Abstract
Human telomerase reverse transcriptase (hTERT) is the catalytic subunit of the human telomerase complex. Growing evidence suggests that hTERT also contributes to the cell physiology independently of telomere elongation. However, its role in bacterial infection is unknown. Here we show that hTERT is critical for Listeria monocytogenes infection, as the depletion of hTERT impaired bacterial intracellular replication. In addition, we observed that L. monocytogenes caused a decrease in hTERT levels at early time points of the infectious process. This effect was mediated by the pore-forming toxin listeriolysin O (LLO) and did not require bacterial entry into host cells. Calcium influx through the LLO pores contributed to a proteasome-independent decrease in hTERT protein levels. Together, our data provide evidence that these bacteria trigger hTERT degradation, an event that is detrimental to bacterial replication.
INTRODUCTION
Telomeres are nucleoprotein complexes with a 3′ single strand overhang that form the ends of chromosomes. They protect the ends of chromosomes from being recognized as double-stranded DNA breaks and also from degradation (40). The inability of the conventional replication machinery to duplicate entirely linear DNA leads to progressive telomere shortening (39, 49). This progressive attrition of telomeres at each cell division has been suggested to lead to a senescent state (22). Cellular senescence is characterized by an irreversible proliferation arrest despite favorable growth conditions (7). In highly proliferative cells, such as stem cells, germ cells, and cancer cells, the shortening of telomeres is counteracted by a ribonucleoprotein complex named telomerase (19). The human telomerase enzyme consists of a catalytic subunit with reverse transcriptase activity (hTERT) and of a telomerase RNA (hTER), which serves as a template for telomeric DNA synthesis (2). Beyond its canonical function in telomere maintenance, the human telomerase reverse transcriptase exhibits several extratelomeric roles, some of which take place outside the nucleus (31). Suppression of hTERT results in the alteration of the chromatin architecture, which adopts a conformation that inhibits the DNA damage response (33). hTERT can also modulate Wnt/β-catenin signaling pathways by physically occupying the promoters of β-catenin target genes to induce their transcription (8, 41). Surprisingly, hTERT was also reported to be present in the mitochondria, where it exhibited hTER-independent reverse transcriptase and RNA-dependent RNA polymerase activities (30, 45). In addition, mitochondrial hTERT was found to protect cells from apoptosis (1, 11, 32, 44). Interestingly, the use of hTERT mutants showed that telomere elongation, the regulation of DNA damage response, and increases in cell proliferation and cellular life span are separable and independent functions of hTERT (37).
Given the multiple roles of hTERT in diverse cellular processes, this enzyme represents an attractive target for pathogens. Indeed, several tumor viruses promote transcription and posttranslational modifications of hTERT during carcinogenesis (3). The virus-induced upregulation of hTERT promotes cell immortalization, preventing the appearance of cellular senescence—one of the antiviral defense strategies of host cells (42). No regulation of hTERT by bacteria has been reported.
Listeria monocytogenes is an invasive bacterial pathogen that infects humans and animals following the ingestion of contaminated food products. The disease listeriosis manifests itself as gastroenteritis, meningitis, encephalitis, and maternofetal and neonatal infections, resulting in a case fatality rate of 20 to 30% (48). The diversity of the clinical symptoms results in part from the ability of L. monocytogenes to cross the intestinal, placental, and blood-brain barriers of the host. At the cellular level, L. monocytogenes employs elaborate strategies to manipulate host cells: it controls endocytosis pathways, hijacks the cytoskeleton to ensure its own propagation to neighboring cells, impairs posttranslational modifications of host proteins, perturbs mitochondrial physiology, and modulates host gene expression (10). The deep knowledge of its infectious process and the availability of a wide range of mutants make this bacterium a powerful tool for investigating the effects of bacterial infection on the catalytic subunit of telomerase.
In the present study, we reveal that downregulation of hTERT impairs Listeria infection and that L. monocytogenes induces a decrease in hTERT levels. This diminution is triggered by listeriolysin O (LLO), a pore-forming toxin secreted by L. monocytogenes. The decrease in hTERT levels is stimulated by calcium influx through LLO pores at the level of the host plasma membrane. Our study demonstrates for the first time a regulation of the catalytic subunit of telomerase by bacteria and highlights a role for telomerase in infection.
MATERIALS AND METHODS
Cell lines and bacterial strains.
HeLa cells (ATCC CCL-2) were maintained in modified Eagle's medium (MEM GlutaMAX; Invitrogen) supplemented with 10% fetal calf serum at 37°C in a 10% CO2 atmosphere. When needed, cells were treated with the following components added in medium without supplements: human tumor necrosis factor alpha (TNF-α) (R&D Systems), 10 ng/ml; trichostatin A (Sigma), 1 μg/ml; cytochalasin D (Sigma), 5 μg/ml; MG132 (Calbiochem), 20 μM; lactacystin (Calbiochem), 20 μM; 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF) (Calbiochem), 300 μM; leupeptin (Calbiochem), 100 μM; loxistatin (Calbiochem), 100 μM; bestatin methyl ester (Calbiochem), 100 μM; pepstatin A methyl ester (Calbiochem), 200 μM; KCl (Sigma), 135 mM; and EGTA (Sigma). The incubation times for each drug are indicated in the legends to Fig. 4 and 6 and to Fig. S2 and S3 in the supplemental material.
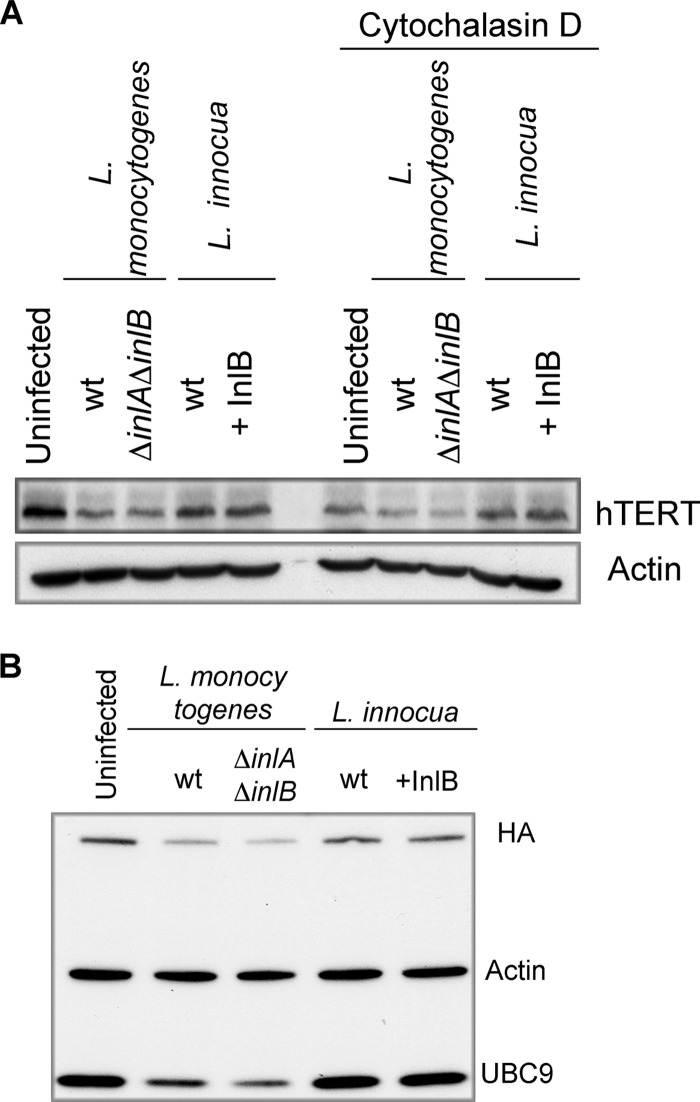
Fig 4.
