Abstract
Background:
The aim of this study was to compare patient-perceived relief of ocular itch, nasal symptoms, and eye drop comfort when allergic conjunctivitis was treated with bepotastine besilate 1.5% versus olopatadine hydrochloride 0.2%.
Methods:
This randomized, observer-masked, single-center, crossover study included 30 patients with ocular itching associated with allergic conjunctivitis accompanied by nasal symptoms. Patients were treated with bepotastine besilate 1.5% twice daily (7 am and 4 pm) or olopatadine hydrochloride 0.2% once daily (7 am) for 14 days. Following a 7-day washout period during which only preservative-free artificial tears were used twice daily, patients were crossed over to the alternative treatment for 14 days. Parameters evaluated by twice-daily patient diaries included each treatment’s ability to relieve ocular itch, ability to relieve itchy/runny nose, ability to relieve ocular allergy symptoms, and eye drop comfort. At the conclusion of the study, patients were also asked to identify which agent provided better all-day relief of ocular itching, better all-day relief of itchy/runny nose, superior comfort, and for which treatment they would prefer a prescription.
Results:
According to the mean daily diary responses, bepotastine besilate 1.5% provided significantly better relief of evening ocular itch, relief of morning and evening itchy/runny nose, and relief of morning and evening ocular allergy symptoms. At study end, 63.3% and 66.7% of patients preferred bepotastine besilate 1.5% for all-day relief of ocular itching and all-day relief of itchy/runny nose, respectively. At study end, there was no significant difference in the number of patients preferring one treatment over the other for comfort. Overall, 66.7% of patients stated that they would prefer to treat their allergic conjunctivitis with bepotastine besilate 1.5% over olopatadine hydrochloride 0.2%.
Conclusion:
Based on their evaluation of therapeutic performance, patients preferred bepotastine besilate 1.5% over olopatadine hydrochloride 0.2% by two-to-one for the treatment of allergic conjunctivitis.
Keywords: allergic conjunctivitis, bepotastine besilate, olopatadine hydrochloride, patient preference
Introduction
Ocular discomfort associated with allergic conjunctivitis is a common patient complaint, responsible for approximately 15% of primary care office and emergency room visits for eye-related problems.1 According to epidemiological surveys, up to 35% of the US population suffers from ocular allergies, though the true prevalence may be higher.2,3 Clinical diagnosis is made by assessing both patient symptoms of intermittent or exposure-related ocular itching and signs of conjunctival papillae, hyperemia, and epiphora. Interestingly, 94% of patients with allergic conjunctivitis also have allergic rhinitis symptoms of nasal itching and rhinorrhea.4 All of these symptoms have a negative impact on patients’ ocular and nasal comfort and may result in disruption and restriction of daily activities, performance, and productivity, as well as increased economic burden.5,6 This reduction in general quality of life underscores the importance of allergic conjunctivitis and its proper treatment.
Allergic conjunctivitis occurs due to a type I hypersensitivity cascade reaction triggered by repeated antigen exposure. After a sensitized individual comes into contact with the allergen (usually grass or tree pollen), cross-linking of complementary IgE molecules on the conjunctival mast cell surface trigger mast cell degranulation and histamine release. This early phase response is characterized by itching, hyperemia, and chemosis. Six to 12 hours later, the early phase response is followed by a late phase reaction, which involves conjunctival infiltration of inflammatory cells, particularly eosinophils. These late phase reaction cells can cause tissue damage and severe allergic inflammation of the conjunctiva.7 Recommended treatments for symptoms of allergic conjunctivitis include avoidance of the offending antigen, topical mast cell stabilizers, antihistamines, and steroids.8
The newest type of topical anti-allergy medication for allergic conjunctivitis is the dual-action agent, which combines strong antihistaminic activity with mast cell-stabilizing properties to provide both rapid and long-lasting relief. Bepotastine besilate 1.5% ophthalmic solution (Bepreve®, Bausch + Lomb, Rochester, NY) and olopatadine hydrochloride 0.2% ophthalmic solution (Pataday®, Alcon Laboratories, Fort Worth, TX) are two popular topical medications prescribed for the treatment of allergic conjunctivitis. Both are dual-action agents. Bepotastine besilate 1.5% was approved by the US Food and Drug Administration in 2009 for the treatment of ocular itching associated with allergic conjunctivitis, with twice a day dosing in patients 2 years of age and older.9 Bepotastine besilate 1.5% has undergone three randomized, placebo-controlled US clinical studies, two conjunctival allergen challenge studies, and a six-week safety study with twice-daily dosing. Both the single and multisite Phase II/III conjunctival allergen challenge studies demonstrated rapid clinical benefit in treating allergen-induced ocular itching that lasted for at least 8 hours (primary efficacy endpoint),10,11 and the single-site trial reported effects lasting 16 hours.11 It has also been shown that bepotastine besilate 1.5% provides complete relief in approximately 3 minutes in 68% of eyes, even if the itching is severe.12 Olopatadine hydrochloride 0.2% was approved by the Food and Drug Administration in 2004 for the treatment of ocular itching associated with allergic conjunctivitis, with once a day dosing in patients 2 years of age and older.13 One conjunctival allergen challenge study revealed that olopatadine significantly reduced the itching score at 3, 5, and 10 minutes after antigen induction for up to 16 hours after dosing.14 In another double-masked conjunctival allergen challenge study, olopatadine was found to suppress ocular itching significantly, while also earning a superior comfort rating.15 These results establish the efficacy and comfort of both bepotastine besilate 1.5% and olopatadine hydrochloride 0.2%.
