Abstract
Uropathogenic Escherichia coli (UPEC) strains suppress the acute inflammatory response in the urinary tract to ensure access to the intracellular uroepithelial niche that supports the propagation of infection. Our understanding of this initial cross talk between host and pathogen is incomplete. Here we report the identification of a previously uncharacterized periplasmic protein, YbcL, encoded by UPEC that contributes to immune modulation in the urinary tract by suppressing acute neutrophil migration. In contrast to wild-type UPEC, an isogenic strain lacking ybcL expression (UTI89 ΔybcL) failed to suppress transepithelial polymorphonuclear leukocyte (PMN) migration in vitro, a defect complemented by expressing ybcL episomally. YbcL homologs are present in many E. coli genomes; expression of the YbcL variant encoded by nonpathogenic E. coli K-12 strain MG1655 (YbcLMG) failed to complement the UTI89 ΔybcL defect, whereas expression of the UPEC YbcL variant (YbcLUTI) in MG1655 conferred the capacity for suppressing PMN migration. This phenotypic difference was due to a single amino acid difference (V78T) between the two YbcL homologs, and a majority of clinical UPEC strains examined were found to encode the suppressive YbcL variant. Purified YbcLUTI protein suppressed PMN migration in response to live or killed MG1655, and YbcLUTI was detected in the supernatant during UPEC infection of bladder epithelial cells or PMNs. Lastly, early PMN influx to murine bladder tissue was augmented upon in vivo infection with UTI89 ΔybcL compared with wild-type UPEC. Our findings demonstrate a role for UPEC YbcL in suppression of the innate immune response during urinary tract infection.
INTRODUCTION
Urinary tract infections (UTI) are among the most common bacterial infections in the United States, resulting in over $2 billion in direct and indirect costs (11). Uncomplicated UTI primarily afflict otherwise healthy women, though anatomical and urodynamic abnormalities, genetic variation, and behavior can predispose individuals to infection. Despite appropriate antibiotic therapy, resolution is often short-lived, and recurrent UTI are a major problem (25% of women experience recurrent infection within 6 months of initial infection) (11). As the gastrointestinal (GI) tract serves as a reservoir for uropathogenic bacteria, recurrent infections are typically thought to arise through reinoculation of the urinary tract with fecal flora. However, recent investigations have identified a bacterial reservoir within the bladder epithelium that is refractory to antibiotic and immune clearance and may also contribute to recurrence (28, 31). The recent emergence of antibiotic-resistant isolates further complicates the effective treatment of UTI (37).
The majority of community-onset UTI are caused by a heterogeneous group of uropathogenic Escherichia coli (UPEC) strains that employ a variety of strategies to effectively colonize and persist within the urinary tract. This is evidenced by an array of disease manifestations, which include asymptomatic bacteriuria, acute and recurrent cystitis, and pyelonephritis. Investigations using a murine model of cystitis and UPEC isolate UTI89 have revealed a complex pathogenic cascade that begins with bacterial binding and invasion of the superficial umbrella cells of the bladder epithelium through type 1 pilus-uroplakin interactions (24, 25, 38). Internalized bacteria rapidly multiply within the epithelial cell cytoplasm to form intracellular bacterial communities (IBCs) that are protected from the mounting immune response (2, 26). Expansion of the IBC and associated epithelial cell rupture release UPEC to initiate binding and invasion events with neighboring cells, leading to additional rounds of IBC formation and propagating the infection (19). The importance of bacterial amplification within the intracellular niche for UPEC pathogenesis is demonstrated by the attenuation of UPEC mutants unable to form mature IBCs (1, 29), the conservation of IBC formation among clinical UPEC isolates in multiple murine backgrounds (12), and the presence of IBCs in samples from human patients (30). Given the significance of the IBC, the events that precede bacterial invasion facilitating intracellular replication likely dictate disease outcome.
As the urinary tract is typically a sterile environment, the proliferation of UPEC within the bladder elicits a robust inflammatory response characterized by the production of cytokines and chemokines and the recruitment of leukocytes, primarily polymorphonuclear leukocytes (PMN) or neutrophils, which are essential for clearance of bacteria from the urinary tract (13). UPEC strains have acquired mechanisms to modulate the innate immune response during acute infection to access the intracellular niche (reviewed in reference 17). Recent studies have demonstrated inhibition of proinflammatory signaling pathways and attenuated cytokine production by cultured bladder epithelial cells during infection with UPEC relative to nonpathogenic E. coli (3, 15, 18, 20). Similarly, UPEC strains inhibit PMN functions such as production of reactive oxygen species, phagocytosis, and chemotaxis (9, 10, 23). Though bacterial effectors responsible for some of these phenotypes have been identified in some UPEC strains, the conservation of innate immune modulation (3, 15) and the considerable genome plasticity among UPEC strains (5, 6, 33) suggest that additional mechanisms of immune modulation exist.
In this study, we identified a previously uncharacterized bacterial protein, YbcL, that contributes to modulation of the host immune response by UPEC during acute UTI. While both nonpathogenic and uropathogenic E. coli strains encode YbcL homologs, only the uropathogenic variant, YbcLUTI, suppressed PMN migration in an in vitro model of acute inflammation, dependent upon a threonine at amino acid 78 (where the nonpathogenic allele encodes a valine). The suppressive phenotype was conferred upon the nonpathogenic strain E. coli K-12 MG1655 by episomal expression of the YbcLUTI variant or by addition of purified YbcLUTI protein to the bacterial inoculum. Furthermore, YbcLUTI was detected in the supernatant during UPEC infection of bladder epithelial cells and PMN in vitro, and YbcLUTI suppressed PMN migration to the bladder at early time points in a murine cystitis model. Taken together, these results describe a novel bacterial product that contributes to UPEC pathogenesis by influencing the innate immune response in the urinary tract.
MATERIALS AND METHODS
Bacterial strains and culture.
E. coli strains were grown statically in Luria-Bertani (LB) broth at 37°C for 18 h. Where indicated, chloramphenicol, ampicillin, or isopropyl β-d-1-thiogalactopyranoside (IPTG) was added at 20 μg/ml, 100 μg/ml, or 100 μM, respectively. UPEC strain UTI89 was isolated from a patient with cystitis (6), and MG1655 is a well-characterized K-12 laboratory strain that is type 1 piliated (4). Heat-killed bacterial suspensions were generated by a 30-min incubation at 55°C, and an aliquot of the suspension was plated to confirm bacterial death. UTI89 ybcL::cat (also denoted UTI89 ΔybcL) was created by linear transformation of UTI89/pKM208 (27) with a fragment amplified from template plasmid pKD3 (8) using the primers JLP266 and JLP267 (primer sequences are given in Table 1); the deletion was verified by direct sequencing. For complementation experiments, a plasmid encoding YbcL with a C-terminal FLAG tag under the control of an IPTG-inducible promoter was constructed. The ybcL open reading frame (ORF) carried by UTI89 was amplified from genomic DNA using primers MEL23 and MEL24, with the reverse primer containing the FLAG epitope sequence. The fragment was digested with BamHI and XbaI and then ligated into pTRC99A (Ampr) which had been similarly digested. Transformed clones of E. coli Top10 (Invitrogen) were selected on ampicillin plates and tested by colony PCR. Accuracy of the resulting pYbcLUTI construct was confirmed by direct sequencing. Using a similar strategy, ybcL carried by MG1655 was amplified using primers MEL62 and MEL24 and ligated into pTRC99A to generate pYbcLMG. The QuikChange site-directed mutagenesis kit (Stratagene) was used to generate point mutations at the ybcL codon for residue 78. Primers MEL69 and MEL70 and template plasmid pYbcLUTI were used to generate pYbcLUTI(T78V). Primers MEL237 and MEL238 and template plasmid pYbcLUTI were used to generate pYbcLUTI(T78A). Primers MEL67 and MEL68 and template plasmid pYbcLMG were used to generate pYbcLMG(V78T). The expected mutations were verified by direct sequencing.
