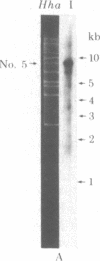
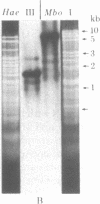
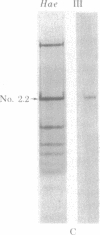
Abstract
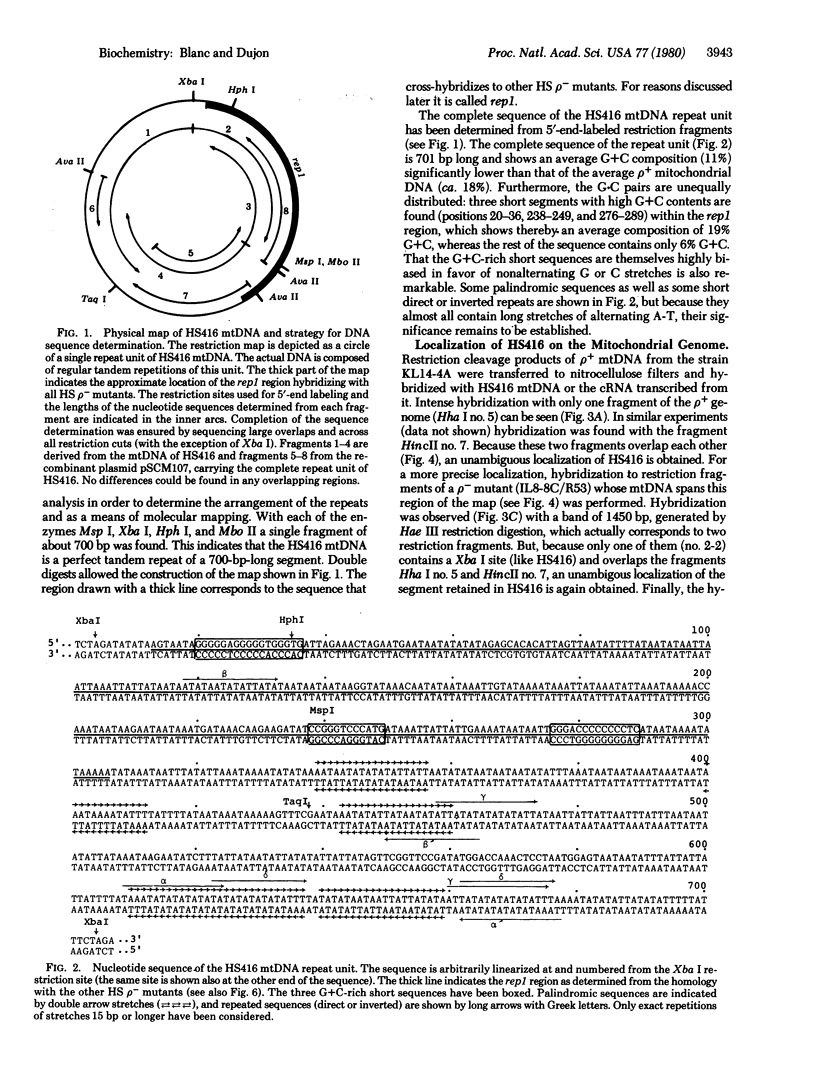
Hypersuppressiveness is a heritable property of some rho- mutants (called HS) that, in crosses to rho+, give rise to about 100% rho- cells. The mtDNAs of all HS rho- mutants reveal a common organization: they all share a homologous region of about 300 base pairs (called rep) and the fragments retained are always short (ca. 1% of the wild-type genome) and tandemly repeated. Using one HS rho- mutant as an example, we show that, after crosses with rho+ strains, the mitochondrial genome of the progeny is indistinguishable from that of the HS parent. This suggests that HS mtDNA molecules have a decisive selective advantage for replication during the transient heteroplasmic stage that follows zygote formation, the rep regions playing a role in the control of replication initiation of the mtDNA molecules. The complete nucleotide sequence of one HS rho- mutant and its localization in the oli1-rib3 segment of the rho+ mitochondrial genome are presented. Comparison of the nucleotide sequences of the rep regions of two different HS rho- mutants reveals that several rep sequences must exist in the wild-type genome, probably as a result of duplications of an originally unique ancestor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carnevali F., Morpurgo G., Tecce G. Cytoplasmic DNA from petite colonies of Saccharomyces cerevisiae: a hypothesis on the nature of the mutation. Science. 1969 Mar 21;163(3873):1331–1333. doi: 10.1126/science.163.3873.1331. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Deutsch J., Dujon B., Netter P., Petrochilo E., Slonimski P. P., Bolotin-Fukuhara M., Coen D. Mitochondrial genetics. VI. The petite mutation in Saccharomyces cerevisiae: interrelations between the loss of the p+ factor and the loss of the drug resistance mitochondrial genetic markers. Genetics. 1974 Feb;76(2):195–219. doi: 10.1093/genetics/76.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell. 1980 May;20(1):185–197. doi: 10.1016/0092-8674(80)90246-9. [DOI] [PubMed] [Google Scholar]
- EPHRUSS B., GRANDCHAMP S. ETUDES SUR LA SUPPRESSIVIT'E DES MUTANTS 'A DEFICIENCE RESPIRATOIRE DE LA LEVURE. I. EXISTENCE AU NIVEAU CELLULAIRE DE DIVERS "DEGR'ES DE SUPPRESSIVIT'E". Heredity (Edinb) 1965 Feb;20:1–7. doi: 10.1038/hdy.1965.1. [DOI] [PubMed] [Google Scholar]
- Ephrussi B., Jakob H., Grandchamp S. Etudes Sur La SuppressivitE Des Mutants a Deficience Respiratoire De La Levure. II. Etapes De La Mutation Grande En Petite Provoquee Par Le Facteur Suppressif. Genetics. 1966 Jul;54(1):1–29. doi: 10.1093/genetics/54.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi B., de Margerie-Hottinguer H., Roman H. SUPPRESSIVENESS: A NEW FACTOR IN THE GENETIC DETERMINISM OF THE SYNTHESIS OF RESPIRATORY ENZYMES IN YEAST. Proc Natl Acad Sci U S A. 1955 Dec 15;41(12):1065–1071. doi: 10.1073/pnas.41.12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldthwaite C. D., Cryer D. R., Marmur J. Effect of carbon source on the replication and transmission of yeast mitochondrial genomes. Mol Gen Genet. 1974;133(2):87–104. doi: 10.1007/BF00264830. [DOI] [PubMed] [Google Scholar]
- Hall R. M., Nagley P., Linnane A. W. Biogenesis of mitochondria. XLII. Genetic analysis of the control of cellular mitochondrial DNA levels in Saccharomyces cerevisiae. Mol Gen Genet. 1976 May 7;145(2):169–175. doi: 10.1007/BF00269590. [DOI] [PubMed] [Google Scholar]
