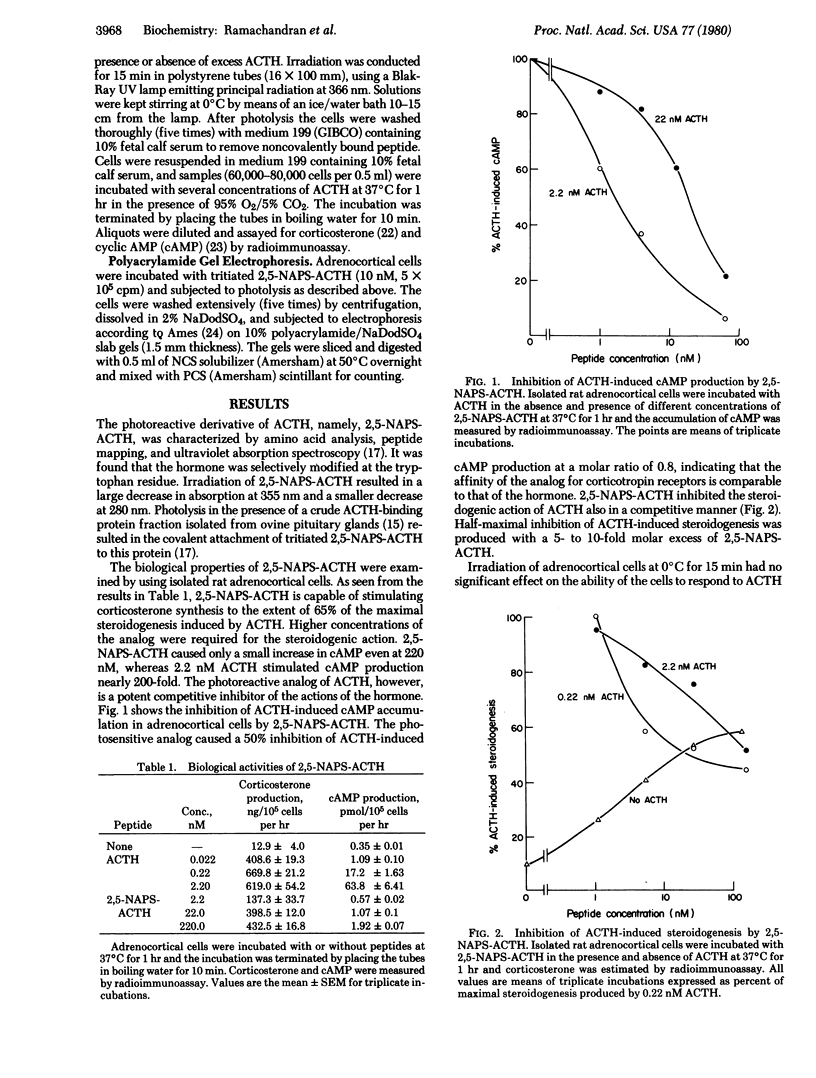
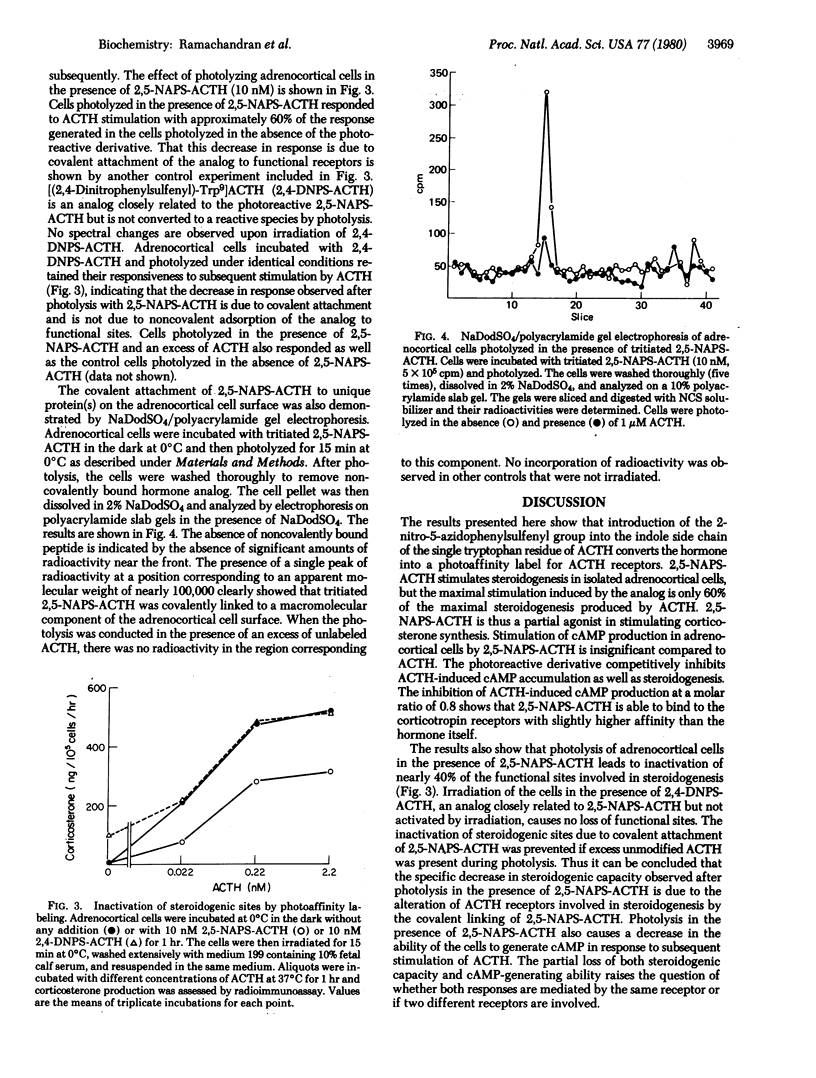
Abstract
A photoaffinity label for corticotropin (ACTH) receptors was prepared by selective chemical modification of the single tryptophan residue in the hormone by reaction with 2-nitro-5-azidophenylsulfenyl chloride. The photoreactive derivative, [(2-nitro-5-azidophenylsulfenyl)-Trp9]ACTH (2,5-NAPS-ACTH), stimulated corticosterone synthesis to 60% of the maximal rate induced by ACTH in isolated rat adrenocortical cells. 2.5-NAPS-ACTH caused only a marginal stimulation of cyclic AMP production compared to the unmodified hormone. Stimulation of corticosterone production and cyclic AMP accumulation induced by ACTH were both inhibited in a competitive manner by 2,5-NAPS-ACTH. Photolysis of adrenocortical cells in the presence of 2,5-NAPS-ACTH resulted in a 40% inactivation of ACTH receptors mediating steroidogenesis, as shown by the decrease in response to subsequent stimulation with ACTH. No loss of function was observed when photolysis was conducted in the presence of the photoresistant analog [(2,4-dinitrophenylsulfenyl)-Trp9]ACTH. Covalent attachment of the hormone to the receptors was also demonstrated by photolyzing adrenocortical cells in the presence of tritiated 2,5-NAPS-ACTH of high specific radioactivity (90 Ci/mmol) and analyzing the cell proteins by sodium dodecyl sulfate/polyacrylamide gel electrophoresis. A protein with an approximate molecular weight of 100,000 was specifically labeled by this procedure. The unique labeling of an adrenocortical cell protein and the concomitant loss of ACTH responsiveness suggest that physiologically relevant receptors are photolabeled by this method.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Bayley H., Knowles J. R. Photoaffinity labeling. Methods Enzymol. 1977;46:69–114. doi: 10.1016/s0076-6879(77)46012-9. [DOI] [PubMed] [Google Scholar]
- Bregman M. D., Levy D. Labeling of glucagon binding components in hepatocyte plasma membranes. Biochem Biophys Res Commun. 1977 Sep 23;78(2):584–590. doi: 10.1016/0006-291x(77)90219-4. [DOI] [PubMed] [Google Scholar]