Interestingly, no head-to-head clinical studies have been published directly comparing bepotastine besilate 1.5% and olopatadine hydrochloride 0.2%. In an effort to determine which agent better controls the symptoms of allergic conjunctivitis in our patient community, we undertook an investigator-initiated, single-center, observer-masked, crossover patient preference study. This study compares patient-perceived relief of ocular itch, relief of nasal symptoms, eye drop comfort, and preferred medicine when treating allergic conjunctivitis with bepotastine besilate 1.5% compared with olopatadine hydrochloride 0.2% for 14 days.
Materials and methods
Thirty consecutive patients presenting in September 2011, during the peak fall allergy season, with ocular itching associated with allergic conjunctivitis and itchy/runny nose participated in this study conducted at McCabe Vision Center, Murfreesboro, TN. All enrolled patients were at least 18 years of age, had a diagnosis of allergic conjunctivitis with no concurrent unrelated ocular diseases, and had no plans to undergo ocular surgery during the study period. Any woman capable of becoming pregnant agreed to have urinary human chorionic gonadotropin pregnancy testing performed at screening and to use a medically acceptable form of birth control throughout the study duration and for at least one week prior to and one week after completion of the study. Patients who had a known hypersensitivity to either agent, a history of alcohol or drug abuse, a positive history of an ocular herpetic infection, an active ocular infection, or any significant illness were not enrolled in the study. Patients who were actively taking steroids or antihistamines within 7 days prior to enrollment, pregnant, planning to become pregnant, or nursing/lactating were also excluded. Investigational review board approval was obtained for this study (Sterling Institutional Review Board, Atlanta, GA). All patients signed an informed consent form.
The enrolled patients were assigned sequentially according to a computer-generated randomization list to receive bepotastine besilate 1.5% or olopatadine hydrochloride 0.2% in a 1:1 ratio. Patients instilled either bepotastine besilate 1.5% twice daily (at approximately 7 am and 4 pm) or olopatadine hydrochloride 0.2% once daily (at approximately 7 am) for 14 days. Following a 7-day washout period during which only preservative-free artificial tears were used twice daily, patients were crossed-over to the other treatment for 14 days. Each treatment was provided in the packaging originally approved by the Food and Drug Administration, but the single investigator was masked as to which treatment the patient was currently using. Patients were instructed to use gentle eye lid closure for at least two minutes after dosing and to repeat instillation of a single drop if there was uncertainty as to whether successful instillation of the treatment had occurred. Patients wearing contact lenses were instructed to remove the lenses prior to application of medication and to replace the lenses 10 minutes following application of medication. In addition, patients wearing contact lenses were encouraged to use glasses during the study period.
Patients completed an office questionnaire at visit 1 (baseline, day 0), visit 2 (day 14), and visit 3 (day 35), as well as a daily home diary at noon and 8 pm. During visit 1, prior to dispensing the treatment, patients were asked to rate the following items on a five-point Likert scale: ocular itching associated with allergies; itchy/runny nose associated with allergies; and satisfaction with over-the-counter allergy medication. Patients were asked whether the itching associated with allergies was more bothersome in the morning or in the evening. Patients were also asked whether they spent more time outside in the morning or the evening, and whether their allergy symptoms were seasonal or perennial.
During visit 2, a different survey questionnaire was administered. Prior to dispensing the second medication, the patients were asked to rate the following items on a five-point Likert scale: ocular itching prior to dosing in the morning; how well the eye drop relieved ocular itching during the day; how well the eye drop relieved itchy/runny nose; and the comfort of the drop. The patients were assessed for adverse events. Following the questionnaire, the patients received preservative-free artificial tear drops and were instructed to use the drops twice daily for one week prior to starting the second treatment.
On day 21, patients were informed to discontinue using the artificial tear drops and to start using the new treatment for 14 days. During visits 2 and 3, the patients were assessed for adverse events. In addition, a final summary questionnaire was given at visit 3. The patients were asked to choose which eye drop provided better all-day relief of ocular itching, provided better all-day relief of itchy/runny nose, and was more comfortable. Lastly, the patients were asked to choose which medication they would like to have as a prescription to continue treating their allergic conjunctivitis.
During the study period, at noon and 8 pm, patients recorded in their daily diary how well the treatment relieved their ocular itch and nasal itchy/runny nose (graded on a 1–5 scale, with 5 being completely relieved) and how well the treatment relieved all of their ocular allergy symptoms (graded on a 1–3 scale, with 3 being completely relieved). Ocular allergy symptoms included ocular itch, epiphora, conjunctival chemosis, hyperemia, and eye lid edema. Nasal allergy symptoms included itchy, runny, or congested nose. At 8 pm, the patients also rated eye drop comfort on a 1–5 scale, with 5 being very comfortable. Comfort was defined by the patient, given that all patients have different tolerability thresholds.
Statistical analysis using the paired t-test, Pearson’s Chi-squared test, and the two-proportion z-test was performed to compare any differences in measured values between the two study medications as captured in the survey and diary data.