Table 1.
Primers used in this study
| Primer | Sequence (5′ → 3′) |
|---|---|
| JLP266 | TCGTTTCAAGTGTATTGGCATTCATAACATTTTCTGCGCAGTGTAGGCTGGAGCTGCTTC |
| JLP267 | TAAACTGGTGTTATCTCAGCGGTTGCAATTTTATTGGCATCATATGAATATCCTCCTTAG |
| MEL23 | GCATGGATCCGGTCACAACAATGAGG |
| MEL24 | GCATTCTAGACTACTTGTCATCGTCGTCCTTGTAGTCCTTTATCTCATAAACT |
| MEL30 | GCATTCTAGACTAGTGGTGATGGTGATGATGCTTTATCTCATAAACT |
| MEL62 | GCATGGATCCGGTCATAACAAAGAGGT |
| MEL67 | GCAACAGTAACATATTTGCCCACTGATGCAGGGAGACGTGATGG |
| MEL68 | CCATCACGTCTCCCTGCATCAGTGGGCAAATATGTTACTGTTGC |
| MEL69 | GCAACTGTAACATATTTGCCCGTTGATGCAGGAAGACGTGATGG |
| MEL70 | CCATCACGTCTTCCTGCATCAACGGGCAAATATGTTACAGTTGC |
| MEL231 | ATGAAAAMACTTATCGTTTCAA |
| MEL232 | CTACTTTATCTCATAAACTGGTG |
| MEL237 | GCAACTGTAACATATTTGCCCGCTGATGCAGGAAGACGTGATGG |
| MEL238 | CCATCACGTCTTCCTGCATCAGCGGGCAAATATGTTACAGTTGC |
| MEL286 | GCAT GGATCC ATGAAACTGACAACACATCATCTACGGGCG |
| MEL287 | GTTGCTCTCCTGTTTTTATTTCATTACTAGTGACCTGAAA TTTAATATGCTTTTCATCGC |
| MEL288 | AGGTTGCGACCAGAGCAGCAGCGATGAAAAGCATATTAAATTTCAGGTCACTAGTAATGA |
| MEL289 | TTCATTACTAGTGACCTGAAA TTTAATATGCTTTTCATCGCTGCTGCTCTGGCTGCAACC |
| MEL290 | AGGTTGCAGCCAGAGCAGCAGCGATGAAAAGCATATTAAATTTCAGGTCACTAGTAATGA |
Membrane-tethered YbcL variants were designed according to the findings of Yamaguchi and colleagues (34). To tether YbcL to the bacterial inner membrane (YbcLIM), we generated a fusion protein between NlpA (an inner membrane lipoprotein) and the mature form of YbcL. Using UTI89 genomic DNA as the template, code for the signal sequence and the first 12 amino acids of NlpA as well as a region homologous to the N terminus of YbcL was amplified by primers MEL286 and MEL287. Sequence encoding the mature form of YbcL, including a region homologous to NlpA, was amplified by primers MEL288 and MEL24. These PCR products were annealed and extended by PCR, digested with BamHI and XbaI, and ligated into pTRC99A. Using a similar approach, we generated an NlpA-YbcL fusion protein that localized to the outer membrane (YbcLOM) by mutating the second amino acid in NlpA from an aspartic acid to a serine (34). Primers MEL286 and MEL289 were used to amplify the NlpA product containing the amino acid mutation, and primers MEL290 and MEL24 were used to amplify the YbcL product. The PCR products were cloned into pTRC99A as described above. The two constructs were verified by direct sequencing. Equivalent expression of all YbcL variants after IPTG induction was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis of bacterial fractions.
Tissue culture.
The 5637 bladder epithelial cell line (derived from a bladder carcinoma; ATCC HTB-9) was obtained from the American Type Culture Collection. Cells were cultured and experiments were conducted in RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum (Sigma) at 37°C in a humidified atmosphere with 5% CO2 unless otherwise noted. Preparation of inverted epithelial monolayers has been previously described (23). Briefly, ∼ 105 5637 cells were seeded on an inverted Transwell insert (0.33-cm2 polycarbonate membranes with 3-μm diameter pores; Corning number 3472) and allowed to adhere to the membrane for 16 h. Transwells were then moved to a 24-well plate containing tissue culture medium, and additional medium was added to the upper reservoir. Fresh medium was applied every 2 days until the epithelial monolayers reached confluence, as assessed by impermeability to liquid (21).
Human PMN isolation.
In accordance with a protocol approved by the Washington University Human Research Protection Office, PMN were isolated from venous blood of healthy adult volunteers after verbal consent was obtained. The isolation of human PMN from blood was adapted from a previously published protocol (14). In short, erythrocyte numbers were reduced by dextran sedimentation, contaminating immune cells (other than PMN) were removed using a Ficoll density gradient (Ficoll-Paque Plus; GE Healthcare), and the remaining erythrocytes were lysed hypotonically. PMN viability was >99% as assessed by trypan blue exclusion, and purity was >99% as determined by visualization of nuclear morphology after staining (Hema3; Fisher Scientific). Purified PMN were resuspended in RPMI 1640 medium (Gibco) to a concentration of 107 PMN/ml and used immediately.
Transepithelial PMN migration assay.
Transepithelial PMN migration assays were conducted in accordance with previously published protocols (23). Briefly, Transwells with confluent 5637 monolayers were washed three times in RPMI. Bacterial cells were washed in phosphate-buffered saline (PBS) and diluted in RPMI. A bacterial inoculum of 6 × 106 CFU/ml (a multiplicity of infection [MOI] of 40 CFU/cell) or an equivalent volume of RPMI was applied to the apical sides of inverted Transwells and incubated for 1 h at 37°C. The Transwells were then righted into 24-well plates (Ultra Low Attachment plates; Corning number 3473) containing 0.6 ml RPMI, and 106 PMN were added to the upper reservoir. After 1 h at 37°C, PMN in the lower reservoir were collected and enumerated using a hemacytometer, and the number of PMN recruited into the lower reservoir was normalized to input PMN. Data represent the mean and standard deviation from at least three independent experiments. Statistically significant differences were determined using an unpaired Student's t test.
To generate conditioned medium, 5637 cells grown to confluence in 15-cm dishes were infected with the indicated strains of E. coli at an MOI of 40. After 1 h of incubation at 37°C, the supernatant was collected and filter sterilized using syringe-driven filter units (0.22-μm pore size; Millipore). The filter-sterilized supernatant (conditioned medium) was used as the inoculum and replaced 0.6 ml RPMI in the lower reservoir in the transepithelial PMN migration assay. PMN migration in response to the conditioned medium was assessed as described above.
YbcL localization by Western blotting.
To mimic the transepithelial PMN migration assay, 5637 cells or freshly isolated PMN were infected with the indicated strains of E. coli at an MOI of 40 or 10, respectively. After 1 h of incubation at 37°C, the supernatant was collected and the eukaryotic cells were washed with PBS and lysed using 0.1% Triton X-100 containing protease inhibitors (Roche). The supernatant and cell lysate samples were filter sterilized using syringe-driven filter units (0.22-μm pore size; Millipore), and protein was precipitated using 15% trichloroacetic acid (TCA) (Sigma). Samples were separated by SDS-PAGE using 4 to 20% precast gels (Bio-Rad) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). After blocking with 2% nonfat milk plus 2% bovine serum albumin (BSA) (Sigma), blots were probed with mouse anti-FLAG antibody (1:1,000; Sigma) followed by goat anti-mouse IgG antibody (1:2,000; Sigma) and were developed using Tropix CDP-Star (Applied Biosystems).
