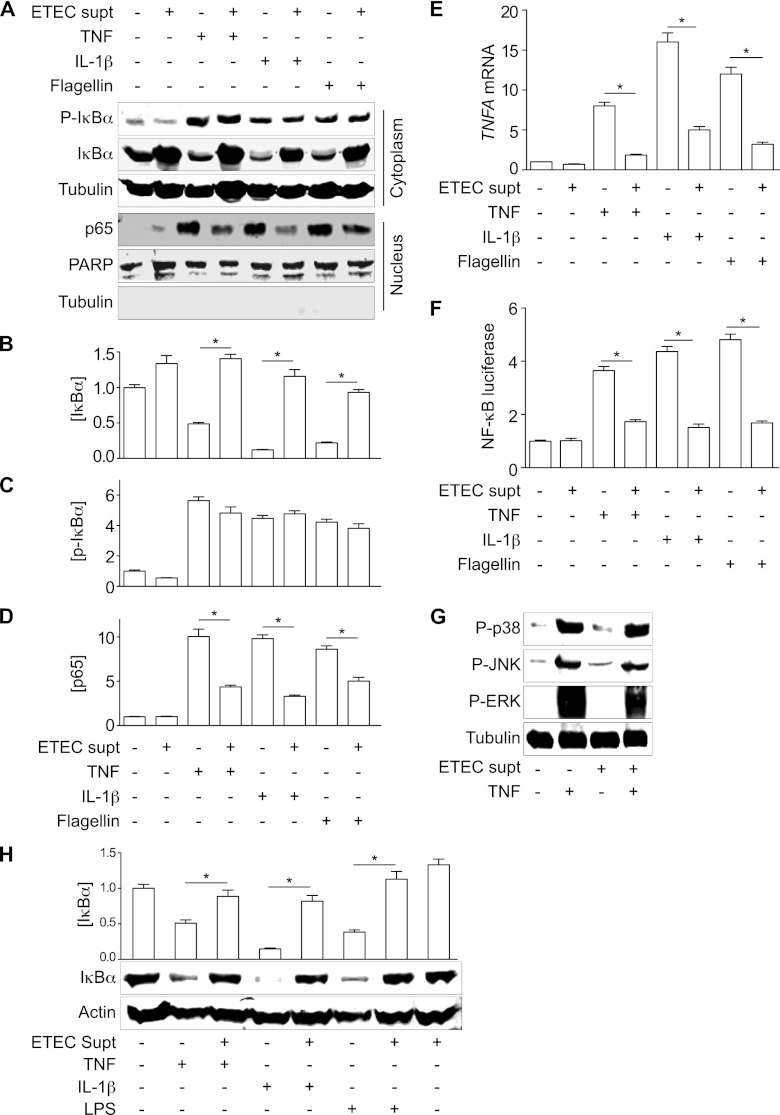
IκBα degradation is required to activate NF-κB. We next examined whether ESF can block NF-κB nuclear translocation and subsequent transcriptional activity. NF-κB can be activated by different stimuli, including TNF, IL-1β, and microbial products such as flagellin (1, 10). We found that ETEC H10407 supernatants blocked the degradation of phospho-IκBα induced by TNF, IL-1, and flagellin (Fig. 3A to D).
Fig 3.
ESF inhibits NF-κB nuclear translocation and transcriptional activity. (A) Immunoblotting of total and phospho(Ser32/36)-IκBα, as well as nucleus-localized NF-κB p65, after pretreating HCT-8 cells with ETEC supernatant for 1.5 h and then stimulating the cells with either TNF (20 ng/ml), IL-1β (10 ng/ml), or flagellin (200 ng/ml) for 30 min. PARP and tubulin immunoblotting were used to normalize nuclear protein concentrations and to demonstrate the absence of contaminating cytoplasmic proteins in the nuclear fractions, respectively. (B to D) Quantification of IκBα, phospho(Ser32/36)-IκBα, and p65 abundance from data shown in panel A. Asterisks indicate significantly different protein abundance between the specified pairwise comparisons. (E) RT-PCR analysis of TNF-α gene (TNFA) transcription after treating cells with TNF, IL-1β, or flagellin, with or without the ETEC supernatant. Asterisks indicate significantly different protein abundance between the specified pairwise comparisons. (F) NF-κB luciferase activity after treating cells with TNF, IL-1β, or flagellin, with or without the ETEC supernatant. Asterisks indicate significantly different protein abundance between the specified pairwise comparisons. (G) Immunoblotting of phospho-p38, phospho-JNK, and phospho-ERK1/2 after ETEC supernatant and TNF treatment. (H) IκBα immunoblotting after treating differentiated THP-1 cells with TNF (20 ng/ml), IL-1β (10 ng/ml), or LPS (1 μg/ml) for 30 min, with or without the ETEC supernatant. Asterisks indicate significantly different protein abundance between the specified pairwise comparisons.