Results
This study screened 36 patients, and 30 patients met the inclusion criteria. The included patients had a mean age of 50 years. The majority (66.7%, 20/30 patients) were women. Table 1 shows the baseline characteristics of the group. Patients reported a trend towards worse allergy symptoms in the morning (ocular itch and itchy/runny nose), even though most (60%) of the patients spent more of their time outside in the afternoon or evening. The patient population was almost equally divided between those claiming seasonal (53.3%) and perennial (46.7%) allergies. The vast majority (86.4%) of the patients were either not satisfied or only partly satisfied with over-the-counter allergy relief medications.
Table 1.
Patient baseline characteristics
| Parameter | Value |
|---|---|
| Age (y) | |
| Mean ± SD | 49.8 ± 2.76 |
| Range | 23–75 |
| Gender, n (%) | |
| Male | 10 (33.3) |
| Female | 20 (66.7) |
| Severity of eye itch (1–5) | |
| Mean ± SD | 3.17 ± 0.16 |
| Time of most severe eye itch, n (%) | |
| AM | 15 (50) |
| PM | 13 (43.3) |
| Neither | 2 (6.7) |
| Severity of itchy/runny nose (1–5) | |
| Mean ± SD | 3.3 ± 0.19 |
| Time of most severe itchy/runny nose, n (%) | |
| AM | 17 (56.7) |
| PM | 11 (36.7) |
| Neither | 2 (6.7) |
| Satisfaction with OTC allergy eye drops, n (%) | |
| Very satisfied | 3 (10) |
| Some what satisfied | 15 (50) |
| Not satisfied | 11 (36.7) |
| Never tried | 1 (3.6) |
| Duration of allergy symptoms, n (%) | |
| Seasonal | 16 (53.3) |
| Perennial | 14 (46.7) |
| Time most outside, n (%) | |
| AM | 12 (40) |
| PM | 18 (60) |
Abbreviations: OTC, over the counter; SD, standard deviation.
Ocular itching
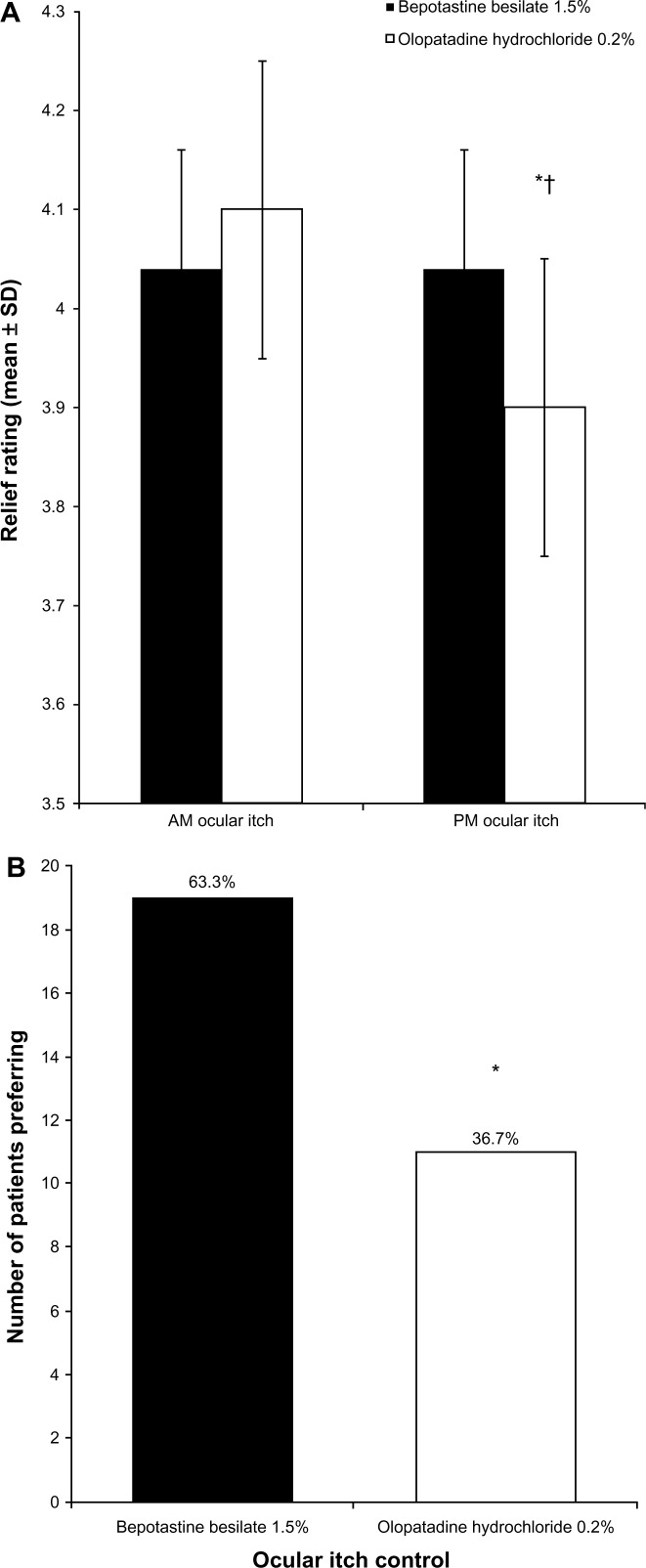
According to the mean daily diary responses, both treatments were equally efficacious at relieving morning ocular itching (Figure 1A). In contrast, bepotastine besilate 1.5% was significantly more effective at relieving ocular itching in the evening (P = 0.011). When comparing ocular itching relief between morning and evening, olopatadine hydrochloride 0.2% was significantly less effective in the evening than in the morning (P < 0.0001), whereas bepotastine besilate 1.5% was equally effective in the morning and evening. At study end, 63.3% (19/30) of patients preferred bepotastine besilate 1.5% for all-day relief of ocular itching (P = 0.04; Figure 1B).