Sequencing of ybcL alleles in clinical isolates.
A collection of 74 UPEC isolates, including strains from women with acute cystitis, recurrent cystitis, asymptomatic bacteriuria, or pyelonephritis, was obtained from Scott Hultgren (6, 12, 30). Chromosomal DNA was isolated from each UPEC strain using the Wizard genomic DNA purification kit (Promega) according to the manufacturer's instructions. Primers MEL231 and MEL232 were designed to bind conserved regions within the ybcL ORF identified through nucleotide alignment of ybcL alleles present in sequenced E. coli genomes. PCR was conducted using Pfu DNA polymerase (Stratagene), and product formation was assessed by agarose gel electrophoresis. Amplicons of the predicted size were purified with the QIAquick PCR purification kit (Qiagen) and submitted for sequencing (SeqWright). Nucleotide alignments were performed using Vector NTI software (Invitrogen). The prevalences of specific amino acids at position 78 in YbcL proteins from various E. coli groups were compared using Fisher's exact test.
Purification of YbcL variants.
ybcL alleles were amplified from the constructs described above using the following primer sets: for pYbcLUTI and pYbcLUTI(T78V), MEL23 and MEL30; for pYbcLMG and pYbcLMG(V78T), MEL62 and MEL30 (where the reverse primer contains a sequence encoding a 6-histidine tag [6×His] in place of the FLAG epitope). The amplicons were cloned into pTRC99A as described above. The constructs were confirmed by direct sequencing, and expression of the YbcL variants was confirmed by SDS-PAGE. Periplasms were prepared from E. coli Top10 carrying these plasmids and dialyzed overnight in PBS before being applied to an Ni-nitrilotriacetic acid (NTA) column (Qiagen). Protein purification was conducted according to the instructions of the manufacturer, using an elution buffer containing 200 mM imidazole. Protein concentrations were determined using a bicinchoninic acid protein assay (Thermo Scientific).
Murine cystitis and tissue MPO activity assay.
All animal procedures were approved in advance by the Animal Studies Committee at Washington University. In accordance with a well-described model of murine cystitis (16), 8-week-old female C3H/HeN mice (Harlan) were transurethrally inoculated with 50 μl of bacterial suspension (2.5 × 107 CFU) or sterile PBS. At 1 h postinfection (p.i.), animals were sacrificed, bladders were harvested and homogenized in 1 ml PBS, and an aliquot of each bladder homogenate was plated on LB agar to determine tissue bacterial burden. The myeloperoxidase (MPO) content of bladder tissue was measured as described previously (23). Aliquots of undiluted bladder homogenates were transferred to a 96-well plate, and a standard curve was generated using purified MPO. Samples were incubated with the reaction buffer for 1 h (Fluoro MPO; Cell Technology) according to the manufacturer's instructions. Enzyme activity was measured by fluorescent detection of an MPO product using a microplate reader (Synergy 2; BioTek). MPO activity in the bladder samples is reported in units/ml, and data points represent the means of triplicate measurements from individual bladders. At least 12 mice were infected for each bacterial strain tested. Differences in MPO levels were examined for significance using an unpaired Student t test, and bacterial loads were compared using the Mann-Whitney U test.
RESULTS
YbcL encoded by UTI89 suppresses transepithelial PMN migration.
Given the ability of UPEC strain UTI89 to suppress innate immune responses by undefined mechanisms (17, 18, 23), we sought to further characterize the early host-pathogen interaction. Guided by preliminary transcriptional profiling data (23; J. Loughman, unpublished data), we identified a periplasmic protein, YbcL, with structural homology to mammalian Raf-1 kinase-inhibitory protein (RKIP) (32), a modulator of eukaryotic signal transduction pathways (22, 35, 36). To investigate a role for YbcL in suppression of innate responses by UTI89, we utilized an in vitro model of acute inflammation that quantifies PMN migration across a bladder epithelial monolayer. Transwells bearing confluent 5637 uroepithelial monolayers were infected with E. coli strains or mock infected for 1 h before freshly isolated human PMN were applied to the upper reservoir, and PMN migration into the lower reservoir was enumerated using a hemacytometer. Consistent with our prior results (23), the nonpathogenic E. coli strain MG1655 stimulated robust PMN migration, while infection with the UPEC strain UTI89 resulted in significantly fewer PMN in the lower reservoir (Fig. 1) (P < 0.0001). The low level of PMN migration upon UPEC infection reflects active suppression of the inflammatory response by UPEC rather than failure to induce inflammatory signaling, as coinfection with MG1655 plus UTI89 yields the uropathogenic phenotype (23). In contrast to wild-type UTI89, UTI89 ΔybcL elicited significantly more PMN (P < 0.0001), and episomal expression of YbcL in the ybcL mutant restored wild-type levels of PMN migration (Fig. 1). The differential PMN migration observed was not the result of differences in either 5637 or PMN viability; both cell types survived equally well in the presence of the E. coli strains used at early time points, as assessed by lactate dehydrogenase (LDH) release (data not shown). These data suggest that YbcL encoded by UTI89 contributes to UPEC-mediated suppression of PMN migration.
Fig 1.
UPEC YbcL suppresses transepithelial PMN migration in vitro. 5637 bladder epithelial cell monolayers grown on Transwell inserts were infected at their apical surfaces with the indicated strains of E. coli or mock infected, and freshly isolated human PMN were added at the basolateral surface. The number of PMN recruited to the apical surface was enumerated at 1 h p.i. and is shown normalized to input PMN. Infection with MG1655 or UTI89 ΔybcL elicited significantly more PMN than that with wild-type UTI89 (*, P < 0.0001).
Suppression of PMN migration by YbcLUTI relies on threonine 78.
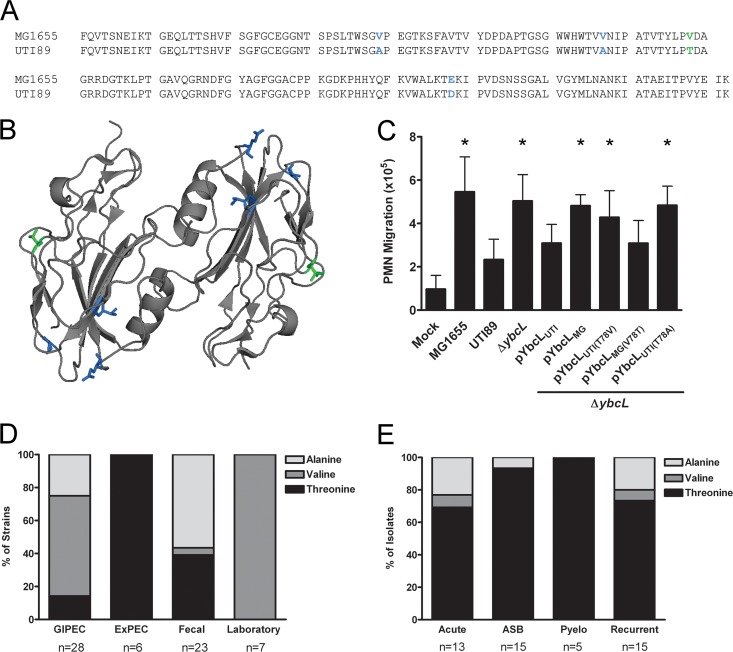
To investigate the properties of YbcL responsible for UPEC-specific suppression of the innate immune response, we first explored sequence conservation among YbcL homologs encoded by E. coli. The nonpathogenic strain MG1655 (4) contains a ybcL allele that is 95% identical at the nucleotide level to the UTI89 allele (6), resulting in six predicted amino acid differences. Four are contained within the mature protein, and three of these amino acid differences represent conservative or semiconservative changes (Fig. 2A and B, blue). In the single nonconservative difference, the UTI89 variant (denoted YbcLUTI) contains a threonine at position 78, while the MG1655 variant (YbcLMG) contains a valine (Fig. 2A and B, green). The crystal structure of YbcL encoded by K-12 strain W3110 (100% identical at the amino acid level to YbcLMG) has been solved (32). However, any effect that these amino acid differences may have on the tertiary structure of YbcLUTI is unclear.