We next determined whether ESF-mediated blockage of IκBα degradation was sufficient to inhibit the nuclear translocation of the NF-κB p65 subunit. Stimulation of HCT-8 cells with TNF, IL-1β, or flagellin induced NF-κB p65 nuclear translocation, whereas pretreating cells with ETEC supernatant significantly blocked p65 nuclear translocation (Fig. 3A to D).
We also examined the impact of ESF on NF-κB-dependent transcriptional activity induced by different stimuli. HCT-8 cells incubated with ETEC supernatants exhibited a lower level of TNF-α gene induction, irrespective of the stimulus (Fig. 3E). Data from luciferase reporter assays also showed that pretreating HCT-8 cells with ETEC supernatant significantly suppressed NF-κB transcriptional activity (Fig. 3F).
MAPK signaling also plays important roles in regulating host innate immunity (8, 38). We also examined whether ESF also targets the host MAPK signaling pathway. Pretreatment of HCT-8 cells with ETEC supernatant did not inhibit TNF-induced MAPK activation (Fig. 3G). Similar to its impact on intestinal epithelial cells, ESF also inhibited the degradation of IκBα in the macrophage-like THP-1 cell line when these cells were stimulated with TNF, IL-1β, or LPS (Fig. 3H). Taken together, these data indicate that one or more ETEC H10407 secreted factors subvert NF-κB activation by blocking IκBα degradation.
ESF utilizes a clathrin-dependent endocytosis pathway.
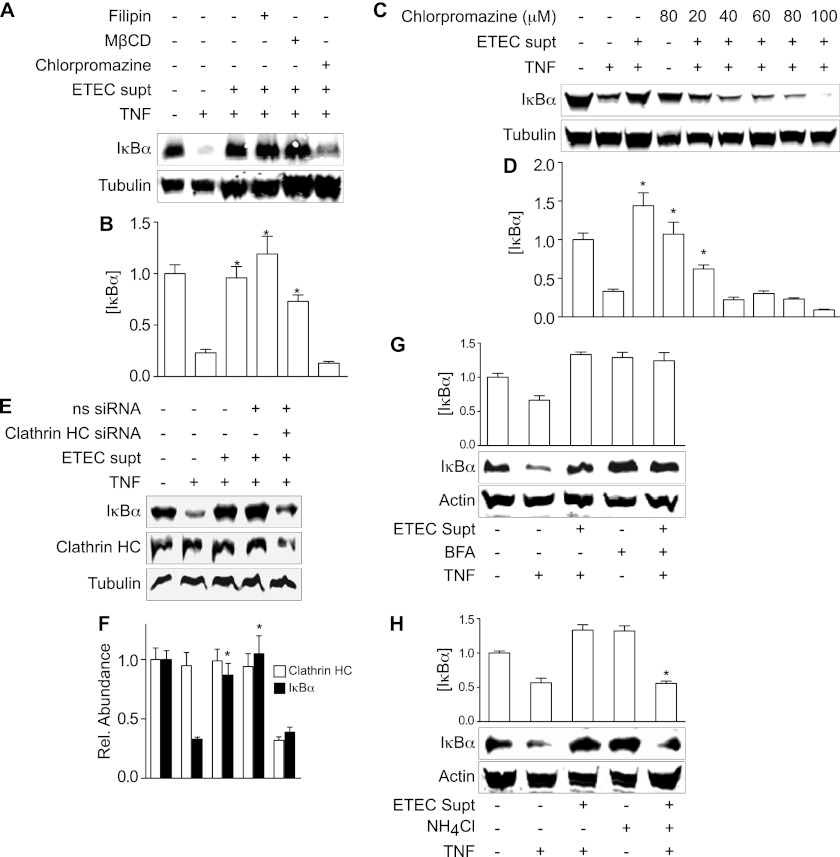
We next examined whether ESF enters intestinal epithelial cells through an endocytic pathway. Treating HCT-8 cells with the clathrin-dependent endocytosis inhibitor chlorpromazine, but not with the lipid raft-dependent endocytosis inhibitors filipin or methyl-β-cyclodextrin (MβCD), prevented the ETEC supernatant from blocking TNF-induced IκBα degradation (Fig. 4A and B). The ability of chlorpromazine to prevent the ETEC supernatant from protecting against TNF-induced IκBα degradation was dose dependent (Fig. 4C and D), suggesting that ESF may enter intestinal epithelial cells through clathrin-dependent endocytosis.