L. monocytogenes causes a decrease in hTERT levels prior to host cell invasion. (A) HeLa cells were treated or not treated with cytochalasin D for 1 h. Then, cells were infected for 3 h with L. monocytogenes EGD or a ΔinlA ΔinlB mutant. Cells were also exposed to wild-type L. innocua or to a mutant expressing InlB (+InlB). The cell lysates were analyzed by immunoblotting with anti-hTERT and anti-actin antibodies. (B) HeLa cells were transiently transfected with a plasmid encoding hTERT-HA. Then, the cells were treated as described for panel A. The immunoblot was probed with anti-HA, anti-UBC9, and anti-actin antibodies.
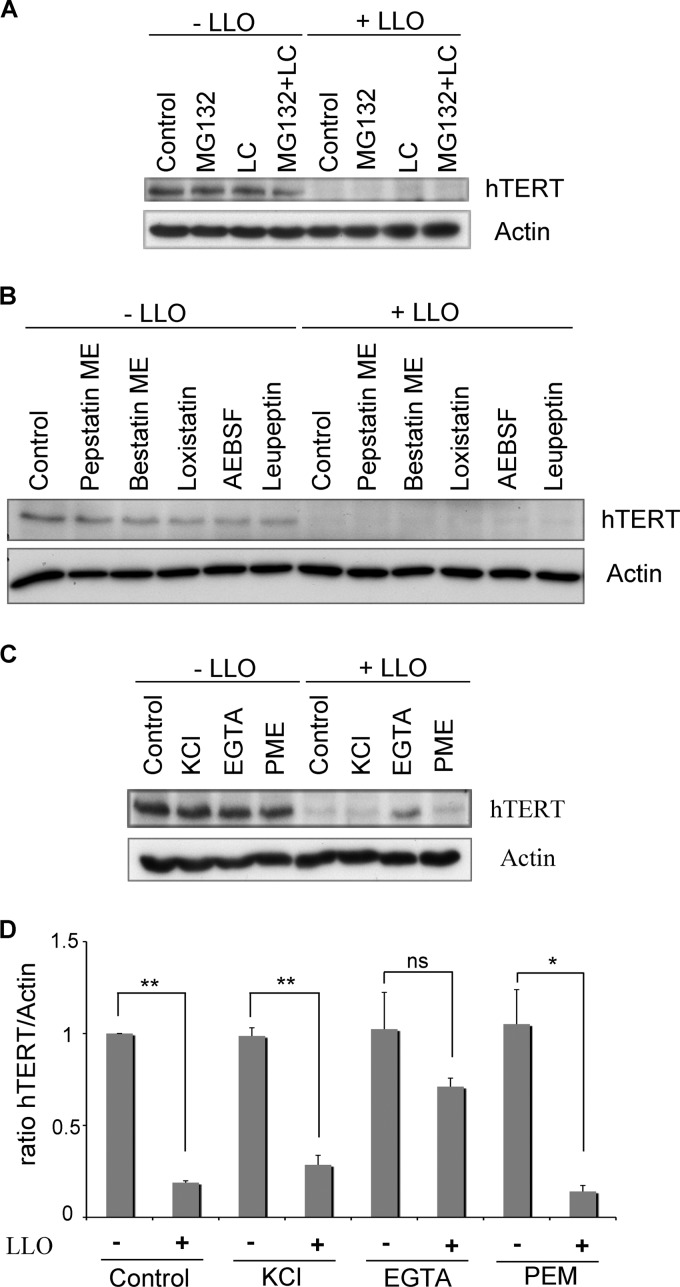
Fig 6.
Calcium signaling contributes to LLO-induced degradation of hTERT. (A) HeLa cells were pretreated with the proteasome inhibitors MG132 or lactacystin (LC) or with both for 5 h. LLO was then added to the cell medium for 20 min. hTERT and actin were monitored by Western blotting. (B) HeLa cells were pretreated for 1.5 h with the indicated protease inhibitors (pepstatin methyl ester [ME], bestatin methyl ester, loxistatin, AEBSF, and leupeptin) before being exposed to LLO for 20 min. The cell extracts were probed with anti-hTERT and anti-actin. (C) HeLa cells were incubated with KCl (with or without LLO) or EGTA (with or without LLO). For pepstatin methyl ester (PEM), the cells were first treated with the inhibitor for 1 h before the addition of LLO. hTERT and actin levels were revealed by Western blotting. (D) Quantifications of the hTERT levels shown in panel C from three independent experiments are normalized to actin and shown relative to uninfected cells. The results are shown as means ± standard errors of the mean. One asterisk marks P values of <0.05, two asterisks mark P values of <0.001, and ns marks a nonsignificant difference.
Listeria strains were grown in brain heart infusion (BHI) medium (BD Difco) at 37°C. The bacterial strains used in this study were Listeria innocua (BUG 499), L. innocua overexpressing InlB (BUG 1642), L. monocytogenes EGD-e (BUG 1600), L. monocytogenes EGD (BUG 600), L. monocytogenes EGD ΔinlA ΔinlB (BUG 949), L. monocytogenes EGD Δhly (BUG 2132), L. monocytogenes L028 (BOF 343), L. monocytogenes L028 Tn::hly (BOF 415), and L. monocytogenes L028 Tn::hly (BOF 415) overexpressing LLO (BUG 210).
LLO wild type (wt) and mutants (C484, Y206A, W492A) were purified as described previously (17) and used at 3 nM.
Infection.
