Abstract
Campylobacter jejuni is the most prevalent cause of food-borne gastroenteritis in the developed world; however, the molecular basis of pathogenesis is unclear. Secretion of virulence factors is a key mechanism by which enteric bacterial pathogens interact with host cells to enhance survival and/or damage the host. However, C. jejuni lacks the virulence-associated secretion systems possessed by other enteric pathogens. Many bacterial pathogens utilize outer membrane vesicles (OMVs) for delivery of virulence factors into host cells. In the absence of prototypical virulence-associated secretion systems, OMVs could be an important alternative for the coordinated delivery of C. jejuni proteins into host cells. Proteomic analysis of C. jejuni 11168H OMVs identified 151 proteins, including periplasmic and outer membrane-associated proteins, but also many determinants known to be important in survival and pathogenesis, including the cytolethal distending toxin (CDT). C. jejuni OMVs contained 16 N-linked glycoproteins, indicating a delivery mechanism by which these periplasm-located yet immunogenic glycoproteins can interact with host cells. C. jejuni OMVs possess cytotoxic activity and induce a host immune response from T84 intestinal epithelial cells (IECs), which was not reduced by OMV pretreatment with proteinase K or polymyxin B prior to coincubation with IECs. Pretreatment of IECs with methyl-beta-cyclodextrin partially blocks OMV-induced host immune responses, indicating a role for lipid rafts in host cell plasma membranes during interactions with C. jejuni OMVs. OMVs isolated from a C. jejuni 11168H cdtA mutant induced interleukin-8 (IL-8) to the same extent as did wild-type OMVs, suggesting OMV induction of IL-8 is independent of CDT.
INTRODUCTION
Campylobacter jejuni is a Gram-negative, flagellated bacterium that is the leading causative agent of food-borne bacterial acute human gastroenteritis worldwide (2). Symptoms range from mild diarrhea to severe inflammatory enteritis. The majority of C. jejuni infections result in uncomplicated acute gastroenteritis; however, C. jejuni is also the most commonly identified infectious agent in peripheral neuropathies such as Guillain-Barré syndrome (GBS) (57). In the developing world, C. jejuni is a leading cause of mortality among young children. In the United Kingdom, it is estimated that 500,000 C. jejuni infections occur each year (34). C. jejuni is one of the most extensively characterized enteric bacterial pathogens at the genetic and phenotypic levels; however, in contrast to most other enteropathogens, the mechanisms by which C. jejuni causes diarrheal disease in humans remain unclear (18, 74). The genome content of C. jejuni strains appears anomalous compared to most Gram-negative pathogens, having no readily identifiable secretion systems to deliver virulence factors into host cells. Clearly, alternative infection mechanisms need to be investigated for this atypical enteropathogen.
The coordinated delivery of virulence factors is a common mechanism by which enteric bacterial pathogens interact with host cells to enhance their survival and/or cause damage to the host cell. Secretion mechanisms utilized by enteric pathogens often require intimate contact with the host cell, such as the type III and type IV secretion systems, which deliver bacterial proteins and nonprotein material (e.g., fragments of peptidoglycan) directly into the host cytoplasm. Surprisingly, C. jejuni lacks many of the virulence-associated secretion systems possessed by other enteric pathogens. The only potential type III secretion system identified in C. jejuni is the flagellar apparatus (60). Previous studies have indicated that the secretion of Campylobacter invasion antigens (Cia) and other virulence factors is dependent on a functional flagellar apparatus (43, 62). However, the secretion of virulence factors via the C. jejuni flagellar is contentious (58) and would not facilitate the direct delivery of bacterial proteins into host cells as is observed with other enteric pathogens.
Rather than secrete virulence factors into the surrounding milieu, where they could be degraded by host proteases, many Gram-negative bacterial pathogens utilize outer membrane vesicles (OMVs) as a mechanism of delivering active proteins and other moieties into host cells (19). In this way, virulence factors are not secreted individually but as multiple factors delivered in a concerted package. OMVs play roles in establishing colonization of the host, delivering virulence factors and modulating the host responses for many Gram-negative bacterial pathogens (47). Indeed, toxin delivery mediated by OMVs is recognized as a potent virulence mechanism for many bacterial pathogens (19).
Both nonpathogenic and pathogenic Gram-negative bacteria constitutively release OMVs (46). Bacterial OMVs are discrete, closed outer membrane blebs produced by growing cells and not a product of cell lysis or cell death (46). OMVs are spherical proteoliposomes with an average diameter ranging from 10 to 500 nm (46). These nanostructures are enriched with outer membrane proteins, phospholipids, lipooligosaccharides (LOS), and numerous periplasmic proteins of wide molecular mass range (54). Many virulence factors that are periplasmic proteins are enriched in OMVs, including enterotoxigenic Escherichia coli (ETEC) heat-labile enterotoxin (LT) (39). Recently, studies have reported a diverse range of Gram-negative bacterial pathogens that readily release various quantities of OMVs into their extracellular milieu and for which OMVs have been associated with pathogenesis (19). For example, Pseudomonas aeruginosa OMVs have been shown to deliver multiple virulence factors, including β-lactamase, alkaline phosphatase, hemolytic phospholipase C, and the Cif toxin, directly into airway epithelial cells (10). The human oral pathogen Porphyromonas gingivalis selectively sorts the gingipain proteases, major virulence factors, into OMVs and excludes other abundant outer membrane proteins (28).
Although OMVs from C. jejuni were identified nearly 30 years ago (8, 52), it is only recently that the biological significance of OMV formation in C. jejuni pathogenesis has become apparent with the discovery that biologically active cytolethal distending toxin (CDT) is released within OMVs produced by C. jejuni 81-176 (49). This was the first evidence that C. jejuni utilizes OMVs to deliver virulence factors to the surrounding environment, including infected host tissue (49). Bacterial CDTs belong to the AB2-type toxins and generally comprise three subunits, CdtA, CdtB, and CdtC (24). CdtA and CdtC act as carriers to deliver the catalytic subunit, CdtB, into host cells (67). CdtB exhibits DNase I-like activity and induces limited DNA damage such as double-strand damage, leading to the activation of DNA repair responses and cell cycle arrest at the G2/M phase (48, 73). C. jejuni CDT exerts multiple effects, including the release of interleukin-8 (IL-8) from intestinal epithelial cells (IECs) (30, 76), promoting host DNA repair responses (27), gastroenteritis in NF-κB-deficient mice (21), and cell death in human monocytic cells (31).
We hypothesized that in the absence of prototypical virulence-associated secretion systems, C. jejuni utilizes OMVs to deliver bacterial proteins to promote cellular adhesion and invasion, while modulating the host response during infection of the human intestinal tract. This study used proteomic techniques to identify C. jejuni OMV proteins and also investigated the interactions of C. jejuni OMVs with IECs, including the role of OMV-associated CDT in inducing the host innate immune response.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The C. jejuni wild-type strains used were strain 11168H, a hypermotile derivative of the sequence strain NCTC11168, which shows higher levels of cecal colonization in a chick colonization model (35), and strain 81-176, a gastroenteritis isolate widely used for C. jejuni infection studies (7). The C. jejuni 11168H pglB, peb3, and cdtA mutants were obtained from the London School of Hygiene & Tropical Medicine (LSHTM) Campylobacter Resource Facility (http://crf.lshtm.ac.uk/index.htm). The mutations were confirmed by PCR and sequencing as described previously (26). C. jejuni strains were grown either on blood agar plates containing Columbia agar base (Oxoid, Basingstoke, United Kingdom) supplemented with 7% (vol/vol) horse blood (TCS Microbiology, Botolph Claydon, United Kingdom) and Campylobacter selective supplement (Oxoid) or in Brucella broth (Oxoid) with shaking at 75 rpm at 37°C in a microaerobic chamber (Don Whitley Scientific, Shipley, United Kingdom) containing 85% N2, 10% CO2, and 5% O2. Unless otherwise stated, C. jejuni strains were grown on blood agar plates for 24 h prior to use in experiments. Kanamycin was added as required at the concentration of 50 μg/ml.
Isolation of C. jejuni OMVs and TEM.