Fig 4.
Cellular uptake of ESF. (A) IκBα immunoblotting after pretreating HCT-8 cells with ETEC supernatant for 1.5 h and then stimulating the cells with TNF (20 ng/ml) for 30 min. Where indicated, HCT-8 cells were first treated with filipin (7 μM), MβCD (15 mM), or chlorpromazine (50 μM) for 30 min before adding ETEC supernatant. (B) Quantification of data shown in panel A (mean ± SEM; n = 3). (C) IκBα protection as a function of chlorpromazine treatment. (D) Quantification of data shown in panel C (mean ± SEM; n = 3). (E) IκBα and clathrin heavy chain (HC) immunoblotting. Where indicated, HCT-8 cells were first transfected with an siRNA duplex targeting clathrin HC or a nonspecific (ns) siRNA duplex. Tubulin immunoblotting was used to normalize protein concentrations. (F) Quantification of data shown in panel E (mean ± SEM; n = 3). For all quantitative data in this figure, asterisks indicate IκBα abundance significantly different from that in the TNF-only lane. (G) IκBα immunoblotting after pretreating SW480 cells with BFA (500 ng/ml) for 6 h and then with ETEC supernatant for 2 h, followed by TNF for 20 min. (H) IκBα immunoblotting after pretreating SW480 cells with NH4Cl (20 mM) for 6 h and then with ETEC supernatant for 2 h, followed by TNF for 20 min.
To substantiate further the role of clathrin in the uptake of ESF, we also used siRNA to knock down clathrin heavy chain (HC) abundance in intestinal epithelial cells. We used the SW480 cell line for siRNA transfection, as this cell line has high transfection efficiency. Knocking down clathrin HC levels by ∼65% significantly abrogated the ability of ETEC supernatant to block TNF-induced IκBα degradation (Fig. 4E and F).
We also performed a preliminary investigation of how ESF might traffic within intestinal epithelial cells. Pretreating cells with brefeldin A (BFA), an inhibitor of Golgi-endoplasmic reticulum (ER) trafficking (25), did not abrogate the ability of ESF to block TNF-induced IκBα degradation (Fig. 4G). In contrast, preincubating cells with NH4Cl, an inhibitor of endosomal acidification (7), significantly reduced the inhibitory activity of ESF (Fig. 4H). Thus, these data suggest that endosomal acidification but not Golgi-ER trafficking is important for ESF translocation after its delivery into host cells.
ESF inhibits IκBα polyubiquitination.
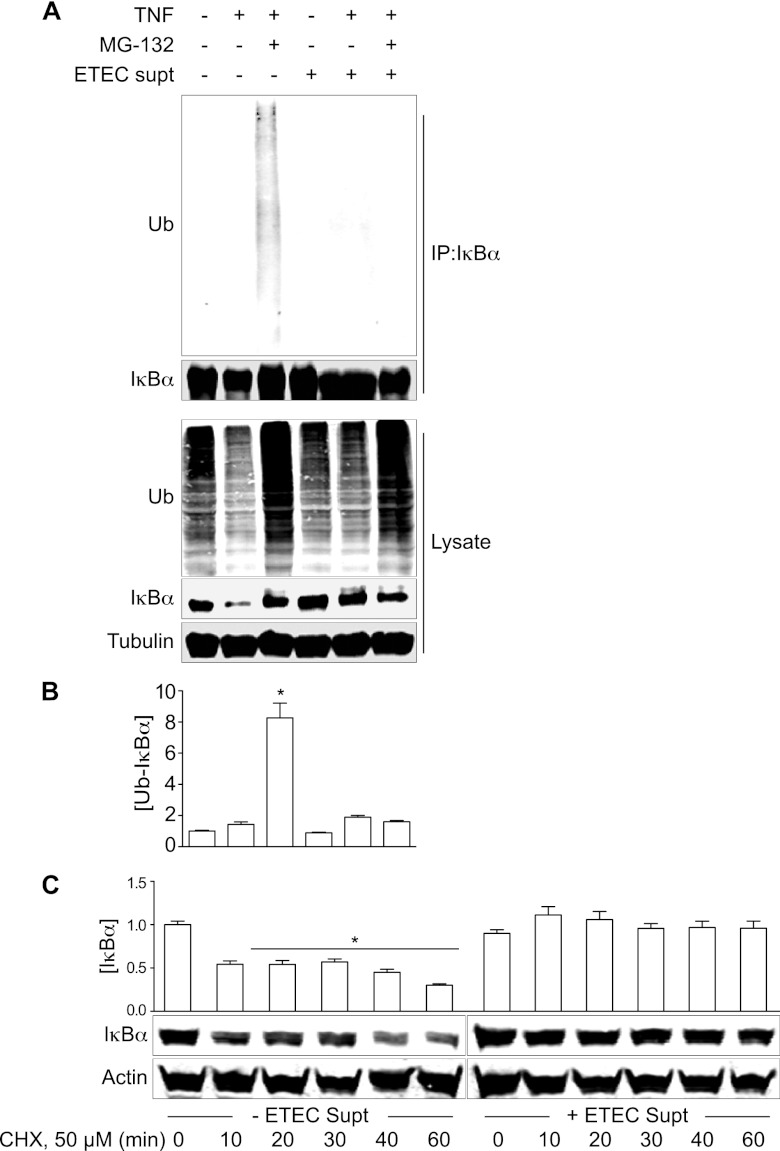