For the infection experiments, bacteria were cultured overnight and then subcultured 1:10 in BHI medium for 2 h at 37°C. Thirty minutes prior to infection, HeLa cells were incubated in medium without serum. After addition of the bacteria, the cells were centrifuged at 1,000 × g for 1 min before an incubation period at 37°C for 1 h. When not specified, infection was achieved with a multiplicity of infection (MOI) of 50 with bacteria that were washed three times in medium without serum prior to infection. For a longer infection process, infected cells were grown in growth medium supplemented with 20 μg/ml gentamicin to kill the extracellular bacteria.
To evaluate the extent of L. monocytogenes infection or Listeria entry by counting the CFU, infected cells were washed and intracellular bacteria were released by 0.1% Triton X-100. The cell lysates were plated on BHI agar plates to quantify the intracellular bacteria.
To quantify intracellular bacteria by immunofluorescence, the infected cells were washed and fixed in 4% paraformaldehyde, and extracellular bacteria were stained with an anti-L. monocytogenes R11 antibody (18) and an Alexa Fluor 546 goat anti-rabbit antibody (Invitrogen) before permeabilization of the host cell plasma membrane. After permeabilization, intracellular bacteria were stained with an anti-L. monocytogenes R11 antibody and an Alexa Fluor 488 goat anti-rabbit antibody (Invitrogen).
Transfection of HeLa cells.
Control small interfering RNA (siRNA) (siGENOME nontargeting siRNA 1) and hTERT1 siRNA (described in reference 32) were purchased from Dharmacon, while hTERT2 siRNA was custom designed (5′-UCAGACAGCACUUGAAGAG-3′) and purchased from Eurofins MWG Operon. For siRNA transfection, we used Oligofectamine (Invitrogen) according to the manufacturer's instructions. To infect transfected cells, we adjusted the multiplicity of infection to the number of viable cells in every well in order to infect them with 50 bacteria per cell in each experiment.
The retroviral plasmids pBABE-puro and pBABE-puro-hTERT-HA were created in Bob Weinberg's laboratory and were purchased from Addgene (plasmids 1764 and 1772, respectively). HeLa cells were transiently transfected with 2 μg of plasmid using the FuGENE HD transfection reagent (Roche) according to the manufacturer's instructions.
Real-time PCR.
Total RNA was extracted using an RNeasy kit (Qiagen). cDNA was synthesized from 500 ng RNA using an iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time PCR (qPCR) was performed by using the SYBR green kit (Bio-Rad). The qPCR protocol and gene expression method (2−ΔΔCt) were as described previously (14).
Immunoblotting.
For Western blot analysis, cells were lysed with 2× Laemmli loading buffer (124 mM Tris-HCl [pH 6.8], 4% SDS, 20% glycerol, 0.02% bromophenol blue, 0.03% dithiothreitol [DTT]), sonicated for 2 s, and then boiled for 5 min. The samples were loaded on 6% gels or 4 to 15% Mini-Protean TGX gradient gel (Bio-Rad). The proteins were transferred on a nitrocellulose membrane (GE Healthcare) that was then blocked in 10% milk. The primary antibodies were anti-actin (catalog no. A5441; Sigma), anti-hTERT (manufacturer part no. 600-401-252; Rockland), anti-phospho-IκB (Cell Signaling Technology), and anti-hemagglutinin (HA) tag (clone 6E2; Cell Signaling Technology). Rabbit polyclonal antibodies against UBC9 (R201) and LLO (R176) were raised by immunizing rabbits with purified recombinant LLO and UBC9 proteins. The sera were then purified against UBC9 and LLO to obtain the purified antibodies (43).
Statistical analyses.
Our results are expressed as the means of three independent experiments. The error bars represent the standard errors of the mean. The analyses were performed with Student's t test, and the statistical significance was established at P values of <0.05 or <0.001 (indicated by one or two asterisks, respectively, in Fig. 1, 2, 3, and 6).
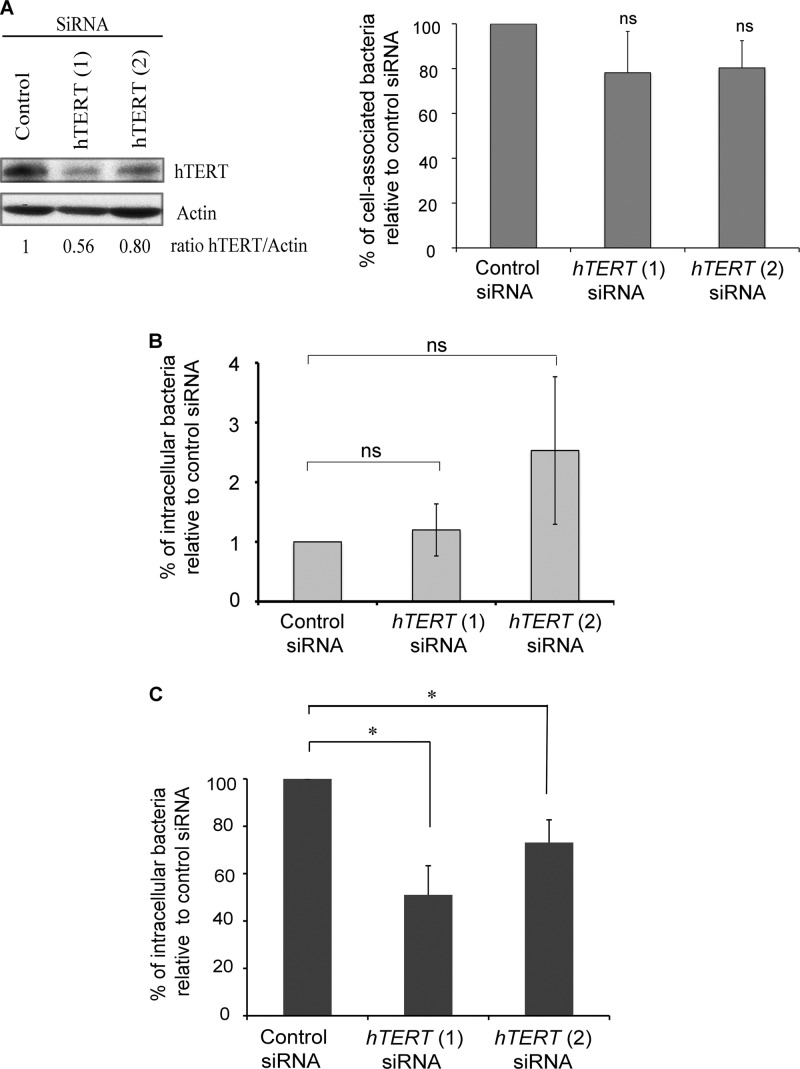
Fig 1.
hTERT is critical for Listeria infection. HeLa cells were treated with control, hTERT1, or hTERT2 siRNAs for 72 h, counted, and then infected with L. monocytogenes EGD. (A) Thirty minutes after the beginning of infection, cell-associated bacteria were quantified and normalized on control siRNA-treated cells. (B) Thirty minutes after the beginning of infection, cells were treated with 100 μg/ml gentamicin for 30 min. The intracellular bacteria were counted and normalized on control siRNA-treated cells. (C) One hour after infection, the cells were treated with 20 μg/ml gentamicin for 4 h. The percentages of intracellular bacteria are relative to the control siRNA-treated cells. The experiments were performed three times, and the results are shown as means ± standard error of the means. The asterisks mark P values of <0.05, and ns marks nonsignificant differences.
