Abstract
An understanding of the immunogenetic basis of naturally acquired immunity to Plasmodium falciparum infection would aid in the designing of a rationally based malaria vaccine. Variants within the Fc gamma receptors (FcγRs) mediate immunity through engagement of immunoglobulin G and other immune mediators, such as gamma interferon (IFN-γ), resulting in erythrophagocytosis and production of inflammatory cytokines in severe malarial anemia (SMA). The Toll-like receptors (TLRs) trigger transcription of proinflammatory cytokines and induce adaptive immune responses. Therefore, these receptors may condition malaria disease pathogenesis through alteration in adaptive and innate immune responses. To further delineate the impacts of FcγRIIIA and TLR9 in SMA pathogenesis, the associations between FcγRIIIA −176F/V and TLR9 −1237T/C variants, SMA (hemoglobin [Hb] < 6.0 g/dl), and circulating IFN-γ levels were investigated in children (n = 301) from western Kenya with acute malaria. Multivariate logistic regression analysis (controlling for potential confounders) revealed that children with the FcγRIIIA −176V/TLR9 −1237C (VC) variant combination had 64% reduced odds of developing SMA (odds ratio [OR], 0.36; 95% confidence interval [CI], 0.20 to 0.64; P = 0.001), while carriers of the FcγRIIIA −176V/TLR9 −1237T (VT) variant combination were twice as susceptible to SMA (OR, 2.04; 95% CI, 1.19 to 3.50; P = 0.009). Children with SMA had higher circulating IFN-γ levels than non-SMA children (P = 0.008). Hemoglobin levels were negatively correlated with IFN-γ levels (r = −0.207, P = 0.022). Consistently, the FcγRIIIA −176V/TLR9 −1237T (VT) carriers had higher levels of circulating IFN-γ (P = 0.011) relative to noncarriers, supporting the observation that higher IFN-γ levels are associated with SMA. These results demonstrate that FcγRIIIA-176F/V and TLR9 −1237T/C variants condition susceptibility to SMA and functional changes in circulating IFN-γ levels.
INTRODUCTION
Plasmodium falciparum malaria is a complex clinical syndrome comprising a milieu of life-threatening conditions, including severe malarial anemia (SMA), cerebral malaria (CM), metabolic acidosis, high-density parasitemia (≥10,000 parasites/μl), respiratory distress, hypoglycemia, and other less frequent complications, such as hypotension (32). Globally, falciparum malaria accounts for the greatest degree of malaria-related morbidity and mortality (63). The majority of this morbidity and mortality occurs in immune-naïve African children under 5 years of age (11). In western Kenya, SMA (hemoglobin [Hb] < 6.0g/dl with any density of parasitemia) is the most common clinical manifestation of severe falciparum malaria in pediatric populations resident in regions of transmission holoendemicity (9, 43).
Changes in the human genome have been influenced by pressure due to malaria endemicity—for example, the observed increase in the sickle cell allele (HbAS) in malaria-exposed populations despite its fatal consequences (58). Even though not completely understood, the pathological mechanisms that underlie SMA may include lysis of infected and uninfected erythrocytes (20, 51), erythrocyte sequestration in the spleen (12, 21), and imbalanced cytokine production in bone marrow suppression (26) and, consequently, dyserythropoiesis (1, 49).
Fc gamma receptors (FcγR) are a heterogeneous group of hematopoietic cell surface glycoproteins that facilitate the efficiency of antibody-antigen interactions with effector cells of the immune system (17, 27, 52). FcγR genes are mapped to chromosome 1q on 1q21-q23 (17, 27, 52). These receptors regulate a variety of humoral and cellular immune responses, including phagocytosis, degranulation, antibody-dependent cellular cytotoxicity (ADCC), regulation of cytokine expression, activation of B cells, and clearance of immune complexes (23). The FcγR family consists of FcγRI, FcγRII, and FcγRIII (61).
The FcγRs have functional allelic polymorphisms that influence their effector capabilities (61). FcγRIIIA is expressed predominantly on macrophages, monocytes, natural killer (NK) cells, and γ/δ T cells where they function as phagocytic and cytotoxic triggers to antigens (15). It has two codominantly expressed alleles, the −176V and −176F alleles, which differ in the amino acid at position −176 in the extracellular domain (valine or phenylalanine, respectively). The existence of dimorphism in the amino acid position −176 (F/V) of FcγRIIIA has been shown to influence the binding of IgG subtypes, with the −176V variant displaying a higher binding affinity for IgG1 and IgG3 compared to the −176F variant (29). In P. falciparum infections, IgG1 and IgG3 antibodies have been shown to be associated with low parasitemia and low risk of malaria infection (6). Despite these investigations, the functional role of FcγR variants in regulation of IFN-γ during malaria pathogenesis remains elusive.
Toll-like receptors (TLRs) are type 1 transmembrane proteins that are differentially expressed among immune cells (4, 28). TLRs recognize and bind to conserved pathogen-associated molecular patterns (PAMPs), triggering activation of signal transduction pathways that induce cytokine production (5). TLR9 occupies 5 kb on chromosome 3p21.3 and consists of two exons and containss 1,028 amino acids (22). The PAMPs for TLR9 are hemozoin and unmethylated CpG-DNA (8, 50). Hemozoin, a heme metabolite secreted during malaria infection, activates the innate immune system via a TLR9-mediated MyD88-dependent pathway, resulting in signals that upregulate tumor necrosis factor alpha (TNF-α), interleukin 12p40 (IL-12p40), monocyte chemoattractant protein 1 (MCP-1), and IL-6 production by dendritic cells (16). Hemozoin is also a carrier that facilitates entry of plasmodial unmethylated CpG-DNA into the host cell, where the latter can bind to and stimulate TLR9 (48).
Several single-nucleotide polymorphisms (SNPs) that alter susceptibility to infectious and inflammatory diseases have been identified in TLRs. For instance, a study carried out in Ugandan children (aged 3 to 12 years) showed that carriers of a C allele at TLR9 −1237CC or the G allele at TLR9 1174GG were associated with an increased risk of cerebral malaria (CM) since these alleles enhanced production of gamma interferon (IFN-γ) following severe P. falciparum infection (53). Studies of pregnant Ghanaian women with P. falciparum infection showed that variations in TLR9 −1486CC increased the risk of maternal malaria (35). Other studies investigating the development of premalignant gastric changes induced by Helicobacter pylori have identified TLR9 (−1237T/C) polymorphism as a risk factor (37). Taken together, these studies demonstrate that TLRs have the capacity to mount acute inflammatory responses against invading pathogens through induction of inflammation.
IFN-γ is a multifunctional cytokine produced by T lymphocytes, B cells, and natural killer cells (NKs). It plays an important role in inflammatory responses and is often associated with the development of overt Th1-like cell-mediated immune responses (25) and hence forms an important part of the immune system. Previous studies with animal models indicated that early production of IFN-γ is necessary for resolution of parasitemia and stimulation of phagocytic cells, leading to clearance of infected erythrocytes (54). Moreover, elevated levels of IFN-γ in the acute phase of uncomplicated P. falciparum malaria has been shown to limit progression to clinical malaria (59). Studies in Thai adults demonstrated significantly higher IFN-γ levels in uncomplicated malaria than in individuals presenting with complicated malaria (56). In addition, a previous longitudinal study with a pediatric population in western Kenya demonstrated that high levels of circulating IFN-γ were associated with enhanced SMA severity in a pediatric population (46). Furthermore, studies with animal models have shown that long-term immunity to malaria infection may be affected by an IFN-γ-mediated depletion of parasite-specific CD4+ T cells during infection (64), further demonstrating the critical role of IFN-γ in malaria pathogenesis.
