Abstract
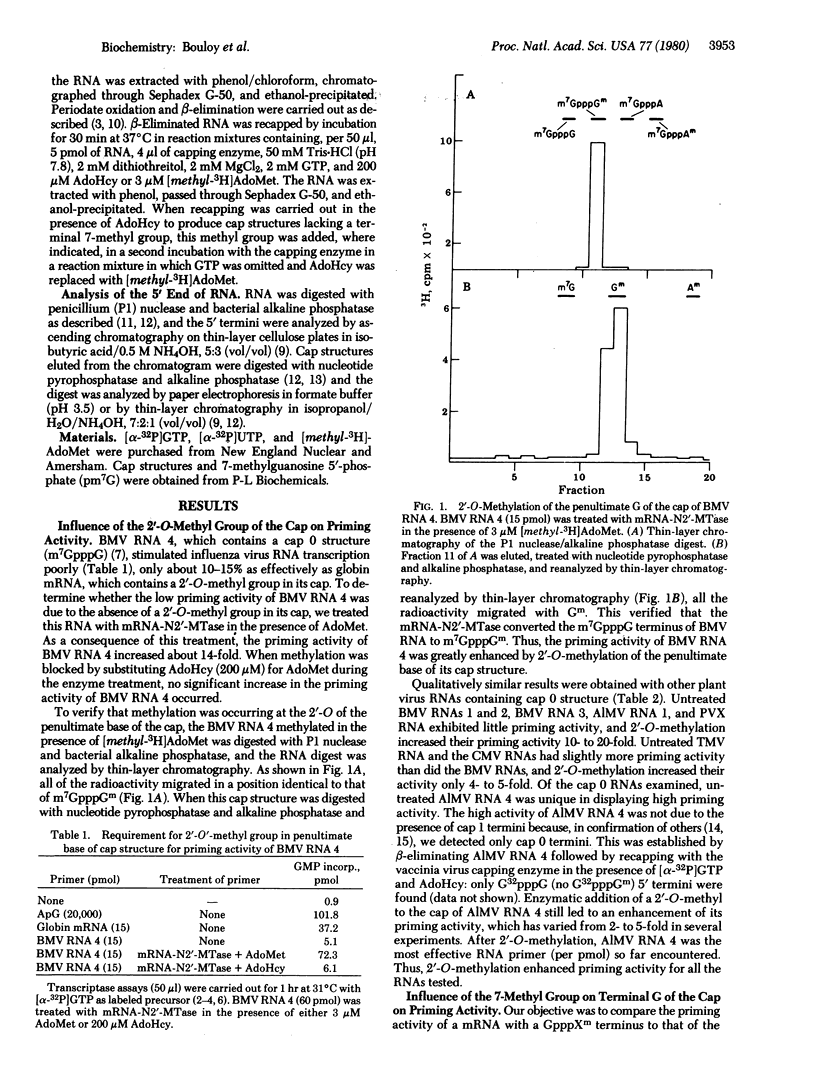
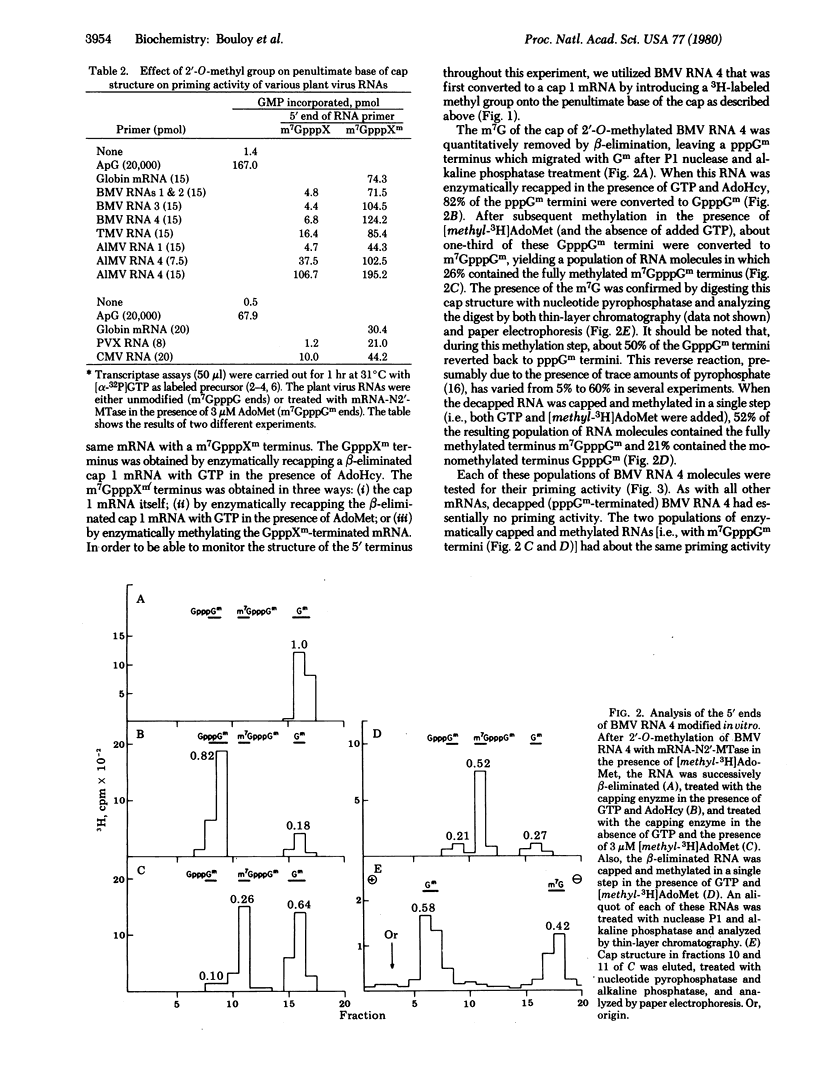
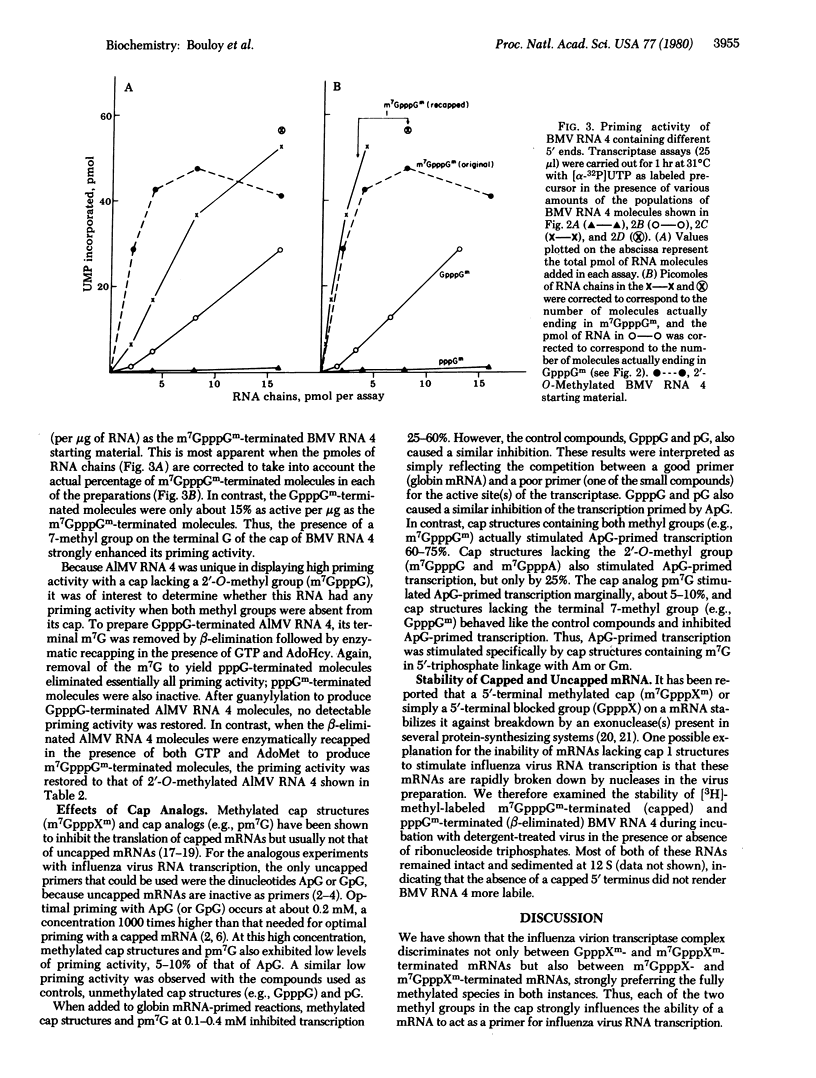
The ability of eukaryotic mRNAs to serve as primers for influenza virus RNA transcription depends on the presence of a 5'-terminal methylated can structure, the absence of which eliminates essentially all priming activity [Plotch, S. J., Bouloy, M. & Krug, R. M. (1979) Proc. Natl. Acad. Sci. USA 76, 1618-1622]. The present study was undertaken to determine the extent to which each of the methyl groups in the cap influences the priming activity of a mRNA. To assess the importance of the 2'-O-methyl group on the penultimate base of the cap, we used several plant viral RNAs containing the monomethylated cap 0 structure, m7GpppG. Brome mosaic virus (BMV) RNA 4 stimulated influenza virus RNA transcription only about 10-15% as effectively as did globin mRNA, which has a cap with a 2'-O-methyl group. When the cap of BMV RNA 4 was enzymatically 2'-O-methylated, its priming activity was increased 14-fold. Qualitatively similar results were obtained with other plant virus RNAs. To assess the importance of the terminal 7-methyl group, BMV RNA 4 containing the cap structure GpppGm was prepared by a series of chemical and enzymatic steps. These molecules were found to be only about 15% as active in priming as BMV RNA 4 molecules containing the fully methylated cap, m7GpppGm, indicating that the terminal 7-methyl group also strongly enhances priming activity. These results indicate that the cap 1 structure (m7GpppXm) found in all mammalian cellular mRNAs is more stringently required for priming influenza virus RNA transcription than for translation in cell-free systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbosa E., Moss B. mRNA(nucleoside-2'-)-methyltransferase from vaccinia virus. Purification and physical properties. J Biol Chem. 1978 Nov 10;253(21):7692–7697. [PubMed] [Google Scholar]
