Abstract
Whooping cough is a reemerging disease caused by two closely related pathogens, Bordetella pertussis and Bordetella parapertussis. The incidence of B. parapertussis in whooping cough cases has been increasing since the introduction of acellular pertussis vaccines containing purified antigens that are common to both strains. Recently published results demonstrated that these vaccines do not protect against B. parapertussis due to the presence of the O antigen on the bacterial surface that impairs antibody access to shared antigens. We have investigated the effect of the lack of opsonization of B. parapertussis on the outcome of its interaction with human neutrophils (polymorphonuclear leukocytes [PMNs]). In the absence of opsonic antibodies, PMN interaction with B. parapertussis resulted in nonbactericidal trafficking upon phagocytosis. A high percentage of nonopsonized B. parapertussis was found in nonacidic lysosome marker (lysosome-associated membrane protein [LAMP])-negative phagosomes with access to the host cell-recycling pathway of external nutrients, allowing bacterial survival as determined by intracellular CFU counts. The lipopolysaccharide (LPS) O antigen was found to be involved in directing B. parapertussis to PMN lipid rafts, eventually determining the nonbactericidal fate inside the PMN. IgG opsonization of B. parapertussis drastically changed this interaction by not only inducing efficient PMN phagocytosis but also promoting PMN bacterial killing. These data provide new insights into the immune mechanisms of hosts against B. parapertussis and document the crucial importance of opsonic antibodies in immunity to this pathogen.
INTRODUCTION
Whooping cough is a reemerging disease caused by two closely related pathogens, Bordetella pertussis and Bordetella parapertussis (11, 22). The exact contribution of each strain to the epidemiological situation is not yet certain, but recent studies have suggested that the incidence of B. parapertussis in whooping cough cases is high and increasing (7, 16). Whooping cough vaccines are still derived solely from B. pertussis. These vaccines were found to be less protective against B. parapertussis (8, 10, 40), eventually leading to a selective advantage of B. parapertussis over B. pertussis (3, 13, 17, 18). Accordingly, B. parapertussis has been found to cause larger proportions of whooping cough cases than before among vaccinated groups, with a significant increase in prevalence after the introduction of the acellular vaccines (4, 17, 19, 35). Although closely related (24), these two strains differ in the structure of their respective lipopolysaccharides (LPS) (2, 26). B. pertussis exhibits a lipooligosaccharide containing lipid A and a core oligosaccharide with a trisaccharide modification. However, due to a deletion of the wbm locus, B. pertussis LPS lacks the O antigen (26). B. parapertussis LPS is similar to B. pertussis LPS but lacks the trisaccharide modification and includes an O antigen (26, 27). According to previous studies, the O antigen is involved in the lack of protection of pertussis vaccines against B. parapertussis. In addition to conferring serum resistance (9), the O antigen interferes with the binding of antibodies induced by the cross-reactive antigens included in pertussis vaccines, preventing bacterial opsonization (39, 40). This shielding property seems particularly effective against antibodies induced by acellular B. pertussis vaccines. In agreement with in vitro findings, in vivo assays have shown antibodies induced by B. parapertussis but not by B. pertussis to be critical in preventing B. parapertussis colonization (40). Both B. parapertussis-induced antibodies and neutrophils were found necessary for immune elimination of this bacterium. Neither neutrophils nor antibodies by themselves seem to play a major role in the dynamics of the infection of naive mice by B. parapertussis. In the absence of either specific antibodies or polymorphonuclear leukocytes (PMNs), B. parapertussis is able to successfully colonize mice (38). Taken together, these findings suggest that bacterial clearance critically depends on cellular bactericidal activity mediated by opsonic antibodies and affected by PMNs. Despite its potential importance in the epidemiology of whooping cough, given the lack of efficient bacterial recognition of antibodies induced by pertussis vaccines, the innate interaction of phagocytes and B. parapertussis has not been fully investigated. Microbial pathogens, such as B. pertussis, can survive the encounter with PMNs by interfering with their attachment, phagocytosis, and trafficking to lysosomal compartments (14, 23, 30). In this study, we examined the outcome of the innate interaction of B. parapertussis with human PMNs, the role of the O antigen in this interaction, and the relevance of the Fc receptor (FcR) in the induction of Ig-triggered cellular effector functions against B. parapertussis.
MATERIALS AND METHODS
Bacterial strains and growth.
B. parapertussis strain CN2591, the isogenic B. parapertussis mutant strain lacking the O antigen, and strain CN2591Δwbm, previously described (1, 26), were used in this study. For phagocytosis experiments, these strains were transformed with plasmid pCW505 (kindly supplied by Alison Weiss, Cincinnati, OH), which induces cytoplasmic expression of green fluorescent protein (GFP) without affecting growth or antigen expression (36). Bacteria were stored at −70°C and recovered by growth on Bordet-Gengou agar (BGA) plates supplemented with 15% defibrinated sheep blood (bBGA) at 36°C. Virulent bacteria were subsequently plated on bBGA, cultured for 20 h at 36°C, and used in all experiments.
Antibodies.
We used polyclonal rabbit antibody against human flotillin-1 (Santa Cruz Biotechnology, Santa Cruz, CA), monoclonal antibody (MAb) against human lysosome-associated membrane protein 1 (LAMP-1) (Pharmingen, San Diego, CA), anti-hFcγRI (CD64) MAb 22 (mIgG1) (Medarex, Annandale, NJ), Cy3-conjugated goat F(ab′)2 fragments of anti-rabbit immunoglobulin and fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 fragments of anti-rabbit immunoglobulin (both from Jackson ImmunoResearch, West Grove, PA), Cy3-conjugated goat F(ab′)2 fragments of anti-mouse immunoglobulin and phycoerythrin (PE)-conjugated goat F(ab′)2 fragments of anti-rabbit IgG (both from Molecular Probes, Eugene, OR), and FITC-conjugated goat F(ab′)2 fragments of anti-mouse immunoglobulin (from Southern Biotechnology).
Immunoglobulin G (IgG) fractions from pooled sera of whooping cough patients with high titers of anti-B. parapertussis antibodies, as measured by enzyme-linked immunosorbent assay (37), were isolated as described previously (30). Polyclonal rabbit anti-B. parapertussis antiserum was obtained as described elsewhere (12).
Cells.
Peripheral blood polymorphonuclear leukocytes (PMNs) were isolated from heparinized venous blood using Ficoll-Histopaque (Sigma, St. Louis, MO) gradient centrifugation. PMNs were harvested, and the remaining erythrocytes were removed by hypotonic lysis. Cell viability was 99% as determined by trypan blue exclusion. Prior to functional assays, PMNs were washed twice with Dulbecco's modified Eagle medium (DMEM) (HyClone) supplemented with 10% heat-inactivated fetal calf serum (FCS) (HyClone), suspended, and used immediately. All experiments were carried out with freshly isolated PMNs lacking FcγRIa (CD64) expression, as monitored by fluorescence-activated cell sorter (FACS) analysis using a FACScalibur flow cytometer with anti-hFcγRI MAb 22 (mIgG1) (28). Data were processed using the CellQuest software (BD Biosciences).