Although FcγRIIIA −176F/V and TLR9 −1237T/C polymorphisms have been implicated in inflammatory diseases (31, 35, 37), to date, no studies have examined the associations between these variants and malaria disease outcomes, specifically in the pediatric population resident in Siaya District, an area of western Kenya where P. falciparum transmission is holoendemic. Since previous genetics-based studies have thoroughly investigated SNPs (41, 47) and haplotypes (40, 46), we investigated the effect of cross-SNP combinations in conditioning SMA. We examined the associations between FcγRIIIA (−176F/V) and TLR9 (−1237T/C) promoter-variant combinations and susceptibility to SMA (Hb < 6.0 g/dl) in children (aged 3 to 36 months) residing in this area of western Kenya where P. falciparum transmission is holoendemic. In addition, we investigated the functional role of these variants in mediating circulating IFN-γ concentrations in children with malaria. The results presented here show that coinheritance of FcγRIIIA −176F/V and TLR9 −1237T/C is associated with susceptibility to SMA and functional changes in circulating IFN-γ levels.
MATERIALS AND METHODS
Study site.
The study was conducted in Siaya District Hospital, western Kenya, and the surrounding community, a region where P. falciparum transmission is holoendemic (43). The region is inhabited by the Luo ethnic tribe (>96%), which is hence a homogenous population for genetic-based studies. The prevalence of falciparum malaria is ∼83% in children aged <4 years, with severe disease manifesting as SMA and/or high-density parasitemia (HDP) (39, 43).
Study participants.
Children (n = 301) of both sexes were recruited in Siaya District Hospital (SDH) in western Kenya during their initial hospitalization for treatment of malaria using questionnaires and existing medical records. Recruitment followed a two-phase tier of screening and enrollment. The parent or guardian of the child received detailed explanation of the study. The enrollment decision was made after initial HIV-1 screening of the child and obtaining informed consent. Questionnaires and written informed consent were administered in the language of choice (i.e., English, Kiswahili, or Dholuo). The children with acute malaria were stratified into two categories. The group with nonsevere malarial anemia (non-SMA) contained children with a positive smear for asexual P. falciparum, parasitemia of any density, and an Hb level of ≥6.0 g/dl. The SMA group contained children with a positive smear for asexual P. falciparum, parasitemia of any density, and an Hb level of <6.0 g/dl (33). Venous blood samples (<3.0 ml) were collected in EDTA-containing Vacutainer tubes at the time of enrollment, prior to provision of treatment or any supportive care. Blood samples were used for malaria diagnosis, hematological measurements, HIV testing, bacterial culture, and genetic analyses. Children were excluded from the study for any one of the following reasons: CM (rare in this area of holoendemicity); a history of any HIV-1-related symptoms, such as oral thrush; clinical evidence of acute respiratory infection; prior hospitalization; intent to relocate during the study period; and unwillingness of the parent or guardian to enroll the child in the study. Participants were treated according to the Ministry of Health (MOH)—Kenya guidelines, which included the use of oral artemether-lumefantrine (Coartem) for uncomplicated malaria and intravenous quinine (and on rare occasions, blood transfusion) for severe malaria. The study approval was obtained from the Ethics Review Committee of the Kenya Medical Research Institute (KEMRI). Informed written consent was obtained from the parent or legal guardian of all children participating in the study.
Laboratory procedures.
Hemoglobin levels and complete blood counts were determined using the Beckman Coulter ACT diff2 (Beckman-Counter Corporation, Miami, FL). To determine parasitemia, 10% Giemsa-stained thick blood smears were prepared and examined under a microscope at high-power magnification. The number of P. falciparum parasites per 300 white blood cells (WBC) was determined, and parasitemia (number of parasites per microliter) was estimated using the total WBC count. In order to delineate severe anemia caused by malaria versus other anemia-promoting conditions, HIV-1, bacteremia, sickle-cell trait (HbAS) status, and glucose-6-phosphate dehydrogenase (G6PD) deficiency were determined. Pre- and posttest HIV counseling was provided for all participants. HIV-1 exposure and infection were determined serologically (i.e., with Unigold and Determine) and through HIV-1 proviral DNA PCR testing, respectively, according to previously published methods (45). Bacteremia was determined using the Wampole Isostat Pediatric 1.5 system (Wampole Laboratories), and blood was processed according to the manufacturer's instructions. API biochemical galleries (bioMérieux, Inc.) and/or serology was used for identification of blood-borne bacterial isolates. The presence of the sickle cell trait (HbAS) was determined by cellulose acetate electrophoresis, while glucose-6-phosphate dehydrogenase (G6PD) deficiency was determined as previously described (47).
Genotyping.
Blood spots were made on FTA Classic cards (Whatman, Inc., Clifton, NJ), air dried, and stored at room temperature until use. DNA was extracted using the Gentra system (Gentra System, Inc., Minneapolis, MN) according to the manufacturer's recommendations. The FcγRIIIA −176F/V (rs396991, assay identification [ID] no. C_25815666_10) and TLR9 −1237C/T (rs5743836, assay ID no. C_32645383_10) promoter polymorphisms were genotyped using the high-throughput TaqMan 5′ allelic discrimination Assay-by-Design method based on the manufacturer's instructions (Applied Biosystems, Foster City, CA).
Quantification of IFN-γ levels.
Plasma samples were obtained from venous blood and stored at −80°C. Batch analysis was performed to restrict experimental variability between assays. Circulating IFN-γ concentrations were determined using the human cytokine 25-plex antibody (Ab) bead kit (BioSource International) according to the manufacturer's instructions. Plates were read on the Luminex 100 system (Luminex Corporation) and analyzed using the Bio-plex Manager software (Bio-Rad Laboratories). The detection limit for IFN-γ was 2.0 pg/ml.
Data analyses.
SPSS statistical software package version 19.0 (IBM SPSS, Inc., Chicago, IL) was used for all statistical analyses. χ2 analysis was used to examine differences between proportions. Comparisons across groups were determined by the Kruskal-Wallis test, while the Mann-Whitney U test was used for comparisons of demographic and clinical characteristics and circulating IFN-γ levels between the two clinical groups and SNP combinations. FcγRIIIA (−176 F/V) and TLR9 (−1237C/T) SNP combinations were constructed using the HPlus software program (version 2.5). The relationships between genotypes, SNP combinations, and SMA were determined by multivariate logistic regression, controlling for the confounding effects of age, gender, HIV-1 status (including HIV-1-exposed and definitively HIV-1+ results), glucose-6-phosphate dehydrogenase (G6PD) deficiency, sickle cell trait (HbAS), and bacteremia. The correlation between IFN-γ concentrations and Hb levels in parasitemic children was determined by Spearman's correlation coefficient. Statistical significance was set at P ≤ 0.05.
