Abstract
cDNA corresponding to the GA4 gene of Arabidopsis thaliana L. (Heynh.) was expressed in Escherichia coli, from which cell lysates converted [14C]gibberellin (GA)9 and [14C]GA20 to radiolabeled GA4 and GA1, respectively, thereby confirming that GA4 encodes a GA 3β-hydroxylase. GA9 was the preferred substrate, with a Michaelis value of 1 μm compared with 15 μm for GA20. Hydroxylation of these GAs was regiospecific, with no indication of 2β-hydroxylation or 2,3-desaturation. The capacity of the recombinant enzyme to hydroxylate a range of other GA substrates was investigated. In general, the preferred substrates contained a polar bridge between C-4 and C-10, and 13-deoxy GAs were preferred to their 13-hydroxylated analogs. Therefore, no activity was detected using GA12-aldehyde, GA12, GA19, GA25, GA53, or GA44 as the open lactone (20-hydroxy-GA53), whereas GA15, GA24, and GA44 were hydroxylated to GA37, GA36, and GA38, respectively. The open lactone of GA15 (20-hydroxy-GA12) was hydroxylated but less efficiently than GA15. In contrast to the free acid, GA25 19,20-anhydride was 3β-hydroxylated to give GA13. 2,3-Didehydro-GA9 and GA5 were converted by recombinant GA4 to the corresponding epoxides 2,3-oxido-GA9 and GA6.
Dwarf mutants with reduced biosynthesis of the GA plant hormones have been valuable tools in studies of the function of these compounds (Ross, 1994). In Arabidopsis thaliana, mutations at six loci (GA1-GA6) that result in reduced GA biosynthesis have been identified (Koorneef and van der Veen, 1980; Sponsel et al., 1997), and three of these loci have recently been cloned. The GA1 locus was isolated by genomic subtraction (Sun et al., 1992) and shown by heterologous expression in Escherichia coli to encode the enzyme that cyclizes geranylgeranyl diphosphate to copalyl diphosphate (Sun and Kamiya, 1994). This enzyme was formerly referred to as ent-kaurene synthase A but has been renamed copalyl diphosphate synthase (Hedden and Kamiya, 1997; MacMillan, 1997). The GA5 locus was shown to correspond to one of the GA 20-oxidase genes (Xu et al., 1995), the products of which catalyze the conversion of GA12 to GA9 and GA53 to GA20 (Phillips et al., 1995; Xu et al., 1995). GA 20-oxidases are 2-oxoglutarate-dependent dioxygenases that are encoded by small multigene families, members of which are differentially expressed in plant tissues (Phillips et al., 1995; Garcia-Martinez et al., 1997).
The GA4 locus was isolated by T-DNA tagging and, on the basis of the derived amino acid sequence, was also shown to encode a dioxygenase (Chiang et al., 1995). Several lines of evidence indicate that the GA4 gene encodes a GA 3β-hydroxylase. Shoots of a ga4 mutant, all alleles of which are semidwarf, contained reduced concentrations of the 3β-hydroxy GAs GA1, GA4, and GA8 compared with the Landsberg erecta wild type, whereas levels of immediate precursors to these GAs were elevated (Talon et al., 1990). Furthermore, metabolism of [13C]GA20 to [13C]GA1 was substantially less in the mutant than in the wild type (Kobayashi et al., 1994). In the present paper we confirm by functional expression of its cDNA in E. coli that GA4 encodes a GA 3β-hydroxylase. In addition, we determine the substrate specificity of recombinant GA4 using a number of C20- and C19-GAs and show by kinetic analysis that the enzyme has a higher affinity for GA9 than for GA20, which is consistent with the non-13-hydroxylation pathway predominating in Arabidopsis (Talon et al., 1990).