Cholesterol sequestration of PMNs.
Cholesterol sequestration was achieved by incubating PMNs with 10 mg/ml of β-methyl cyclodextrin (Sigma) (15 min at 37°C) or 35 μg/ml of nystatin (Sigma) (30 min at 37°C) in serum-free DMEM plus bovine serum albumin (BSA) (0.2%) and lovastatin (5 μg/ml) (Sigma) (DMEM-BSA-L). Cells were then washed, suspended in DMEM-BSA-L, and used immediately. No decrease in PMN viability was detected after treatment.
Quantification of phagocytosis.
Bacterial phagocytosis was evaluated by confocal microscopy. PMNs were incubated with nonopsonized B. parapertussis at a multiplicity of infection (MOI) of 30 or 300 (bacteria per cell) for 15 min at 37°C to allow bacterial interaction with the PMNs. In select experiments, 200 ng/ml cytochalasin D (Sigma) was added to inhibit phagocytosis (34). PMNs were extensively washed to remove nonattached bacteria and further incubated for 1 h at 37°C. Phagocytosis was stopped by placing the PMNs on ice. Cells were then fixed using 4% paraformaldehyde. After fixation, the PMNs were washed once with phosphate-buffered saline (PBS) and incubated for 10 min at room temperature with PBS containing 50 mM NH4Cl. PMN surface-bound bacteria were detected by a two-step labeling procedure. The PMNs were incubated with polyclonal rabbit anti-B. parapertussis antiserum (30 min at 4°C), followed by incubation with Cy3-conjugated goat F(ab′)2 fragments of anti-rabbit immunoglobulin for another 30 min at 4°C. In order to determine the number of intracellular bacteria after two washing steps, the cells were permeabilized by incubation with PBS containing 0.1% saponin (Sigma-Aldrich) and 0.2% BSA for 30 min and further incubated for another 30 min with rabbit anti-B. parapertussis antiserum in the presence of 0.1% saponin and 0.2% BSA. After washing three times, the PMNs were incubated (30 min) with FITC-conjugated F(ab′)2 fragments of goat anti-rabbit IgG1. Labeling of the bacteria with FITC-conjugated antibodies was performed to minimize the loss of read-out sensitivity due to the quenching of GFP fluorescence after internalization. Finally, the cells were spun on microscope slides. Microscopic analyses were performed using a confocal laser scanning microscope (model TCS SP5; Leica, Germany). The number of extracellular (red and green fluorescent) and intracellular (green fluorescent) bacteria per cell was determined by microscopic examination of 20 randomly selected fields showing a minimum of 5 cells per field. In select experiments, B. parapertussis was opsonized with human IgG (200 μg/ml) prior to incubation with PMNs at an MOI of 30.
Respiratory burst determination.
Reactive oxygen species (ROS) production by PMNs was determined as described previously (32). Briefly, tubes containing 50 μl with 3 × 106 IgG-opsonized bacteria or 3 ×107 nonopsonized bacteria were transferred to a luminometer (Luminoskan TL Plus; Thermo Lab Systems, Finland), in which the chemoluminescence response of 105 PMNs was measured every minute for 30 min at 37°C, after the injection of 600 μl of 180 μM luminol (Sigma).
Confocal microscopy analysis.
Colocalization studies were performed as described previously (15) with minor modifications. Briefly, PMNs were incubated with nonopsonized wild-type B. parapertussis (MOI, 30 or 300), nonopsonized B. parapertussis Δwbm (MOI, 300), or IgG-opsonized B. parapertussis (MOI, 30) for 15 min at 37°C, washed to remove nonattached bacteria, and further incubated for another hour at 37°C. In select experiments, cell samples were taken every 20 min during a 2-h incubation at 37°C. The infected cells were incubated with or without 200 nM LysoTracker DND-99 (Molecular Probes) (5 min at 37°C), followed by fixation with paraformaldehyde. Those samples that were not incubated with LysoTracker stain were washed twice with PBS and incubated for 10 min at room temperature with PBS containing 50 mM NH4Cl. After two washing steps, the cells were incubated for 30 min with PBS containing 0.1% saponin (Sigma) and 0.2% BSA, followed by incubation with polyclonal rabbit anti-B. parapertussis antiserum (30 min at 4°C) in the presence of 0.1% saponin and 0.2% BSA. After three washing steps, the PMNs were incubated with FITC-conjugated goat F(ab′)2 fragments of anti-rabbit immunoglobulin for another 30 min at 4°C. Samples of infected cells incubated for 1 h at 37°C that were not incubated with LysoTracker stain were washed twice with PBS and incubated for 10 min at room temperature with PBS containing 50 mM NH4Cl. After two washing steps, the cells were incubated for 30 min with PBS containing 0.1% saponin (Sigma) and 0.5% BSA. Next, the cells were incubated for 30 min at 4°C with either mouse anti-human LAMP-1 monoclonal antibodies plus polyclonal rabbit anti-B. parapertussis antiserum or rabbit anti-human flotillin-1 plus polyclonal mouse anti-B. parapertussis antiserum antibodies (30 min at 4°C) in the presence of 0.1% saponin and 0.2% BSA. After three washing steps, the cells were incubated with either Cy3-conjugated F(ab′)2 fragments of goat anti-mouse antibodies plus FITC-conjugated goat F(ab′)2 fragments of anti-rabbit immunoglobulin or Cy3-conjugated F(ab′)2 fragments of goat anti-rabbit antibodies plus FITC-conjugated goat F(ab′)2 fragments of anti-mouse immunoglobulin for another 30 min at 4°C. To avoid cytophilic binding of antibodies to the FcγR, all incubations were done in the presence of 25% heat-inactivated human serum. Additionally, isotype controls were run in parallel.
Microscopic analyses were performed using a confocal laser scanning microscope (model TCS SP5; Leica, Germany). The percentage of phagosomes containing the bacterium that colocalized with a given marker was calculated from the number of total intracellular bacteria by analyzing at least 50 phagosomes per donor.
Transferrin uptake.
The uptake of transferrin by PMNs was assayed as described previously (33), with minor modifications. Briefly, PMNs were incubated with nonopsonized wild-type B. parapertussis (MOI, 300), nonopsonized B. parapertussis Δwbm (MOI, 300), or IgG-opsonized B. parapertussis (MOI, 30) for 15 min at 37°C, depleted of transferrin by incubation in DMEM containing 1% BSA for 1 h at 37°C, and further incubated for 10 min at 4°C with 10 μg/ml Alexa transferrin-594 (Molecular Probes) in an excess of BSA (1%) to saturate nonspecific endocytosis. Next, the cells were incubated for 5 min at 37°C to allow internalization of the ligand, washed with DMEM containing 1% BSA, and further incubated for another 45 min at 37°C. Finally, the cells were fixed, and microscopic analyses were performed using a confocal laser scanning microscope. At least 50 bacteria per donor were analyzed for colocalization with transferrin in each experiment.