Because ESF blocks IκBα degradation, but not its phosphorylation, we hypothesized that it might disrupt the polyubiquitination of phosphorylated IκBα, thus affecting the subsequent recognition and degradation of IκBα by the 26S proteasome. We used immunoprecipitation to examine the levels of polyubiquitinated IκBα in treated intestinal epithelial cells. Pretreatment of HCT-8 cells with MG132, a potent inhibitor of the 26S proteasome, significantly blocked TNF-induced IκBα degradation and thus resulted in the accumulation of polyubiquitinated IκBα (Fig. 5A and B, lane 3). In contrast, pretreating the HCT-8 cells with ETEC supernatant did not increase IκBα polyubiquitination, although IκBα degradation was significantly blocked (Fig. 5A and B, lane 5). Coincubation with both ESF and MG132 failed to result in the accumulation of polyubiquitinated IκBα (Fig. 5A and B, lane 6).
Fig 5.
ESF prevents IκBα ubiquitination. (A) HCT-8 cells were pretreated with ETEC supernatant and then stimulated with TNF (20 ng/ml) for 30 min. Cell lysates were immunoprecipitated using an IκBα antibody and immunoblotted for both ubiquitin (Ub) and IκBα. Where indicated, HCT-8 cells were first treated with MG132 (50 μg/ml). (B) Quantification of ubiquitinated IκBα from immunoprecipitated samples (mean ± SEM; n = 3). Ubiquitinated IκBα intensity was normalized to immunoprecipitated IκBα abundance. Asterisks indicate IκBα abundance significantly different from that in the TNF-only lane. (C) IκBα immunoblotting after pretreating SW480 with 50 μM cycloheximide (CHX) for the indicated times. Where indicated, cells were first incubated with ETEC supernatant (2 h) before the addition of CHX.
We also considered the possibility that ESF might act by increasing IκBα synthesis rather than by blocking IκBα degradation. To test this idea, we treated HCT-8 cells with the protein synthesis inhibitor cycloheximide (CHX) in the presence or absence of ESF. As expected, treating cells with 50 μM CHX significantly reduced IκBα protein abundance as a function of time (Fig. 5C, left). However, in the presence of ESF, IκBα protein levels were maintained over the course of CHX treatment, suggesting that ESF prevented the degradation of previously synthesized IκBα (Fig. 5C, right) rather than promoting IκBα synthesis. Overall, these data suggest that ESF affects the NF-κB pathway at a point involving IκBα polyubiquitination.