RESULTS
Clinical, demographic, and laboratory characteristics of the study participants.
A cross-sectional analysis in children (n = 301, aged 3 to 36 months) presenting with acute P. falciparum malaria (any density of parasitemia) was performed. Clinical stratification of the study groups was done based on a previous age- and geographically defined reference population from western Kenya (33), i.e., nonsevere malaria (non-SMA) (Hb ≥ 6.0g/dl; n = 163) and severe malaria anemia (SMA) (Hb < 6.0 g/dl; n = 138). The distribution of gender, parasitemia (parasites/μl), proportions of subjects with high-density parasitemia (HDP) (≥10,000 parasites/μl), and axillary temperature (°C) were not significantly different between the groups (P = 0.668, P = 0.508, P = 0.456, and P = 0.109, respectively) (Table 1). Children presenting at the hospital with SMA were younger than those with non-SMA (P = 0.010). With reference to previous grouping, the Hb (g/dl) concentration and erythrocyte counts (1012/liter) were lower in the SMA group (P < 0.001) for the two clinical parameters (Table 1).
Table 1.
Clinical, demographic, and laboratory characteristics of the study participantsa
| Characteristic | Result for: |
P value | |
|---|---|---|---|
| Non-SMA (Hb ≥ 6.0 g/dl) | SMA (Hb < 6.0 g/dl) | ||
| No. of participants (n = 301) | 163 | 138 | |
| Gender, n (%) | |||
| Male | 82 (50.3) | 66 (47.8) | 0.668b |
| Female | 81 (49.7) | 72 (52.2) | |
| Age, mo | 11.0 (10.0) | 8.0 (8.0) | 0.010c |
| Hemoglobin level, g/dl | 7.9 (3.0) | 4.9 (1.0) | <0.001c |
| Parasite density, parasites/μl | 18,957.0 (43,921.5) | 17,261.55 (36,272.0) | 0.508c |
| HDP, no. (%) of subjects with ≥10,000 parasites/μl | 106/163 (65.0) | 84/138 (60.9) | 0.456b |
| Red blood count, 1012/liter | 3.65 (1.2) | 2.13 (0.8) | <0.001c |
| Axillary temp, °C | 37.6 (2.0) | 37.4 (2.0) | 0.109c |
Data are shown as the median (with interquartile range [IQR] in parentheses) unless otherwise noted. Children with parasitemia (n = 301) were stratified according to a modified definition of SMA based on age- and geographically matched Hb concentrations (i.e., Hb, <6.0 g/dl with any density of parasitemia) (33) into non-SMA (n = 163) and SMA (n = 138). HDP, high-density parasitemia.
Statistical significance determined by χ2 analysis.
Statistical significance determined by the Mann-Whitney U test.
Distribution of FcγRIIIA (−176F/V) and TLR9 (−1237T/C) genotypes and alleles in the clinical groups.
To investigate the role played by the polymorphic variation in the FcγRIIIA (−176F/V) and TLR9 (−1237T/C) promoters in conditioning susceptibility to SMA, their allelic distributions were compared between the clinical groups (Table 2). The distributions of FcγRIIIA −176F/V genotypes in the overall population were 54.5% FF, 36.5% FV, and 9.0% VV, with overall allele frequencies of 0.72 for F and 0.28 for V. However, the overall allele frequency for FcγRIIIA −176F/V did not deviate from the Hardy-Weinberg equilibrium (HWE) (χ2 = 1.83, P = 0.180). The prevalences of FcγRIIIA −176 F/V genotypes in non-SMA were 52.2% FF, 36.8% FV, and 11.0% VV with allele frequencies of 0.71 for F and 0.29 for V, while the genotypic distributions of FcγRIIIA −176F/V in the SMA group were 57.3% FF, 36.2% FV, and 6.5% VV, with allele frequencies of 0.75 for F and 0.25 for V. The allele frequencies in the non-SMA (χ2 = 2.20, P = 0.140) and SMA (χ2 = 0.07, P = 0.800) groups for FcγRIIIA −176F/V did not significantly deviate from HWE.
Table 2.
Distribution of the FcγRIIIA (−176F/V) and TLR9 (−1237T/C) genotypes in the clinical groups
| Genotype | No. (%) with genotype in groupa: |
P valueb | |
|---|---|---|---|
| Non-SMA (Hb ≥ 6.0 g/dl) (n = 163) | SMA (Hb < 6.0 g/dl) (n = 138) | ||
| FcγRIIIA −176F/V | |||
| FF | 85 (52.2) | 79 (57.3) | |
| FV | 60 (36.8) | 50 (36.2) | 0.356 |
| VV | 18 (11.0) | 9 (6.5) | |
| TLR9 (−1237T/C) | |||
| TT | 60 (36.8) | 63 (45.7) | |
| TC | 87 (53.4) | 63 (45.7) | 0.297 |
| CC | 16 (9.8) | 12 (8.6) | |
Data are presented as n (%) children. Children with parasitemia were categorized on the basis of the presence or absence of severe malarial anemia (SMA) (defined as Hb < 6.0 g/dl with any density of parasitemia) (33).
Statistical significance determined by χ2 analysis.
The distribution of the TLR9 (−1237T/C) genotype in the overall study population was 40.9% TT, 49.8% TC, and 9.3% CC (Table 2), with overall allele frequencies of 0.70 for T and 0.30 for C. There was no significant departure from the HWE in the overall study group (χ2 = 0.60, P = 0.350). The genotypic distributions in the non-SMA group were 36.8% TT, 54.4% TC, and 9.8% CC (Table 2), with allele frequencies of 0.63 for T and 0.36 for C. The allele frequency in the non-SMA group demonstrated a significant departure from the HWE (χ2 = 4.00, P = 0.040). The genotypic prevalences in the SMA group were 45.7% TT, 45.7% TC, and 8.6% CC (Table 2), with allele frequencies of 0.69 for T and 0.31 for C. However, there was no departure from HWE in the SMA group (χ2 = 0.60, P = 0.400).
Additional χ2 analysis showed that the distributions of the individual FcγRIIIA −176F/V and TLR9 (−1237 T/C) genotypes were comparable between the SMA and non-SMA groups (P = 0.356 and P = 0.297, respectively).
Influence of polymorphic variability in FcγRIIIA −176F/V and TLR9 (−1237T/C) on SMA.
The association between individual genotypes of FcγRIIIA −176F/V and TLR9 (−1237T/C) and susceptibility to SMA was determined using multivariate logistic regression analyses while controlling for the confounding effects of age, gender, HIV-1 status, sickle cell trait (HbAS), bacteremia, and G6PD deficiency (3, 45, 62). No significant associations were observed between the variations at individual loci of FcγRIIIA (−176F/V) and TLR9 (−1237T/C) and susceptibility to SMA (Table 3).
Table 3.