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Morgan M. A., Shatkin A. J., Krug R. M. Cap and internal nucleotides of reovirus mRNA primers are incorporated into influenza viral complementary RNA during transcription in vitro. J Virol. 1979 Dec;32(3):895–904. doi: 10.1128/jvi.32.3.895-904.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4886–4890. doi: 10.1073/pnas.75.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani D., Revel M., Groner Y. Translational discrimination of 'capped' and 'non-capped' mRNAS: inhibition of a series of chemical analogs of m7GpppX. FEBS Lett. 1976 May 1;64(2):326–331. doi: 10.1016/0014-5793(76)80321-3. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Implications of RNA-RNA splicing in evolution of eukaryotic cells. Science. 1978 Dec 22;202(4374):1257–1260. doi: 10.1126/science.364651. [DOI] [PubMed] [Google Scholar]
- Dasgupta R., Harada F., Kaesberg P. Blocked 5' termini in brome mosaic virus RNA. J Virol. 1976 Apr;18(1):260–267. doi: 10.1128/jvi.18.1.260-267.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A. J. Reovirus messenger RNA contains a methylated, blocked 5'-terminal structure: m-7G(5')ppp(5')G-MpCp-. Proc Natl Acad Sci U S A. 1975 Jan;72(1):362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Shatkin A. J., Jelinek W., Salditt-Georgieff M., Darnell J. E. Methylated, blocked 5 termini in HeLa cell mRNA. Proc Natl Acad Sci U S A. 1975 May;72(5):1904–1908. doi: 10.1073/pnas.72.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Stolarsky L. Dependence on potassium concentration of the inhibition of the translation of messenger ribonucleic acid by 7-methylguanosine 5'-phosphate. Biochemistry. 1977 Dec 27;16(26):5676–5680. doi: 10.1021/bi00645a004. [DOI] [PubMed] [Google Scholar]