OMVs were isolated as described previously (49). C. jejuni cells from a 24-h plate were resuspended in 1 ml Brucella broth and inoculated into 100 ml Brucella broth to an optical density at 600 nm (OD600) of 0.1. Cultures were grown for 14 h to mid-log phase and then centrifuged at 10,000 × g for 15 min, and the resulting supernatant was filtered through a 0.22-μm membrane (Millipore, Watford, United Kingdom). The filtrate was concentrated to 2 ml using an Ultra-4 centrifugal filter unit with a nominal 10-kDa cutoff (Millipore). The concentrated filtrate was ultracentrifuged at 150,000 × g for 3 h at 4°C using a TLS 55 rotor (Beckman Instruments, Palo Alto, CA). The absence of viable bacteria was confirmed by plating OMV samples on blood agar plates and incubating under both microaerobic and aerobic conditions for 48 h. All isolation steps were carried out at 4°C, and the resulting OMV pellet was resuspended in phosphate-buffered saline (PBS) or 50 mM HEPES buffer (pH 7.4) and stored at −20°C. Approximately 200 μg OMVs by protein content was isolated from 100 ml of culture supernatant, determined by bicinchoninic acid (BCA) assay (Thermo Fisher Scientific, Loughborough, United Kingdom). The amounts of OMVs used in subsequent experiments are always quantified by protein amount. For subsequent experiments, OMVs were pretreated with either proteinase K (100 μg/ml for 60 min at 37°C) or polymyxin B (100 μg/ml for 30 min at room temperature), or alternatively, OMVs were heat treated (100°C for 20 min). For transmission electron microscopy (TEM), C. jejuni OMVs were fixed, dehydrated, and embedded in Epon (38). Samples were examined using a Jeol 1200EX transmission electron microscope at 80 kV (Welwyn Garden City, United Kingdom).
Liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS).
Three biological replicates of 25 μg OMVs were separated by 12% (wt/vol) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Invitrogen, Paisley, United Kingdom) and then stained with Coomassie brilliant blue. Each stained gel lane was excised and sliced horizontally from top to bottom to yield a series of 28 equal gel slices 2.5 mm deep. Each of the resulting gel slices was subjected to standard in-gel destaining, reduction, alkylation, and trypsinolysis procedures (66). Digests were transferred to high-performance LC (HPLC) sample vials and stored at 4°C until required for LC-ESI-MS/MS analysis. LC was performed using an Ultimate 3000 nano-HPLC system (Dionex, Camberley, United Kingdom) comprising a WPS-3000 well-plate micro-auto-sampler, an FLM-3000 flow manager and column compartment, a UVD-3000 UV detector, an LPG-3600 dual-gradient micropump, and an SRD-3600 solvent rack controlled by Chromeleon chromatography software. A micropump flow rate of 246 μl/min−1 was used in combination with a cap-flow splitter cartridge, affording a flow split of 1/82 and a final flow rate of 3 μl/min−1 through a 5-cm by 200-μm ID monolithic reversed-phase column (Dionex) maintained at 50°C. Samples of 4 μl were applied to the column by direct injection. Peptides were eluted by the application of a 15-min linear gradient from 8 to 45% solvent B (80% [vol/vol] acetonitrile, 0.1% [vol/vol] formic acid) and directed through a 3-nl UV detector flow cell. LC was interfaced directly with a three-dimensional (3-D) high-capacity ion trap mass spectrometer (Esquire HCTplus; Bruker Daltonics, Coventry, United Kingdom) via a low-volume (50 μl/min maximum) stainless steel nebulizer (Agilent, Wokingham, United Kingdom) and ESI. Parameters for MS/MS analysis were set as previously described (5).
Database mining.
Deconvoluted MS/MS data in mgf (Mascot Generic) format were imported into ProteinScape V2.1 (Bruker Daltronics) proteomics data analysis software for downstream data mining of a cognate C. jejuni 11168 genomic database utilizing the Mascot V2.2 (Matrix Science, London, United Kingdom) search algorithm. The protein content of individual gel slices was established using the “protein search” feature of ProteinScape, while separate compilations of the proteins contained in all 28 gel slices of each of the three biological replicates were produced using the “protein extractor” feature of the software. Mascot search parameters were set in accordance with published guidelines (71), and to this end, the fixed and variable modifications selected were carbamidomethyl (C) and oxidation (M), respectively, and mass tolerance values for MS and MS/MS were set at 1.5 Da and 0.5 Da, respectively. Molecular weight search (MOWSE) scores attained for individual protein identifications were inspected manually and considered significant only if (i) two peptides were matched for each protein and (ii) each peptide contained an unbroken “b” or “y” ion series of a minimum of four amino acid residues.
SDS-PAGE and Western blot analysis.
Purified C. jejuni OMVs were mixed with Laemmli sample buffer (1:1) and separated by SDS-PAGE (NuPage Novex Bis-Tris 4-12%; Invitrogen). Briefly, 20 μg of each OMV sample was added to 2× Laemmli buffer and boiled for 10 min prior to electrophoresis. Gels were stained with Coomassie brilliant blue stain or blotted onto Immun-Blot polyvinylidene difluoride (PVDF) membranes (GE Healthcare, Chalfont St Giles, United Kingdom). For Western blots, the separated proteins were transferred onto nitrocellulose membranes (GE Healthcare) using the Mini Trans-Blot system (GE Healthcare). The membrane was blocked with 10% (wt/vol) nonfat dried milk and incubated overnight at 4°C. Following incubation, the membrane was washed three times and incubated with an anti-Peb3 antibody (1:2,000 dilution in 10% [wt/vol] nonfat dried milk), followed by 1 h of incubation at room temperature with a 1:10,000 dilution of anti-rabbit horseradish peroxidase-conjugated secondary antibody (Sigma-Aldrich, Poole, United Kingdom). The blots were visualized with ECL Western blotting detection reagent (GE Healthcare). For soybean agglutinin (SBA) lectin blotting, the PVDF membrane was blocked with 0.5% (vol/vol) Tween 20 in PBS for 30 min at room temperature and probed with 5 μg of peroxidase-labeled SBA (Sigma-Aldrich) for 1 h. The membrane was then washed with 0.5% (vol/vol) Tween 20 in PBS and subjected to detection using the DAB peroxidase substrate kit (Vector Laboratories, Peterborough, United Kingdom).
Cell lines, media, and culture conditions.
The human Caco-2 colon adenocarcinoma cells and human T84 colon cancer epithelial cells were obtained from the National Type Culture Collection. Caco-2 cells were maintained at subconfluence in Dulbecco's modified Eagle medium (DMEM) with 10% (vol/vol) heat-inactivated fetal calf serum (FCS) supplemented with 1% (wt/vol) nonessential amino acids and 1% (wt/vol) penicillin-streptomycin (Sigma-Aldrich) at 37°C in a 5% CO2 humidified atmosphere. T84 cells were maintained under the same conditions in DMEM/F-12 (Invitrogen) supplemented with 10% (vol/vol) FCS, 1% (wt/vol) nonessential amino acids, and 1% (wt/vol) penicillin-streptomycin. Cells were split around 80 to 90% confluence and seeded at 5 × 105 cells per well into 24-well tissue culture plates (Corning Glass Works, Corning, Netherlands) using 1-ml volumes of cell culture medium per well. Medium was replenished every 2 days. For enzyme-linked immunosorbent assays (ELISAs), the medium from T84 cells was removed, and the monolayers were washed twice with PBS and then maintained in antibiotic-free, serum-free DMEM/F-12 medium.
Cytotoxicity detection assay.
The ability of OMVs isolated from C. jejuni wild-type strain 11168H to induce cell damage was assessed using the CytoTox 96 nonradioactive cytotoxicity assay (Promega, Southampton, United Kingdom). Briefly, 7 days prior to infection, Caco-2 cells were seeded into 24-well plates, grown to confluence, and challenged with 100 μg of 11168H OMVs, proteinase K-treated OMVs, or polymyxin B-treated OMVs. After coincubation at 37°C for 24 h, cell supernatants were analyzed for the release of lactate dehydrogenase (LDH). Nonchallenged cells represented the 0% cytotoxicity negative control. Total lysis of cells following treatment with 1% (vol/vol) Triton X-100 represented the 100% cytotoxicity positive control.
Coincubation of C. jejuni OMVs with intestinal epithelial cell lines and analysis of proinflammatory cytokines levels by ELISA.
T84 cells were infected with live C. jejuni wild-type strain 11168H or cdtA mutant at a multiplicity of infection (MOI) of 100:1 or cocultured with 100 μg or 10 μg of 11168H OMVs or cdtA mutant OMVs or proteinase K-treated or polymyxin B-treated 11168H OMVs for 24 h. The levels of IL-8, IL-6, tumor necrosis factor alpha (TNF-α), and human beta-defensin-3 (hBD) secretion were assessed using a commercially available sandwich ELISA kit according to the manufacturer's instructions (Peprotech, London, United Kingdom). Detection was performed using a Dynex MRX II 96-well plate reader at an absorbance of 405 nm (A405) and analyzed using Revelation software (Dynex).
Pretreatment of T84 cells with methyl-β-cyclodextrin treatment to produce cholesterol depletion prior to coculture with C. jejuni OMVs.