DISCUSSION
We have discovered that ETEC suppresses host innate immune responses by targeting the NF-κB signaling pathway. An ETEC secreted factor (ESF) with heat-stable, protein-like qualities blocks NF-κB activation by disrupting the polyubiquitination and subsequent degradation of IκBα. NF-κB plays a crucial role in mediating intestinal immune response to enteric pathogens (32). Activation of this immune signaling pathway by bacterial infection elicits a large number of protective mechanisms against infection (5). Many pathogens have evolved strategies to modulate NF-κB signaling (32), and our data provide evidence that ETEC has also evolved a strategy to evade innate responses by targeting NF-κB.
ETEC H10407 is a highly virulent strain originally isolated from a patient with severe diarrhea in Bangladesh (12) that has been commonly used in challenge experiments for studying ETEC pathogenesis (20). ETEC H10407 inhibition of innate responses might contribute to the high virulence of this strain. We also found that two other human ETEC isolates, but not any porcine or bovine isolates, block TNF-induced IκBα degradation. Although we examined a limited number of ETEC isolates, including some that have not been extensively characterized, we suggest that the factor responsible for suppressing NF-κB activation is not conserved in all ETEC strains. However, our data do not distinguish between the possibilities that ESF is either unique to ETEC or encoded by other enteric pathogens.
The boiling of ETEC supernatants did not abrogate their ability to inhibit TNF-induced IκBα degradation, suggesting that ESF is a heat-resistant factor. Most human ETEC isolates encode colonization factor antigens (CFAs), LT, and heat-stable toxins, such as ST-H (ST-1b) and ST-P (ST-1a). Most of these characterized virulence factors are encoded on multiple plasmids (12, 13), although some virulence determinants, such as the type two secretion system (T2SS), are chromosomally encoded. Curing the large pCS1 virulence plasmid from H10407 (H10407-P) did not affect the ability of ETEC to block TNF-induced IκBα degradation, suggesting that ESF is not encoded on this virulence plasmid. In addition, some ETEC isolates tested in our study that did not block TNF-induced IκBα degradation express ST-1a/ST-P, suggesting that this heat-stable toxin may not be involved in producing the activity we measured. Furthermore, fractionating the supernatant to remove small proteins (<3 kDa) also failed to abrogate ESF activity. Taken together, our data suggest the possibility that ESF may be a previously uncharacterized ETEC protein. However, they do not definitively rule out the possibility that some of the phenotypes we observe are due to the activity of one or more previously characterized heat-stable toxins.
Not only ETEC infection but also cell-free ETEC culture supernatants blocked TNF-induced IκBα degradation. Further investigation showed that clathrin-mediated endocytosis is involved in the uptake of ESF into intestinal epithelial cells. Although ETEC lacks a T3SS, this pathogen has evolved other mechanisms to deliver virulence proteins into host cells. ETEC uses a T2SS to secrete LT and other virulence factors (40). However, deleting T2SS function from ETEC had no effect on its ability to inhibit IκBα degradation (data not shown).
Many bacterial toxins enter host cells by receptor-mediated endocytosis and then translocate to the cytoplasm by exploiting intracellular trafficking pathways to reach their cytosolic targets (33). Disrupting Golgi-ER trafficking with BFA did not alter the ability of ESF to block TNF-induced IκBα degradation, suggesting that ESF trafficking is independent of Golgi-ER trafficking. However, inhibiting endosomal acidification with NH4Cl significantly reduced ESF activity, suggesting that endosomal acidification may be involved in ESF trafficking. These studies are limited by the fact that we have not yet identified the source(s) of the ESF activity.
ETEC supernatants blocked TNF-, IL-1β-, and flagellin-induced IκBα degradation but not IκBα phosphorylation, suggesting that the secreted protein is a general inhibitor of the NF-κB pathway that prevents IκBα ubiquitination. Additionally, ETEC supernatants were active against NF-κB pathway agonists (TNF, IL-1β, LPS) in multiple cell types, including the macrophage-like THP-1 cell line.
Upon stimulation with microbial products or cytokines, phosphorylated IκBα is recognized by the β-TrCP E3 ubiquitin ligase and is polyubiquitinated by the host ubiquitination machinery (23, 36). It is possible that ESF disrupts IκBα polyubiquitination by interacting with the host ubiquitination machinery. The Shigella virulence protein OspG also blocks IκBα polyubiquitination by interacting with multiple ubiquitin-conjugating enzymes (24). However, we cannot rule out the possibility that ESF might instead affect IκBα ubiquitination indirectly. Identifying and characterizing the biochemical mechanism of action of ESF in future studies will undoubtedly clarify these issues. Dysregulated NF-κB signaling is involved in the pathogenesis of numerous chronic inflammatory diseases. Ultimately, one might envision utilizing bacterial or viral proteins as starting points for developing novel anti-inflammatory compounds.