Association between individual FcγRIIIA −176F/V and TLR9 (−1237T/C) genotypes and severe malarial anemia
| Genotype | Association with SMA (Hb ≤ 6.0 g/dl)a |
||
|---|---|---|---|
| OR | 95% CI | P value | |
| FcγRIIIA −176F/V | |||
| FF | 1.00 (reference) | ||
| FV | 0.88 | 0.53–1.45 | 0.163 |
| VV | 0.58 | 0.24–1.40 | 0.235 |
| TLR9 −1237T/C | |||
| TT | 1.00 (reference) | ||
| TC | 0.67 | 0.41–1.10 | 0.110 |
| CC | 0.72 | 0.31–1.68 | 0.450 |
Children with acute malaria (n = 301) were stratified according to the modified definition of severe malarial anemia (SMA) based on age- and geographically matched Hb concentration (i.e., Hb < 6.0g/dl with any density of parasitemia) (33). Odds ratios (OR) and 95% confidence intervals (CI) were determined using multivariate logistic regression, controlling for age, gender, HIV-1 infection, sickle cell trait (HbAS), bacteremia, and G6PD deficiency. The reference groups in the multivariate logistic regression analysis were the homozygous wild-type genotypes.
Distribution of FcγRIIIA −176F/V and TLR9 (−1237T/C) SNP combinations in the clinical groups.
As shown in Table 4, cross-gene SNP combination for the receptor polymorphisms yielded the following overall prevalences in the non-SMA and SMA groups (33): FcγRIIIA −176F/TLR9 −1237T (FT), 80.1% (241/301); FcγRIIIA −176F/TLR9 −1237C (FC), 38.2% (115/301); FcγRIIIA −176V/TLR9 −1237T (VT), 26.6% (80/301); and FcγRIIIA −176V/TLR9 −1237C (VC), 24.0% (74/301). Additional comparison showed a higher proportion of carriers of the −176V/−1237T (VT) SNP combination in the SMA group (33.3%) compared to the non-SMA group (20.9%; P = 0.015). Consistent with this observation, the VT carriers presented with significantly lower Hb levels (median, 5.70 g/dl; interquartile range [IQR], 3) relative to the non-VT carriers' median (6.70 g/dl; IQR, 3) (P = 0.014). Further analysis revealed a significantly lower proportion of the −176V/−1237C (VC) SNPs in the SMA group (15.2%) compared to the non-SMA group (32.5%; P = 0.001). In agreement with this finding, carriers of this SNP combination also had significantly higher Hb levels (median, 6.70 g/dl; IQR, 3) compared to the noncarriers (median, 5.60 g/dl; IQR, 3) (P = 0.002). Further analysis revealed comparable proportions and Hb levels for the −176F/−1237T (P = 0.887 and P = 0.588) and −176F/−1237C combinations (P = 0.209 and P = 0.064) (Table 4). These results show that carriage of FcγRIIIA −176F/V and TLR9 (−1237T/C) SNP combinations may condition susceptibility to SMA in children with acute malaria.
Table 4.
Distribution of FcγRIIIA −176F/V and TLR9 (−1237T/C) SNP combinations in the clinical groupsa
| FcγRIIIA/TLR9 −1237T/C SNP combination (n) | Median (IQR) Hb level (g/dl) | Pb | Result for: |
Pc | |
|---|---|---|---|---|---|
| Non-SMA (n = 163) | SMA (n = 138) | ||||
| −176F/−1237T (241) | |||||
| Carriers | 6.20 (3.00) | 0.588 | 131 (80.6) | 110 (79.7) | 0.887b |
| Noncarriers | 6.25 (3.00) | 32 (19.6) | 28 (20.3) | ||
| −176F/−1237C (115) | |||||
| Carriers | 5.90 (3.00) | 0.064 | 57 (35.0) | 58 (42.0) | 0.209 |
| Noncarriers | 6.30 (3.00) | 106 (65.0) | 80 (58.0) | ||
| −176V/−1237T (80) | |||||
| Carriers | 5.70 (3.00) | 0.014 | 34 (20.9) | 46 (33.3) | 0.015 |
| Noncarriers | 6.30 (3.00) | 129 (79.1) | 92 (66.7) | ||
| −176V/−1237C (74) | |||||
| Carriers | 6.70 (3.00) | 0.002 | 53 (32.5) | 21 (15.2) | 0.001 |
| Noncarriers | 5.60 (3.00) | 110 (67.5) | 117 (84.8) | ||
Data are presented as proportions (n with percentage in parentheses), and the Hb levels are medians (with IQR in parentheses). The comparisons between carriers and noncarriers of the SNP combinations were computed using the Mann-Whitney U test. SMA, Hb < 6.0g/dl with any density of parasitemia (33); non-SMA, Hb ≥ 6.0g/dl with any density of parasitemia. Values in boldface are statistically significant at P ≤ 0.005.
Statistical significance determined by the Mann-Whitney U test.
Statistical significance determined by χ2 analysis.
Associations between FcγRIIIA −176F/V and TLR9 −1237T/C gene SNP combinations and SMA.
Prior to the determination of the influence of the SNP combinations on circulating plasma IFN-γ levels, multivariate logistic regression analysis controlling for covariates (3, 45, 62) was performed. The analysis demonstrated that carriers of the −176V/−1237T (VT) SNP combination were at an increased risk of developing SMA (odds ratio [OR], 2.04; 95% CI, 1.19 to 3.50; P = 0.009) relative to noncarriers, while carriers of the −176V/−1237C (VC) combination were at reduced risk of SMA (OR, 0.36; 95% CI, 0.20 to 0.64; P = 0.001) (Table 5). Further analysis did not reveal any association between the −176F/−1237T (OR, 0.94; 95% CI, 0.52 to 1.68; P = 0.830) and the −176F/−1237C (OR, 1.30; 95% CI, 0.80 to 2.10; P = 0.288) SNP combinations and SMA (Table 5). Given a possible dilution effect of each SNP combination in heterozygous individuals, additional construction of SNPs was carried out based on the carriage of F/V (at −176F/V) and T/C (at −1237T/C)—i.e., FV/TC and associations with SMA. Results revealed that heterozygous individuals were peripherally at an increased risk for the development of SMA (OR, 1.89; 95% CI, 0.99 to 3.64; P = 0.055). However, low numbers could not allow determination of associations between the dominant (FF/TT) and recessive (VV/CC) SNP combination models and SMA.
Table 5.
Relationship between FcγRIIIA −176F/V and TLR9 −1237T/C SNP combinations and severe malarial anemiaa
| SNP combination | SMA (Hb < 6.0g/dl) |
||
|---|---|---|---|
| OR | 95% CI | P value | |
| FcγRIIIA −176F/TLR9 −1237T | 0.94 | 0.52–1.68 | 0.830 |
| FcγRIIIA −176F/TLR9 −1237C | 1.30 | 0.80–2.10 | 0.288 |
| FcγRIIIA −176V/TLR9 −1237T | 2.04 | 1.19–3.50 | 0.009 |
| FcγRIIIA −176V/TLR9 −1237C | 0.36 | 0.20–0.64 | 0.001 |
Children with acute malaria (n = 301) were stratified according to the modified definition of severe malarial anemia (SMA) based on age- and geographically matched Hb concentration (i.e., Hb < 6.0g/dl with any density of parasitemia) (33). Odds ratios (OR) and 95% confidence intervals (CI) were determined using multivariate logistic regression, controlling for age, gender, sickle cell trait (HbAS), bacteremia, and G6PD deficiency. The reference groups in this multivariate logistic regression analysis were those without the respective SNP combinations.