To evaluate the role of cholesterol-rich membrane rafts in C. jejuni OMV interactions with host cells, T84 cells were treated with 10 mM methyl-β-cyclodextrin (MβCD) (Sigma-Aldrich), a cholesterol-depleting agent, for 1 h at 37°C before cells were washed twice with 1 ml of PBS. Following cholesterol depletion, T84 cells were infected with the live C. jejuni 11168H wild-type strain at an MOI of 100:1 or cocultured with 100 μg C. jejuni 11168H OMVs in 1% (vol/vol) serum-containing growth medium at 37°C for the indicated periods.
Galleria mellonella model of C. jejuni infection.
G. mellonella larvae were obtained from LiveFoods Direct (Sheffield, United Kingdom) and kept on wood chips at 16°C. Experiments were performed with slight modifications from the original published method (13) as described previously (26). Briefly, 5 μg or 0.5 μg of 11168H OMVs or heat-treated OMVs were injected into the right foremost leg of the G. mellonella larvae by microinjection (Hamilton, Switzerland). As a positive control, larvae were injected with a 10-μl inoculum of a 24-h C. jejuni 11168H culture diluted to an OD600 of 0.1, giving an infectious dose of approximately 106 CFU (26). For each experiment, 10 G. mellonella larvae were infected, and the experiments (for which larvae of the same approximate weight were chosen) were repeated three times. Larvae were incubated at 37°C, with survival recorded at 24-h intervals for 72 h.
Statistical analysis.
All experiments represent at least three biological replicates, with each experiment performed in triplicate. All data were analyzed using Prism statistical software (Version 5; GraphPad Software, San Diego, CA). Values were expressed as means ± standard errors of the means (SEM). Variables were compared for significance using two-way analysis of variance (ANOVA) and the Bonferroni test, with one asterisk (*) indicating a P value between 0.01 and 0.05, two asterisks (**) indicating a P value between 0.001 and 0.01, and three asterisks (***) indicating a P value of <0.001.
RESULTS
Campylobacter jejuni 11168H produces pleiomorphic OMVs of variable sizes.
Previously it was demonstrated that the C. jejuni wild-type strain 81-176 produces distinct OMVs of various sizes and shapes (49). OMVs were isolated from the C. jejuni wild-type strain 11168H using a similar protocol (49). TEM analysis of purified OMVs revealed the presence of typical spherical vesicles with a pleiomorphic bilayer (Fig. 1A). These OMVs were found to differ in size from 10 nm to 250 nm. The apparent density of the isolated OMVs was mixed, suggesting variation in the contents among individual OMVs. To eliminate the possibility of OMVs arising from dead cells, bacterial viability assays were performed on exponential-growth-phase cultures prior to vesicle isolation. The results indicated that >99% of cells prior to OMV isolation were viable (data not shown). OMVs isolated from wild-type strain 81-176 displayed the same characteristics (Fig. 1B).
Fig 1.
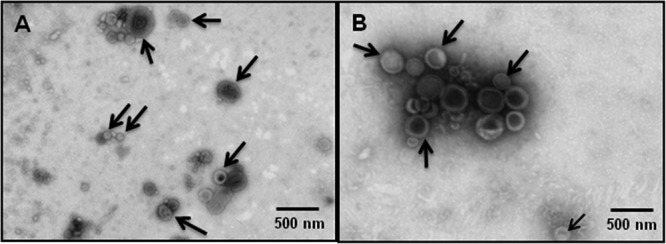
Transmission electron micrographs of negatively stained C. jejuni outer membrane vesicles (OMVs). C. jejuni wild-type strain 11168H OMVs (A) and 81-176 OMVs (B) with intact bilayer membranes. OMVs have an irregular spherical shape and are not uniform in size. Arrows indicate differences in the apparent density of the isolated OMVs. The diameters of OMVs range from 10 to 250 nm (scale bar, 500 nm).
Proteomic analysis of C. jejuni 11168H OMVs.
The presence of virulence-associated proteins in bacterial OMVs is widespread (19). The major protein components of triplicate samples of C. jejuni 11168H OMVs were analyzed using one-dimensional (1D) SDS-PAGE followed by LC-ESI-MS/MS. Proteomic analysis reproducibly identified 151 proteins in at least two of the three biological replicate samples. The proportions of proteins from each cluster of orthologous groups (COG) categorization (70) is presented in Fig. 2. Proteins with unknown function represented the largest proportion (26%). Sixteen further COG categories were represented in the OMV proteome, a finding that is in line with OMV content in other pathogenic bacteria. Many membrane-associated proteins were detected (see Table S1 in the supplemental material) including several characterized lipoproteins and outer membrane proteins. The presence of a large number of periplasmic and cytoplasmic proteins supports the observations that OMV composition derives from multiple bacterial compartments and not solely from the outer membrane. The presence of previously identified virulence factors such as CDT was confirmed, and 16 C. jejuni glycoproteins (65) were also identified in OMVs. LipoP (http://www.cbs.dtu.dk/services/LipoP/) was used to predict signal peptides and lipoprotein signal peptides (36).
Fig 2.
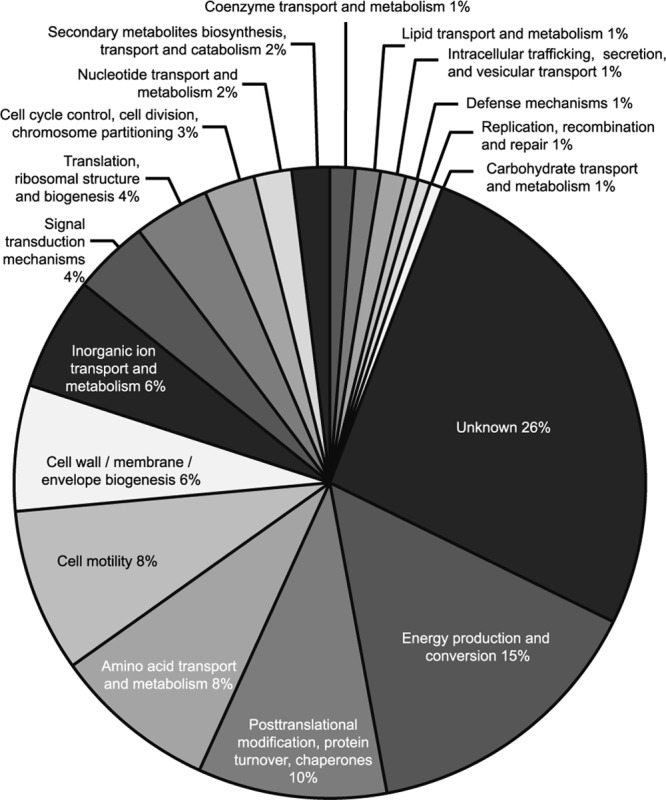
Major protein functional categories (COGs) identified in C. jejuni 11168H outer membrane vesicles following proteomic analysis.
N-linked glycoproteins are associated with C. jejuni 11168H OMVs.
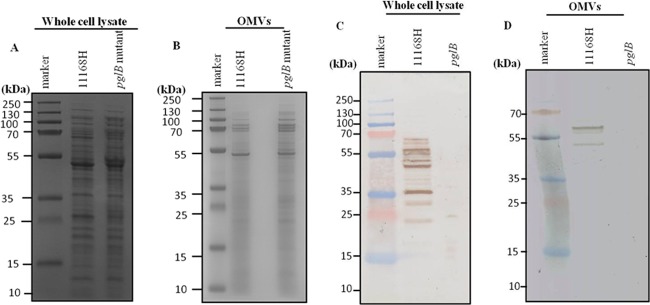
All C. jejuni genomes sequenced to date encode an N-linked protein glycosylation (Pgl) pathway (69). N-linked protein glycosylation occurs in the periplasm, where many of the proteins associated with OMVs are also localized. N-linked glycosylation may be involved in the evasion of the host immune response, as mutation of pgl genes that encode the glycan biosynthesis system results in changes in immunoreactivity (68). Proteomic analysis of 11168H OMVs identified 16 proteins that have been shown to be C. jejuni glycoproteins (65). To confirm the presence of N-linked glycoproteins associated with OMVs, 11168H OMVs were separated by 1D SDS-PAGE and probed with SBA lectin to identify the C. jejuni heptasaccharide glycan that has a terminal GalNac residue specifically recognized by SBA lectin (51). SBA lectin bound to multiple bands (Fig. 3) indicating that 11168H OMVs contain numerous glycoproteins. A C. jejuni 11168H pglB mutant deficient for N-linked glycosylation was used as a control. SBA lectin did not bind to OMVs isolated from the 11168H pglB mutant, which lacks the oligosaccharyltransferase PglB to attach the heptasaccharide glycan to proteins in the periplasm. This is the first indirect evidence indicating that C. jejuni OMVs may deliver antigenic N-linked glycoproteins to host cells. One of the 16 N-linked glycoproteins associated with 11168H OMVs identified by the proteomic analysis was Peb3. Peb3 was first identified as one of four major antigenic C. jejuni proteins that cross-reacted with antiserum from convalescent patients (61), later identified as a glycoprotein (50) and more recently as a transport protein involved with the utilization of phosphate-containing molecules from the host (55). The association of Peb3 with C. jejuni 11168H wild-type and pglB and peb3 mutant OMVs was investigated by immunoblotting using an anti-Peb3 antibody and compared with whole-cell lysates. This indicated the presence of both glycosylated and unglycosylated Peb3 associated with 11168H OMVs (Fig. 4). A reduced amount of unglycosylated Peb3 and no glycosylated Peb3 were detected with pglB mutant OMVs compared to wild-type OMVs. Peb3 was undetectable in peb3 mutant OMVs. In addition, analysis of wild-type strain 11168H outer membrane and periplasmic fractions indicated that Peb3 was located within the periplasm and not associated with the outer membrane (Fig. 4).