Fig 2.
Threonine 78 is required for suppression of PMN migration by YbcLUTI. (A and B) Amino acid alignment of mature YbcL homologs encoded by MG1655 and UTI89 (A) and a ribbon diagram of YbcL encoded by K-12 strain W3110 (dimeric as in its crystal structure [32]) (B). Conservative and semiconservative differences are depicted in blue, and the nonconservative difference is depicted in green. (C) Complementation of UTI89 ΔybcL by episomal expression of YbcL variants was assessed using an in vitro model of transepithelial PMN migration. Experiments were conducted and data are represented as described for Fig. 1. An asterisk denotes a statistically significant (P < 0.005) increase in PMN migration compared to that with wild-type UTI89. (D and E) The presence of threonine at position 78 correlates with the suppression of PMN migration by YbcL. The distribution of amino acids at position 78 in YbcL homologs encoded by sequenced E. coli strains (D) or clinical UPEC isolates (E) (asymptomatic bacteriuria [ASB], acute cystitis [Acute], recurrent cystitis [Recurrent], and pyelonephritis [Pyelo]) is shown. Overall, threonine 78 is present in 83% of UPEC strains (43/52) compared to 25% of other E. coli strains (15/60) (P < 0.0001).
Because MG1655 was unable to suppress in vitro PMN migration and YbcL contributed to this phenotype during infection with UTI89, we hypothesized that the YbcL variants encoded by these E. coli strains were functionally divergent. In accordance with this hypothesis, episomal expression of the YbcLMG variant failed to complement UTI89 ΔybcL in the transepithelial PMN migration model (Fig. 2C) (P < 0.0001 compared to UTI89). To assess the importance of the nonconservative amino acid substitution in suppression of PMN migration by YbcLUTI, we generated additional YbcL variants. Expression of YbcLUTI(T78V) (containing a threonine-to-valine mutation at position 78) in UTI89 ΔybcL did not suppress PMN migration (Fig. 2C) (P < 0.005), demonstrating that this mutation resulted in a loss of function for the uropathogenic variant. Conversely, expression of YbcLMG(V78T) (containing a valine-to-threonine mutation at position 78) in UTI89 ΔybcL reduced PMN levels in the lower reservoir (Fig. 2C), demonstrating a gain of function for the nonpathogenic variant. These data demonstrate the functional divergence of the YbcL variants encoded by nonpathogenic and uropathogenic E. coli strains and highlight the importance of threonine 78 in YbcL for UPEC-mediated suppression of PMN migration.
Given the functional consequence of the nonconservative amino acid difference between MG1655 and UTI89 YbcL variants, we hypothesized that threonine 78 would be conserved among UPEC. We therefore assessed the distribution of YbcL homologs among sequenced E. coli strains, focusing on the amino acid at position 78. A BLAST search using the full UTI89 YbcL amino acid sequence revealed YbcL homologs in many but not all sequenced E. coli genomes, including laboratory strains, uncharacterized fecal isolates, and human pathogens classified as either gastrointestinal E. coli (GIPEC) (including adherent-invasive E. coli [AIEC], enteroaggregative E. coli [EAEC], and enterohemorrhagic E. coli [EHEC]) or extraintestinal E. coli (ExPEC) (including neonatal meningitis E. coli [NMEC], avian-pathogenic E. coli [APEC] and UPEC). Among the sequenced strains encoding YbcL homologs, position 78 contained a threonine in 100% of ExPEC isolates, compared to 39% of uncharacterized fecal isolates, 14% of GIPEC isolates (all that contained T78 were AIEC), and 0% of laboratory strains (Fig. 2D) (P < 0.05 for ExPEC versus each group). To further examine the correlation between threonine 78 in YbcL and ExPEC, we amplified and sequenced ybcL alleles from clinical UPEC isolates associated with a range of disease manifestations (6, 12, 30). We were unable to generate an amplicon from 26 of the 74 isolates despite using multiple primer sets, suggesting that like sequenced E. coli strains, clinical isolates also vary in their genomic content. Among 48 clinical isolates from which a ybcL homolog could be amplified, 39 (81%) contained a threonine at position 78 (Fig. 2E). In total, 83% of UPEC strains (including both sequenced and clinical strains), compared to 25% of other E. coli strains, encoded a threonine at position 78 (P < 0.0001).
In addition to threonine and valine, alanine was also found at position 78 in some YbcL homologs encoded by these various E. coli strains. As with valine, episomal expression of the alanine-containing variant YbcLUTI(T78A) failed to complement UTI89 ΔybcL in the transepithelial PMN migration model (Fig. 2C) (P < 0.0001). Taken together, these data demonstrate the prevalence of threonine 78 in YbcL among UPEC strains and illustrate its importance in suppression of the innate immune response by these diverse uropathogens.
YbcLUTI confers suppressive activity on nonpathogenic E. coli MG1655.
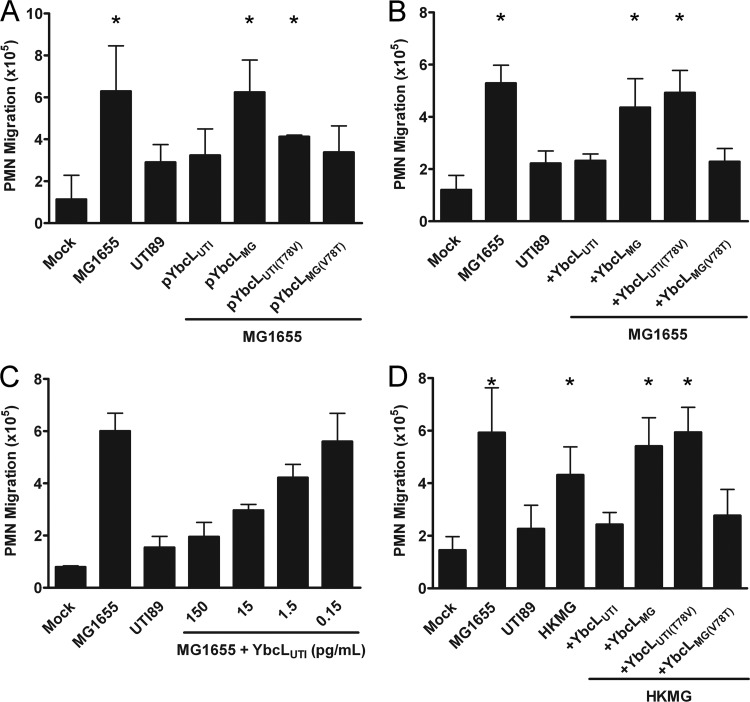
We next aimed to investigate whether other UPEC-encoded factors were required for YbcLUTI-mediated suppression of PMN migration. To define the bacterial context required for this phenotype, we assessed PMN migration in response to MG1655 episomally expressing a panel of YbcL variants. Expression of YbcLUTI or YbcLMG(V78T) in MG1655 yielded PMN migration levels similar to those for wild-type UTI89 (Fig. 3A), demonstrating conferral of the uropathogenic phenotype upon the nonpathogenic strain. In contrast, episomal expression of YbcLMG or YbcLUTI(T78V) in MG1655 allowed significantly more PMN migration than UTI89 (Fig. 3A) (P < 0.05), consistent with the nonpathogenic phenotype.