Figure 1.
(A) Morning and evening relief of ocular itch over 14 days of treatment (mean ± standard deviation). Ocular itch was graded on a 1–5 scale, with 5 being completely relieved. *P = 0.011 versus bepotastine besilate 1.5% in the evening; †P < 0.0001 versus olopatadine hydrochloride 0.2% in the morning. (B) Patient preference for all-day relief of ocular itching at study end (visit 3, day 35).
Notes: Each bar represents the number of patients who stated that the medication was superior. *P = 0.04.
Abbreviation: SD, standard deviation.
Itchy/runny nose
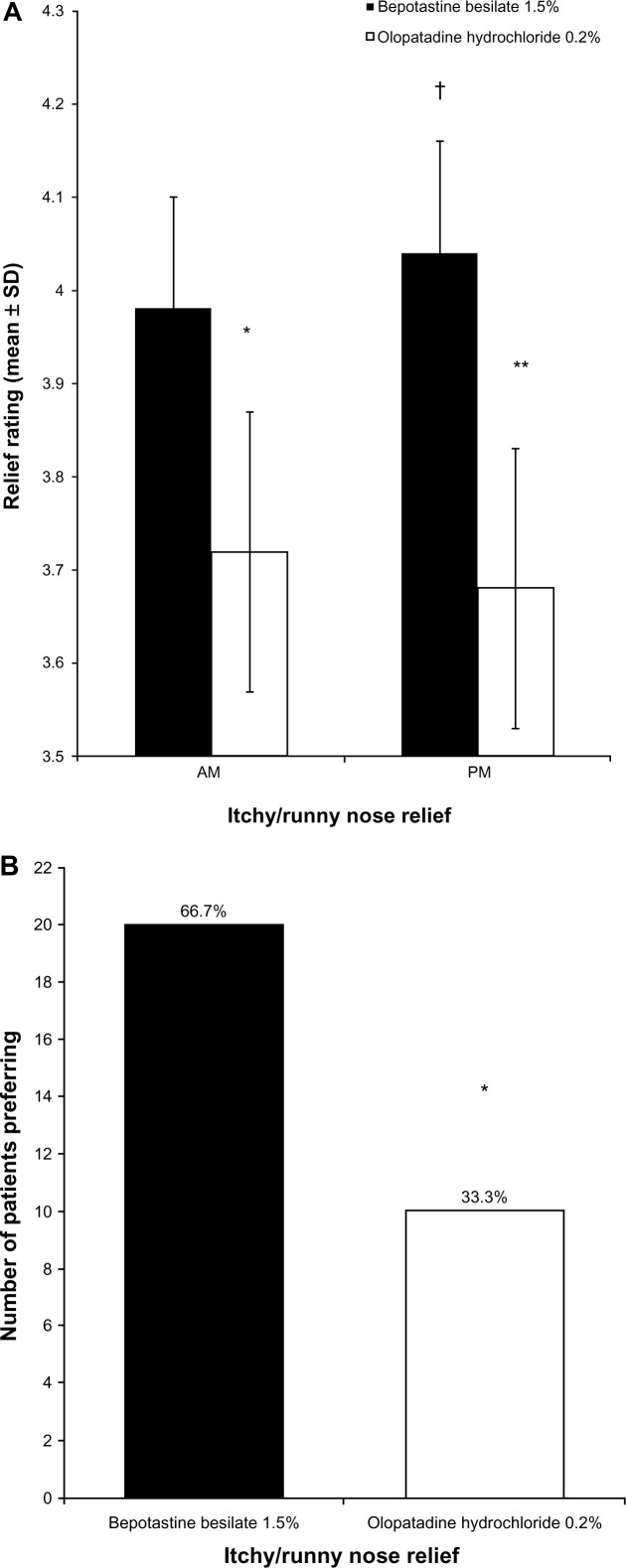
According to the mean daily diary responses, bepotastine besilate 1.5% was significantly more effective at relieving morning and evening itchy/runny nose (P = 0.0001) compared with olopatadine hydrochloride 0.2% (Figure 2A). In addition, when comparing itchy/runny nose relief between morning and evening, bepotastine besilate 1.5% provided significantly more relief in the evening than in the morning (P < 0.035), whereas the relief associated with olopatadine hydrochloride 0.2% did not change between morning and evening. At study end, 66.7% (20/30) of patients preferred bepotastine besilate 1.5% for all-day relief of itchy/runny nose (P = 0.01; Figure 2B).
Figure 2.
(A) Morning and evening relief of itchy/runny nose over 14 days of treatment (mean ± standard deviation). Nasal symptoms were graded on a 1–5 scale, with 5 being completely relieved. *P < 0.0001 versus bepotastine besilate 1.5% in the morning; **P < 0.0001 versus bepotastine besilate 1.5% in the evening; †P = 0.035 versus bepotastine besilate 1.5% in the morning. (B) Patient preference for all-day relief of itchy/runny nose at study end (visit 3, day 35).