Relationship between circulating IFN-γ and SMA.
To determine whether the changes in the circulating levels of IFN-γ are associated with severity of acute malaria, the levels were compared between the SMA (n = 69) and non-SMA (n = 70) groups. It is critical to note that after the first screening (to determine Hb levels), we were unable to collect additional blood samples to carry out measurements of IFN-γ levels in some study participants due to the fact that the children either were too anemic or were too sick to ethically allow collection of additional blood samples, hence the reduction in numbers in this analysis. As presented in Fig. 1, the results demonstrate that children with SMA had significantly higher levels of circulating plasma IFN-γ concentrations (median, 21.5; IQR, 34.9 pg/ml) compared to non-SMA children (median, 14.8; IQR, 27.1 pg/ml) (P = 0.008). Additional analyses demonstrated that IFN-γ levels were negatively correlated with Hb levels (r = −0.207, P = 0.022).
Fig 1.
Relationship between circulating IFN-γ levels and SMA (Hb < 6.0g/dl). Data are represented in box plots for the non-SMA (n = 70) and SMA (n = 69) groups. The boxes represent the interquartile ranges; the lines through the boxes are the medians, while the whiskers show the 10th and the 90th percentiles. Shaded boxes show children with SMA, while open boxes are non-SMA children. Non-SMA children had significantly lower circulating IFN-γ levels relative to SMA children (P = 0.008, Mann-Whitney U test).
Association between circulating IFN-γ and FcγRIIIA (−176F/V) and TLR9 (−1237T/C) promoter polymorphisms.
To determine whether these genotypes were associated with functional changes in concentrations of IFN-γ levels, plasma levels of IFN-γ were compared across the genotypic groups. As presented in Fig. 2A and B, there were no significant differences in the concentrations of plasma IFN-γ across genotypes for both FcγRIIIA (−176F/V; FF, 77; FV, 53; and VV, 9) (P = 0.480) and TLR9 (−1237T/C; TT, 65; TC, 65; and CC, 9) (P = 0.559). The distributions of IFN-γ in the FcγRIIIA −176F/V genotype were as follows: FF, median, 19.6, and IQR, 30.6; FV, median, 15.9, and IQR, 28.1; and VV, median, 19.6, and IQR, 27.3. The distributions of IFN-γ in the TLR9 (−1237T/C) genotype were as follows: TT, median, 16.2, and IQR, 34.6; TC, median, 19.6, and IQR, 33.6; and CC, median, 14.0, and IQR, 27.5.
Fig 2.
Association between circulating IFN-γ and FcγRIIIA −176F/V (A) and TLR9 (−1237T/C) (B). Data are represented in box plots for FcγRIIIA −176F/V (FF, 77; FV, 53; and VV, 9) and TLR9 −1237T/C (TT, 65; TC, 65; and CC, 9). The boxes represent interquartile ranges; the lines through the boxes are the medians, while the whiskers show the 10th and the 90th percentiles. Across-group comparisons were determined using Kruskal-Wallis test. The circulating IFN-γ levels were comparable across the FcγRIIIA −176F/V (P = 0.480) and TLR9 −1237T/C (P = 0.559) genotypic groups.
Functional associations between FcγRIIIA −176F/V and TLR9 −1237T/C SNP combinations and circulating IFN-γ levels.
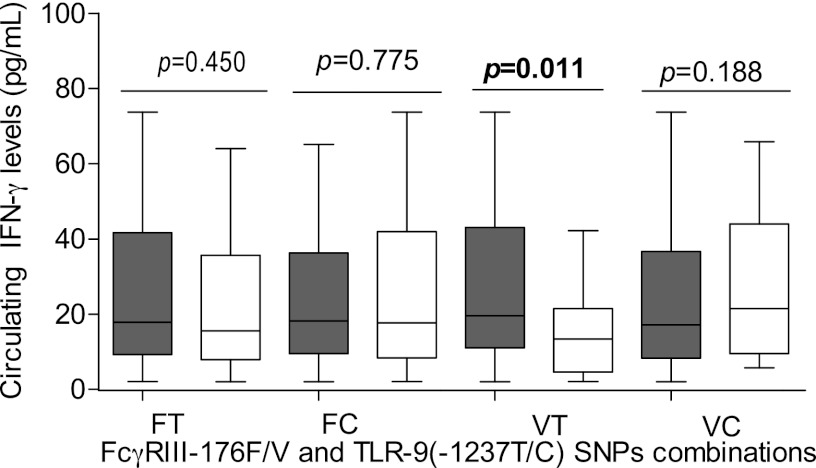
To determine whether coinheritance of these receptor polymorphisms was associated with changes in IFN-γ levels, circulating concentrations of IFN-γ were compared within SNP combinations in FcγRIIIA −176F/V and TLR9 −1237T/C. As shown in Fig. 3, the individuals with the FcγRIIIA −176V/TLR9 −1237T SNP combination had significantly higher levels of IFN-γ (median, 19.6 pg/ml; IQR, 32.1) than those without this combination (median, 13.4 pg/ml; IQR, 16.9) (P = 0.011). However, the concentrations of IFN-γ were comparable between those with the FcγRIIIA −176F/TLR9 −1237T (P = 0.450), FcγRIIIA −176F/TLR9 −1237C (P = 0.775), and FcγRIIIA −176V/TLR9 −1237C (P = 0.188) SNP combinations.
Fig 3.
Association between circulating IFN-γ levels and FcγRIIIA −176F/V and TLR9 (−1237T/C) SNP combinations. Data are represented in box plots for FcγRIIIA −176F and TLR9 −1237T (FT, 121), FcγRIIIA −176F and TLR9 −1237C (FC, 45), FcγRIIIA −176V and TLR9 −1237T (VT, 104), and FcγRIIIA −176V and TLR9 −1237C (VC, 106). The boxes represent interquartile ranges; the lines through the boxes are the medians, while the whiskers show the 10th and the 90th percentiles. Pairwise comparisons in those with and without the combination were done using the Mann-Whitney U test. Shaded boxes show children with the indicated SNP combination, while open boxes show those without the combination. Carriage of FcγRIIIA −176V/TLR9 −1237T (VT) SNP combination was associated with significantly higher levels of circulating IFN-γ levels relative to that in noncarriers (P = 0.011).
DISCUSSION
To describe the role of receptors in susceptibility to severe malarial anemia in children, we performed a cross-sectional analysis of the impacts of FcγRIIIA −176F/V and TLR9 (−1237T/C) promoter variants in a phenotypically well-defined cohort of children aged 3 to 36 months resident in a region of P. falciparum transmission holoendemicity. The results presented here demonstrate that carriage of the FcγRIIIA −176V/TLR9 −1237C (VC) combination confers protection against SMA (Hb < 6.0 g/dl) (33) and is associated with significantly higher Hb levels in this population, whereas carriage of FcγRIIIA −176V and TLR9 −1237T (VT) increases susceptibility to SMA and produces significantly higher levels of circulating IFN-γ. Consistent with previous observations, children with SMA had significantly high levels of circulating IFN-γ (46).