Fig 3.
YbcLUTI confers suppressive activity on nonpathogenic E. coli. (A) The suppression of PMN migration by MG1655 episomally expressing the YbcL variants was evaluated using the transepithelial PMN migration model. (B to D) This model was also used to assess changes in PMN migration caused by the addition of purified YbcL variants to the bacterial stimulus, live MG1655 (B and C), or heat-killed MG1655 (HKMG) (D). Purified YbcL variants were added to the bacterial stimulus immediately before infection of the epithelial layer at a final concentration of 225 ng/ml unless otherwise indicated. YbcL variants containing a threonine at position 78, YbcLUTI and YbcLMG(V78T), suppressed PMN migration, while YbcL variants containing a valine at this position, YbcLMG and YbcLUTI(T78V), had no effect on PMN migration. Asterisks in panels A, B, and D indicate statistically significant (P < 0.05) increases in PMN migration compared to that with wild-type UTI89.
To demonstrate that suppression of PMN migration was mediated directly by YbcL, we added purified YbcL protein to MG1655 immediately before infection of the epithelial layer. An initial concentration of 225 ng/ml was chosen to approximate the amount of YbcL present in bacterial inocula used above that contained pYbcLUTI, as determined by Western blotting (data not shown). The addition of purified YbcLUTI or YbcLMG(V78T) upon infection with MG1655 resulted in PMN levels similar to those seen upon infection with UTI89 (Fig. 3B). Conversely, MG1655 plus purified YbcLMG or YbcLUTI(T78V) stimulated significantly more PMN migration than the uropathogen UTI89 (Fig. 3B) (P < 0.01). Analogous experiments conducted using these purified YbcL variants and UTI89 ΔybcL as the bacterial stimulus resulted in the same trends in PMN migration (data not shown). Furthermore, YbcLUTI maintained migration-suppressing potency at concentrations as low as 150 pg/ml or 8 pM, and a decrement in effect was observed with further dilution (Fig. 3C).
To explore whether YbcL activity required live bacteria (i.e., an intact periplasm), we next used heat-killed MG1655 (HKMG) as the bacterial stimulus, which elicited robust PMN migration in contrast to UTI89 in the transepithelial PMN migration model (Fig. 3D) (P < 0.05). Infection with HKMG plus purified YbcLUTI or YbcLMG(V78T) yielded the uropathogenic phenotype, eliciting low levels of PMN migration similar to those for UTI89 (Fig. 3D), while the addition of YbcLMG or YbcLUTI(T78V) to the same bacterial stimulus had no effect on PMN migration (Fig. 3D) (P < 0.005), in agreement with data generated using live MG1655. Taken together, these data demonstrate that YbcLUTI confers the capacity to suppress PMN migration upon nonpathogenic E. coli and that this activity is independent of other pathogen-specific attributes or active bacterial processes.
YbcL is secreted from the bacterial periplasm.
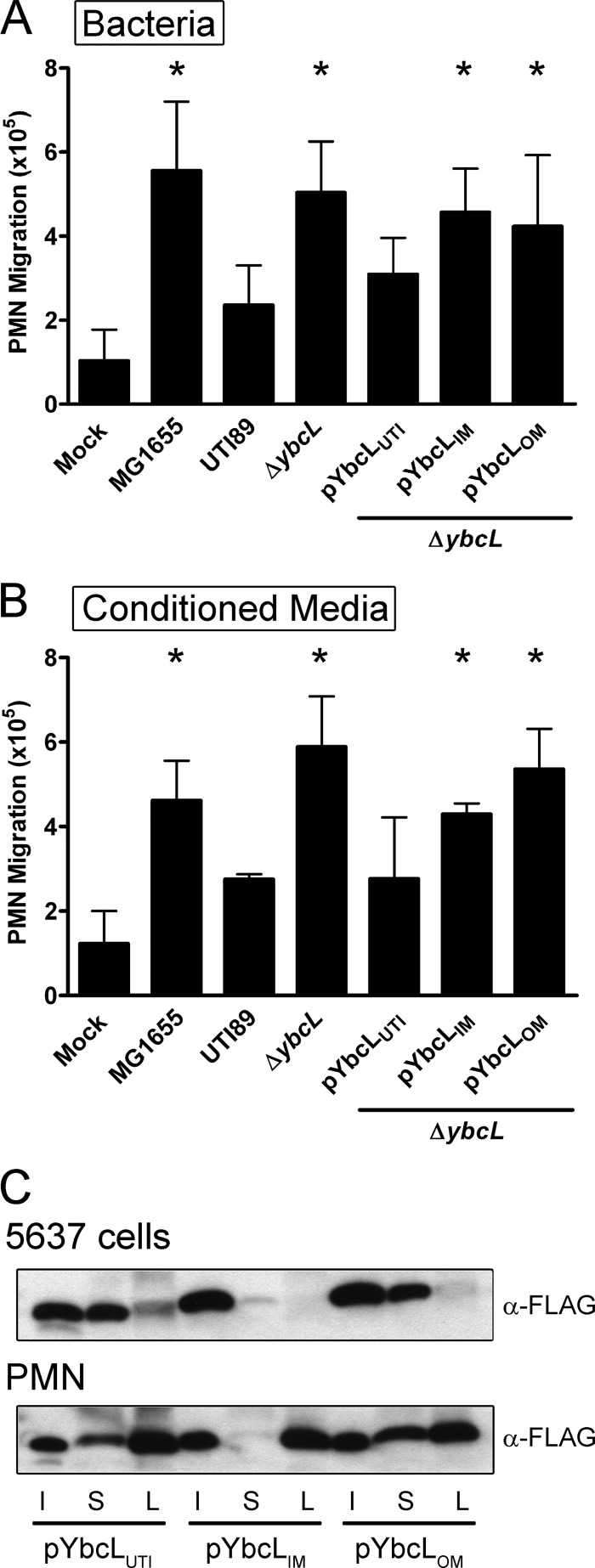
As purified YbcLUTI suppressed PMN migration elicited by both live and heat-killed bacteria in the transepithelial PMN migration model, we hypothesized that YbcLUTI was secreted from the bacterial periplasm during UPEC infection. To demonstrate a requirement for YbcLUTI secretion in the suppression of PMN migration by UTI89, we engineered two fusion proteins composed of the lipoprotein NlpA and YbcL (36) (see Materials and Methods) to tether YbcL to the inner or outer bacterial membrane (YbcLIM and YbcLOM, respectively) and assessed the ability of these variants to complement UTI89 ΔybcL in the transepithelial PMN migration model. UTI89 ΔybcL episomally expressing either YbcLIM or YbcLOM stimulated significantly more PMN migration than wild-type UTI89 (Fig. 4A) (P ≤ 0.005). The membrane-tethered YbcL variants failed to complement the ybcL mutation, suggesting that YbcLUTI does not act to suppress PMN migration from within the bacterial periplasm.