Killing assay.
PMNs were incubated with nonopsonized wild-type B. parapertussis (MOI, 300), nonopsonized B. parapertussis Δwbm (MOI, 300), or IgG-opsonized B. parapertussis (MOI, 30). Bacterial inocula were quantified by plating appropriate dilutions on bBGA. After 15 min of incubation at 37°C with 5% CO2, nonadherent bacteria were removed through three washing steps. Then, 100 μg/ml polymyxin B sulfate (Sigma) was added for 1 h to kill the extracellular bacteria (5). The PMNs were washed, and B. parapertussis intracellular survival was determined as follows. Infected PMNs were pelleted, and the number of viable eukaryotic cells was determined by trypan blue dye exclusion. Next, the PMNs were lysed with 0.1% saponin in sterile water, and serial dilutions of lysates were rapidly plated onto bBGA to enumerate the CFU. Control experiments to assess bacterial phagocytosis under each condition by confocal microscopy were run in parallel to be used to calculate the percentage of intracellular bacteria that were still alive 1 h after phagocytosis. Additionally, control experiments to assess the efficacy of antibiotic bactericidal activity were also performed in parallel. Briefly, samples of 5 × 108 bacteria were incubated with antibiotics for 1 h at 37°C and plated on bBGA. This resulted in a 99.999% decrease in the CFU. No significant differences in bacterial sensitivity to the antibiotics were detected among the strains tested, either opsonized or nonopsonized. Additionally, the number of CFU in the cell culture supernatants was examined. No viable bacteria were detected at any time postinfection.
Statistical analysis.
Student's t test (95% confidence level) or analysis of variance (ANOVA) was used for statistical data evaluation. The significance of the differences between the mean values of the data evaluated by ANOVA was determined with the least-significant-difference (LSD) test at a 95% confidence level. Results are shown as means and standard deviations (SD).
RESULTS
The O antigen is involved in PMN phagocytosis of nonopsonized B. parapertussis.
The LPS O antigen is considered a virulence determinant for other Gram-negative bacteria because, among other reasons, it shapes bacterial uptake by immune cells (21). In this study, we evaluated the role of LPS O antigen in PMN phagocytosis of B. parapertussis. We used two-color confocal microscopy in order to quantify the actual number of bacteria associated with PMNs while discriminating between intracellular and extracellular bacteria. Figure 1A shows that the O antigen of B. parapertussis affects bacterial interaction with human PMNs. PMN uptake of a B. parapertussis mutant defective in the O antigen was significantly higher than that of the wild-type strain. In order to investigate whether the O antigen of B. parapertussis is involved in bacterial attachment or bacterial internalization, we evaluated the interaction of B. parapertussis and a B. parapertussis mutant defective in O antigen with human PMNs in the presence of cytochalasin D, an inhibitor of microfilament polymerization. The differences shown in Fig. 1B resemble the differences shown in Fig. 1A. These results, together with the finding that after 1 h at 37°C most of the bacteria were found inside the cell in both cases (data not shown), suggest that the O antigen is implicated mainly in bacterial attachment, which might indicate different PMN targets for O antigen-expressing and O antigen-lacking B. parapertussis.
Fig 1.
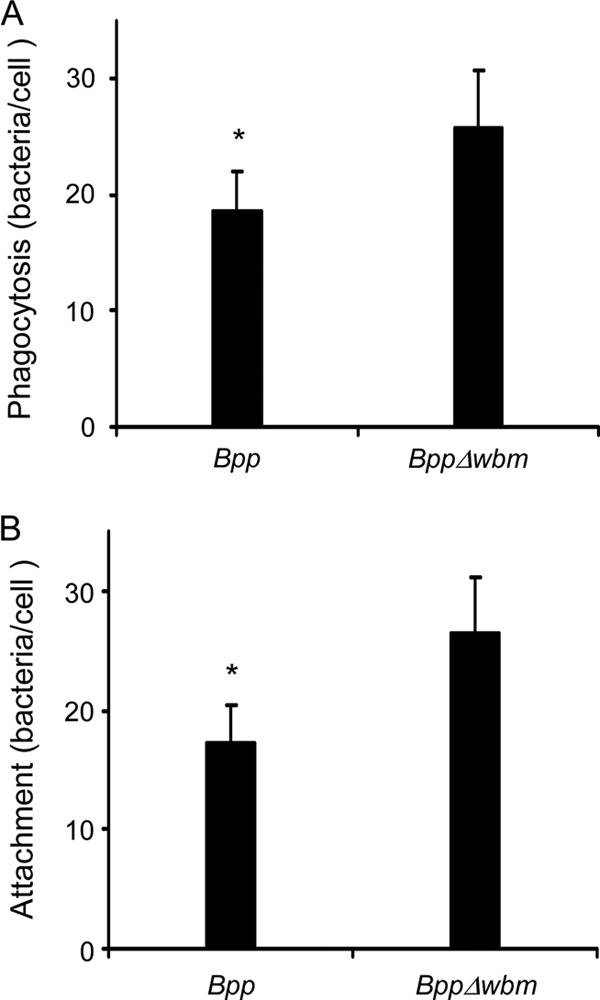
PMN attachment and phagocytosis of Bordetella parapertussis. (A) PMN phagocytosis of wild-type B. parapertussis (Bpp) and O antigen-deficient B. parapertussis (BppΔwbm). The bacteria were incubated with PMNs (MOI, 300) for 15 min at 37°C. After attachment, the PMNs were washed and further incubated for 1 h at 37°C to allow internalization. The cells were fixed and permeabilized prior to labeling the intracellular bacteria with green fluorescent dye and the extracellular bacteria with both green and red fluorescent dyes. Bacterial phagocytosis was assessed by confocal laser scan fluorescence microscopy. To assess the number of phagocytosed bacteria, at least 100 cells were counted per slide. The data represent the means ± SD of four experiments with PMNs from different donors. Phagocytosis of nonopsonized B. parapertussis by PMNs differed significantly from the PMN phagocytosis of B. parapertussis Δwbm. The asterisk indicates a P value of <0.05. (B) PMN attachment of B. parapertussis. Wild-type B. parapertussis and O antigen-deficient B. parapertussis were incubated with PMNs (MOI, 300) in the presence of cytochalasin D for 15 min at 37°C. PMNs were then washed and further incubated for 1 h at 37°C in the presence of cytochalasin D. The cells were fixed and permeabilized prior to labeling the intracellular bacteria in green fluorescent dye and the extracellular bacteria with both green and red fluorescent dyes. Bacterial attachment was assessed by confocal laser scan fluorescence microscopy. To assess the number of PMN-attached bacteria, at least 100 cells per slide were counted. No bacteria exhibiting only green fluorescence were observed in any cells after 1 h of incubation at 37°C, indicating that phagocytosis was efficiently blocked. The data represent the means ± SD of four experiments with PMNs from different donors. The attachment of B. parapertussis by PMNs differed significantly from PMN attachment of B. parapertussis Δwbm. The asterisk indicates a P value of <0.05.