Notes: Each bar represents the number of patients who stated that the medication was superior. *P = 0.01.
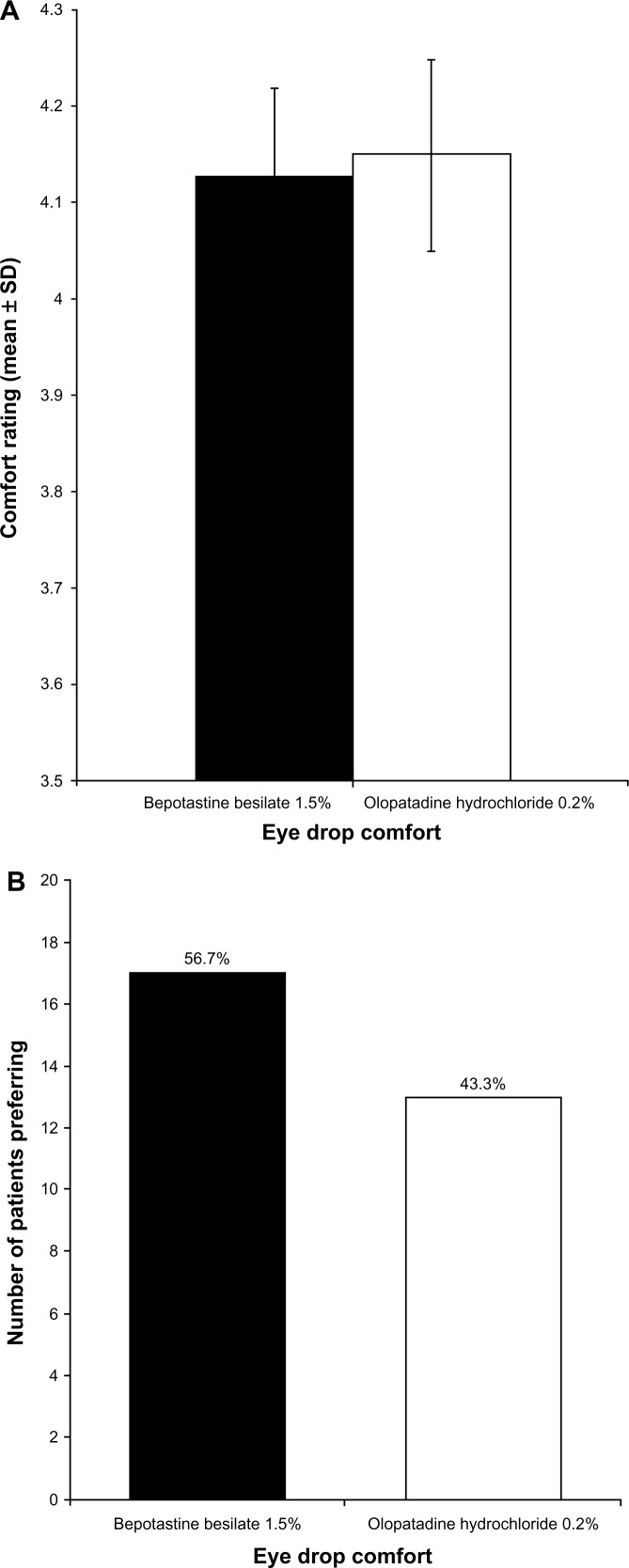
Comfort
Every evening, patients rated eye drop comfort, and these results are presented in Figure 3. According to the mean daily diary responses, both eye drops were rated similarly, with both being very comfortable (Figure 3A). At study end, there was no significant difference in the number of patients preferring one treatment over the other for comfort, with 56.7% (17/30) of patients choosing bepotastine besilate 1.5% as the more comfortable agent and 43.3% (13/30) of patients choosing olopatadine hydrochloride 0.2% (Figure 3B).
Figure 3.
(A) Evening eye drop comfort over 14 days of treatment (mean ± standard deviation). Eye drop comfort was graded on a 1–5 scale, with 5 being very comfortable. (B) Patient preference for eye drop comfort at study end (visit 3, day 35).
Note: Each bar represents the number of patients who stated that the medication was superior.