Fig 4.
YbcLUTI is secreted. (A and B) The level of PMN migration elicited by various strains of E. coli (A) or conditioned medium (B) was evaluated using the transepithelial PMN migration model. YbcL variants tethered to either the inner or outer bacterial membrane (YbcLIM or YbcLOM, respectively) were unable to suppress PMN migration when expressed episomally in UTI89 ΔybcL. The trends in PMN migration observed with conditioned medium mimicked those observed when the inoculum included live bacteria. Asterisks in panels A and B indicate statistically significant (P < 0.05) increases in PMN migration compared to that with wild-type UTI89. (C) Localization of YbcL variants was assessed by Western blotting. After 1 h of infection of 5637 cells or PMN with the indicated strains of E. coli, the supernatant (S) and eukaryotic cell lysate (L) fractions were filter sterilized, TCA precipitated and resolved by SDS-PAGE. During infection of either cell type, YbcLUTI and YbcLOM were clearly detected in the supernatant fractions, while YbcLIM was minimally detected in those fractions. All three variants were detected in the PMN lysate; however, only YbcLUTI was detected in the 5637 cell lysate. An equivalent volume of each bacterial inoculum (I) is shown for comparison across strains.
To support these data, we sought to demonstrate secretion of YbcLUTI by wild-type UTI89 during infection of bladder epithelial cells using a biochemical approach. 5637 cells in 10-cm dishes were infected with the indicated strains of E. coli at an MOI of 40 for 1 h at 37°C. The supernatant (conditioned medium) was filter sterilized and used in place of the bacterial inoculum in the transepithelial PMN migration model. Conditioned medium from infection of 5637 cells with UTI89 stimulated a low level of PMN migration (Fig. 4B). In contrast, conditioned medium generated during UTI89 ΔybcL infection stimulated significantly more PMN migration (Fig. 4B) (P < 0.01). This phenotype could be reversed by expression of YbcLUTI in UTI89 ΔybcL but not by expression of either of the membrane-tethered YbcL variants, YbcLIM or YbcLOM (P < 0.01 compared to UTI89). These data suggest that YbcLUTI is secreted from the bacterial periplasm and mediates suppression of PMN migration from the exterior of the bacterial cell.
To corroborate evidence from the transepithelial PMN migration model suggesting that YbcLUTI is secreted, we assessed localization of the YbcL variants during UPEC infection of bladder epithelial cells or neutrophils. 5637 cells or PMN were infected with the indicated strains of E. coli at an MOI of 40 or 10, respectively, for 1 h at 37°C. The supernatant and eukaryotic cell lysate fractions were filter sterilized, concentrated by TCA precipitation, and resolved using SDS-PAGE. During UPEC infection of 5637 cells or PMN, YbcLUTI and YbcLOM were clearly detected in the supernatant, in contrast to YbcLIM, which was minimally detected in that fraction (Fig. 4C). All three YbcL variants were detected in the PMN lysate. However, only YbcLUTI was detected in the 5637 cell lysate (Fig. 4C). When these cell types were infected with either MG1655 or UTI89 ΔybcL episomally expressing the MG1655 YbcL variant, YbcLMG exhibited the same localization pattern as YbcLUTI (data not shown), confirming that the differential PMN migration observed in the transepithelial PMN migration model was not the result of differences in secretion of the YbcL variants. These data demonstrate that YbcLUTI is secreted from the bacterial periplasm during infection of bladder epithelial cells and PMN. Although it was detected in the supernatant, YbcLOM did not complement the ybcL mutant in the transepithelial PMN migration model, suggesting that localization to the supernatant is not sufficient for suppression of PMN migration by YbcLUTI.
YbcLUTI suppresses acute PMN migration in vivo.
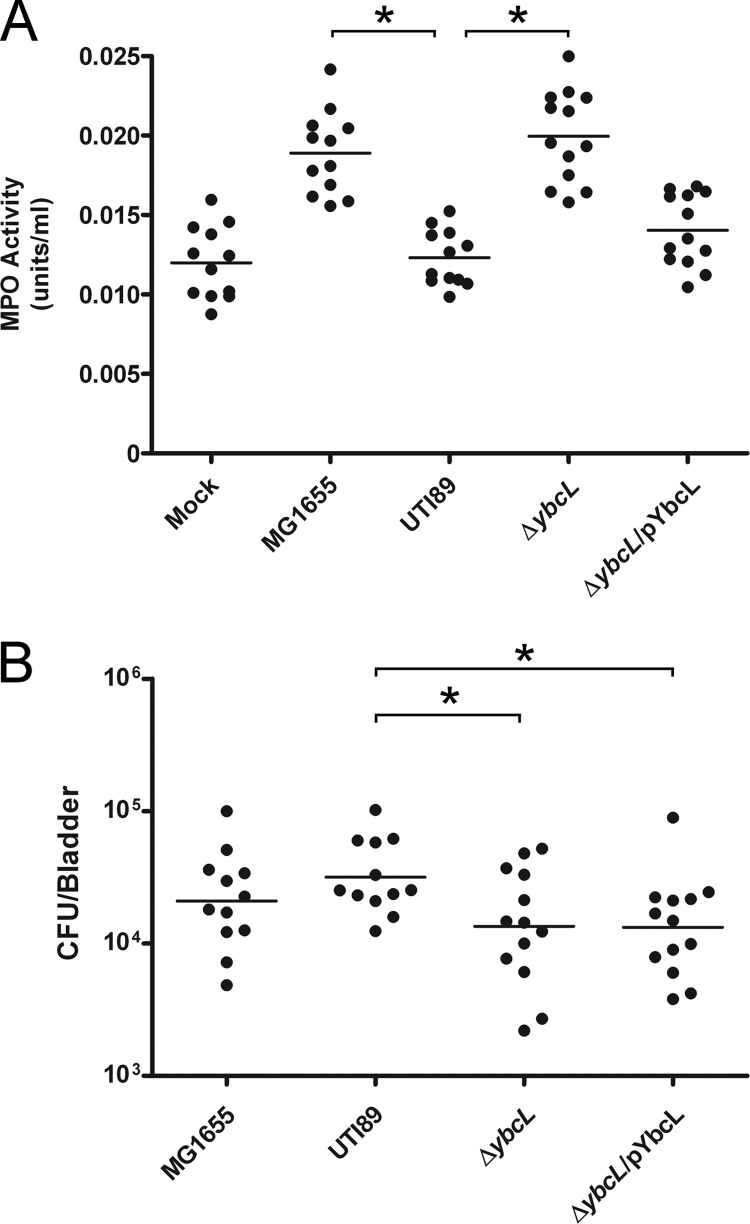
We used a murine model of cystitis to assess a potential contribution by YbcLUTI to UPEC-mediated suppression of the innate response in vivo (16, 23). Female C3H/HeN mice were transurethrally inoculated with the indicated strains of E. coli or PBS, and myeloperoxidase (MPO) activity in bladder homogenates was determined at 1 h p.i. as a surrogate for PMN influx into bladder tissue. In accordance with our in vitro observations, MG1655 and UTI89 ΔybcL elicited significantly more PMN than wild-type UTI89 (Fig. 5A) (P < 0.0001). Suppression of PMN migration was nearly completely restored to the ybcL mutant upon complementation with pYbcLUTI (Fig. 5A). Modestly lower bacterial titers were recovered after infection with the ybcL mutant or the complemented strain compared to wild-type UTI89 (Fig. 5B) (P < 0.05). It is unlikely that these two strains exhibited a defect in colonization at this early time point, as both assembled levels of type 1 pili similar to those for wild-type UTI89 as assessed by microscopy, hemagglutination titers, and in vitro binding and invasion assays using 5637 cells (data not shown). In addition, UTI89 ΔybcL formed IBCs that were indistinguishable from those of wild-type UTI89 as assessed by confocal fluorescence microscopy (data not shown), and bacterial titers recovered from wild-type- or ybcL mutant-infected mice were similar at 6, 16, 24, and 48 hours p.i. and at 1 and 2 weeks p.i. (data not shown). In agreement with results obtained using the in vitro model of inflammation, these in vivo data argue that UPEC-encoded YbcL suppresses early PMN migration in a murine model of cystitis.