- Burleigh B. D., Liu W. K., Ward D. N. Photocoupling of the subunits of ovine lutropin using a specific aryl azide derivative of the beta subunit. J Biol Chem. 1978 Oct 25;253(20):7179–7185. [PubMed] [Google Scholar]
- Canova-Davis E., Ramachandran J. Chemical modification of the tryptophan residue in adrenocorticotropin. Biochemistry. 1976 Feb 24;15(4):921–927. doi: 10.1021/bi00649a030. [DOI] [PubMed] [Google Scholar]
- Das M., Miyakawa T., Fox C. F., Pruss R. M., Aharonov A., Herschman H. R. Specific radiolabeling of a cell surface receptor for epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2790–2794. doi: 10.1073/pnas.74.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galardy R. E., Jamieson J. D. Photoaffinity labeling of a peptide secretagogue receptor in the exocrine pancreas. Mol Pharmacol. 1977 Sep;13(5):852–863. [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Ji T. H. A novel approach to the identification of surface receptors. The use of photosensitive hetero-bifunctional cross-linking reagent. J Biol Chem. 1977 Mar 10;252(5):1566–1570. [PubMed] [Google Scholar]
- Lefkowitz R. J., Roth J., Pricer W., Pastan I. ACTH receptors in the adrenal: specific binding of ACTH-125I and its relation to adenyl cyclase. Proc Natl Acad Sci U S A. 1970 Mar;65(3):745–752. doi: 10.1073/pnas.65.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. Preparation of photo-affinity probes for the insulin receptor site in adipose and liver cell membranes. Biochim Biophys Acta. 1973 Oct 18;322(2):329–336. doi: 10.1016/0005-2795(73)90308-5. [DOI] [PubMed] [Google Scholar]
- Moyle W. R., Kong Y. C., Ramachandran J. Steroidogenesis and cyclic adenosine 3',5'-monophosphate accumulation in rat adrenal cells. Divergent effects of adrenocorticotropin and its o-nitrophenyl sulfenyl derivative. J Biol Chem. 1973 Apr 10;248(7):2409–2417. [PubMed] [Google Scholar]