Overall ocular allergy symptoms
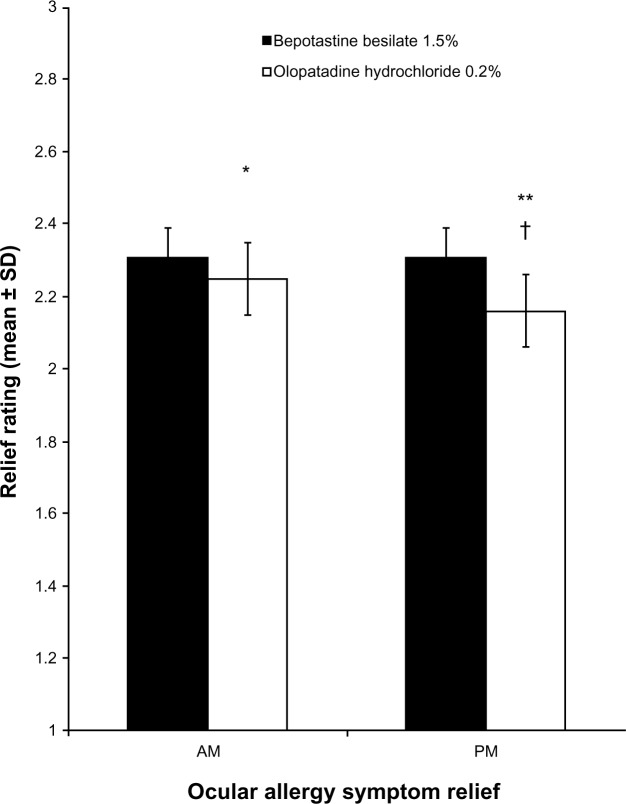
Figure 4 compares the mean rating of each treatment’s ability to relieve all ocular-related allergy symptoms in the morning and evening. According to the mean daily diary responses, bepotastine besilate 1.5% was significantly more effective at relieving morning and evening ocular allergy symptoms (P = 0.032 and P < 0.0001, respectively) compared with olopatadine hydrochloride 0.2% (Figure 4). In addition, when comparing ocular allergy symptom relief between morning and evening, the relief associated with bepotastine besilate 1.5% did not change between morning and evening, whereas the relief associated with olopatadine hydrochloride 0.2% was significantly greater in the morning than the evening (P < 0.001).
Figure 4.
Morning and afternoon/evening relief of overall ocular allergy symptoms over 14 days of treatment (mean ± standard deviation).
Notes: Overall ocular allergy symptoms graded on a 1–3 scale, with 3 being completely relieved. Ocular allergy symptoms included ocular itch, epiphora, conjunctival chemosis, hyperemia, and eyelid edema. *P = 0.032 versus bepotastine besilate 1.5% in the morning; **P < 0.0001 versus bepotastine besilate 1.5% in the evening; †P < 0.001 versus olopatadine hydrochloride 0.2% in the morning.
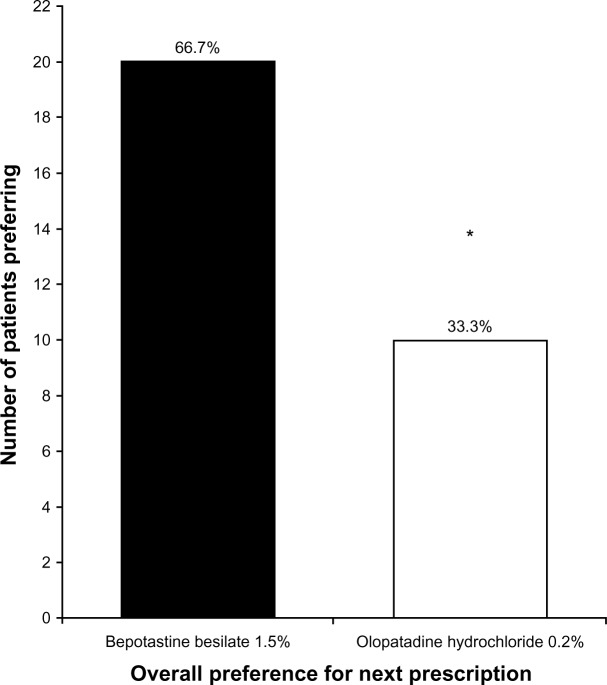
Prescription preference
At study end, patients were asked to name which eye drop they wanted a prescription for, so that they could continue treatment. A total of 66.7% (20/30) of patients stated that they would prefer to treat their allergic conjunctivitis with bepotastine besilate 1.5% compared with 33.3% (10/30) of patients who would prefer to treat with olopatadine hydrochloride 0.2% (P = 0.01, Figure 5).
Figure 5.
Overall patient preference for next prescription to treat allergic conjunctivitis at study end (visit 3, day 35). *P = 0.01.
Using Pearson’s Chi-squared test, patient preferences for ocular itch relief, itchy/runny nose relief, and better all-day relief of both treatments were examined as they related to the data in Table 1. Selection of medication preference was shown to be independent of gender, duration of allergy symptoms, time spent outside, time of most severe eye itch, and time of most severe itchy/runny nose (P ≥ 0.40 for all variables).
Adverse events
Approximately 10% of the patients treated with bepotastine besilate 1.5% reported a mild adverse taste. None of the patients were discontinued from the study. No other adverse events were reported.
Discussion
The increasing prevalence of allergic conjunctivitis and its deleterious effects on vision and ocular comfort necessitates the use of a safe, highly effective, and comfortable topical medicine. However, the current literature is lacking comparative data to assist the eye care professional in selecting the appropriate initial topical treatment. The majority of patients presenting at the clinical site of this study report that over-the-counter agents are not very effective. This observation prompted initiation of this single-center, randomized, observer-masked, crossover trial to examine the differences in patient satisfaction regarding symptom relief and comfort of two commonly prescribed allergy eye drops, bepotastine besilate 1.5% twice daily and olopatadine hydrochloride 0.2% once daily.