Fig 5.
YbcLUTI suppresses acute PMN migration in vivo. C3H/HeN mice were infected with the indicated strains of E. coli or PBS, and bladders were harvested at 1 h p.i. (A) A surrogate for PMN infiltration in the bladder, myeloperoxidase (MPO) activity, was measured in bladder homogenates by fluorescent detection of an MPO product and is represented in units/ml. MG1655 and UTI89 ΔybcL elicited significantly more PMN than wild-type UTI89 (*, P < 0.0001). (B) Bladders infected with UTI89 ΔybcL or the complemented strain showed a small but statistically significant decrease in bacterial load compared to those infected with UTI89 (*, P < 0.05). Horizontal lines indicate the means in both panels.
DISCUSSION
The present study identifies a novel bacterial protein encoded by UPEC that contributes to modulation of the innate immune response during UTI. UPEC-encoded YbcL suppressed early PMN migration in an in vitro model of acute inflammation and an in vivo model of murine cystitis. Examination of the YbcL homolog encoded by the nonpathogenic E. coli K-12 strain MG1655 revealed three conservative or semiconservative and one nonconservative amino acid difference compared to the UTI89 homolog. We demonstrated that threonine at the nonconservative position 78 is required for suppression of PMN migration by the uropathogenic variant YbcLUTI. In contrast, the nonpathogenic variant YbcLMG contains a valine at this position and has no effect on PMN migration. We hypothesize that threonine 78 is required directly or indirectly for protein-protein interactions. Future work will investigate how the identity of a single amino acid dictates YbcL functionality in this model of transepithelial PMN migration.
The presence of YbcL homologs in many but not all E. coli strains exemplifies both the genomic heterogeneity within the species and the variation in mechanisms of immune modulation among pathogenic strains. We were not surprised to find YbcL homologs containing threonine 78 in some uncharacterized fecal isolates and GIPEC strains, as the GI tract serves as a reservoir for UPEC in addition to the resident (commensal) microflora and supports a considerable amount of horizontal gene transfer. Like UPEC in the urinary tract, GIPEC influences local immune responses within the GI tract (7), although it is unclear if YbcL homologs contribute to this phenotype in the gut. Given that the majority of GIPEC strains encode valine- or alanine-containing YbcL homologs, immune modulation in the GI tract by these strains more likely occurs independently of YbcL. In contrast, the prevalence of threonine 78 among UPEC-encoded YbcL homologs suggests that suppression of PMN migration by YbcL is a conserved mechanism of innate immune modulation within the urinary tract. As threonine-containing YbcL homologs are present in UPEC strains that cause asymptomatic bacteriuria and pyelonephritis in addition to acute and recurrent cystitis, it is likely that YbcL contributes to pathogenesis throughout the urinary tract.
Using a murine cystitis model, we demonstrated that YbcL encoded by UTI89 suppresses acute PMN migration to the bladder. Compared to wild-type UTI89, both the ybcL mutant and the complemented strain yielded modestly lower bacterial titers at 1 h p.i. We hypothesize that the lower ybcL mutant titers may be the result of increased PMN recruitment to those bladders, as evidenced by elevated MPO levels. In agreement with that hypothesis, MG1655 titers also trended lower than UTI89 titers at 1 h p.i. The slightly lower titers in the complement-infected bladders might relate to decreased bacterial fitness caused by maintenance of the plasmid or overexpression of YbcLUTI, as PMN levels were similar to those measured in wild-type infection. Examination of IBC formation and bacterial titers at subsequent time points revealed no significant differences between wild-type- and ΔybcL-infected mice, suggesting that YbcL facilitates the establishment of UTI rather than persistence. Considering the large bacterial inoculum (∼107 CFU) and the capacity of IBCs to amplify and propagate infection, it is not surprising that increased PMN recruitment in the UTI89 ΔybcL-infected bladders early did not adversely affect bacterial titers at later time points. In the human urinary tract, where the inoculum is likely to be significantly lower and varying host genetics influence susceptibility to UTI, the activity of YbcLUTI may significantly favor bacterial survival prior to epithelial invasion, tipping the balance toward infection rather than clearance.
Suppression of PMN migration by YbcLUTI was conferred by episomal expression or the addition of purified protein to either live or nonviable MG1655, demonstrating that YbcLUTI functions independently of bacterial context. Using multiple approaches, we demonstrated that YbcL was secreted by UTI89 during infection of bladder epithelial cells or PMN. We were unable to detect YbcLUTI by Western blotting in filter-sterilized, TCA-precipitated conditioned medium from UTI89/pYbcLUTI ΔybcL grown in LB (M. Lau and D. Hunstad, unpublished data), suggesting that secretion of YbcLUTI is regulated. Given that the localization pattern of YbcLMG mimicked the pattern of YbcLUTI during infection of eukaryotic cells, it is unlikely that the amino acid at position 78 regulates secretion. While YbcL was detected in the supernatant, the mode of delivery from the bacterial cell remains unclear, although it is unlikely to be pathogen specific, as the localization pattern of the YbcL variants (YbcLUTI and YbcLMG) was independent of the bacterial strain, MG1655 or UTI89 ΔybcL. In light of these observations, we hypothesize that secretion of YbcLUTI, a periplasmic protein, occurs through outer membrane proteins (such as secretins) or via outer membrane vesicles (OMVs). Given its presence in the bacterial outer membrane, we hypothesize that YbcLOM in the supernatant fraction during UPEC infection is associated with OMVs and that the membrane tether prevents that YbcL variant from suppressing PMN migration. As periplasmic proteins as well as outer membrane proteins are packaged in OMVs and precedent exists for the delivery of UPEC effectors via OMVs (e.g., cytotoxic necrotizing factor 1) (9), it is possible that these vesicles mediate YbcLUTI secretion. Future work will address these hypotheses.
In addition to localization to the supernatant during UPEC infection, similar levels of the three YbcL variants were also detected in the filtered PMN lysate. It is unlikely that the PMN-associated YbcL signal originated from internalization of supernatant YbcL, as YbcLIM was not present in the supernatant but was detected in the PMN lysate. Rather, as PMN are professional phagocytes, we hypothesize that the PMN-associated YbcL signal was generated via bacterial lysis within the phagolysosome. In addition to the supernatant and PMN lysate, YbcLUTI also was detected in the 5637 cell lysate. As the membrane-tethered YbcL variants were not 5637 cell associated and were unable to complement the ybcL mutant in the transepithelial PMN migration model, it is possible that association with epithelial cells is required for suppression of PMN migration by YbcLUTI. Future experimentation will focus on specifying the relative contribution of YbcL activity on these cell types to the suppression of PMN migration in our models.