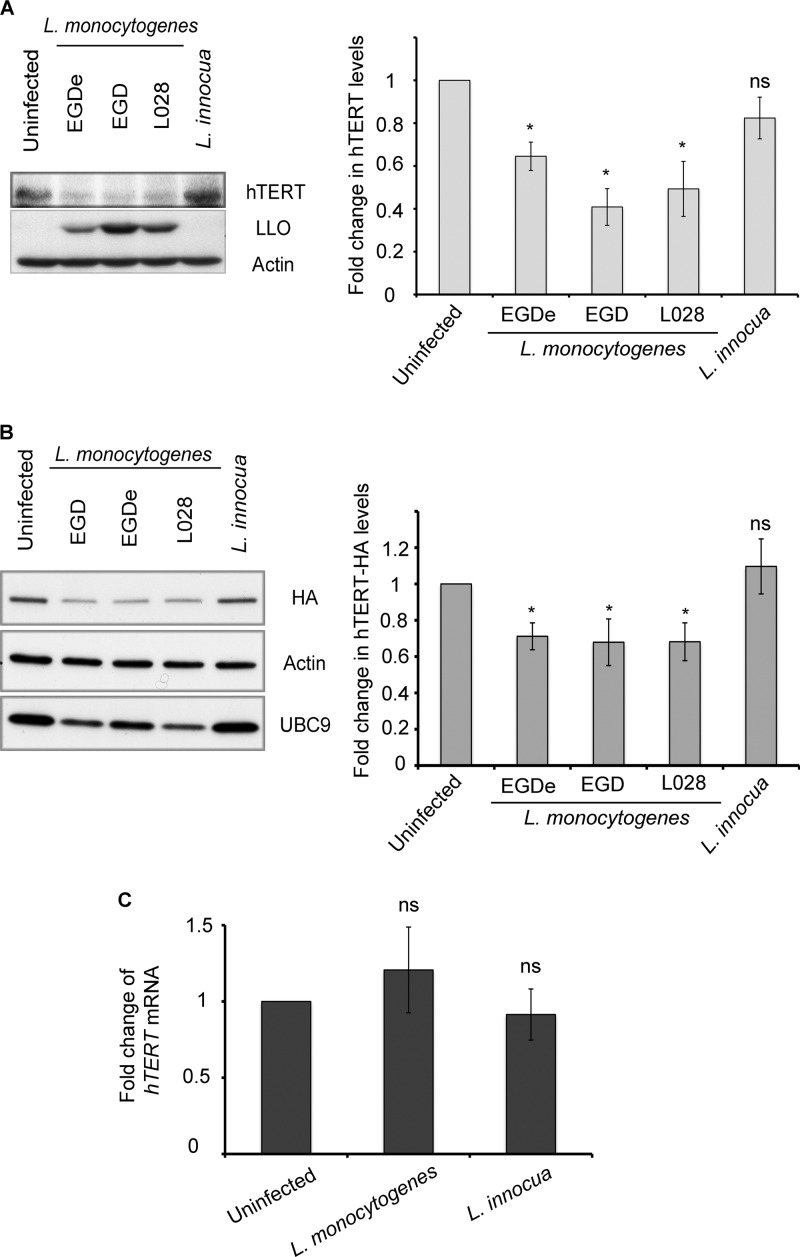
Fig 2.
L. monocytogenes induces a decrease in hTERT levels. (A) HeLa cells were infected for 1 h with L. monocytogenes strains (EGD-e, EGD, and L028) or incubated with L. innocua. hTERT, LLO, and actin were monitored by Western blotting. (B) HeLa cells transiently expressing hTERT-HA were infected for 1 h with a wild-type L. monocytogenes strain (EGD-e, EGD, or L028) or incubated with L. innocua. The cell lysates were analyzed by immunoblotting with anti-HA, anti-actin, and anti-UBC9 antibodies. The quantifications of hTERT and HA levels shown in panels A and B were from three independent experiments and are normalized to actin and shown relative to uninfected cells. The results are shown as mean ± standard errors of the mean. The asterisks mark P values of <0.05, and ns marks nonsignificant differences. (C) hTERT mRNA was extracted 1 h after the infection of HeLa cells with L. monocytogenes EGD or L. innocua. Reverse transcription was performed, followed by real-time PCR. hTERT expression levels were normalized to actin and are presented as levels relative to uninfected cells. The data are the average of data from three independent experiments, and error bars represent the standard errors of the mean.
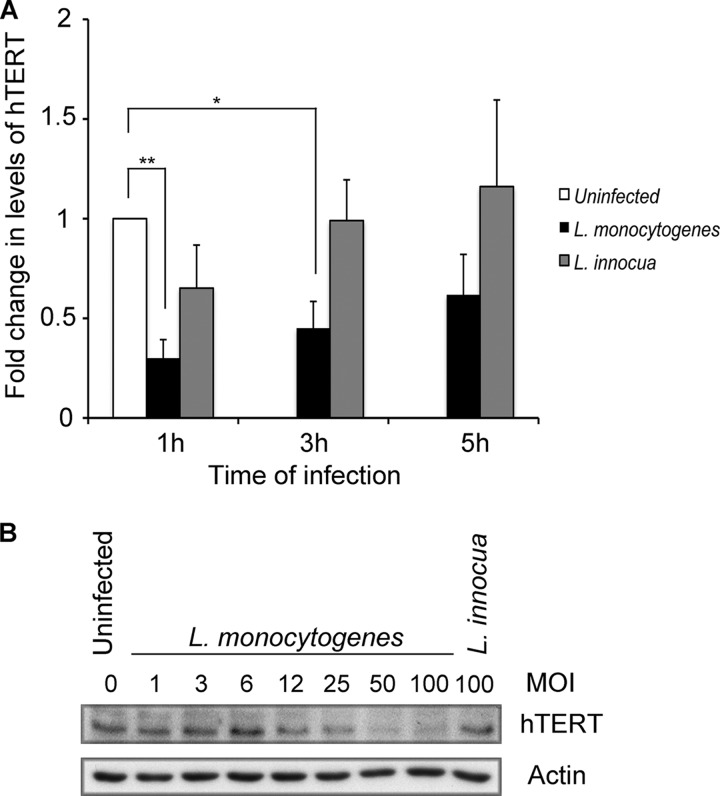
Fig 3.
hTERT decrease depends on the duration of infection and the number of bacteria. (A) HeLa cells were infected with L. monocytogenes EGD or incubated with L. innocua for the indicated times. Immunoblotting was performed to monitor hTERT and actin levels. The hTERT signal was quantified and normalized to actin, and data from three independent experiments were pooled. The results are shown as means and standard errors of the mean. The latter were relative to uninfected cells. One asterisk marks P values of 0.007, and two asterisks mark P values of 0.0008. (B) HeLa cells were infected for 1 h with L. monocytogenes EGD at different multiplicities of infection or incubated with L. innocua, and hTERT and actin levels were revealed by Western blotting.