LPS O antigen-mediated interaction of B. parapertussis with PMNs delivers the bacteria to compartments that do not fuse with lysosomes.
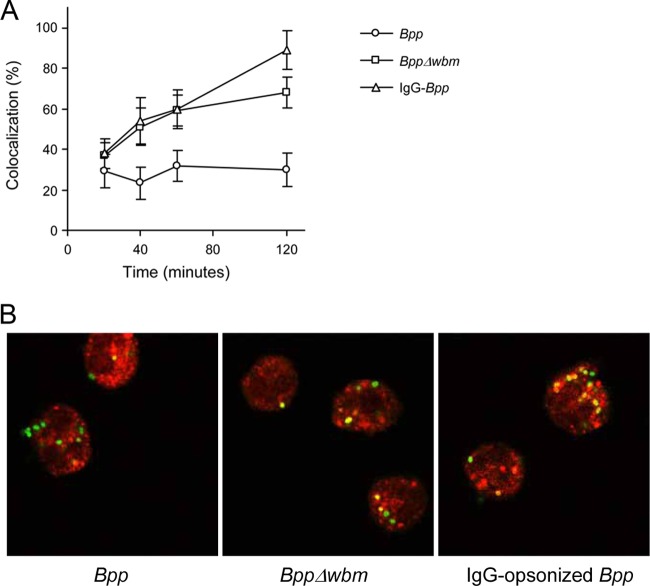
O antigen-mediated phagocytosis was found previously to be associated with the delivery of bacteria through a pathway that avoids phagosome-lysosome fusion, eventually preventing cellular bactericidal activity (25). The intracellular routing of nonopsonized B. parapertussis was investigated by confocal microscopy. We first evaluated nonopsonized B. parapertussis colocalization with the acidotropic LysoTracker dye at different time points postinfection. Figure 2 shows that 1 h after infection, most B. parapertussis-containing vacuoles were nonacidic, as attested by the lack of accumulation of the acidotropic dye in 63% ± 4% of the Bordetella-containing phagosomes. Importantly, this percentage did not change at later time points (Fig. 2).
Fig 2.
PMN intracellular trafficking of B. parapertussis. Nonopsonized B. parapertussis (Bpp), nonopsonized B. parapertussis Δwbm, or IgG-opsonized B. parapertussis were incubated with human PMNs (MOI, 300 for nonopsonized bacteria and 30 for IgG-opsonized bacteria) for 15 min at 37°C. After washing, samples were taken at different time points, and bacterially infected PMNs were incubated with LysoTracker and fixed prior to being subjected to confocal microscopic analysis. (A) Time course of the respective percentages of LysoTracker-positive bacteria. The data represent the means ± SD of three independent experiments. (B) PMNs with green fluorescent bacteria 1 h after infection. Colocalization is reflected by the yellow areas. Representative confocal microscopy images of one of three independent experiments are shown.
We next investigated the relevance of the O antigen in this transportation to nonacidic compartments. Figure 2 shows that the percentage of O antigen-deficient B. parapertussis found in LysoTracker-positive phagosomes was significantly higher than that observed in the nonopsonized wild-type strain, indicating that the O antigen is involved in the ability of B. parapertussis to impair phagolysosome maturation.
We then evaluated whether the intracellular fate of B. parapertussis could be modified by bacterial opsonization with specific antibodies. PMN uptake of B. parapertussis drastically increased in the presence of opsonins (data not shown). The MOI had to be reduced 10 times in order to achieve a level of infection comparable to that obtained with nonopsonized bacteria. Figure 2B shows that IgG-opsonized bacteria were mainly transported to acidic organelles, indicating that the ability to impair phagolysosome maturation was abrogated by IgG opsonization of the bacteria. In order to investigate whether the multiplicity of infection was involved in the differences in intracellular trafficking, we evaluated the fate of nonopsonized B. parapertussis incubated with PMNs at the MOI used for opsonized bacteria (MOI, 30). Although a lower number of bacteria were found inside human PMNs, the results showed that most intracellular bacteria were not colocalized with LysoTracker (data not shown).
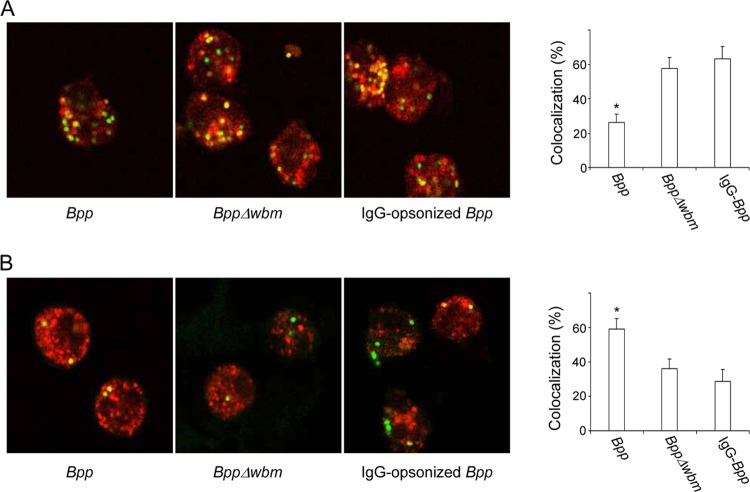
The characteristics of the B. parapertussis-containing compartments were then investigated. As can be seen in Fig. 3A and consistent with the low percentage of acidic phagosomes found 1 h postinfection (Fig. 2), nonopsonized B. parapertussis-containing phagosomes were mainly negative for the late-endosomal and -lysosomal marker LAMP-1 at this time point. These results suggest that those bacteria that successfully evaded transportation to acidic compartments eventually remained in the phagosomes lacking lysosomal or late endosomal characteristics. In agreement with the higher volume of bacterial trafficking to lysosomal compartments shown in Fig. 2, IgG opsonization as well as the lack of O antigen resulted in a significant increase in bacterial colocalization with the late-endosomal and -lysosomal marker LAMP-1 (Fig. 3A).
Fig 3.