Since FcγRIIIA is mainly expressed on the macrophages and monocytes, they play a primary role in phagocytosis of unparasitized erythrocytes and induction of proinflammatory cytokines, which play a role in SMA pathogenesis (10). On macrophages, FcγRIIIA is also involved in the clearance of immune complex (15). Consistent with a previous study with Thai adults (41), the individual FcγRIIIA −176F/V polymorphism failed to show any association with SMA. However, it is important to note that this polymorphism influences preferential binding of IgG in which FcγRIIIA (−176V/V) has higher binding affinity to IgG1 and IgG3, which are associated with low parasitemia and low risk of malaria (6, 57). We are currently exploring this model to test whether carriage of this variant is associated with higher IgG binding in children naturally exposed to P. falciparum malaria in a region of holoendemicity in western Kenya, in which the primary clinical outcome of severe malaria is SMA.
There is accumulating evidence on the potential role of TLR9 polymorphisms in clinical malaria (13, 30, 65). Consistent with our study, two separate studies, carried out in Brazil and Iran, have recently revealed no impact of individual TLR9 (−1237T/C) promoter polymorphism on susceptibility to mild malaria in their respective populations (30, 65). Moreover, the TLR9 (−1237TT) genotype has only been associated with low parasitemia but not increased susceptibility to clinical malaria in Ghanaian children aged 3 to 11 years (40). Investigations with Gambian and Malawian children less than 5 years old characterized by mixed clinical phenotypes (cerebral and/or severe malaria anemia) did not show any association between the TLR9 (−1237T/C) polymorphisms and severe malaria (13). However, a study of Ugandan children (aged 4 to 12 years) showed that the TLR9 (−1237CC) genotype was associated with elevated levels of plasma IFN-γ and enhanced cerebral malaria (53), emphasizing the fact that these variants may individually be associated with CM rather than SMA. Moreover, other studies have revealed that individuals infected by malaria have upregulated TLR9 and elevated IFN-γ and that mice with TLR9 gene knockout produce low IFN-γ levels in response to Plasmodium chabaudi AS (24). The discrepancies observed between our study and others may in part be explained by the difference in clinical phenotypes since in our study population, the main clinical manifestation is SMA in pediatric populations, while the earlier studies focused on heterogeneous populations in which the most severe clinical manifestation was CM. In addition, pathways of TLR9 signaling involve polymorphisms in the downstream molecules (for instance, NF-κB and MyD88) that were not investigated in the present study. Furthermore, due to the high prevalence of malaria in our population and the fact that TLRs only act in recognition and induction of the immune response, we assume that polymorphisms in the TLRs are not the primary determinants of clinical malaria. We are currently investigating additional genes that may significantly alter TLR pathways and alter malaria disease susceptibility.
Since susceptibility to infectious disease occurs through multifactorial, complex, and even contradictory selective pressures (7), our laboratory constructed cross-SNP combinations between FcγRIIIA (−176F/V) and TLR9 (−1237T/C) in an attempt to determine whether coinheritance of these receptor-SNP combinations could influence susceptibility to SMA. Based on results from this study, carriage of the −176V/−1237C (VC) SNP combination was associated with reduced susceptibility to the development of SMA relative to noncarriers. Consistent with this observation, carriers of VC had concomitantly higher levels of hemoglobin, suggesting a potential protective role against SMA pathogenesis through increased erythropoietic responses. In addition, the carriers of the −176V/−1237T (VT) SNP combination were almost twice at risk of developing SMA and had relatively lower hemoglobin levels. In the present study, we demonstrated that SMA in this population is characterized in part by elevated circulating IFN-γ levels, as previously shown (46). Furthermore, the −176V/−1237T SNP combination, which was associated with an increased risk of SMA, was also associated with higher circulating IFN-γ levels. This is not surprising given that elevated circulating IFN-γ levels are associated with SMA in this population. As such, any gene combination that may be associated with higher circulating IFN-γ levels may promote SMA. It would be plausible to explore how different cytokine milieus in relation to IFN-γ levels and IgG production promote the development of SMA over time in this pediatric population resident in western Kenya. This approach will address the inherent limitation in examining cytokine production at a single time point (time of admission) and in circulation rather than in the local microenvironments, which in essence complicates the clear understanding of the exact role of immune mediators such as IFN-γ in SMA (34). This study underscores the importance of the use of cross-SNP combinations in genetic association studies of infectious diseases such as malaria because it reveals associations that are not identifiable with just single gene polymorphisms since such disease outcome(s) are dictated by genes functioning in concert (2).
It is worth noting that substitution of a T for a C in TLR9 −1237 in the SNP combination (VC versus VT) significantly determined whether individuals were increasingly susceptible or had reduced risk to SMA in children who presented with acute malaria. Activation of gene transcription depends upon the binding of regulatory and transcription factors to specific recognition sequences in the promoter. As such, variation in the TLR9 −1237T/C promoter sequences likely alters specific transcription factor recognition sites and consequently affects transcriptional activation of IFN-γ and production of other effectors during acute disease. For example, the presence of a T at the TLR9 −1237 locus (VT in the SNP combination) may favor enhanced binding of transcriptional factors (or cause disruption of repressor binding sites) that lead to higher IFN-γ production, whereas, the presence of the C in the promoter (VC in the SNP combination) may create sites for enhanced binding of repressors that favor reduced IFN-γ production. Although the impact of the surface receptor-SNP combinations examined here on promoter binding elements is largely unknown, our laboratory is currently investigating the mechanism(s) by which these cross-SNP combinations may alter IFN-γ production.
Despite continued investigations, the exact role of IFN-γ in the pathogenesis of SMA continues to be baffling. For example, high early IFN-γ production has been shown to confer protection against symptomatic malaria episodes in children aged 5 to 14 years from a region of malaria endemicity of Papua New Guinea (19). An additional study in an area of holoendemic perennial falciparum malaria transmission in southern Ghana reported that malaria-specific production of IFN-γ was associated with reduced clinical malaria and fever (18). Collectively, these studies implicate increased IFN-γ production in clinical malaria. However, certain studies have reported an association between higher levels of IFN-γ and severe malaria. For instance, a study in Uganda reported a positive association between increased IFN-γ levels and CM (53). Furthermore, a previous report on a population of children resident in western Kenya demonstrated that IFN-γ was a positive predictor of SMA (42). The results presented in the present study versus those from the previous study (42) likely differ due to differences in the stratification of the cohort groups. In the present study, we stratified our study population into the SMA group (Hb < 6.0 g/dl with any density of parasitemia) and non-SMA groups (Hb ≥ 6.0g/dl with any density of parasitemia), while the previous study (42) further stratified the overall non-SMA group (Hb > 6.0 g/dl with any density of parasitemia) into uncomplicated malaria (UM) (Hb levels of >11.0 g/dl; n = 31) and non-SMA (Hb levels of 6.0 to 10.9 g/dl; n = 37) for the least-angle regression (LAR) analyses. In addition, potential underlying genetic variations that may potentially contribute to differences in functional changes (e.g., IFN-γ) during disease in the population were never controlled for as a variable in the LAR analyses. In the present study, we demonstrate that children with SMA had significantly higher IFN-γ concentrations, a finding consistent with a previous study of the same population (46). Even though not explicitly explored, the pathogenic mechanisms of elevated IFN-γ in SMA may in part be a consequence of overstimulation of monocytes by IFN-γ to secrete TNF-α (44). This stimulation would lead to the formation of toxic oxides and free radicals, such as reactive oxygen species (H2O2 and inducible nitric oxide synthase [iNOS]), by liver cells against intrahepatic parasites and erythrocytic-stage parasites (38, 55, 60), as well as enhanced phagocytic activities of monocytes/macrophages against parasitized and nonparasitized erythrocytes (36). Moreover, overproduction of IFN-γ also promotes enhanced malarial anemia pathogenesis through bone marrow suppression, dyserythropoiesis, and erythrophagocytosis (14). However, for enhanced immunity to be accomplished, milieus of balance of both proinflammatory and anti-inflammatory cytokines are involved (18) and should be considered in future study designs.