Fig 3.
N-linked glycoproteins are associated with C. jejuni 11168H outer membrane vesicles (OMVs). C. jejuni wild-type strain 11168H and pglB mutant whole-cell lysates (A) or OMVs (B) separated by SDS-PAGE and stained with Coomassie brilliant blue stain. Corresponding SBA lectin binding to whole-cell lysates (C) or OMVs (D) to probe for the N-linked glycan attached to C. jejuni proteins by the oligosaccharyltransferase PglB in the bacterial periplasm. Molecular mass sizes (kDa) are indicated.
Fig 4.
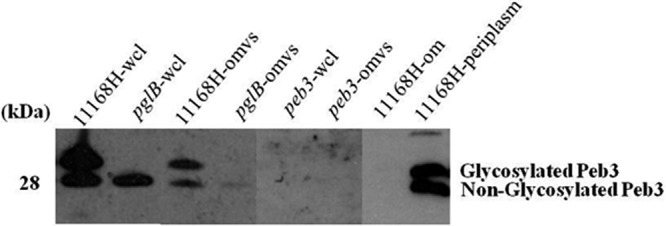
Immunoblot analysis of purified C. jejuni wild-type strain 11168H, pglB mutant, and peb3 mutant outer membrane vesicles (OMVs) with an anti-Peb3 antibody. Purified OMVs (omvs) were separated by SDS-PAGE followed by immunoblotting with an anti-Peb3 antibody and compared with wild-type strain 11168H, pglB mutant, and peb3 mutant whole-cell lysates (wcl) and also purified outer membrane (om) and periplasmic (periplasm) fractions from the wild-type strain 11168H.
C. jejuni 11168H OMVs are cytotoxic to Caco-2 intestinal epithelial cells.
The cytotoxicity of OMVs on Caco-2 cells was measured by quantifying the release of cytosolic lactate dehydrogenase (LDH) as a measure of cell damage. Live and heat-killed C. jejuni 11168H cells (MOI, 100:1), untreated 11168H OMVs (100 μg by protein content), and proteinase K- or polymyxin B-pretreated 11168H OMVs (100 μg) were coincubated with Caco-2 cells for 24 h. The cytotoxic effect of untreated 11168H OMVs (100 μg) was not statistically different from the cytotoxic effect of live wild-type 11168H cells (MOI, 100:1) over 24 h, while heat-killed 11168H cells (MOI, 100:1) showed a statistically significant decrease in cytotoxicity (P < 0.05) (Fig. 5). The cytotoxicity of OMVs pretreated with either proteinase K or polymyxin B was similar to that of untreated OMVs and was not statistically different from the cytotoxic effect of live wild-type 11168H cells (MOI, 100:1) over 24 h.
Fig 5.
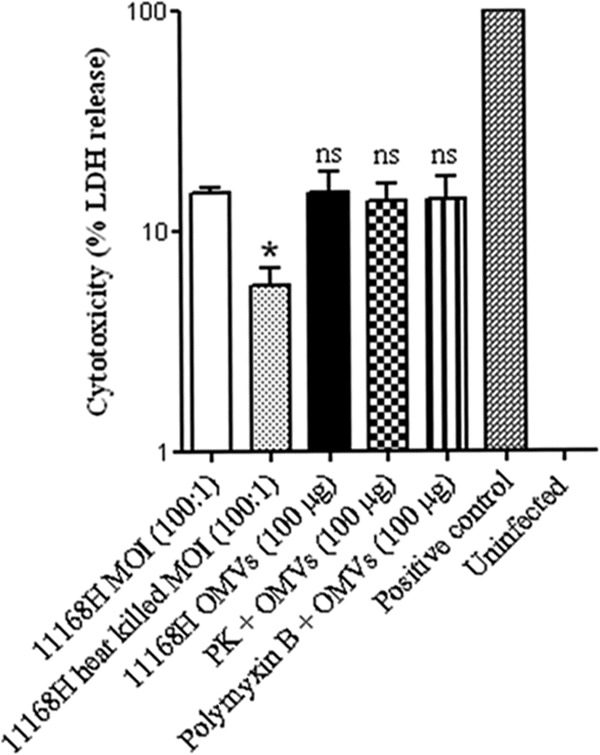
Cytotoxic effect of C. jejuni 11168H outer membrane vesicles (OMVs) on Caco-2 intestinal epithelial cells. Live C. jejuni 11168H (MOI, 100:1), heat-killed C. jejuni 11168H (MOI, 100:1), untreated 11168H OMVs (100 μg), or 11168H OMVs (100 μg) pretreated with either proteinase K (PK) or polymyxin B were coincubated with Caco-2 cells for 24 h. The cytotoxic effect on the Caco-2 cells was measured by quantifying the release of cytosolic lactate dehydrogenase (LDH) as a measure of cell damage. Nonchallenged Caco-2 cells represented 0% cytotoxicity (Uninfected), and total lysis of Caco-2 cells following treatment with 1% (vol/vol) Triton X-100 represented 100% cytotoxicity (Positive control). *, P < 0.05; ns, no significant difference in cytotoxicity compared to live 11168H cells.
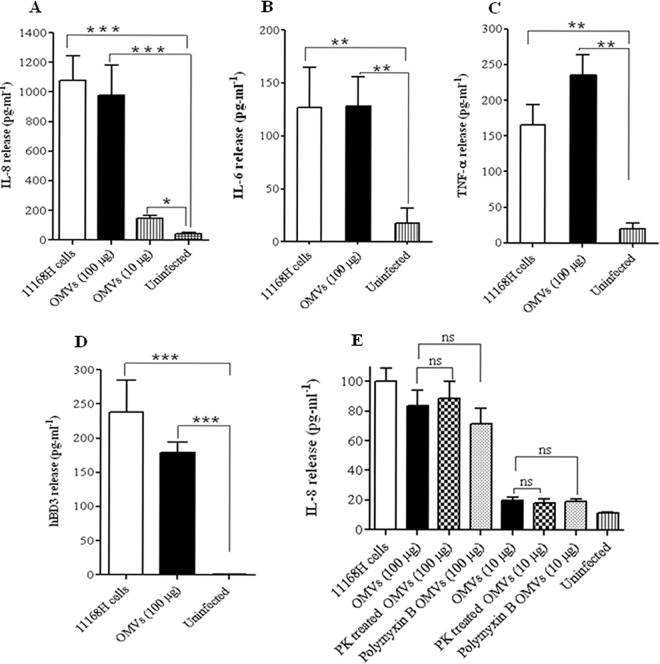
C. jejuni 11168H OMVs induced an IL-8, IL-6, TNF-α, and hBD-3 response from T84 intestinal epithelial cells.
C. jejuni interactions with IECs modulate both the innate and the adaptive immune responses (14, 53, 63). However, the role of C. jejuni OMVs in inducing such host responses has not been reported. IEC-derived IL-8, IL-6, TNF-α, and hBD-3 are well-characterized markers denoting an early host response against C. jejuni (56). T84 cells were coincubated with C. jejuni 11168H OMVs (10 μg or 100 μg) for 24 h, and the levels of IL-8, IL-6, TNF-α, and hBD-3 secreted were measured by ELISA (Fig. 6A to D). The levels of IL-8, IL-6, TNF-α, and hBD-3 induced by 11168H OMVs (100 μg) were significantly increased and similar to those of live C. jejuni wild-type strain 11168H (MOI, 100:1). A reduced but still significant level of IL-8 was induced by 10 μg OMVs compared to 100 μg OMVs (Fig. 6A). The host immune response induced by C. jejuni OMVs was not significantly reduced following pretreatment of OMVs with either proteinase K or polymyxin B (Fig. 6E). Together, these data demonstrate that C. jejuni OMVs alone can induce production of significant levels of IL-8, IL-6, TNF-α, and hBD-3 from T84 cells, independently of live bacterial cells.