- Klessig D. F. Two adenovirus mRNAs have a common 5' terminal leader sequence encoded at least 10 kb upstream from their main coding regions. Cell. 1977 Sep;12(1):9–21. doi: 10.1016/0092-8674(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Koper-Zwarthoff E. C., Lockard R. E., Alzner-deWeerd B., RajBhandary U. L., Bol J. F. Nucleotide sequence of 5' terminus of alfalfa mosaic virus RNA 4 leading into coat protein cistron. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5504–5508. doi: 10.1073/pnas.74.12.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M., Morgan M. A., Shatkin A. J. Influenza viral mRNA contains internal N6-methyladenosine and 5'-terminal 7-methylguanosine in cap structures. J Virol. 1976 Oct;20(1):45–53. doi: 10.1128/jvi.20.1.45-53.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Moss B. Modification of RNA by mRNA guanylyltransferase and mRNA (guanine-7-)methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9330–9335. [PubMed] [Google Scholar]
- Martin S. A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9322–9329. [PubMed] [Google Scholar]
- Muthukrishnan S., Morgan M., Banerjee A. K., Shatkin A. J. Influence of 5'-terminal m7G and 2'--O-methylated residues on messenger ribonucleic acid binding to ribosomes. Biochemistry. 1976 Dec 28;15(26):5761–5768. doi: 10.1021/bi00671a012. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Moss B., Cooper J. A., Maxwell E. S. Influence of 5'-terminal cap structure on the initiation of translation of vaccinia virus mRNA. J Biol Chem. 1978 Mar 10;253(5):1710–1715. [PubMed] [Google Scholar]