RESULTS
hTERT is important for intracellular Listeria replication.
To determine the role of hTERT in the infectious process, we treated HeLa cells with hTERT or control siRNAs and then infected them with L. monocytogenes for either 30 min to assess bacterial adhesion and entry or for 5 h to measure intracellular replication. After 30 min of infection, we observed that the number of cell-associated bacteria in hTERT-depleted cells was not significantly different from that of control cells (Fig. 1A). In order to assess the effect on bacterial entry, we infected HeLa cells for 30 min and then treated them with 100 μg/ml gentamicin for another 30 min. No significant differences in bacterial entry were observed when comparing control cells with hTERT1 or hTERT2 siRNA-treated cells (Fig. 1B). However, 5 h after infection, the number of intracellular bacteria was significantly reduced in the cells treated with hTERT siRNA compared to those in the control (Fig. 1C). We confirmed this effect by quantifying intracellular bacteria via an immunofluorescence approach (see Fig. S1 in the supplemental material). These results suggest that hTERT is important for the intracellular replication of Listeria.
Listeria monocytogenes induces a decrease in hTERT levels.
Based on the observation discussed above that hTERT plays a role in Listeria infection, we examined whether infection with different wild-type strains of L. monocytogenes or incubation with L. innocua, a nonpathogenic Listeria species, would affect hTERT at the protein level. The level of hTERT was analyzed by Western blotting. We observed a decrease in hTERT levels upon infection with L. monocytogenes for 1 h (Fig. 2A). To confirm the results obtained with the antibody against hTERT (50), we transfected HeLa cells with a plasmid expressing hTERT-HA and infected these cells. LLO expression and the degradation of UBC9, a host enzyme, were employed as markers of L. monocytogenes infection (43). As observed with the endogenous protein, the level of hTERT-HA decreased in the cells infected with L. monocytogenes (Fig. 2B).
We next examined the levels of hTERT mRNA 1 h after infection of HeLa cells by L. monocytogenes or incubation with L. innocua. We did not observe a significant difference in hTERT mRNA levels between the infected and uninfected cells (Fig. 2C). Moreover, we performed a similar experiment 3 h after infection and found that Listeria infection did not modify hTERT mRNA levels in those host cells. Trichostatin A (TSA), which is known to inhibit histone deacetylases and to activate the hTERT promoter, was used as a positive control for hTERT transcription activation (9). As expected, and in contrast to Listeria infection, TSA induced an approximately 1.72-fold increase in hTERT mRNA levels (see Fig. S2 in the supplemental material). Taken together, our data show that L. monocytogenes decreases the levels of the hTERT mRNA without affecting its transcription.
The decrease of hTERT occurs early after infection.
We next examined the kinetics of the decrease in hTERT protein levels. A time course analysis conducted 5 h after infection indicated that L. monocytogenes reduces the levels of hTERT as soon as 1 h after the addition of bacteria to HeLa cells (Fig. 3A). This effect was transient, as the hTERT levels in infected and uninfected cells were similar 5 h after infection. We also observed that the decrease in hTERT levels was dependent on the multiplicity of infection used to infect the cells (Fig. 3B). These results definitively establish that L. monocytogenes induces a decrease in hTERT levels during the initial stages of infection.
L. monocytogenes induces a decrease in hTERT levels before bacterial entry.
We next examined whether a diminution in hTERT levels depends on bacterial invasion. We infected cells with wild-type L. monocytogenes or a ΔinlA ΔinlB mutant that is strongly impaired in the invasion of HeLa cells. Both bacterial strains induced a decrease in the hTERT levels, as assessed by Western blotting (Fig. 4A). In contrast, the cells exposed to wild-type L. innocua or L. innocua expressing InlB (which confers to L. innocua the capacity to invade cells [6]) did not exhibit a decrease in hTERT levels (Fig. 4A), suggesting that hTERT levels do not decrease upon uptake of the nonpathogenic bacterium L. innocua. Similar results were observed with hTERT-HA-expressing cells (Fig. 4B). In addition, the pretreatment of cells with cytochalasin D, an inhibitor of actin polymerization that prevents L. monocytogenes entry (15), did not block hTERT decrease (Fig. 4A). These results demonstrate that the decrease in hTERT levels does not depend on bacterial invasion and is specific to L. monocytogenes.
Listeriolysin O is responsible for the reduction in hTERT levels.
Because hTERT was degraded in the presence of extracellular bacteria, we next investigated whether a secreted factor could promote this effect. We tested the pore-forming toxin listeriolysin O (LLO), a toxin first described as crucial for the escape of Listeria from the internalization vacuole. This toxin is also secreted by extracellular Listeria, and an increasing number of studies have shown that it also acts at the level of the host plasma membrane (21). We infected hTERT-HA-expressing HeLa cells or control cells with wild-type L. monocytogenes or a mutant defective for LLO, the Tn::hly mutant. Whereas a decrease of the hTERT or hTERT-HA levels was observed in the cells infected with wild-type L. monocytogenes, no effect was observed with the Tn::hly mutant (Fig. 5A and B). The infection of cells with the complemented strain (Tn::hly mutant with a plasmid coding for hly) induced a decrease in hTERT (Fig. 5A and B). These results suggest that LLO is required to reduce hTERT protein levels.