Confocal laser scanning fluorescence microscopic analyses of B. parapertussis colocalization with LAMP-1 and transferrin. Nonopsonized B. parapertussis (Bpp), nonopsonized B. parapertussis Δwbm, or IgG-opsonized B. parapertussis was incubated with human PMNs (MOI, 300 for nonopsonized bacteria and 30 for IgG-opsonized bacteria) for 15 min at 37°C. After washing, the bacterially infected PMNs were incubated for 1 hour more at 37°C and fixed and permeabilized prior to incubation with antibodies against LAMP-1 (A) or incubated with Alexa transferrin-594 before fixing (B). Shown are green fluorescent bacteria inside PMNs. Colocalization is reflected by the yellow areas. The bars indicate the respective percentages of LAMP-1-positive or transferrin-positive phagosomes. The data represent the means ± SD of three independent experiments. Representative confocal microscopy images of one of three independent experiments are shown. The asterisks indicate significance (P < 0.05).
Since most of the nonopsonized B. parapertussis bacteria were found outside late-endosomal and -lysosomal compartments, they might have access to nutrients via the recycling pathway. In order to evaluate this issue, we pulsed the PMNs with Alexa transferrin-594 to assess the bacterial access to extracellular material. As can be seen in Fig. 3B, 59% ± 5% of nonopsonized B. parapertussis-containing phagosomes were positive for transferrin 1 h postinfection, suggesting that these bacteria are located in vacuoles in which they have access to recycling endosomes. Consistent with the higher percentage of bacteria located in lysosomal compartments (Fig. 2), the percentage of colocalization of both opsonized B. parapertussis and the O antigen-deficient B. parapertussis mutant with transferrin was significantly lower than that observed for nonopsonized B. parapertussis (Fig. 3B).
The LPS O antigen precludes PMN activation during interaction with nonopsonized B. parapertussis.
As the differences in intracellular bacterial trafficking might be indicating differences in the PMN activation state, we evaluated the effect of the O antigen on the respiratory burst response of PMNs. As shown in Fig. 4, nonopsonized O antigen-deficient B. parapertussis induced a significant respiratory burst response in human PMNs, but no activity was detected with the nonopsonized wild-type strain, suggesting that the O antigen prevents PMN activation. As expected, IgG-opsonized B. parapertussis induced a high respiratory burst response in PMNs (Fig. 4).
Fig 4.
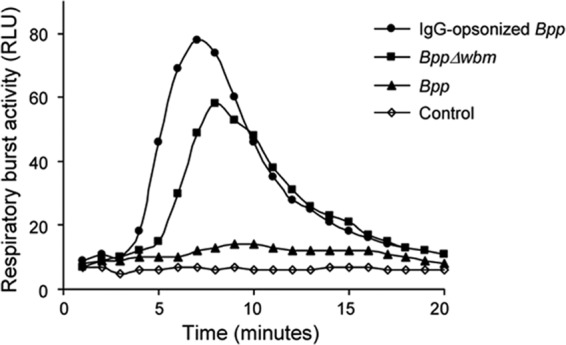
Bacterial induction of PMN respiratory burst. Nonopsonized B. parapertussis (Bpp), nonopsonized B. parapertussis Δwbm, or IgG-opsonized B. parapertussis was incubated with PMNs (MOI, 30 for opsonized bacteria and 300 for nonopsonized bacteria) and luminol at 37°C. PMNs incubated with buffer and luminol served as a control. Chemiluminescence responses were measured every minute for 20 min. Data are representative of four independent experiments. RLU, relative luminometric units.
PMN intracellular survival of B. parapertussis depends on the O antigen.
Given that the lack of opsonization seems to favor intracellular trafficking of B. parapertussis to nonbactericidal compartments, and no PMN activation was detected in the absence of opsonins, we next investigated the intracellular survival of B. parapertussis upon interaction with PMNs in both the presence and the absence of opsonic antibodies. Nonopsonized B. parapertussis survival upon phagocytosis was significantly higher than opsonized bacterial survival (Fig. 5). Moreover, in agreement with the confocal microscopy studies and the induction of PMN ROS production described above, the lack of expression of the O antigen led to a significant reduction of intracellular survival of nonopsonized B. parapertussis, confirming that the O antigen is involved in innate PMN-bacterium interaction, eventually decreasing cellular bacterial killing.
Fig 5.
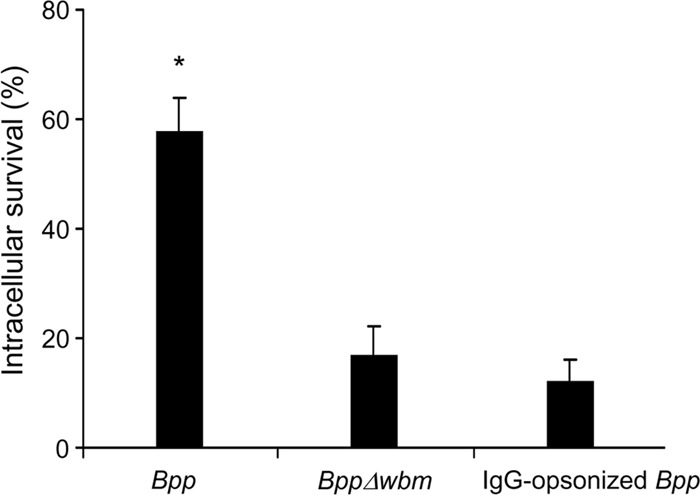
B. parapertussis survival in human PMNs. Nonopsonized B. parapertussis (Bpp), nonopsonized B. parapertussis Δwbm, or IgG-opsonized B. parapertussis was incubated with human PMNs (MOI, 300 for nonopsonized bacteria and 30 for IgG-opsonized bacteria) for 15 min at 37°C, washed, and further incubated with polymyxin B to kill the extracellular bacteria. The number of CFU of B. parapertussis per cell was determined. Bacterial killing was expressed as the percentage of intracellular bacteria that were still alive 1 h after phagocytosis. The data represent the means ± SD of three independent experiments. The number of viable intracellular nonopsonized B. parapertussis per cell was significantly different from the number of viable intracellular opsonized B. parapertussis and O antigen-deficient B. parapertussis (the asterisk indicates a P value of <0.05).
Lipid raft domains are involved in O antigen-dependent B. parapertussis survival to PMN phagocytosis.
LPS O antigen was found previously to be involved in directing the pathogen through an endocytic pathway that avoids fusion with lysosomes by targeting the host cell lipid rafts (25). Since our results suggested an essential role of the O side chain in avoiding the lysosomal pathway and bacterial killing, we next investigated the role of cholesterol-rich domains in the innate interaction of PMNs and nonopsonized B. parapertussis. Treatment of PMNs with β-methyl cyclodextrin, a compound that disrupts cholesterol-rich domains by extracting cholesterol (29), led to a significant decrease in the level of phagocytosis of nonopsonized B. parapertussis.
The experiments were performed in the presence of lovastatin to inhibit de novo cholesterol synthesis. Similar results were obtained with PMNs incubated with nystatin, a cholesterol-binding drug (Fig. 6). Conversely, phagocytosis of O antigen-defective B. parapertussis to PMNs did not change significantly after the incubation of PMNs with either nystatin or β-methyl cyclodextrin (Fig. 6), indicating that the O antigen is involved in directing B. parapertussis to cholesterol-rich domains.