In summary, our results demonstrate that SMA in this pediatric population is conditioned by functional variations in FcγRIIIA (−176F/V) and TLR9 (−1237T/C) promoter polymorphisms. To exhaustively describe the impacts of surface receptors in development of naturally acquired immunity against malaria, further longitudinal studies aimed at examination of an all-inclusive panel of receptor polymorphisms that influence innate immune response and disease outcome are required as this may provide an immunogenetic basis for the development of vaccines that modulate receptor functions.
ACKNOWLEDGMENTS
We are grateful to the Siaya District Hospital for clinical support. We are indebted to the parents/guardians of the study participants and children who took part in the study. This work was published with the approval of the Director, KEMRI.
This work was supported financially by grants from the National Institutes of Health (7R01-TW008306-05).
Footnotes
Published ahead of print 8 October 2012
REFERENCES
- 1. Abdalla S, Weatherall DJ, Wickramasinghe SN, Hughes M. 1980. The anaemia of P. falciparum malaria. Br. J. Haematol. 46:171–183 [DOI] [PubMed] [Google Scholar]
- 2. Adler AJ, Scheller A, Robins DM. 1993. The stringency and magnitude of androgen-specific gene activation are combinatorial functions of receptor and nonreceptor binding site sequences. Mol. Cell. Biol. 13:6326–6335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aidoo M, et al. 2002. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet 359:1311–1312 [DOI] [PubMed] [Google Scholar]
- 4. Akira S. 2003. Mammalian Toll-like receptors. Curr. Opin. Immunol. 15:5–11 [DOI] [PubMed] [Google Scholar]
- 5. Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801 [DOI] [PubMed] [Google Scholar]
- 6. Aribot G, et al. 1996. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, west Africa). Am. J. Trop. Med. Hyg. 54:449–457 [DOI] [PubMed] [Google Scholar]
- 7. Balaresque PL, Ballereau SJ, Jobling MA. 2007. Challenges in human genetic diversity: demographic history and adaptation. Hum. Mol. Genet. 16(special issue 2):R134–R139 [DOI] [PubMed] [Google Scholar]
- 8. Bauer S, et al. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. U. S. A. 98:9237–9242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bloland PB, et al. 1999. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission II. Descriptive epidemiology of malaria infection and disease among children. Am. J. Trop. Med. Hyg. 60:641–648 [DOI] [PubMed] [Google Scholar]
- 10. Brattig NW, et al. 2008. Plasmodium falciparum glycosylphosphatidylinositol toxin interacts with the membrane of non-parasitized red blood cells: a putative mechanism contributing to malaria anemia. Microbes Infect. 10:885–891 [DOI] [PubMed] [Google Scholar]
- 11. Breman JG, Alilio MS, Mills A. 2004. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am. J. Trop. Med. Hyg. 71:1–15 [PubMed] [Google Scholar]
- 12. Buffet PA, Safeukui I, Milon G, Mercereau-Puijalon O, David PH. 2009. Retention of erythrocytes in the spleen: a double-edged process in human malaria. Curr. Opin. Hematol. 16:157–164 [DOI] [PubMed] [Google Scholar]
- 13. Campino S, et al. 2009. TLR9 polymorphisms in African populations: no association with severe malaria, but evidence of cis-variants acting on gene expression. Malar. J. 8:44 doi:10.1186/1475-2875-8-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark IA, Cowden WB. 2003. The pathophysiology of falciparum malaria. Pharmacol. Ther. 99:221–260 [DOI] [PubMed] [Google Scholar]
- 15. Clarkson SB, et al. 1986. Blockade of clearance of immune complexes by an anti-Fc gamma receptor monoclonal antibody. J. Exp. Med. 164:474–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coban C, et al. 2005. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J. Exp. Med. 201:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daeron M. 1997. Fc receptor biology. Annu. Rev. Immunol. 15:203–234 [DOI] [PubMed] [Google Scholar]
- 18. Dodoo D, et al. 2002. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J. Infect. Dis. 185:971–979 [DOI] [PubMed] [Google Scholar]
- 19. D'Ombrain MC, et al. 2008. Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin. Infect. Dis. 47:1380–1387 [DOI] [PubMed] [Google Scholar]
- 20. Dondorp AM, et al. 1999. Red blood cell deformability as a predictor of anemia in severe falciparum malaria. Am. J. Trop. Med. Hyg. 60:733–737 [DOI] [PubMed] [Google Scholar]
- 21. Dondorp AM, et al. 1999. Red cell deformability, splenic function and anaemia in thalassaemia. Br. J. Haematol. 105:505–508 [DOI] [PubMed] [Google Scholar]
- 22. Du X, Poltorak A, Wei Y, Beutler B. 2000. Three novel mammalian Toll-like receptors: gene structure, expression, and evolution. Eur. Cytokine Netw. 11:362–371 [PubMed] [Google Scholar]
- 23. Fanger MW, Shen L, Graziano RF, Guyre PM. 1989. Cytotoxicity mediated by human Fc receptors for IgG. Immunol. Today 10:92–99 [DOI] [PubMed] [Google Scholar]
- 24. Franklin BS, et al. 2009. Malaria primes the innate immune response due to interferon-gamma induced enhancement of Toll-like receptor expression and function. Proc. Natl. Acad. Sci. U. S. A. 106:5789–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gajewski TF, Joyce J, Fitch FW. 1989. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J. Immunol. 143:15–22 [PubMed] [Google Scholar]
- 26. Helleberg M, et al. 2005. Bone marrow suppression and severe anaemia associated with persistent Plasmodium falciparum infection in African children with microscopically undetectable parasitaemia. Malar. J. 4:56 doi:10.1186/1475-2875-4-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Indik ZK, Park JG, Hunter S, Schreiber AD. 1995. The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood 86:4389–4399 [PubMed] [Google Scholar]
- 28. Janeway CA, Jr, Medzhitov R. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216 [DOI] [PubMed] [Google Scholar]
- 29. Koene HR, et al. 1997. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood 90:1109–1114 [PubMed] [Google Scholar]
- 30. Leoratti FM, et al. 2008. Variants in the Toll-like receptor signaling pathway and clinical outcomes of malaria. J. Infect. Dis. 198:772–780 [DOI] [PubMed] [Google Scholar]
- 31. Li X, Ptacek TS, Brown EE, Edberg JC. 2009. Fcgamma receptors: structure, function and role as genetic risk factors in SLE. Genes Immun. 10:380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marsh K, et al. 1995. Indicators of life-threatening malaria in African children. N. Engl. J. Med. 332:1399–1404 [DOI] [PubMed] [Google Scholar]