- Pinck L. The 5'-end groups of alfalfa mosaic virus RNAs are m7G5'ppp5'Gp. FEBS Lett. 1975 Nov 1;59(1):24–28. doi: 10.1016/0014-5793(75)80332-2. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Krug R. M. Transfer of 5'-terminal cap of globin mRNA to influenza viral complementary RNA during transcription in vitro. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1618–1622. doi: 10.1073/pnas.76.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Influenza virion transcriptase: synthesis in vitro of large, polyadenylic acid-containing complementary RNA. J Virol. 1977 Jan;21(1):24–34. doi: 10.1128/jvi.21.1.24-34.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Plotch S. J., Krug R. M. Identification of the RNA region transferred from a representative primer, beta-globin mRNA, to influenza mRNA during in vitro transcription. Nucleic Acids Res. 1980 Mar 11;8(5):925–942. doi: 10.1093/nar/8.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Kaesberg P. Translation of brome mosaic viral ribonucleic acid in a cell-free system derived from wheat embryo. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1799–1803. doi: 10.1073/pnas.70.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Kodama Y., Hashimoto J., Miura K. I. Importance of 5'-terminal blocking structure to stabilize mRNA in eukaryotic protein synthesis. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2734–2738. doi: 10.1073/pnas.74.7.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Rupprecht K. M., Hecht S. M., Shatkin A. J. Eukaryotic mRNA cap binding protein: purification by affinity chromatography on sepharose-coupled m7GDP. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4345–4349. doi: 10.1073/pnas.76.9.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber L. A., Feman E. R., Hickey E. D., Williams M. C., Baglioni C. Inhibition of HeLa cell messenger RNA translation by 7-methylguanosine 5'-monophosphate. J Biol Chem. 1976 Sep 25;251(18):5657–5662. [PubMed] [Google Scholar]