Fig 6.
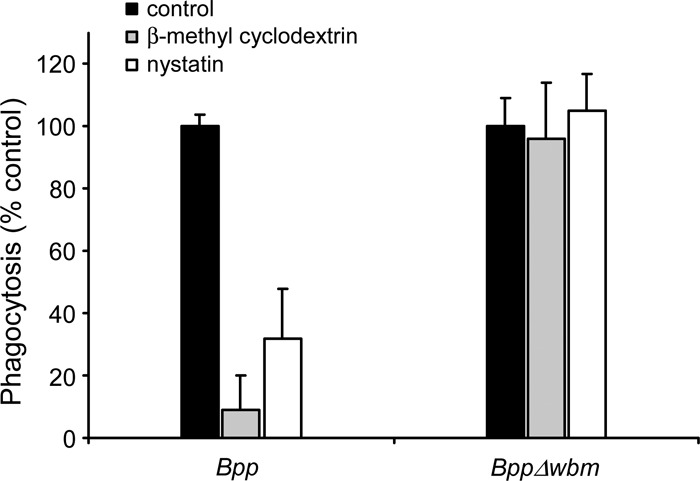
Effect of β-methyl cyclodextrin and nystatin on PMN phagocytosis of B. parapertussis. Nonopsonized B. parapertussis (Bpp) and nonopsonized B. parapertussis Δwbm were treated with or without cholesterol-depleting (β-methyl cyclodextrin, 10 mg/ml) or cholesterol-binding (nystatin, 35 μg/ml) drugs prior to incubation with nonopsonized B. parapertussis or nonopsonized B. parapertussis Δwbm (both at MOIs of 300) for 15 min at 37°C. After attachment, the PMNs were washed and further incubated for 1 h at 37°C to allow internalization. The cells were fixed and permeabilized prior to labeling the intracellular bacteria with green fluorescent dye and the extracellular bacteria with both green and red fluorescent dyes. To assess the number of phagocytosed bacteria, at least 100 cells per slide were counted. For each strain, the control (100% value) was obtained with untreated PMNs. The data represent the means ± SD of four experiments with PMNs from different donors. Phagocytosis of nonopsonized B. parapertussis by β-methyl cyclodextrin or nystatin-treated PMNs was significantly different from phagocytosis by untreated PMNs (P < 0.05).
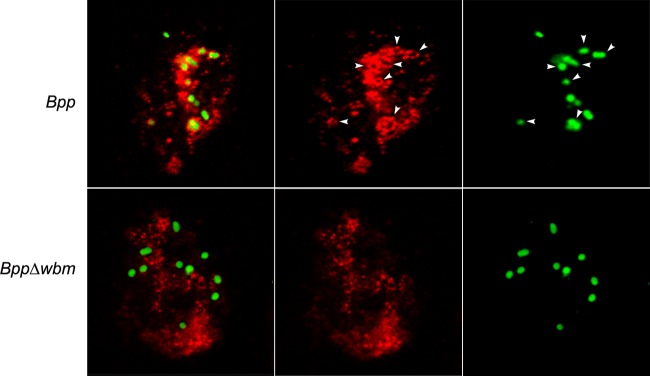
We further investigated this association by confocal microscopy using a lipid raft marker, flotillin. Around 80% of the wild type but not the O antigen-defective B. parapertussis mutant bacteria were found colocalizing with flotillin-enriched areas (Fig. 7), confirming the involvement of lipid rafts in the interaction of B. parapertussis with PMNs.
Fig 7.
B. parapertussis colocalization with flotillin. Nonopsonized B. parapertussis (Bpp) or nonopsonized B. parapertussis Δwbm was incubated with human PMNs (MOI, 300) for 15 min at 37°C. After washing, the bacteria-infected PMNs were incubated for 1 hour more at 37°C, fixed, and permeabilized prior to incubation with antibodies against flotillin-1. Colocalization is reflected by green-fluorescent B. parapertussis surrounded by Cy3-labeled flotillin (indicated by arrowheads). Representative panels of one of three independent experiments are shown.
Next, we examined whether the entry of B. parapertussis through the lipid rafts is required for bacterial survival of PMN phagocytosis. Treatment of PMNs with β-methyl cyclodextrin led to a significant reduction of intracellular survival of nonopsonized wild-type B. parapertussis, whereas the survival of the O antigen-defective mutant of B. parapertussis was unaffected, indicating that the uptake of B. parapertussis through cholesterol-rich domains decreases cellular bacterial killing. Similar results were obtained with PMNs incubated with nystatin (Fig. 8).
Fig 8.
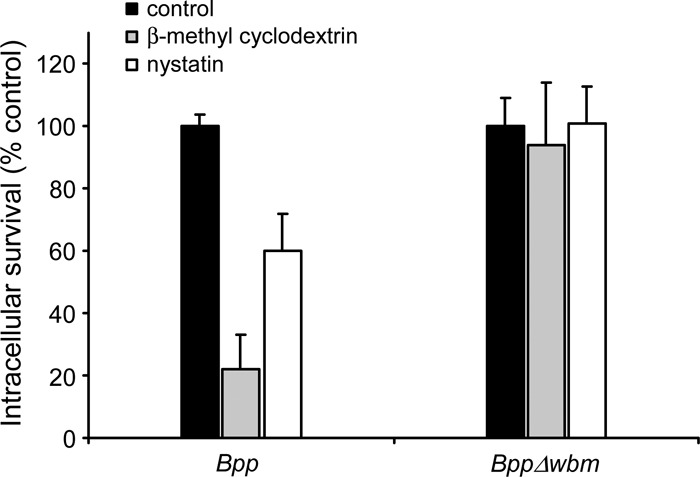
Effect of β-methyl cyclodextrin and nystatin on intracellular survival of B. parapertussis inside PMNs. Nonopsonized B. parapertussis (Bpp) and nonopsonized B. parapertussis Δwbm were treated with or without cholesterol-depleting (β-methyl cyclodextrin, 10 mg/ml) or cholesterol-binding (nystatin, 35 μg/ml) drugs prior to incubation with nonopsonized B. parapertussis or nonopsonized B. parapertussis Δwbm (both at MOIs of 300) for 15 min at 37°C, washed, and further incubated with polymyxin B to kill the extracellular bacteria. The number of CFU of B. parapertussis per cell was determined. The intracellular survival rate was calculated considering the number of phagocytosed bacteria under each condition. For each strain, the control (100% value) was obtained with untreated PMNs. Data represent the means ± SD of three independent experiments. The B. parapertussis intracellular survival rate in PMNs treated with either β-methyl cyclodextrin or nystatin was significantly different from the intracellular survival rate in untreated PMNs (P < 0.05).