- 33. McElroy PD, et al. 1999. Analysis of repeated hemoglobin measures in full-term, normal birth weight Kenyan children between birth and four years of age. III. The Asemobo Bay Cohort Project. Am. J. Trop. Med. Hyg. 61:932–940 [DOI] [PubMed] [Google Scholar]
- 34. Mirghani HA, Eltahir HGAETM, Mirghani YA, Elbashir MI, Adam I. 2011. Cytokine profiles in children with severe Plasmodium falciparum malaria in an area of unstable malaria transmission in central Sudan. J. Trop. Pediatr. 57:392–395 [DOI] [PubMed] [Google Scholar]
- 35. Mockenhaupt FP, et al. 2006. Common polymorphisms of Toll-like receptors 4 and 9 are associated with the clinical manifestation of malaria during pregnancy. J. Infect. Dis. 194:184–188 [DOI] [PubMed] [Google Scholar]
- 36. Naotunne TS, et al. 1991. Cytokines kill malaria parasites during infection crisis: extracellular complementary factors are essential. J. Exp. Med. 173:523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ng MT, et al. 2010. Increase in NF-kappaB binding affinity of the variant C allele of the Toll-like receptor 9 −1237T/C polymorphism is associated with Helicobacter pylori-induced gastric disease. Infect. Immun. 78:1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nussler AK, et al. 1993. In vivo induction of the nitric oxide pathway in hepatocytes after injection with irradiated malaria sporozoites, malaria blood parasites or adjuvants. Eur. J. Immunol. 23:882–887 [DOI] [PubMed] [Google Scholar]
- 39. Obonyo CO, Vulule J, Akhwale WS, Grobbee DE. 2007. In-hospital morbidity and mortality due to severe malarial anemia in western Kenya. Am. J. Trop. Med. Hyg. 77:23–28 [PubMed] [Google Scholar]
- 40. Omar AH, et al. 2012. Toll-like receptor 9 (TLR9) polymorphism associated with symptomatic malaria: a cohort study. Malar. J. 11:168 doi:10.1186/1475-2875-11-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Omi K, et al. 2002. Absence of association between the Fc gamma receptor IIIA-176F/V polymorphism and the severity of malaria in Thai. Jpn. J. Infect. Dis. 55:167–169 [PubMed] [Google Scholar]
- 42. Ong'echa JM, Davenport GC, Vulule JM, Hittner JB, Perkins DJ. 2011. Identification of inflammatory biomarkers for pediatric malarial anemia severity using novel statistical methods. Infect. Immun. 79:4674–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ong'echa JM, et al. 2006. Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic Plasmodium falciparum transmission area. Am. J. Trop. Med. Hyg. 74:376–385 [PubMed] [Google Scholar]
- 44. Oswald IP, Wynn TA, Sher A, James SL. 1992. Interleukin 10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor alpha required as a costimulatory factor for interferon gamma-induced activation. Proc. Natl. Acad. Sci. U. S. A. 89:8676–8680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Otieno RO, et al. 2006. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS 20:275–280 [DOI] [PubMed] [Google Scholar]
- 46. Ouma C, et al. 2012. Functional haplotypes of Fc gamma (Fcgamma) receptor (FcgammaRIIA and FcgammaRIIIB) predict risk to repeated episodes of severe malarial anemia and mortality in Kenyan children. Hum. Genet. 131:289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ouma C, et al. 2006. Association of FCgamma receptor IIA (CD32) polymorphism with malarial anemia and high-density parasitemia in infants and young children. Am. J. Trop. Med. Hyg. 74:573–577 [PubMed] [Google Scholar]
- 48. Parroche P, et al. 2007. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc. Natl. Acad. Sci. U. S. A. 104:1919–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Phillips RE, et al. 1986. The importance of anaemia in cerebral and uncomplicated falciparum malaria: role of complications, dyserythropoiesis and iron sequestration. Q. J. Med. 58:305–323 [PubMed] [Google Scholar]
- 50. Pichyangkul S, et al. 2004. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J. Immunol. 172:4926–4933 [DOI] [PubMed] [Google Scholar]
- 51. Price RN, et al. 2001. Factors contributing to anemia after uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 65:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ravetch JV, Kinet JP. 1991. Fc receptors. Annu. Rev. Immunol. 9:457–492 [DOI] [PubMed] [Google Scholar]
- 53. Sam-Agudu NA, et al. 2010. TLR9 polymorphisms are associated with altered IFN-gamma levels in children with cerebral malaria. Am. J. Trop. Med. Hyg. 82:548–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stevenson MM, Tam MF, Wolf SF, Sher A. 1995. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J. Immunol. 155:2545–2556 [PubMed] [Google Scholar]
- 55. Su Z, Stevenson MM. 2000. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect. Immun. 68:4399–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tangteerawatana P, et al. 2007. Relative levels of IL4 and IFN-gamma in complicated malaria: association with IL4 polymorphism and peripheral parasitemia. Acta Trop. 101:258–265 [DOI] [PubMed] [Google Scholar]
- 57. Taylor RR, Allen SJ, Greenwood BM, Riley EM. 1998. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am. J. Trop. Med. Hyg. 58:406–413 [DOI] [PubMed] [Google Scholar]
- 58. Tishkoff SA, Williams SM. 2002. Genetic analysis of African populations: human evolution and complex disease. Nat. Rev. Genet. 3:611–621 [DOI] [PubMed] [Google Scholar]
- 59. Torre D, et al. 2002. Role of Th1 and Th2 cytokines in immune response to uncomplicated Plasmodium falciparum malaria. Clin. Diagn. Lab. Immunol. 9:348–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tsuji M, et al. 1995. Development of antimalaria immunity in mice lacking IFN-gamma receptor. J. Immunol. 154:5338–5344 [PubMed] [Google Scholar]
- 61. van Sorge NM, van der Pol WL, van de Winkel JG. 2003. FcgammaR polymorphisms: implications for function, disease susceptibility and immunotherapy. Tissue Antigens 61:189–202 [DOI] [PubMed] [Google Scholar]
- 62. Were T, et al. 2011. Bacteremia in Kenyan children presenting with malaria. J. Clin. Microbiol. 49:671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. WHO 2010. World malaria report. WHO Press, Geneva Switzerland [Google Scholar]
- 64. Xu H, et al. 2002. The mechanism and significance of deletion of parasite-specific CD4(+) T cells in malaria infection. J. Exp. Med. 195:881–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zakeri S, Pirahmadi S, Mehrizi AA, Djadid ND. 2011. Genetic variation of TLR-4, TLR-9 and TIRAP genes in Iranian malaria patients. Malar. J. 10:77 doi:10.1186/1475-2875-10-77 [DOI] [PMC free article] [PubMed] [Google Scholar]