Fig 6.
C. jejuni 11168H outer membrane vesicles (OMVs) induce an innate immune response from T84 intestinal epithelial cells (IECs). (A to D) T84 IEC responses to 24 h of coincubation with C. jejuni wild-type strain 11168H (MOI, 100:1) or OMVs (100 μg or 10 μg) were assessed. Levels of IL-8, IL-6, TNF-α, and hBD-3 secreted during C. jejuni OMV interactions with T84 cells were quantified using a human IL-8 ELISA (A), IL-6 ELISA (B), TNF-α ELISA (C), or hBD-3 ELISA (D). (E) T84 IEC responses to 24 h of coincubation with C. jejuni 11168H (MOI, 100:1), untreated 11168H OMVs (100 μg), or 11168H OMVs (100 μg) pretreated with either proteinase K (PK) or polymyxin B. Levels of IL-8 secreted were quantified using a human IL-8 ELISA. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, no significant difference.
MβCD can partially block the innate immune response induced by C. jejuni 11168H OMVs.
The detailed mechanisms and host cell receptor(s) leading to the signaling cascade(s) that result in the host response to coincubation with bacterial OMVs have not yet been identified. OMV-induced signaling has been shown to occur via the specific glycolipid and cholesterol-enriched domains in the cell plasma membrane termed “lipid rafts” (10, 17, 23, 25, 32, 37). To investigate the role of lipid rafts in the host cellular response to C. jejuni OMVs, T84 IECs were treated with 10 mM methyl-beta-cyclodextrin (MβCD), a lipid-raft-disrupting agent, for 1 h before coincubation with C. jejuni 11168H OMVs for 24 h. This pretreatment of T84 cells with MβCD resulted in a significant (P < 0.05) inhibition of the OMV-stimulated induction of IL-8 (Fig. 7, left) and hBD3 (Fig. 7, right). Live C. jejuni wild-type strain 11168H cells stimulated T84 cells pretreated with MβCD showed a more significant reduction in IL-8 induction (Fig. 7, left; P < 0.01) and hBD3 (Fig. 7, right; P < 0.001), consistent with published data (37, 72). These results indicate a role for membrane lipid rafts as important determinants of C. jejuni OMV-mediated signal transduction. However, the exact mechanism of MβCD-induced inhibition of C. jejuni OMV interactions with host cells remains unknown. MβCD did not affect cell integrity or viability at the concentration used as determined using inverted microscopy and the cytotoxicity assay (data not shown).
Fig 7.
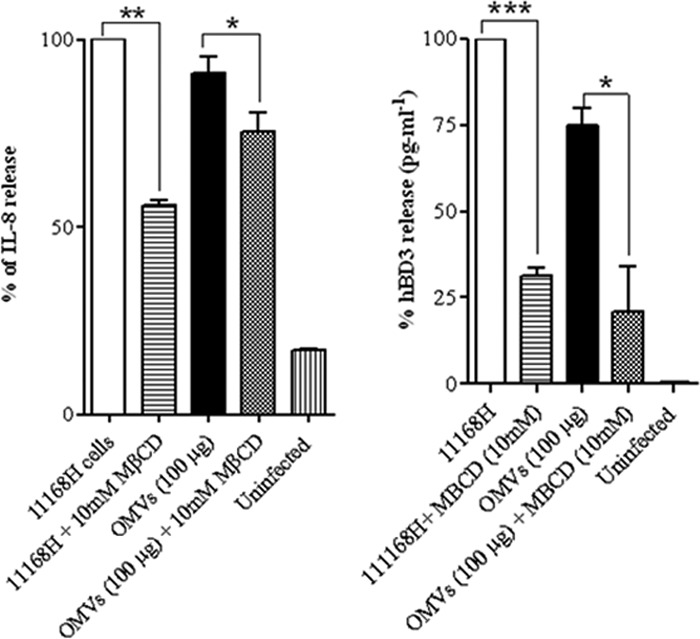
Preincubation of T84 cells with methyl-beta-cyclodextrin (MβCD) can partially block the innate immune response induced by C. jejuni 11168H outer membrane vesicles (OMVs). T84 intestinal epithelial cells were pretreated with 10 mM MβCD for 1 h and then coincubated with C. jejuni wild-type strain 11168H (MOI, 100:1) or OMVs (100 μg). Levels of IL-8 and hBD-3 secreted were quantified using a human IL-8 ELISA (left) or hBD-3 ELISA (right) and presented as the percentage of IL-8 induced by C. jejuni wild-type strain 11168H (MOI, 100:1). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
C. jejuni OMVs induced IL-8 from T84 intestinal epithelial cells via a CDT-independent mechanism.
C. jejuni induction of IL-8 from IECs has been shown to be both CDT dependent and CDT independent (30). The mechanism of IL-8 induction by C. jejuni 11168H OMVs was investigated further, using OMVs isolated from a 11168H cdtA mutant (Fig. 8). The levels of IL-8 induced by the live cdtA mutant were not significantly reduced compared to those induced by live wild-type strain 11168H (MOI, 100:1). The levels of IL-8 induced by cdtA mutant OMVs were also not significantly reduced compared to those induced by 11168H OMVs, indicating that induction of IL-8 by C. jejuni OMVs occurs via a CDT-independent mechanism.
Fig 8.
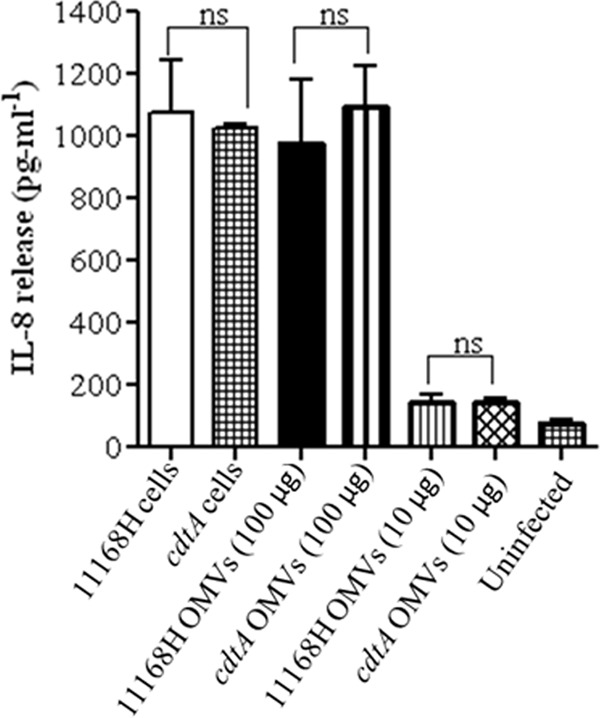
C. jejuni outer membrane vesicles (OMVs) induce IL-8 from T84 intestinal epithelial cells via a CDT-independent mechanism. T84 IEC responses to 24 h of coincubation with C. jejuni wild-type strain 11168H or 11168H cdtA mutant (each at a MOI of 100:1) or with 11168H OMVs or 11168H cdtA mutant OMVs (each at 100 μg and 10 μg) were assessed. Levels of IL-8 secreted were quantified using a human IL-8 ELISA. ns, no significant difference.
G. mellonella larvae exhibit decreased survival after challenge with C. jejuni 11168H OMVs.
The cytotoxic potential of C. jejuni OMVs was further investigated using the Galleria mellonella larvae model of infection. This invertebrate model has been used to assess the virulence of numerous human bacterial pathogens, including C. jejuni (13). The larvae do not have an adaptive immune system but have potent innate immunity and are resistant to microbial infections via cellular and humoral defenses. G. mellonella larvae were injected with either C. jejuni wild-type strain 11168H or the cdtA mutant strain (106 CFU) or OMVs (5 μg or 0.5 μg). At 24 h postinfection, the mortality of G. mellonella larvae challenged with 11168H OMVs (5 μg) was significantly increased (P < 0.001) and was similar to that of larvae infected with the live wild-type strain 11168H (P < 0.001) (Fig. 9). Larvae challenged with 11168H OMVs (0.5 μg) also showed a significant increase in mortality (P < 0.05) but showed reduced mortality compared to larvae challenged with 11168H OMVs (5 μg) (Fig. 9). No further mortality was observed at either 48 h or 72 h postinfection (data not shown). Larvae either infected with the cdtA mutant or challenged with OMVs isolated from the cdtA mutant showed no significant difference in mortality compared to those infected with the wild-type strain or 11168H OMVs, respectively (Fig. 9). However, no mortality was observed with G. mellonella larvae infected with heat-treated 11168H OMVs (5 μg or 0.5 μg).