DISCUSSION
B. parapertussis is a successful pathogen that has persisted within vaccinated populations for decades. A substantial mass of evidence shows that the contribution of B. parapertussis infection to whooping cough has been increasing over the last years (7, 17, 35). Recent surveys have shown that the growing incidence of this pathogen roughly coincides with the introduction of the acellular vaccine. Whooping cough vaccines have little, if any, efficacy against this bacterium (8, 10, 17, 40). Acellular vaccines were found particularly ineffective. Recent studies have shown that the O antigen is responsible for the lack of cross protection between these two strains. O antigen shields B. parapertussis from B. pertussis vaccine-induced antibodies, preventing antibody-mediated phagocytosis in vitro and antibody-mediated clearance in vivo (40). The ability of professional phagocytes to ingest and kill microorganisms is central to innate immunity and host defense. Many persistent pathogens, B. pertussis among them, have evolved strategies to avoid killing by immune cells (14, 23, 31). Since current pertussis vaccines fail to induce opsonic activity against B. parapertussis, the outcome of the innate interaction of this pathogen with human PMNs might be of particular relevance in understanding whooping cough epidemiology. The data shown here demonstrate that in the absence of opsonic antibodies, PMN uptake of B. parapertussis does not result in effective cellular bactericidal activity. Unlike bacterial phagocytosis via the FcR, in the absence of antibodies B. parapertussis seems able to disrupt normal endosomal maturation and fusion with lysosomes. A high percentage of nonopsonized bacteria exhibits nonbactericidal trafficking inside the PMNs once phagocytosed. Our data indicate that B. parapertussis is able to inhibit its own trafficking to lysosomes and remain viable inside the immune cell. The LPS O antigen was found to be involved in this immune evasion mechanism. Our results showed that the O antigen not only reduces B. parapertussis uptake by PMNs but is also involved in precluding phagolysosome maturation. A B. parapertussis mutant strain lacking the expression of the O antigen showed a significant increase in PMN uptake and in the percentage of colocalization with lysosomal markers 1 h after phagocytosis. Accordingly, the O antigen-deficient mutant, unlike the wild-type strain of B. parapertussis, activated the PMNs and was more efficiently killed by the immune cell. The O antigen of other bacterial pathogens has been found to be involved in phagolysosome fusion inhibition by targeting host cell lipid rafts. Bacterial raft association usually generates a phagosome enriched in raft components which eventually preclude phagolysosome fusion (20). Our results suggest that a similar mechanism might underlie the O antigen dependency of B. parapertussis survival to the native interaction with the PMNs. Uptake of the wild-type but not the O antigen-deficient mutant of B. parapertussis was found to be dependent on intact lipid rafts. O antigen dependency of bacterial association with these cholesterol-rich domains was confirmed by confocal microscopy using a lipid raft marker, flotillin. B. parapertussis wild type but not the O antigen-deficient mutant was found colocalizing with flotillin-enriched areas. Importantly, B. parapertussis survival was found to be dependent on the presence of both the O antigen and the intact lipid rafts. The lack of O antigen, or PMN treatment with raft-disrupting drugs, significantly increased B. parapertussis killing by the immune cell. Our results suggest a key role of the PMN lipid raft in the entry and survival of B. parapertussis under nonopsonic conditions and a central role of the O side chain of the LPS in directing the bacteria to these lipid platforms. Taken together, these data identify the O antigen expression as an innate immune evasion mechanism during B. parapertussis infection. These results might explain the dependence on O antigen for B. parapertussis respiratory tract colonization (6). Interestingly, although both B. parapertussis and B. pertussis (14) strains are able to survive the interaction with this aggressive immune cell, only B. parapertussis seems to critically depend on the O antigen. B. pertussis lacks this LPS side chain, which may reflect O antigen-independent host-bacterium interactions that are unique to B. pertussis.
B. parapertussis uptake by PMNs, ROS production, and bactericidal activity drastically increase in the presence of opsonic antibodies. Bacterial phagocytosis mediated by FcR led to a significant enhancement in the proportion of bacteria colocalizing with lysosomal markers 1 h after phagocytosis, eventually leading to efficient bacterial killing. In fact, the presence of antibodies increases the overall bacterial killing 200 times. These results are in agreement with previous in vivo data showing the critical role of both antibodies and PMNs, but not PMNs alone (38), for B. parapertussis clearance and underline the critical importance of the presence of opsonic antibodies at the site of infection.
In summary, according to previous data and the data presented here, the O antigen protects B. parapertussis not only from B. pertussis vaccine or B. pertussis infection-induced antibodies (39, 40) but also from being killed by innate interaction with PMNs in nonimmune hosts. These results reinforce prior calls to consider inclusion of the O antigen in whooping cough vaccines.
ACKNOWLEDGMENTS
This study was supported by MINCyT-FONCyT grants PICT 0559 and PICT 0413 (to M.E.R.) and NIH grant GM083113 (to E.T.H.).
M.E.R. and Y.L. are members of the Scientific Career of the CONICET, J.G. is a doctoral fellow of the MINCyT, and H.V. is a postdoctoral fellow of the CONICET.