Elucidation of the YbcL crystal structure by Serre and colleagues revealed structural homology to the mammalian protein RKIP (32), which modulates signal transduction pathways, including the mitogen-activated protein (MAP) kinase and NF-κB pathways (35, 36). Klumpp and colleagues demonstrated that UPEC strain NU14 inhibits signaling through the MAP kinase and NF-κB pathways during in vitro infection of cultured bladder epithelial cells (20), although the mechanism underlying this inhibition remains unclear. Like NU14, UTI89 also inhibits signaling through these pathways, though this occurs independent of YbcLUTI (M. Lau and D. Hunstad, unpublished data), demonstrating that YbcLUTI and RKIP have distinct functions despite their structural homology. Furthermore, UTI89 ΔybcL, like wild-type UTI89, elicits minimal interleukin-6 (IL-6) and IL-8 from cultured bladder epithelial cells or human PMN relative to that elicited by MG1655 (Lau and Hunstad, unpublished data), suggesting that differences in the induction of these cytokines are not responsible for the increased PMN migration observed with ybcL deletion. Given the structural homology between YbcL and RKIP, the low concentration of YbcLUTI required to suppress PMN migration, and the presence of YbcLUTI in eukaryotic cell lysates, we hypothesize that YbcLUTI inhibits a eukaryotic signaling cascade that promotes transepithelial PMN migration. Ongoing work aims to elucidate the mechanism underlying the differential PMN migration and the role that YbcLUTI plays in mediating this phenotype, with specific attention to the importance of threonine 78.
The success of many mucosal pathogens relies on strategies to modulate host immune processes at the epithelial interface. By suppressing acute PMN recruitment, YbcL may extend the window in which UPEC can accomplish epithelial invasion and establish the protected intracellular niche required for propagating infection. YbcL represents a novel example of a bacterial exoprotein that influences early host-pathogen interactions within the urinary tract.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) grants R01-DK080752 and R03-DK076556 (to D.A.H.). Clinical isolates were collected under NIH grant P50-DK064540.
We thank K. Tiemann for technical assistance and D. Haslam and D. Berg for critical review of the manuscript.
Footnotes
Published ahead of print 10 September 2012
REFERENCES
- 1. Anderson GG, Goller CC, Justice S, Hultgren SJ, Seed PC. 2010. Polysaccharide capsule and sialic acid-mediated regulation promote biofilm-like intracellular bacterial communities during cystitis. Infect. Immun. 78:963–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson GG, et al. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105–107 [DOI] [PubMed] [Google Scholar]
- 3. Billips BK, et al. 2007. Modulation of host innate immune response in the bladder by uropathogenic Escherichia coli. Infect. Immun. 75:5353–5360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blattner FR, et al. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1474 [DOI] [PubMed] [Google Scholar]
- 5. Brzuszkiewicz E, et al. 2006. How to become a uropathogen: comparative genomic analysis of extraintestinal pathogenic Escherichia coli strains. Proc. Natl. Acad. Sci. U. S. A. 103:12879–12884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen SL, et al. 2006. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc. Natl. Acad. Sci. U. S. A. 103:5977–5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Croxen MA, Finlay BB. 2010. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 8:26–38 [DOI] [PubMed] [Google Scholar]
- 8. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis JM, Carvalho HM, Rasmussen SB, O'Brien AD. 2006. Cytotoxic necrotizing factor type 1 delivered by outer membrane vesicles of uropathogenic Escherichia coli attenuates polymorphonuclear leukocyte antimicrobial activity and chemotaxis. Infect. Immun. 74:4401–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis JM, Rasmussen SB, O'Brien AD. 2005. Cytotoxic necrotizing factor type 1 production by uropathogenic Escherichia coli modulates polymorphonuclear leukocyte function. Infect. Immun. 73:5301–5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foxman B. 2010. The epidemiology of urinary tract infection. Nat. Rev. Urol. 7:653–660 [DOI] [PubMed] [Google Scholar]
- 12. Garofalo CK, et al. 2007. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infect. Immun. 75:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haraoka M, et al. 1999. Neutrophil recruitment and resistance to urinary tract infection. J. Infect. Dis. 180:1220–1229 [DOI] [PubMed] [Google Scholar]
- 14. Henson PM, Oades ZG. 1975. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. Secretion of granule constituents and increased oxidation of glucose. J. Clin. Invest. 56:1053–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hilbert DW, et al. 2008. Uropathogenic Escherichia coli dominantly suppress the innate immune response of bladder epithelial cells by a lipopolysaccharide- and Toll-like receptor 4-independent pathway. Microbes Infect. 10:114–121 [DOI] [PubMed] [Google Scholar]
- 16. Hung CS, Dodson KW, Hultgren SJ. 2009. A murine model of urinary tract infection. Nat. Protoc. 4:1230–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hunstad DA, Justice SS. 2010. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu. Rev. Microbiol. 64:203–221 [DOI] [PubMed] [Google Scholar]
- 18. Hunstad DA, Justice SS, Hung CS, Lauer SR, Hultgren SJ. 2005. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect. Immun. 73:3999–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Justice SS, et al. 2004. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 101:1333–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klumpp DJ, et al. 2001. Uropathogenic Escherichia coli potentiates type 1 pilus-induced apoptosis by suppressing NF-κB. Infect. Immun. 69:6689–6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lipschutz JH, et al. 2001. Analysis of membrane traffic in polarized epithelial cells. Curr. Protoc. Cell Biol. Chapter 15:Unit 15.5 [DOI] [PubMed] [Google Scholar]
- 22. Lorenz K, Lohse MJ, Quitterer U. 2003. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature 426:574–579 [DOI] [PubMed] [Google Scholar]
- 23. Loughman JA, Hunstad DA. 2011. Attenuation of human neutrophil migration and function by uropathogenic bacteria. Microbes Infect. 13:555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19:2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mulvey MA, et al. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494–1497 [DOI] [PubMed] [Google Scholar]
- 26. Mulvey MA, Schilling JD, Hultgren SJ. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69:4572–4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murphy KC, Campellone KG. 2003. Lambda Red-mediated recombinogenic engineering of enterohemorrhagic and enteropathogenic E. coli. BMC Mol. Biol. 4:11 doi:10.1186/1471-2199-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mysorekar IU, Hultgren SJ. 2006. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl. Acad. Sci. U. S. A. 103:14170–14175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nicholson TF, Watts KM, Hunstad DA. 2009. OmpA of uropathogenic Escherichia coli promotes postinvasion pathogenesis of cystitis. Infect. Immun. 77:5245–5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. 2007. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 4:e329 doi:10.1371/journal.pmed.0040329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schilling JD, Lorenz RG, Hultgren SJ. 2002. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect. Immun. 70:7042–7049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Serre L, et al. 2001. Crystal structures of YbhB and YbcL from Escherichia coli, two bacterial homologues to a Raf kinase inhibitor protein. J. Mol. Biol. 310:617–634 [DOI] [PubMed] [Google Scholar]
- 33. Welch RA, et al. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:17020–17024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamaguchi K, Yu F, Inouye M. 1988. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell 53:423–432 [DOI] [PubMed] [Google Scholar]
- 35. Yeung K, et al. 1999. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 401:173–177 [DOI] [PubMed] [Google Scholar]
- 36. Yeung KC, et al. 2001. Raf kinase inhibitor protein interacts with NF-κB-inducing kinase and TAK1 and inhibits NF-κB activation. Mol. Cell. Biol. 21:7207–7217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhanel GG, et al. 2005. Antibiotic resistance in outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int. J. Antimicrob. Agents 26:380–388 [DOI] [PubMed] [Google Scholar]
- 38. Zhou G, et al. 2001. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J. Cell Sci. 114:4095–4103 [DOI] [PubMed] [Google Scholar]