According to mean daily diary responses after the 7 am eye drop instillation, both medications provided significant relief from ocular itch, which lasted all morning. However, most patients stated that they were exposed to more outdoor allergens during the afternoon and evening hours. Patients using bepotastine besilate 1.5%, but not olopatadine hydrochloride 0.2%, applied a second drop at 4 pm. Accordingly, when patients were treated with bepotastine besilate 1.5%, the mean ocular itch relief reported in the evening was similar to the relief reported in the morning. In contrast, when patients were treated with olopatadine hydrochloride 0.2%, the evening ocular itch relief was significantly lower than the morning ocular itch relief (P < 0.0001). Indeed, patients reported better all-day relief of ocular itch when using bepotastine besilate 1.5% instead of olopatadine hydrochloride 0.2%. This suggests that although olopatadine hydrochloride 0.2% provides hours of ocular itching relief, it may perform better in the evening if it were dosed twice daily like bepotastine besilate 1.5%; however, olopatadine hydrochloride 0.2% is not indicated for twice-daily dosing.
Although neither of these ocular allergy eye drops is indicated for the relief of nasal symptoms in patients with allergic conjunctivitis, both medications reduced the severity of nasal symptoms in this study. A 10 mg tablet formulation of bepotastine besilate was developed in Japan as a treatment for allergic rhinitis16 and Patanase® nasal spray (olopatadine hydrochloride 0.6%; Alcon Laboratories, Fort Worth, TX) is indicated for the relief of symptoms of seasonal allergic rhinitis.17 It is logical that bepotastine besilate 1.5% ophthalmic solution and olopatadine hydrochloride 2.0% ophthalmic solution could relieve the nasal allergy symptoms associated with allergic conjunctivitis, considering that topically applied medications present in the tear film on the ocular surface can migrate through the nasolacrimal duct. This mechanism explains how both treatments reduce the nasal symptoms of patients treated for allergic conjunctivitis. However, this study demonstrated that patients reported greater relief of itchy/runny nose symptoms with bepotastine besilate 1.5% than olopatadine hydrochloride 0.2% in both the morning and evening during the 2-week dosing period.
There was no significant difference between the comfort ratings of the treatments. These data agree with the conclusion of a previous study, where bepotastine besilate 1.5% and olopatadine hydrochloride 0.2% also had similar comfort ratings.18 Olopatadine hydrochloride 0.2% has been rated as more comfortable than many other allergy eye drops,19,20 suggesting that bepotastine besilate 1.5% may also be one of the more comfortable agents for the treatment of allergic conjunctivitis.
Overall, 66.7% of patients reported that they would prefer their next prescription for the treatment of ocular allergies to be written for bepotastine besilate 1.5% rather than for olopatadine hydrochloride 0.2%. This finding is based solely on study observations, irrespective of product cost (patients were not made aware of any potential product price difference). The preference is especially noteworthy due to the difference in dosing schedules between the two medications. As demonstrated by Reginster et al, the number of required treatment doses is negatively related to patient compliance.21 Because poor treatment adherence leads to reduced clinical benefit, as has been demonstrated with chronic medications, it would be assumed that olopatadine hydrochloride 0.2% would be preferred by patients, because of both its convenience and efficacy. The present results contradict this assumption. Our patients reported greater relief of evening ocular itch, evening itchy/runny nose, and evening ocular allergy symptoms with bepotastine besilate 1.5%. Thus, patients suffering from allergic conjunctivitis will choose and comply with a twice-daily, rather than once-daily, dosing schedule because they feel that better relief of their ocular itching, itchy/runny nose, and other ocular allergy symptoms in the evening is worth the effort of instilling a second dose of their allergy eye drop.
Several limitations of this study must be considered. Because this was a single-center trial, the efficacy of bepotastine besilate 1.5% and olopatadine hydrochloride 0.2% was only evaluated for geographical site-specific allergens. Also, the study was conducted during the fall allergy season, which does not allow for determination of each medicine’s ability to alleviate symptoms of all seasonal allergies. The majority of the study population was older women. In our clinical practice, more women than men presented with complaints of symptoms of allergic conjunctivitis. Larger epidemiological studies would be needed to determine whether, like dry eye, more women suffer from allergic conjunctivitis. Finally, the study was observer-masked rather than double-masked. The study was designed with on-label medication administration to evaluate preference in a real-world scenario. There is the possibility that patients could have been influenced by product marketing or previous brand experience, but this too simulates a real-world scenario. We recommend that larger, randomized, double-masked, patient-preference, crossover studies be performed during different months of the year and at different geographical locations to better define the best initial topical therapy for patients with allergic conjunctivitis in these regions.
In conclusion, this study demonstrated that the patients at this site preferred bepotastine besilate 1.5% over olopatadine hydrochloride 0.2% for the treatment of ocular itch, itchy/runny nose, and ocular allergy symptoms associated with allergic conjunctivitis. Although this study did not include the investigator’s evaluation of clinical efficacy, it provides novel information from the patient’s perspective concerning treatment of this prevalent ocular disease which reduces performance and quality of life. Interestingly, patients chose efficacy over convenience to relieve the multiple symptoms of allergic conjunctivitis.
Footnotes
Disclosure
No author has a financial or proprietary interest in any material or method mentioned in this work.