- Ramachandran J., Behrens C. Preparation and characterization of specifically tritiated adrenocorticotropin. Biochim Biophys Acta. 1977 Feb 28;496(2):321–328. doi: 10.1016/0304-4165(77)90314-2. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Lee V. Divergent effects of o-nitrophenyl sulfenyl ACTH on rat and rabbit fat cell adenyl cyclases. Biochem Biophys Res Commun. 1970 Oct 23;41(2):358–366. doi: 10.1016/0006-291x(70)90512-7. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Lee V., Li C. H. Stimulation of lipolysis and cyclic AMP accumulation in rabbit fat cells by human growth hormone. Biochem Biophys Res Commun. 1972 Jul 25;48(2):274–279. doi: 10.1016/s0006-291x(72)80046-9. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Lee V. Preparation and properties of the o-nitrophenyl sulfenyl derivative of ACTH: an inhibitor of the lipolytic action of the hormone. Biochem Biophys Res Commun. 1970 Feb 6;38(3):507–512. doi: 10.1016/0006-291x(70)90743-6. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Suyama A. T. Inhibition of replication of normal adrenocortical cells in culture by adrenocorticotropin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):113–117. doi: 10.1073/pnas.72.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. J., Long J. A., Ramachandran J. Effects of antiserum to adrenocorticotropin on adrenal growth and function. Endocrinology. 1978 Feb;102(2):371–378. doi: 10.1210/endo-102-2-371. [DOI] [PubMed] [Google Scholar]
- Schwyzer R., Caviezel M. p-Azido-L-phenylalanine: a photo-affinity 'probe' related to tyrosine. Helv Chim Acta. 1971;54(5):1395–1400. doi: 10.1002/hlca.19710540521. [DOI] [PubMed] [Google Scholar]
- YAMASHIRO D. PARTITION CHROMATOGRAPHY OF OXYTOCIN ON 'SEPHADEX'. Nature. 1964 Jan 4;201:76–77. doi: 10.1038/201076a0. [DOI] [PubMed] [Google Scholar]
- Yamashiro D., Li C. H. Adrenocorticotropins. 44. Total synthesis of the human hormone by the solid-phase method. J Am Chem Soc. 1973 Feb 21;95(4):1310–1315. doi: 10.1021/ja00785a049. [DOI] [PubMed] [Google Scholar]
- Yip C. C., Yeung C. W., Moule M. L. Photoaffinity labeling of insulin receptor of rat adiopocyte plasma membrane. J Biol Chem. 1978 Mar 25;253(6):1743–1745. [PubMed] [Google Scholar]