ACKNOWLEDGMENTS
The project described was supported by a subaward of grant P20 RR016443 from the National Center for Research Resources (NCRR) of the U.S. National Institutes of Health (NIH) to P.R.H.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Published ahead of print 1 October 2012
REFERENCES
- 1. Akira S, Takeda K. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511 [DOI] [PubMed] [Google Scholar]
- 2. Baeuerle PA, Henkel T. 1994. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 12:141–179 [DOI] [PubMed] [Google Scholar]
- 3. Baker DR, Billey LO, Francis DH. 1997. Distribution of K88 Escherichia coli-adhesive and nonadhesive phenotypes among pigs of four breeds. Vet. Microbiol. 54:123–132 [DOI] [PubMed] [Google Scholar]
- 4. Berberov EM, et al. 2004. Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect. Immun. 72:3914–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caamano J, Hunter CA. 2002. NF-κB family of transcription factors: central regulators of innate and adaptive immune functions. Clin. Microbiol. Rev. 15:414–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chutkan H, Kuehn MJ. 2011. Context-dependent activation kinetics elicited by soluble versus outer membrane vesicle-associated heat-labile enterotoxin. Infect. Immun. 79:3760–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Duve C, et al. 1974. Commentary. Lysosomotropic agents. Biochem. Pharmacol. 23:2495–2531 [DOI] [PubMed] [Google Scholar]
- 8. Dong C, Davis RJ, Flavell RA. 2002. MAP kinases in the immune response. Annu. Rev. Immunol. 20:55–72 [DOI] [PubMed] [Google Scholar]
- 9. Dorsey FC, Fischer JF, Fleckenstein JM. 2006. Directed delivery of heat-labile enterotoxin by enterotoxigenic Escherichia coli. Cell. Microbiol. 8:1516–1527 [DOI] [PubMed] [Google Scholar]
- 10. Doyle SL, O'Neill LA. 2006. Toll-like receptors: from the discovery of NFκB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 72:1102–1113 doi:10.1126/stke.3572006re13 [DOI] [PubMed] [Google Scholar]
- 11. DuPont HL, et al. 1971. Pathogenesis of Escherichia coli diarrhea. N. Engl. J. Med. 285:1–9 [DOI] [PubMed] [Google Scholar]
- 12. Evans DG, Silver RP, Evans DJ, Jr, Chase DG, Gorbach SL. 1975. Plasmid-controlled colonization factor associated with virulence in Escherichia coli enterotoxigenic for humans. Infect. Immun. 12:656–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fleckenstein JM, et al. 2010. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect. 12:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fleckenstein JM, Roy K, Fischer JF, Burkitt M. 2006. Identification of a two-partner secretion locus of enterotoxigenic Escherichia coli. Infect. Immun. 74:2245–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Francis DH, Willgohs JA. 1991. Evaluation of a live avirulent Escherichia coli vaccine for K88+, LT+ enterotoxigenic colibacillosis in weaned pigs. Am. J. Vet. Res. 52:1051–1055 [PubMed] [Google Scholar]
- 16. Freter R. 1956. Experimental enteric Shigella and Vibrio infections in mice and guinea pigs. J. Exp. Med. 104:411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao X, et al. 2009. Bacterial effector binding to ribosomal protein s3 subverts NF-κB function. PLoS Pathog. 5:e1000708 doi:10.1371/journal.ppat.1000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greenberg DE, Jiang ZD, Steffen R, Verenker MP, DuPont HL. 2002. Markers of inflammation in bacterial diarrhea among travelers, with a focus on enteroaggregative Escherichia coli pathogenicity. J. Infect. Dis. 185:944–949 [DOI] [PubMed] [Google Scholar]
- 19. Hacker H, Karin M. 2006. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006:re13 doi:10.1126/stke.3572006re13 [DOI] [PubMed] [Google Scholar]
- 20. Harro C, et al. 2011. Refinement of a human challenge model for evaluation of enterotoxigenic Escherichia coli vaccines. Clin. Vaccine Immunol. 18:1719–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson AM, Kaushik RS, Francis DH, Fleckenstein JM, Hardwidge PR. 2009. Heat-labile enterotoxin promotes Escherichia coli adherence to intestinal epithelial cells. J. Bacteriol. 191:178–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson AM, Kaushik RS, Rotella NJ, Hardwidge PR. 2009. Enterotoxigenic Escherichia coli modulates host intestinal cell membrane asymmetry and metabolic activity. Infect. Immun. 77:341–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karin M, Ben-Neriah Y. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621–663 [DOI] [PubMed] [Google Scholar]