Fig 9.
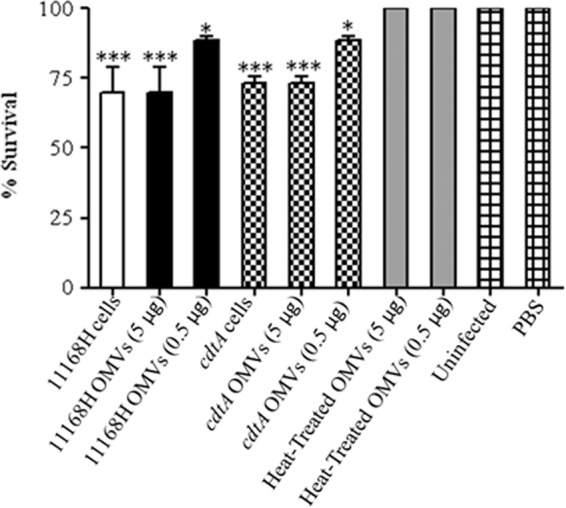
Effect of C. jejuni outer membrane vesicles (OMVs) in the Galleria mellonella infection model. G. mellonella larvae were injected with a 10-μl inoculum of either C. jejuni wild-type strain 11168H (106 CFU) or 11168H OMVs (5 μg or 0.5 μg) or heat-treated 11168H OMVs (5 μg or 0.5 μg) by microinjection in the right foremost leg. Larvae were incubated at 37°C, with survival and appearance recorded after 24 h. PBS and no-injection controls were used. For each experiment, 10 G. mellonella larvae were infected, and experiments were repeated in triplicate. *, P < 0.05; ***, P < 0.001.
DISCUSSION
One of the many conundrums regarding C. jejuni pathogenesis is the lack of classical virulence-associated bacterial secretion systems possessed by other enteric pathogens. As such, the mechanisms used by C. jejuni to deliver virulence factors into host cells are still unclear and have become a matter of conjecture (74). OMVs are increasingly recognized as key determinants for bacterial virulence (47) and play a major role in host-pathogen interactions, including the trafficking and eventual release of diverse virulence factors from many pathogenic bacteria (19). Recently, it was shown that the C. jejuni wild-type strain 81-176 produces OMVs that contain biologically active CDT (49). This indicated that C. jejuni might utilize OMVs as a delivery system during pathogenesis. In this study, we used proteomic techniques to identify proteins associated with C. jejuni 11168H OMVs and investigated the effects of the coordinated delivery to human IECs of C. jejuni virulence factors via OMVs in eliciting inflammatory and cytotoxic responses.
In this study, OMVs isolated from both C. jejuni wild-type strains 11168H and 81-176 varied in size from 10 to 250 nm. Previously, it was reported that OMVs isolated from strain 81-176 varied in size from 10 to 50 nm (49). The isolation of OMVs of different sizes may be explained by the use of a different growth temperature (42°C) and different medium (Muller-Hinton biphasic agar/broth) in the previous study (49). Indeed, the size range of OMVs isolated in this study is consistent with that of OMVs isolated from other Gram-negative bacteria (16). Variation in the size, shape, and electron density of C. jejuni OMVs suggests that there may be differential sorting of OMV cargo proteins, as is the case for P. gingivalis OMVs (28).
Proteome analysis of OMVs from C. jejuni 11168H identified at least 151 proteins derived mainly from the outer membrane and periplasm but also associated with the inner membrane and cytoplasm. The virulence-associated proteins identified included all three components of CDT (CdtA, CdtB, and CdtC), the fibronectin binding proteins CadF and FlpA, and the HtrA and Cj0511 proteases, as well as 16 N-linked glycoproteins, including the major antigenic protein Peb3. Apart from CDT, this is the first demonstration that virulence-associated C. jejuni proteins are delivered via OMVs. None of the three characterized secreted Cia proteins CiaB, CiaC, and CiaI (11, 15, 42) were found to be associated with C. jejuni OMVs, suggesting that OMVs are not the mechanism by which these virulence factors are secreted. The identification of a large number of N-linked glycoproteins associated with C. jejuni OMVs is particularly significant. The role of N-linked glycosylation in the biology of C. jejuni is still not fully understood. N-linked glycosylation may be involved in the evasion of the host immune response, as mutation of pgl genes that encode the glycan biosynthesis system results in changes in immunoreactivity (68). However, as most N-linked glycoproteins are not predicted to be surface exposed but rather located in the periplasm (75), it has been unclear why posttranslational modification of these proteins should enhance immune evasion. The identification of N-linked glycoproteins associated with C. jejuni OMVs indicates a mechanism by which these periplasm-located proteins may be delivered into human IECs to which the human immune system responds. The fact that Peb3 appears to be localized in the periplasm coupled with the reduction in the amount of Peb3 in pglB mutant OMVs compared to wild-type OMVs suggests that N-linked glycosylation may also play a role in OMV cargo selection. This is supported by the previous observation that when the C. jejuni N-linked glycosylation pathway is functionally transferred into E. coli, N-linked glycoproteins are identified within E. coli OMVs (20).
Many OMV-associated toxins have been identified, and OMVs have been shown to deliver active toxins to host cells from a variety of enteric pathogens, including ETEC, Shiga toxin-producing E. coli, and Helicobacter pylori (19). Virtually all the CDT secreted from C. jejuni wild-type strain 81-176 was found to be OMV associated, and coculture of 81-176 OMVs with the HCT8 human ileocecum carcinoma cell line induced a distinct cell enlargement and caused cell cycle arrest associated with the cytolethal distending effects of C. jejuni CDT (49). In this study, C. jejuni 11168H OMVs were shown to be cytotoxic to Caco-2 cells after a 24-h exposure, as measured by the presence of extracellular LDH, suggesting a role for other cytotoxic C. jejuni factors in addition to CDT, such as bacterial exotoxic proteases that can damage extracellular structures, as the primary effect of CDT is eukaryotic cell cycle arrest at the G2/M stage, which results in a cessation of cell division rather than cell lysis (49). The two serine proteases identified as associated with C. jejuni 11168H OMVs by proteomic analysis were HtrA (4, 9) and Cj0511. Further studies are required to establish whether this is a result of OMV-induced reduction in host cell membrane integrity or actual host cell lysis, though it is tempting to speculate that C. jejuni OMVs could modulate changes in human IEC membranes that enhance bacterial invasion.
Bacterial OMVs have been shown to be activators of the host innate and acquired immune response pathways (19). The effect of OMV-associated virulence factors can modulate the host immune response resulting in promotion of immune/inflammation-mediated damage or stimulation of clearance of the pathogen (19). A proinflammatory response to OMVs has been observed for several bacterial species, including H. pylori (33) and P. aeruginosa (6). Human IECs produce the proinflammatory cytokines IL-6 (22) and TNF-α (3) as well as the proinflammatory chemokine IL-8 (53) in response to C. jejuni infection in vitro. hBDs are antimicrobial peptides involved in innate immune defenses against pathogenic bacteria (59). Infection with C. jejuni leads to increased transcription, translation, and secretion of hBD-2 and hBD-3 from Caco-2 and HT-29 human IECs (77). This study is the first to show that C. jejuni OMVs act as initiators of the host immune response, inducing IL-8, IL-6, TNF-α, and hBD-3. The induction of IL-8 was also shown to be dependent on the concentration of C. jejuni 11168H OMVs incubated with T84 cells. The observation that OMVs pretreated with proteinase K show no reduction in the ability to induce both IL-8 and hBD-3 indicates that the C. jejuni virulence factors involved in the induction of these host responses are intravesicular components of OMVs, since proteinase K will degrade proteins externally associated with OMVs but not those within the lumen of the OMVs (10). LOS are a constituent of C. jejuni OMVs, so this could play a role in the induction of the innate immune response. The observation that OMVs pretreated with polymyxin B show no reduction in the ability to induce IL-8 indicates that C. jejuni LOS is not a major determinant in the host response to OMVs observed in this study. LOS activates proinflammatory cytokine and chemokines such as IL-8 through the signaling of Toll-like receptor 4 (TLR4) expressed on the surface of human IECs. However, T84 cells express only low levels of TLR4 (1), so a role for OMV-associated LOS in inducing the innate immune response should not be dismissed. One result of the proinflammatory response is the alteration of epithelial barrier function (64), and the induction of such a host cell response by C. jejuni OMVs may be a cause of the loss of host cell membrane integrity indicated by the cytotoxicity assay.