Footnotes
Published ahead of print 1 October 2012
REFERENCES
- 1. Allen A, Maskell D. 1996. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol. Microbiol. 19:37–52 [DOI] [PubMed] [Google Scholar]
- 2. Allen AG, Thomas RM, Cadisch JT, Maskell DJ. 1998. Molecular and functional analysis of the lipopolysaccharide biosynthesis locus wlb from Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Mol. Microbiol. 29:27–38 [DOI] [PubMed] [Google Scholar]
- 3. Bokhari H, et al. 2011. Molecular typing of Bordetella parapertussis isolates circulating in Pakistan. FEMS Immunol. Med. Microbiol. 63:373–380 [DOI] [PubMed] [Google Scholar]
- 4. Borska K, Simkovicova M. 1972. Studies on the circulation of Bordetella pertussis and Bordetella parapertussis in populations of children. J. Hyg. Epidemiol. Microbiol. Immunol. 16:159–172 [PubMed] [Google Scholar]
- 5. Brockmeier SL, Register KB. 2000. Effect of temperature modulation and bvg mutation of Bordetella bronchiseptica on adhesion, intracellular survival and cytotoxicity for swine alveolar macrophages. Vet. Microbiol. 73:1–12 [DOI] [PubMed] [Google Scholar]
- 6. Burns VC, Pishko EJ, Preston A, Maskell DJ, Harvill ET. 2003. Role of Bordetella O antigen in respiratory tract infection. Infect. Immun. 71:86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cherry JD, Seaton BL. 2012. Patterns of Bordetella parapertussis respiratory illnesses: 2008–2010. Clin. Infect. Dis. 54:534–537 [DOI] [PubMed] [Google Scholar]
- 8. David S, van Furth R, Mooi FR. 2004. Efficacies of whole cell and acellular pertussis vaccines against Bordetella parapertussis in a mouse model. Vaccine 22:1892–1898 [DOI] [PubMed] [Google Scholar]
- 9. Goebel EM, Wolfe DN, Elder K, Stibitz S, Harvill ET. 2008. O antigen protects Bordetella parapertussis from complement. Infect. Immun. 76:1774–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He Q, Viljanen MK, Arvilommi H, Aittanen B, Mertsola J. 1998. Whooping cough caused by Bordetella pertussis and Bordetella parapertussis in an immunized population. JAMA 280:635–637 [DOI] [PubMed] [Google Scholar]
- 11. Heininger U, et al. 1994. Clinical characteristics of illness caused by Bordetella parapertussis compared with illness caused by Bordetella pertussis. Pediatr. Infect. Dis. J. 13:306–309 [DOI] [PubMed] [Google Scholar]
- 12. Hellwig SM, et al. 2001. Targeting to Fcγ receptors, but not CR3 (CD11b/CD18), increases clearance of Bordetella pertussis. J. Infect. Dis. 183:871–879 [DOI] [PubMed] [Google Scholar]
- 13. Kurova N, Njamkepo E, Brun D, Tseneva G, Guiso N. 2010. Monitoring of Bordetella isolates circulating in Saint Petersburg, Russia between 2001 and 2009. Res. Microbiol. 161:810–815 [DOI] [PubMed] [Google Scholar]
- 14. Lamberti Y, Perez Vidakovics ML, van der Pol LW, Rodriguez ME. 2008. Cholesterol-rich domains are involved in Bordetella pertussis phagocytosis and intracellular survival in neutrophils. Microb. Pathog. 44:501–511 [DOI] [PubMed] [Google Scholar]
- 15. Lamberti YA, Hayes JA, Perez Vidakovics ML, Harvill ET, Rodriguez ME. 2010. Intracellular trafficking of Bordetella pertussis in human macrophages. Infect. Immun. 78:907–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lavine J, Broutin H, Harvill ET, Bjornstad ON. 2010. Imperfect vaccine-induced immunity and whooping cough transmission to infants. Vaccine 29:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liese JG, Renner C, Stojanov S, Belohradsky BH. 2003. Clinical and epidemiological picture of B. pertussis and B. parapertussis infections after introduction of acellular pertussis vaccines. Arch. Dis. Child. 88:684–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Long GH, Karanikas AT, Harvill ET, Read AF, Hudson PJ. 2010. Acellular pertussis vaccination facilitates Bordetella parapertussis infection in a rodent model of bordetellosis. Proc. Biol. Sci. 277:2017–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maixnerova M. 2003. The 2001 serological survey in the Czech Republic—parapertussis. Cent. Eur. J. Public Health 11(Suppl):S23–S24 [PubMed] [Google Scholar]
- 20. Mañes S, del Real G, Martinez-AC 2003. Pathogens: raft hijackers. Nat. Rev. Immunol. 3:557–568 [DOI] [PubMed] [Google Scholar]
- 21. Matiasovic J, et al. 2011. Influence of the lipopolysaccharide structure of Salmonella enterica serovar Enteritidis on interactions with pig neutrophils. Vet. Microbiol. 150:167–172 [DOI] [PubMed] [Google Scholar]
- 22. Mattoo S, Cherry JD. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18:326–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moulder JW. 1985. Comparative biology of intracellular parasitism. Microbiol. Rev. 49:298–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parkhill J, et al. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35:32–40 [DOI] [PubMed] [Google Scholar]
- 25. Porte F, Naroeni A, Ouahrani-Bettache S, Liautard JP. 2003. Role of the Brucella suis lipopolysaccharide O antigen in phagosomal genesis and in inhibition of phagosome-lysosome fusion in murine macrophages. Infect. Immun. 71:1481–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Preston A, et al. 1999. Genetic basis for lipopolysaccharide O antigen biosynthesis in Bordetellae. Infect. Immun. 67:3763–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Preston A, et al. 2006. Complete structures of Bordetella bronchiseptica and Bordetella parapertussis lipopolysaccharides. J. Biol. Chem. 281:18135–18144 [DOI] [PubMed] [Google Scholar]
- 28. Repp R, et al. 1991. Neutrophils express the high affinity receptor for IgG (Fcγ RI, CD64) after in vivo application of recombinant human granulocyte colony-stimulating factor. Blood 78:885–889 [PubMed] [Google Scholar]
- 29. Rodal SK, et al. 1999. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10:961–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodriguez ME, et al. 2001. Fc receptor-mediated immunity against Bordetella pertussis. J. Immunol. 167:6545–6551 [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez ME, van der Pol WL, Sanders LA, van de Winkel JG. 1999. Crucial role of FcγRIIa (CD32) in assessment of functional anti-Streptococcus pneumoniae antibody activity in human sera. J. Infect. Dis. 179:423–433 [DOI] [PubMed] [Google Scholar]
- 32. Schmitz FJ, Veldkamp KE, Van Kessel KP, Verhoef J, Van Strijp JA. 1997. Delta-toxin from Staphylococcus aureus as a costimulator of human neutrophil oxidative burst. J. Infect. Dis. 176:1531–1537 [DOI] [PubMed] [Google Scholar]
- 33. Sturgill-Koszycki S, Schaible UE, Russell DG. 1996. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 15:6960–6968 [PMC free article] [PubMed] [Google Scholar]
- 34. van den Herik-Oudijk IE, Capel PJA, van der Bruggen T, van de Winkel JGJ. 1995. Identification of signaling motifs within human Fc_RIIa and Fc_RIIb isoforms. Blood 85:2202–2211 [PubMed] [Google Scholar]
- 35. Watanabe M, Nagai M. 2004. Whooping cough due to Bordetella parapertussis: an unresolved problem. Expert Rev. Anti Infect. Ther. 2:447–454 [DOI] [PubMed] [Google Scholar]
- 36. Weingart CL, et al. 1999. Fluorescent labels influence phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 67:4264–4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wolfe DN, Goebel EM, Bjornstad ON, Restif O, Harvill ET. 2007. The O antigen enables Bordetella parapertussis to avoid Bordetella pertussis-induced immunity. Infect. Immun. 75:4972–4979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolfe DN, Kirimanjeswara GS, Harvill ET. 2005. Clearance of Bordetella parapertussis from the lower respiratory tract requires humoral and cellular immunity. Infect. Immun. 73:6508–6513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang X, Goebel EM, Rodriguez ME, Preston A, Harvill ET. 2009. The O antigen is a critical antigen for the development of a protective immune response to Bordetella parapertussis. Infect. Immun. 77:5050–5058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang X, Rodriguez ME, Harvill ET. 2009. O antigen allows B. parapertussis to evade B. pertussis vaccine-induced immunity by blocking binding and functions of cross-reactive antibodies. PLoS One 4:e6989 doi:10.1371/journal.pone.0006989 [DOI] [PMC free article] [PubMed] [Google Scholar]