- 24. Kim DW, et al. 2005. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. U. S. A. 102:14046–14051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klausner RD, Donaldson JG, Lippincott-Schwartz J. 1992. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116:1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Labrec EH, Schneider H, Magnani TJ, Formal SB. 1964. Epithelial cell penetration as an essential step in the pathogenesis of bacillary dysentery. J. Bacteriol. 88:1503–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le Negrate G. 2012. Subversion of innate immune responses by bacterial hindrance of NF-κB pathway. Cell. Microbiol. 14:155–167 [DOI] [PubMed] [Google Scholar]
- 28. Luo ZQ. 2012. Legionella secreted effectors and innate immune responses. Cell. Microbiol. 14:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mercado EH, et al. 2011. Fecal leukocytes in children infected with diarrheagenic Escherichia coli. J. Clin. Microbiol. 49:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nagy B. 1980. Vaccination of cows with a K99 extract to protect newborn calves against experimental enterotoxic colibacillosis. Infect. Immun. 27:21–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Newton HJ, et al. 2010. The type III effectors NleE and NleB from enteropathogenic E. coli and OspZ from Shigella block nuclear translocation of NF-κB p65. PLoS Pathog. 6:e1000898 doi:10.1371/journal.ppat.1000898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rahman MM, McFadden G. 2011. Modulation of NF-κB signalling by microbial pathogens. Nat. Rev. Microbiol. 9:291–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Repella TL, Ho M, Chong TP, Bannai Y, Wilson BA. 2011. Arf6-dependent intracellular trafficking of Pasteurella multocida toxin and pH-dependent translocation from late endosomes. Toxins 3:218–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sears CL, Kaper JB. 1996. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol. Rev. 60:167–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sham HP, et al. 2011. Attaching and effacing bacterial effector NleC suppresses epithelial inflammatory responses by inhibiting NF-κB and p38 mitogen-activated protein kinase activation. Infect. Immun. 79:3552–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Skaug B, Jiang X, Chen ZJ. 2009. The role of ubiquitin in NF-κB regulatory pathways. Annu. Rev. Biochem. 78:769–796 [DOI] [PubMed] [Google Scholar]
- 37. Starr T, et al. 2012. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 11:33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Symons A, Beinke S, Ley SC. 2006. MAP kinase kinase kinases and innate immunity. Trends Immunol. 27:40–48 [DOI] [PubMed] [Google Scholar]
- 39. Tacket CO, et al. 1994. Enteral immunization and challenge of volunteers given enterotoxigenic E. coli CFA/II encapsulated in biodegradable microspheres. Vaccine 12:1270–1274 [DOI] [PubMed] [Google Scholar]
- 40. Tauschek M, Gorrell RJ, Strugnell RA, Robins-Browne RM. 2002. Identification of a protein secretory pathway for the secretion of heat-labile enterotoxin by an enterotoxigenic strain of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:7066–7071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Trosky JE, et al. 2004. Inhibition of MAPK signaling pathways by VopA from Vibrio parahaemolyticus. J. Biol. Chem. 279:51953–51957 [DOI] [PubMed] [Google Scholar]
- 42. Wan F, et al. 2011. IKKβ phosphorylation regulates RPS3 nuclear translocation and NF-κB function during infection with Escherichia coli strain O157:H7. Nat. Immunol. 12:335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang X, Gao X, Hardwidge PR. 2012. Heat-labile enterotoxin-induced activation of NF-κB and MAPK pathways in intestinal epithelial cells impacts enterotoxigenic Escherichia coli (ETEC) adherence. Cell. Microbiol. 14:1231–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yen H, et al. 2010. NleC, a type III secretion protease, compromises NF-κB activation by targeting p65/RelA. PLoS Pathog. 6:e1001231 doi:10.1371/journal.ppat.1001231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang W, et al. 2006. Significance of heat-stable and heat-labile enterotoxins in porcine colibacillosis in an additive model for pathogenicity studies. Infect. Immun. 74:3107–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]