References
- 1.Manners T. Managing eye conditions in general practice. BMJ. 1997;315:816–817. [PMC free article] [PubMed] [Google Scholar]
- 2.Abelson MB, Loeffler O. Conjunctival allergen challenge: models in the investigation of ocular allergy. Curr Allergy Asthma Rep. 2003;3:363–368. doi: 10.1007/s11882-003-0100-z. [DOI] [PubMed] [Google Scholar]
- 3.Bielory L. Ocular allergy overview. Immunol Allergy Clin North Am. 2008;28:1–23. doi: 10.1016/j.iac.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Wüthrich B, Brignoli R, Canevascini M, Gerber M. Epidemiological survey in hay fever patients: symptom prevalence and severity and influence on patient management. Schweiz Med Wochenschr. 1998;128:139–143. German. [PubMed] [Google Scholar]
- 5.Pitt AD, Smith AF, Lindsell L, Voon LW, Rose PW, Bron AJ. Economic and quality-of-life impact of seasonal allergic conjunctivitis in Oxfordshire. Ophthalmic Epidemiol. 2004;11:17–33. doi: 10.1076/opep.11.1.17.26437. [DOI] [PubMed] [Google Scholar]
- 6.Blaiss MS. Allergic rhinoconjunctivitis: burden of disease. Allergy Asthma Proc. 2007;28:393–397. doi: 10.2500/aap.2007.28.3013. [DOI] [PubMed] [Google Scholar]
- 7.Niederkorn JY. Immune regulatory mechanisms in allergic conjunctivitis: insights from mouse models. Curr Opin Allergy Clin Immunol. 2008;8:472–476. doi: 10.1097/ACI.0b013e32830edbcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leibowitz HM. The red eye. N Engl J Med. 2000;343:345–351. doi: 10.1056/NEJM200008033430507. [DOI] [PubMed] [Google Scholar]
- 9.Bepreve®. [Package insert] Irvine, CA: ISTA Pharmaceuticals Inc; 2009. [Google Scholar]
- 10.Abelson MB, Torkildsen GL, Williams JI, et al. Time to onset and duration of action of the antihistamine bepotastine besilate ophthalmic solutions 1.0% and 1.5% in allergic conjunctivitis: a phase III, single center, prospective, randomized, double masked, placebo controlled, conjunctival allergen challenge assessment in adults and children. Clin Ther. 2009;31:1908–1921. doi: 10.1016/j.clinthera.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Williams JI, Schooley GL, Gow JA, McNamara TR. Bepreve 1.5% provides clinically meaningful reduction in allergen-induced ocular itching for subjects in an analysis of two phase 3 conjunctival allergen challenge (CAC) clinical trials. [AAAI abstract 151] J Allergy Clin Immunol. 2010;125(2 Suppl 1):AB38. [Google Scholar]
- 12.Clark JC, Williams JI, Gow JA, et al. Bepotastine besilate ophthalmic solution 1.5% rapidly eliminates ocular itching in more severely allergic subjects in the conjunctival allergen challenge model of allergic conjunctivitis. Poster presented at the Eastern Allergy Conference; Palm Beach, FL. May 6–9, 2010. [Google Scholar]
- 13.Pataday™. [Package insert] Fort Worth, TX: Laboratories Inc; 2010. [Google Scholar]
- 14.Berdy GJ, Spangler DL, Bensch G, et al. A comparison of the relative efficacy and clinical performance of olopatadine hydrochloride 0.1% ophthalmic solution and ketotifen fumarate 0.025% ophthalmic solution in the conjunctival antigen challenge model. Clin Ther. 2000;22:826–833. doi: 10.1016/S0149-2918(00)80055-7. [DOI] [PubMed] [Google Scholar]
- 15.Abelson MB, Greiner JV. Comparative efficacy of olopatadine 0.1% ophthalmic solution versus levocabastine 0.05% ophthalmic suspension using the conjunctival allergen challenge model. Curr Med Res Opin. 2004;20:1953–1958. doi: 10.1185/030079904X5724. [DOI] [PubMed] [Google Scholar]
- 16.Hashiguchi K, Tang H, Fujita T, Suematsu K, Gotoh M, Okubo K. Bepotastine besilate tablets suppress nasal symptoms caused by Japanese cedar pollen exposure in an artificial exposure chamber (OHIO Chamber) Expert Opin Pharmacother. 2009;10:523–529. doi: 10.1517/14656560902758368. [DOI] [PubMed] [Google Scholar]
- 17.Patanase® Nasal Spray [Prescribing information] Fort Worth, TX: Alcon Laboratories Inc; 2008. [Google Scholar]
- 18.Spears K, McKee E, Edmonson W. Anti-allergy drop comfort and cost. Optometry. 2011;82:386. [Google Scholar]
- 19.Artal MN, Luna JD, Discepola M. A forced choice comfort study of olopatadine hydrochloride 0.1% versus ketotifen fumarate 0.05% Acta Ophthalmol Scand Suppl. 2000;230:64–65. doi: 10.1034/j.1600-0420.2000.078s230064.x. [DOI] [PubMed] [Google Scholar]
- 20.Epstein AB, van Hoven PT, Kaufman A, Carr WW. Management of allergic conjunctivitis: an evaluation of the perceived comfort and therapeutic efficacy of olopatadine 0.2% and azelastine 0.05% from two prospective studies. Clin Ophthalmol. 2009;3:329–336. doi: 10.2147/opth.s5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reginster JY, Rabenda V, Neuprez A. Adherence, patient preference and dosing frequency: understanding the relationship. Bone. 2006;38:S2–S6. doi: 10.1016/j.bone.2006.01.150. [DOI] [PubMed] [Google Scholar]