OMVs have been shown to deliver bacterial virulence factors into host cells by fusion with the host cell plasma membrane or via receptor-mediated endocytic pathways (19, 47). Binding of OMVs to lipid rafts has been reported for a number of bacterial pathogens, including ETEC (40), H. pylori (37), and P. aeruginosa (10). Disruption of the lipid rafts from the cell membranes of T84 cells with the cholesterol-depleting agent MβCD resulted in a reduction of the ability of C. jejuni OMVs to induce secretion of both IL-8 and hBD-3, indicating that these cholesterol-enriched domains may be similarly involved in the delivery of C. jejuni virulence factors by OMVs into human IECs. However, C. jejuni OMVs still induced secretion of both IL-8 and hBD-3 from MβCD-treated T84 cells, well above the levels observed in the controls. This would suggest that other pathways are also involved in OMV delivery of bacterial virulence factors into host cells. The identification of the fibronectin binding proteins CadF (44) and FlpA (45) as well as the major antigenic protein Peb3 (61) and the serine protease HtrA (4) associated with C. jejuni OMVs would indicate further possible mechanisms for the adherence of OMVs to human IECs, which could lead to internalization of OMVs by receptor-mediated endocytosis.
In this study, the levels of IL-8 induced by live 11168H cdtA mutant were not significantly reduced compared to those induced by live wild-type strain 11168H (MOI, 100:1). This is in support of the findings in a previous study that showed that 81-176 cdtA, cdtB, and cdtC mutants induce IL-8 from INT407 cells to an extent similar to that of the wild-type strain (30). In addition, the levels of IL-8 induced by cdtA OMVs were similar to those induced by 11168H OMVs (for both 100-μg and 10-μg amounts). Previously, membrane fractions isolated from 81-176 cdtA, cdtB, and cdtC mutants have been shown not to induce IL-8 from INT407 cells, in contrast to those from the wild-type strain (29, 30), suggesting a different activity for CDT within OMVs compared to membrane-bound CDT. The data in the present study indicate that induction of IL-8 by C. jejuni OMVs occurs via a CDT-independent mechanism.
G. mellonella larvae have been reported to be susceptible to C. jejuni infection as well as other enteric pathogens (12, 13). OMVs from Xenorhabdus nematophila and Photorhabdus luminescens have been shown to be cytotoxic both in tissue culture and to insect larvae (41). Inoculation of G. mellonella larvae with either C. jejuni 11168H or cdtA mutant OMVs resulted in killing of the larvae to the same extent. The cytotoxicity of 11168H OMVs was abolished by heat treatment, suggesting cytotoxicity is mediated by proteins within the OMVs. This is the first report of C. jejuni OMVs killing larvae and is also further evidence of the cytotoxicity of C. jejuni OMVs, which occurs via a CDT-independent mechanism.
In this study, we have demonstrated the importance of OMVs as a delivery vehicle for C. jejuni virulence factors and highlighted a number of effects on human IECs, including cytotoxicity and induction of the innate immune response, including the secretion of IL-8 from T84 cells via a CDT-independent mechanism. The identification of N-linked glycoproteins associated with C. jejuni OMVs indicates a mechanism for delivering these modified proteins, which are usually located in the bacterial periplasm, into host cells and could thus account for the reported immunogenicity of these proteins. Further studies are required to elucidate the role of the individual C. jejuni virulence factors secreted within OMVs during pathogenesis and the modulation of the host cell response to C. jejuni infection.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Marta Neto and Fatma Dalgakiran for technical assistance. The anti-Peb3 antibody was a generous gift from Shaun Cawthraw (Veterinary Laboratories Agency, United Kingdom).
Moredun Research Institute receives funding from the Scottish Government's Rural and Environment Science and Analytical Services Division (RESAS).
Footnotes
Published ahead of print 10 September 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Abreu MT, et al. 2001. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167:1609–1616 [DOI] [PubMed] [Google Scholar]
- 2. Allos BM. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 32:1201–1206 [DOI] [PubMed] [Google Scholar]
- 3. Al-Salloom FS, Al Mahmeed A, Ismaeel A, Botta GA, Bakhiet M. 2003. Campylobacter-stimulated INT407 cells produce dissociated cytokine profiles. J. Infect. 47:217–224 [DOI] [PubMed] [Google Scholar]
- 4. Baek KT, Vegge CS, Brondsted L. 2011. HtrA chaperone activity contributes to host cell binding in Campylobacter jejuni. Gut Pathog. 3:13 doi:10.1186/1757-4749-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Batycka M, et al. 2006. Ultra-fast tandem mass spectrometry scanning combined with monolithic column liquid chromatography increases throughput in proteomic analysis. Rapid Commun. Mass Spectrom. 20:2074–2080 [DOI] [PubMed] [Google Scholar]
- 6. Bauman SJ, Kuehn MJ. 2006. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 8:2400–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472–479 [DOI] [PubMed] [Google Scholar]
- 8. Blaser MJ, Hopkins JA, Berka RM, Vasil ML, Wang WL. 1983. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect. Immun. 42:276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boehm M, et al. 2012. Rapid paracellular transmigration of Campylobacter jejuni across polarized epithelial cells without affecting TER: role of proteolytic-active HtrA cleaving E-cadherin but not fibronectin. Gut Pathog. 4:3 doi:10.1186/1757-4749-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bomberger JM, et al. 2009. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5:e1000382 doi:10.1371/journal.ppat.1000382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buelow DR, Christensen JE, Neal-McKinney JM, Konkel ME. 2011. Campylobacter jejuni survival within human epithelial cells is enhanced by the secreted protein CiaI. Mol. Microbiol. 80:1296–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Champion OL, et al. 2009. Galleria mellonella as an alternative infection model for Yersinia pseudotuberculosis. Microbiology 155:1516–1522 [DOI] [PubMed] [Google Scholar]
- 13. Champion OL, et al. 2010. Insect infection model for Campylobacter jejuni reveals that O-methyl phosphoramidate has insecticidal activity. J. Infect. Dis. 201:776–782 [DOI] [PubMed] [Google Scholar]
- 14. Chen ML, Ge Z, Fox JG, Schauer DB. 2006. Disruption of tight junctions and induction of proinflammatory cytokine responses in colonic epithelial cells by Campylobacter jejuni. Infect. Immun. 74:6581–6589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Christensen JE, Pacheco SA, Konkel ME. 2009. Identification of a Campylobacter jejuni-secreted protein required for maximal invasion of host cells. Mol. Microbiol. 73:650–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deatherage BL, et al. 2009. Biogenesis of bacterial membrane vesicles. Mol. Microbiol. 72:1395–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ding SZ, et al. 2010. Helicobacter pylori-induced histone modification, associated gene expression in gastric epithelial cells, and its implication in pathogenesis. PLoS One 5:e9875 doi:10.1371/journal.pone.0009875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dorrell N, Wren BW. 2007. The second century of Campylobacter research: recent advances, new opportunities and old problems. Curr. Opin. Infect. Dis. 20:514–518 [DOI] [PubMed] [Google Scholar]
- 19. Ellis TN, Kuehn MJ. 2010. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74:81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher AC, et al. 2011. Production of secretory and extracellular N-linked glycoproteins in Escherichia coli. Appl. Environ. Microbiol. 77:871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fox JG, et al. 2004. Gastroenteritis in NF-kappaB-deficient mice is produced with wild-type Campylobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect. Immun. 72:1116–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friis LM, Keelan M, Taylor DE. 2009. Campylobacter jejuni drives MyD88-independent interleukin-6 secretion via Toll-like receptor 2. Infect. Immun. 77:1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gantier MP, et al. 2010. Genetic modulation of TLR8 response following bacterial phagocytosis. Hum. Mutat. 31:1069–1079 [DOI] [PubMed] [Google Scholar]
- 24. Ge Z, Schauer DB, Fox JG. 2008. In vivo virulence properties of bacterial cytolethal-distending toxin. Cell. Microbiol. 10:1599–1607 [DOI] [PubMed] [Google Scholar]
- 25. Grubman A, et al. 2010. The innate immune molecule, NOD1, regulates direct killing of Helicobacter pylori by antimicrobial peptides. Cell. Microbiol. 12:626–639 [DOI] [PubMed] [Google Scholar]