- Heyting C., Talen J. L., Weijers P. J., Borst P. Fine structure of the 21S ribosomal RNA region on yeast mitochondrial DNA. II. The organization of sequences in petite mitochondrial DNAs carrying genetic markers from the 21S region. Mol Gen Genet. 1979 Jan 11;168(3):251–277. doi: 10.1007/BF00271497. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Michaelis G., Douglass S., Tsai M. J., Criddle R. S. Mitochondrial DNA and suppressiveness of petite mutants in Saccharomyces cerevisiae. Biochem Genet. 1971 Oct;5(5):487–495. doi: 10.1007/BF00487138. [DOI] [PubMed] [Google Scholar]
- Michaelis G., Petrochilo E., Slonimski P. P. Mitochondrial genetics. 3. Recombined molecules of mitochondrial DNA obtained from crosses between cytoplasmic petite mutants of Saccharomyces cerevisiae: physical and genetic characterization. Mol Gen Genet. 1973;123(1):51–65. doi: 10.1007/BF00282988. [DOI] [PubMed] [Google Scholar]
- Michel F., Grandchamp C., Dujon B. Genetic and physical characterization of a segment of yeast mitochondrial DNA involved in the control of genetic recombination. Biochimie. 1979;61(9):985–1010. doi: 10.1016/s0300-9084(80)80254-9. [DOI] [PubMed] [Google Scholar]
- Morimoto R., Merten S., Lewin A., Martin N. C., Rabinowitz M. Physical mapping of genes on yeast mitochondrial DNA: localization of antibiotic resistance loci, and rRNA and tRNA genes. Mol Gen Genet. 1978 Jul 25;163(3):241–255. doi: 10.1007/BF00271954. [DOI] [PubMed] [Google Scholar]
- Newlon C. S., Fangman W. L. Mitochondrial DNA synthesis in cell cycle mutants of Saccharomyces cerevisiae. Cell. 1975 Aug;5(4):423–428. doi: 10.1016/0092-8674(75)90061-6. [DOI] [PubMed] [Google Scholar]
- Perlman P. S., Birky C. W., Jr Mitochondrial genetics in Bakers' yeast: a molecular mechanism for recombinational polarity and suppressiveness. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4612–4616. doi: 10.1073/pnas.71.11.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Fangman W. L. Preferential synthesis of yeast mitochondrial DNA in alpha factor-arrested cells. Biochem Biophys Res Commun. 1973 Dec 10;55(3):603–609. doi: 10.1016/0006-291x(73)91186-8. [DOI] [PubMed] [Google Scholar]
- Rank G. H., Bech-Hansen N. T. Genetic evidence for "Darwinian" selection at the molecular level. 3. The effect of the suppressive factor on nuclearly and cytoplasmically inherited chloramphenicol resistance in S. cerevisiae. Can J Microbiol. 1972 Jan;18(1):1–7. doi: 10.1139/m72-001. [DOI] [PubMed] [Google Scholar]
- Rank G. H. Genetic evidence for 'Darwinian' selection at the molecular level. I. The effect of the suppressive factor on cytoplasmically-inherited erythromycin-resistance in Saccharomyces cerevisiae. Can J Genet Cytol. 1970 Mar;12(1):129–136. doi: 10.1139/g70-019. [DOI] [PubMed] [Google Scholar]
- Rank G. H. Genetic evidence for 'Darwinian' selection at the molecular level. II. Genetic analysis of cytoplasmically-inherited high and low suppressitivity in Saccharomyces cervisiae. Can J Genet Cytol. 1970 Jun;12(2):340–346. doi: 10.1139/g70-050. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanders J. P., Weijers P. J., Groot G. S., Borst P. Properties of mitochondrial DNA from Kluyveromyces lactis. Biochim Biophys Acta. 1974 Dec 6;374(2):136–144. doi: 10.1016/0005-2787(74)90357-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Dujon B., Slonimski P. P. Mitochondrial genetics. V. Multifactorial mitochondrial crosses involving a mutation conferring paromomycin-resistance in Saccharomyces cerevisiae. Mol Gen Genet. 1973 Sep 5;125(1):53–90. doi: 10.1007/BF00292983. [DOI] [PubMed] [Google Scholar]