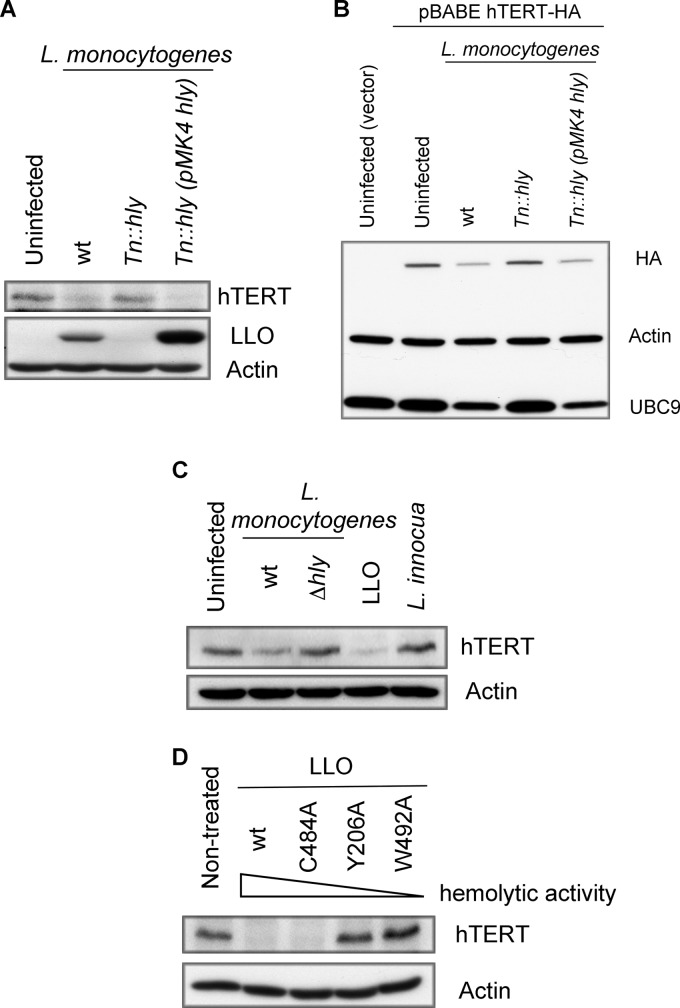
Fig 5.
The pore-forming activity of listeriolysin O (LLO) is responsible for hTERT degradation. Western blots probed for endogenous hTERT (A) or for overexpressed hTERT-HA (or the empty vector as a control) (B). In both panels (A and B), cells were infected for 1 h with L. monocytogenes strain L028 wild type (wt), with a mutant of the hly gene (Tn::hly), or with a complemented strain expressing LLO [Tn::hly(pMK4 hly)]. Actin, HA, hTERT, LLO, and UBC9 were monitored by immunoblotting. (C) HeLa cells were infected for 1 h with L. monocytogenes EGD wild type (wt) or with a mutant deleted for the hly gene (Δhly). The cells were also exposed to L. innocua for 1 h or incubated for 20 min with LLO. The cellular protein extracts were probed with anti-hTERT and anti-actin antibodies. (D) HeLa cells were treated for 20 min with LLOwt or the following mutants: LLOC484A, LLOY206A, and LLOW492A. Immunoblotting was performed with anti-hTERT and anti-actin antibodies.
To determine whether LLO alone was sufficient to induce a reduction in hTERT levels, we treated cells with purified LLO at a sublytic concentration, i.e., 3 nM for 20 min. Strikingly, LLO was able to induce a significant decrease in hTERT levels (Fig. 5C).
To determine whether the pore-forming activity of LLO was required to trigger hTERT degradation, we treated cells with mutants of LLO that are affected in their hemolytic activity. The wild-type version of LLO, LLOwt, has the highest hemolytic activity, followed in a decreasing manner by LLOC484A, LLOY206A, and LLOW492A (43). As shown in Fig. 5D, a decrease of the hTERT level correlated with an increase in the hemolytic activity of LLO. This result suggests that pores formed by LLO trigger hTERT degradation.
Calcium contributes to hTERT degradation induced by L. monocytogenes.
To address the mechanism by which LLO induced the decrease in hTERT levels, we examined whether it could be prevented by inhibiting the activity of the proteasome with the inhibitors MG132 and lactacystin (LC). The inhibition of proteasome activity had no effect on the LLO-induced decrease of hTERT (Fig. 6A), although as expected, the proteasome inhibitors provoked accumulation of ubiquitin-protein conjugates in treated cells (see Fig. S3A in the supplemental material).
To further investigate the molecular basis of the decrease in hTERT levels, we pretreated cells with inhibitors of aspartyl proteases (pepstatin methyl ester), cysteine proteases (loxistatin and leupeptin), metalloproteases (bestatin methyl ester), and serine proteases (AEBSF and leupeptin). We monitored the inhibitory activity of pepstatin methyl ester through the partial resistance to degradation that it conferred to UBC9 in the presence of LLO (see Fig. S3B in the supplemental material) (43). The activities of leupeptin and loxistatin were tested through the inhibition of phospho-IκB degradation, as reported previously (see Fig. S3C and D in the supplemental material) (34), while AEBSF inhibited the activation of serine proteases, as shown by the decreased activation (phosphorylation) of IκB (see Fig. S3C in the supplemental material) (24, 34) and the inhibitory effect on the degradation of phospho-IκB (see Fig. S3D in the supplemental material). The addition of LLO decreased the levels of hTERT in the presence of all tested protease inhibitors (Fig. 6B). We conclude that, under the conditions that we examined, the proteolytic activity necessary for the reduction in hTERT levels in the presence of LLO could not be impaired.
LLO pore formation provokes the permeability of the plasma membrane to K+ and Ca2+ ions (4). K+ efflux leads to histone H3 dephosphorylation (20), while Ca2+ influx plays a role during bacterial entry and induces mitochondrial fragmentation (13, 47). To study the implication of the two ions in the decrease of hTERT levels, we prevented K+ efflux by incubating cells in high extracellular concentrations of KCl, while Ca2+ influx was blocked by incubating cells with EGTA, a calcium chelator. As shown in Fig. 6C and D, the pretreatment of cells with EGTA impaired the decrease in hTERT levels induced by LLO, while blocking K+ efflux had no effect. These results suggest that Ca2+ influx, rather than K+ efflux, contributes to the decrease in hTERT levels in the presence of LLO.
DISCUSSION
In the present study, we provide the first evidence that hTERT is important for Listeria infection. In addition, we show that L. monocytogenes is able to induce a decrease in hTERT levels through the formation of LLO pores in the plasma membrane prior to host cell invasion. The pores formed by LLO induce a decrease of the hTERT level that is proteasome independent but requires Ca2+ influx.