- 26. Gundogdu O, et al. 2011. The Campylobacter jejuni transcriptional regulator Cj1556 plays a role in the oxidative and aerobic stress response and is important for bacterial survival in vivo. J. Bacteriol. 193:4238–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hassane DC, Lee RB, Pickett CL. 2003. Campylobacter jejuni cytolethal distending toxin promotes DNA repair responses in normal human cells. Infect. Immun. 71:541–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haurat MF, et al. 2011. Selective sorting of cargo proteins into bacterial membrane vesicles. J. Biol. Chem. 286:1269–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hickey TE, Baqar S, Bourgeois AL, Ewing CP, Guerry P. 1999. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT407 cells. Infect. Immun. 67:88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hickey TE, et al. 2000. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect. Immun. 68:6535–6541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hickey TE, Majam G, Guerry P. 2005. Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytolethal distending toxin. Infect. Immun. 73:5194–5197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hutton ML, et al. 2010. Helicobacter pylori exploits cholesterol-rich microdomains for induction of NF-kappaB-dependent responses and peptidoglycan delivery in epithelial cells. Infect. Immun. 78:4523–4531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ismail S, Hampton MB, Keenan JI. 2003. Helicobacter pylori outer membrane vesicles modulate proliferation and interleukin-8 production by gastric epithelial cells. Infect. Immun. 71:5670–5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Janssen R, et al. 2008. Host-pathogen interactions in Campylobacter infections: the host perspective. Clin. Microbiol. Rev. 21:505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jones MA, et al. 2004. Adaptation of Campylobacter jejuni NCTC11168 to high-level colonization of the avian gastrointestinal tract. Infect. Immun. 72:3769–3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Juncker AS, et al. 2003. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 12:1652–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaparakis M, et al. 2010. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell. Microbiol. 12:372–385 [DOI] [PubMed] [Google Scholar]
- 38. Karlyshev AV, McCrossan MV, Wren BW. 2001. Demonstration of polysaccharide capsule in Campylobacter jejuni using electron microscopy. Infect. Immun. 69:5921–5924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kesty NC, Kuehn MJ. 2004. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J. Biol. Chem. 279:2069–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. 2004. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 23:4538–4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khandelwal P, Banerjee-Bhatnagar N. 2003. Insecticidal activity associated with the outer membrane vesicles of Xenorhabdus nematophilus. Appl. Environ. Microbiol. 69:2032–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Konkel ME, Kim BJ, Rivera-Amill V, Garvis SG. 1999. Bacterial secreted proteins are required for the internalization of Campylobacter jejuni into cultured mammalian cells. Mol. Microbiol. 32:691–701 [DOI] [PubMed] [Google Scholar]
- 43. Konkel ME, et al. 2004. Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J. Bacteriol. 186:3296–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Konkel ME, et al. 2005. Identification of a fibronectin-binding domain within the Campylobacter jejuni CadF protein. Mol. Microbiol. 57:1022–1035 [DOI] [PubMed] [Google Scholar]
- 45. Konkel ME, Larson CL, Flanagan RC. 2010. Campylobacter jejuni FlpA binds fibronectin and is required for maximal host cell adherence. J. Bacteriol. 192:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kuehn MJ, Kesty NC. 2005. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 19:2645–2655 [DOI] [PubMed] [Google Scholar]
- 47. Kulp A, Kuehn MJ. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64:163–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lara-Tejero M, Galan JE. 2001. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect. Immun. 69:4358–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lindmark B, et al. 2009. Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiol. 9:220 doi:10.1186/1471-2180-9-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Linton D, Allan E, Karlyshev AV, Cronshaw AD, Wren BW. 2002. Identification of N-acetylgalactosamine-containing glycoproteins PEB3 and CgpA in Campylobacter jejuni. Mol. Microbiol. 43:497–508 [DOI] [PubMed] [Google Scholar]
- 51. Linton D, et al. 2005. Functional analysis of the Campylobacter jejuni N-linked protein glycosylation pathway. Mol. Microbiol. 55:1695–1703 [DOI] [PubMed] [Google Scholar]
- 52. Logan SM, Trust TJ. 1982. Outer membrane characteristics of Campylobacter jejuni. Infect. Immun. 38:898–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. MacCallum AJ, Harris D, Haddock G, Everest PH. 2006. Campylobacter jejuni-infected human epithelial cell lines vary in their ability to secrete interleukin-8 compared to in vitro-infected primary human intestinal tissue. Microbiology 152:3661–3665 [DOI] [PubMed] [Google Scholar]
- 54. Mashburn-Warren L, McLean RJ, Whiteley M. 2008. Gram-negative outer membrane vesicles: beyond the cell surface. Geobiology 6:214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Min T, et al. 2009. Specificity of Campylobacter jejuni adhesin PEB3 for phosphates and structural differences among its ligand complexes. Biochemistry 48:3057–3067 [DOI] [PubMed] [Google Scholar]
- 56. Moorhead SM, Griffiths MW. 2011. Expression and characterization of cell-signalling molecules in Campylobacter jejuni. J. Appl. Microbiol. 110:786–800 [DOI] [PubMed] [Google Scholar]
- 57. Nachamkin I, Allos BM, Ho T. 1998. Campylobacter species and Guillain-Barre syndrome. Clin. Microbiol. Rev. 11:555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Novik V, Hofreuter D, Galan JE. 2010. Identification of Campylobacter jejuni genes involved in its interaction with epithelial cells. Infect. Immun. 78:3540–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ouellette AJ. 2004. Defensin-mediated innate immunity in the small intestine. Best Pract. Res. Clin. Gastroenterol. 18:405–419 [DOI] [PubMed] [Google Scholar]
- 60. Parkhill J, et al. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665–668 [DOI] [PubMed] [Google Scholar]
- 61. Pei ZH, Ellison RT, III, Blaser MJ. 1991. Identification, purification, and characterization of major antigenic proteins of Campylobacter jejuni. J. Biol. Chem. 266:16363–16369 [PubMed] [Google Scholar]
- 62. Poly F, et al. 2007. Heterogeneity of a Campylobacter jejuni protein that is secreted through the flagellar filament. Infect. Immun. 75:3859–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rathinam VA, Hoag KA, Mansfield LS. 2008. Dendritic cells from C57BL/6 mice undergo activation and induce Th1-effector cell responses against Campylobacter jejuni. Microbes Infect. 10:1316–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rees LE, et al. 2008. Campylobacter and IFNgamma interact to cause a rapid loss of epithelial barrier integrity. Inflamm. Bowel Dis. 14:303–309 [DOI] [PubMed] [Google Scholar]
- 65. Scott NE, et al. 2011. Simultaneous glycan-peptide characterization using hydrophilic interaction chromatography and parallel fragmentation by CID, higher energy collisional dissociation, and electron transfer dissociation MS applied to the N-linked glycoproteome of Campylobacter jejuni. Mol. Cell. Proteomics 10(2):M000031–MCP201 doi:10.1074/mcp.M000031-MCP201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shevchenko A, Wilm M, Vorm O, Mann M. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850–858 [DOI] [PubMed] [Google Scholar]
- 67. Smith JL, Bayles DO. 2006. The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit. Rev. Microbiol. 32:227–248 [DOI] [PubMed] [Google Scholar]
- 68. Szymanski CM, Yao R, Ewing CP, Trust TJ, Guerry P. 1999. Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol. Microbiol. 32:1022–1030 [DOI] [PubMed] [Google Scholar]
- 69. Szymanski CM, Logan SM, Linton D, Wren BW. 2003. Campylobacter—a tale of two protein glycosylation systems. Trends Microbiol. 11:233–238 [DOI] [PubMed] [Google Scholar]
- 70. Tatusov RL, et al. 2003. The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4:41 doi:10.1186/1471-2105-4-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Taylor GK, Goodlett DR. 2005. Rules governing protein identification by mass spectrometry. Rapid Commun. Mass Spectrom. 19:3420 doi:10.1002/rcm.2225 [DOI] [PubMed] [Google Scholar]
- 72. Watson RO, Galan JE. 2008. Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog. 4:e14 doi:10.1371/journal.ppat.0040014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Whitehouse CA, et al. 1998. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect. Immun. 66:1934–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Young KT, Davis LM, Dirita VJ. 2007. Campylobacter jejuni: molecular biology and pathogenesis. Nat. Rev. Microbiol. 5:665–679 [DOI] [PubMed] [Google Scholar]
- 75. Young NM, et al. 2002. Structure of the N-linked glycan present on multiple glycoproteins in the Gram-negative bacterium, Campylobacter jejuni. J. Biol. Chem. 277:42530–42539 [DOI] [PubMed] [Google Scholar]
- 76. Zheng J, Meng J, Zhao S, Singh R, Song W. 2008. Campylobacter-induced interleukin-8 secretion in polarized human intestinal epithelial cells requires Campylobacter-secreted cytolethal distending toxin- and Toll-like receptor-mediated activation of NF-kappaB. Infect. Immun. 76:4498–4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zilbauer M, et al. 2005. Intestinal innate immunity to Campylobacter jejuni results in induction of bactericidal human beta-defensins 2 and 3. Infect. Immun. 73:7281–7289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.