L. monocytogenes induced an hTERT decrease without affecting transcription of the hTERT gene. Because the half-life of hTERT is around 6 h (28), the decrease of the hTERT level observed 20 min after LLO treatment probably does not result from a decrease in hTERT translation but, rather, from posttranslational regulation of the hTERT protein. Several studies have reported that hTERT is degraded through the ubiquitin-proteasome pathway (27, 29, 38). Here, we observed a decrease in hTERT levels that was proteasome independent. Similarly, a proteasome-independent pathway was suggested previously for the degradation of UBC9 upon treatment with LLO (43). However, the degradation of UBC9 involves an aspartyl protease and is calcium independent (43). In contrast, we observed a contribution of calcium to the pathway leading to the decrease of hTERT levels. Calcium is known to contribute to the activation of cysteine proteases, such as calpains (46). However, other proteins belonging to serine proteases, aspartyl proteases, or metalloproteases can also be activated by the presence of calcium (16, 23, 35). We blocked these classes of proteases using well-characterized inhibitors, but under the conditions tested, none of the protease inhibitors impaired the decrease in hTERT levels. Two possible explanations exist for the inability to prevent the reduction in hTERT levels induced by LLO: (i) the doses of protease inhibitors and the duration of the treatment were not sufficient to prevent the decrease in hTERT levels, and (ii) proteolytic cleavage can be very specific. For example, identification of the protease responsible for the cleavage of paxillin upon treatment with the pore-forming toxin α-hemolysin from uropathogenic Escherichia coli relied on a highly specific trypsin-like serine protease inhibitor (tosyl-l-lysine-chloromethyl ketone), while it was insensitive to the chymotrypsin-like serine protease inhibitor tosyl-l-phenylalanine-chloromethyl ketone (12). Our current work is focusing on identifying the molecular basis of the degradative pathways activated by LLO via proteomic analysis and genome-wide siRNA screening approaches.
We found that hTERT was important for Listeria infection. Our siRNA experiments show that reduced hTERT expression early during infection does not impair bacterial adhesion and entry, yet it results in a decrease of the intracellular bacterial load at later time points. The degradation of hTERT could therefore represent an event that protects host cells at a specific stage of the Listeria infectious cycle. In the absence of siRNA treatment, hTERT levels start recovering 5 h after infection, allowing for the full intracellular replication of L. monocytogenes, further suggesting that this antibacterial effect is time restricted. In addition, the recovery of hTERT levels may contribute to cell survival, given the proposed antiapoptotic role of hTERT.
A decrease in hTERT levels could have important consequences during an in vivo infection. Telomerase activity has been detected in human adult stem cells, including hematopoietic and nonhematopoietic stem cells (25). Secreted LLO could diffuse to such progenitor cells or stem hematopoietic cells in colonized organs. An LLO-induced decrease in hTERT levels in LLO-targeted cells would lower their self-renewal capacity and therefore impair the immune response and promote L. monocytogenes infection (26, 36). It remains to be tested whether hTERT levels are affected in vivo.
The first characterized role of hTERT concerns telomere elongation that contributes to the extension of cellular life span (5). To detect this effect, cells have to be followed through several generations. However, given that long-term infections (for more than 48 h) are toxic in HeLa cells and the observed L. monocytogenes-induced reduction of hTERT levels was followed by a recovery process, we did not expect to provoke detectable effects on telomere length in HeLa cells. In agreement with this, all of our attempts to detect a change in telomere length were unsuccessful.
Most virus-induced tumor cells possess high telomerase activity but short telomeres (3). Indeed, hTERT can extend the cellular life span without inducing net telomere lengthening (37, 51). It is possible that L. monocytogenes affects the recently described noncanonical functions of hTERT (31). As mentioned, hTERT was shown to play roles in processes as diverse as DNA damage, Wnt signaling, and the decrease of the RNA component of a mitochondrial RNA-processing endoribonuclease (RMRP). These roles of hTERT seem to be at least partially independent of each other (37). Indeed, while hTERT induced cell proliferation independently of an increase in Wnt signaling, it was associated with a decrease in RMRP levels (37). The next challenge will thus be to determine whether noncanonical functions of hTERT are necessary for bacterial infection and which specific function of hTERT is targeted by bacterial infection.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Olivier Dussurget and David Ribet for comments and for critical reading of the manuscript, Edith Gouin and Mélanie Hamon for help in the protease inhibitor activity tests, and all members of the Unité des Interactions Bactéries-Cellules laboratory for helpful discussion. We thank Titia de Lange for discussion, for advice, and for hosting A.S.-L.
This work was supported by the Pasteur Institute, INSERM (U604), INRA (USC2020), the Fondation Louis Jeantet, the European Research Council (Advanced grant 233348, MODELIST), the Fondation pour la Recherche Médicale (fellowship to F.S.), and the Fondation Le Roch Les Mousquetaires. P.C. is an HHMI senior international research scholar.
Footnotes
Published ahead of print 24 September 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Ahmed S, et al. 2008. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J. Cell Sci. 121:1046–1053 [DOI] [PubMed] [Google Scholar]
- 2. Autexier C, Lue NF. 2006. The structure and function of telomerase reverse transcriptase. Annu. Rev. Biochem. 75:493–517 [DOI] [PubMed] [Google Scholar]
- 3. Bellon M, Nicot C. 2008. Regulation of telomerase and telomeres: human tumor viruses take control. J. Natl. Cancer Inst. 100:98–108 [DOI] [PubMed] [Google Scholar]
- 4. Bischofberger M, Gonzalez MR, van der Goot FG. 2009. Membrane injury by pore-forming proteins. Curr. Opin. Cell Biol. 21:589–595 [DOI] [PubMed] [Google Scholar]
- 5. Bodnar AG, et al. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349–352 [DOI] [PubMed] [Google Scholar]
- 6. Braun L, Ohayon H, Cossart P. 1998. The InIB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol. Microbiol. 27:1077–1087 [DOI] [PubMed] [Google Scholar]
- 7. Campisi J, d'Adda di Fagagna F. 2007. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8:729–740 [DOI] [PubMed] [Google Scholar]
- 8. Choi J, et al. 2008. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 4:e10 doi:10.1371/journal.pgen.0040010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cong YS, Bacchetti S. 2000. Histone deacetylation is involved in the transcriptional repression of hTERT in normal human cells. J. Biol. Chem. 275:35665–35668 [DOI] [PubMed] [Google Scholar]
- 10. Cossart P. 2011. Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc. Natl. Acad. Sci. U. S. A. 108:19484–19491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Del Bufalo D, et al. 2005. Involvement of hTERT in apoptosis induced by interference with Bcl-2 expression and function. Cell Death Differ. 12:1429–1438 [DOI] [PubMed] [Google Scholar]
- 12. Dhakal BK, Mulvey MA. 2012. The UPEC pore-forming toxin alpha-hemolysin triggers proteolysis of host proteins to disrupt cell adhesion, inflammatory, and survival pathways. Cell Host Microbe 11:58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dramsi S, Cossart P. 2003. Listeriolysin O-mediated calcium influx potentiates entry of Listeria monocytogenes into the human Hep-2 epithelial cell line. Infect. Immun. 71:3614–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elliott KA, Rickords LF, Labrum JM. 2008. Transduction of E2F-1 TAT fusion proteins represses expression of hTERT in primary ductal breast carcinoma cell lines. Mol. Cancer 7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaillard JL, Berche P, Mounier J, Richard S, Sansonetti P. 1987. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55:2822–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gardner MD, et al. 2009. A functional calcium-binding site in the metalloprotease domain of ADAMTS13. Blood 113:1149–1157 [DOI] [PubMed] [Google Scholar]
- 17. Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. 2002. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J. Cell Biol. 156:1029–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gouin E, Dehoux P, Mengaud J, Kocks C, Cossart P. 1995. iactA of Listeria ivanovii, although distantly related to Listeria monocytogenes actA, restores actin tail formation in an L. monocytogenes actA mutant. Infect. Immun. 63:2729–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greider CW, Blackburn EH. 1985. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43:405–413 [DOI] [PubMed] [Google Scholar]
- 20. Hamon MA, Cossart P. 2011. K+ efflux is required for histone H3 dephosphorylation by Listeria monocytogenes listeriolysin O and other pore-forming toxins. Infect. Immun. 79:2839–2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamon MA, Ribet D, Stavru F, Cossart P. 2012. Listeriolysin O: the Swiss army knife of Listeria. Trends Microbiol. 20:360–368 [DOI] [PubMed] [Google Scholar]
- 22. Harley CB. 1991. Telomere loss: mitotic clock or genetic time bomb? Mutat. Res. 256:271–282 [DOI] [PubMed] [Google Scholar]
- 23. Hayley M, Perspicace S, Schulthess T, Seelig J. 2009. Calcium enhances the proteolytic activity of BACE1: an in vitro biophysical and biochemical characterization of the BACE1-calcium interaction. Biochim. Biophys. Acta 1788:1933–1938 [DOI] [PubMed] [Google Scholar]
- 24. Henkel T, et al. 1993. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature 365:182–185 [DOI] [PubMed] [Google Scholar]
- 25. Hiyama E, Hiyama K. 2007. Telomere and telomerase in stem cells. Br. J. Cancer 96:1020–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hiyama K, et al. 1995. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J. Immunol. 155:3711–3715 [PubMed] [Google Scholar]
- 27. Kim JH, et al. 2005. Ubiquitin ligase MKRN1 modulates telomere length homeostasis through a proteolysis of hTERT. Genes Dev. 19:776–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee JH, Chung IK. 2010. Curcumin inhibits nuclear localization of telomerase by dissociating the Hsp90 co-chaperone p23 from hTERT. Cancer Lett. 290:76–86 [DOI] [PubMed] [Google Scholar]
- 29. Lee JH, Khadka P, Baek SH, Chung IK. 2010. CHIP promotes human telomerase reverse transcriptase degradation and negatively regulates telomerase activity. J. Biol. Chem. 285:42033–42045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maida Y, et al. 2009. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature 461:230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martinez P, Blasco MA. 2011. Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nat. Rev. Cancer 11:161–176 [DOI] [PubMed] [Google Scholar]
- 32. Massard C, et al. 2006. hTERT: a novel endogenous inhibitor of the mitochondrial cell death pathway. Oncogene 25:4505–4514 [DOI] [PubMed] [Google Scholar]
- 33. Masutomi K, et al. 2005. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc. Natl. Acad. Sci. U. S. A. 102:8222–8227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miyamoto S, Maki M, Schmitt MJ, Hatanaka M, Verma IM. 1994. Tumor necrosis factor alpha-induced phosphorylation of I kappa B alpha is a signal for its degradation but not dissociation from NF-kappa B. Proc. Natl. Acad. Sci. U. S. A. 91:12740–12744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Molloy SS, Bresnahan PA, Leppla SH, Klimpel KR, Thomas G. 1992. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J. Biol. Chem. 267:16396–16402 [PubMed] [Google Scholar]
- 36. Morrison SJ, Prowse KR, Ho P, Weissman IL. 1996. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity 5:207–216 [DOI] [PubMed] [Google Scholar]
- 37. Mukherjee S, Firpo EJ, Wang Y, Roberts JM. 2011. Separation of telomerase functions by reverse genetics. Proc. Natl. Acad. Sci. U. S. A. 108:E1363–E1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oh W, et al. 2010. Hdm2 negatively regulates telomerase activity by functioning as an E3 ligase of hTERT. Oncogene 29:4101–4112 [DOI] [PubMed] [Google Scholar]
- 39. Olovnikov AM. 1973. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 41:181–190 [DOI] [PubMed] [Google Scholar]
- 40. Palm W, de Lange T. 2008. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 42:301–334 [DOI] [PubMed] [Google Scholar]
- 41. Park JI, et al. 2009. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reddel RR. 2010. Senescence: an antiviral defense that is tumor suppressive? Carcinogenesis 31:19–26 [DOI] [PubMed] [Google Scholar]
- 43. Ribet D, et al. 2010. Listeria monocytogenes impairs SUMOylation for efficient infection. Nature 464:1192–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saretzki G, Ludwig A, von Zglinicki T, Runnebaum IB. 2001. Ribozyme-mediated telomerase inhibition induces immediate cell loss but not telomere shortening in ovarian cancer cells. Cancer Gene Ther. 8:827–834 [DOI] [PubMed] [Google Scholar]
- 45. Sharma NK, et al. 2012. Human telomerase acts as a hTR-independent reverse transcriptase in mitochondria. Nucleic Acids Res. 40:712–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sorimachi H, Hata S, Ono Y. 2011. Impact of genetic insights into calpain biology. J. Biochem. 150:23–37 [DOI] [PubMed] [Google Scholar]
- 47. Stavru F, Bouillaud F, Sartori A, Ricquier D, Cossart P. 2011. Listeria monocytogenes transiently alters mitochondrial dynamics during infection. Proc. Natl. Acad. Sci. U. S. A. 108:3612–3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Swaminathan B, Gerner-Smidt P. 2007. The epidemiology of human listeriosis. Microbes Infect. 9:1236–1243 [DOI] [PubMed] [Google Scholar]
- 49. Watson JD. 1972. Origin of concatemeric T7 DNA. Nat. New Biol. 239:197–201 [DOI] [PubMed] [Google Scholar]
- 50. Wu YL, et al. 2006. Immunodetection of human telomerase reverse-transcriptase (hTERT) re-appraised: nucleolin and telomerase cross paths. J. Cell Sci. 119:2797–2806 [DOI] [PubMed] [Google Scholar]
- 51. Zhu J, Wang H, Bishop JM, Blackburn EH. 1999. Telomerase extends the lifespan of virus-transformed human cells without net telomere lengthening. Proc. Natl. Acad. Sci. U. S. A. 96:3723–3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.