MATERIALS AND METHODS
Expression of GA4 in Escherichia coli
The coding region of GA4 was amplified by reverse transcription-PCR from mRNA extracted from floral apices of the ga1–2 mutant of Arabidopsis thaliana (L.) Heynh. as described previously (Phillips et al., 1995) using oligonucleotide primers with NcoI and BamHI sites: forward primer, CAACCATGGCTGCTATGTTAACAGA; reverse primer, CAAGGATCCTCATTCTTCTCTGTGATTT.
The cloned PCR product was transferred into pET3d and pET9d E. coli expression vectors (Pharmacia). Although the expression products contained 3β-hydroxylase activity, sequencing of the PCR product identified a point mutation, resulting in a change of amino acid within the coding region. To prepare expression constructs with the correct sequence (Chiang et al., 1997), a SacI-BamHI fragment containing the mutation was replaced in both vectors with the corresponding fragment from the GA4 cDNA clone pCD7 (Chiang et al., 1995). Cultures (50 mL) of E. coli BL21 transformed with the recombinant plasmids were grown with shaking at 37°C in 2× YT broth (1.6% [w/v] bactotryptone, 1% [w/v] yeast extract, and 0.5% [w/v] NaCl) containing 200 μg L−1 carbenicillin (pET3d) or 100 μg mL−1 kanamycin (pET9d). At the mid-logarithmic stage, cultures were transferred to 30°C and shaken for 30 min, after which expression was induced by the addition of IPTG (final concentration, 5 mm). At the same time, more antibiotics were added (400 μg mL−1 carbenicillin or 200 μg mL−1 kanamycin). Cultures were grown for another 3 h at 30°C and then placed on ice for 10 min, and cells were harvested by centrifugation at 5000 rpm at 4°C for 5 min. Pellets were resuspended in 25 mL of 100 mm Tris-HCl, pH 7.1, at 25°C containing 4 mm DTT, and recentrifuged as described above. Pellets were then resuspended in the same buffer (2 mL) containing lysozyme at 1 mg/mL, incubated at 30°C with shaking for 15 min, and, after cooling on ice, sonicated (three 5-s pulses). After centrifugation of the lysates at 12,000g for 15 min at 4°C, the supernatants were frozen in liquid N2 and stored at −80°C.
Analysis of Expressed Protein by SDS-PAGE
Proteins from cultures induced with IPTG, described above, were analyzed by SDS-PAGE. For comparison, proteins were analyzed from cells grown under the same conditions but without the addition of IPTG. Induced cells contained typically 0.5 to 1.0 mg protein mL−1 culture, whereas noninduced cells contained approximately 30% of the protein concentration present in induced cells. Soluble and insoluble protein fractions were obtained after lysis of cells (1 mL) using lysozyme, as described above. After the sample was centrifuged, the supernatant and resuspended pellet, in 100 mm Tris-HCl buffer, pH 7.5 (0.1 mL), were diluted 1:1 with loading buffer. Equal quantities of protein for each sample were loaded onto the gel.
Enzyme Assays with Recombinant Protein
Provision of Substrates
[17-14C]GA9 (specific radioactivity 2.10 TBq mol−1) and [17-13C,3H]GA5 (1.51 TBq mol−1) were gifts from Dr I. Yamaguchi (University of Tokyo) and Prof. J. MacMillan (Long Ashton Research Station), respectively. [17-14C]GA24 (1.72 TBq mol−1) and [17-14C]GA19 (1.72 TBq mol−1) were obtained from Prof. L.N. Mander (Australian National University, Canberra). 2,3-Didehydro[17-14C]GA9 (1.75 TBq mol−1) and [17-14C]GA20 (1.84 TBq mol−1) were synthesized as described by MacMillan et al. (1997). [1,7,12,18-14C4]GA12 (5.74 TBq mol−1), −GA12-aldehyde (6.90 TBq mol−1), and −GA15 (6.32 TBq mol−1) were prepared from R-[2-14C]mevalonic acid using a cell-free system from pumpkin endosperm, as described by Graebe et al. (1974). [1,7,12,18-14C4]GA53 (5.59 TBq mol−1) and [17-14C]GA44 (1.42 TBq mol−1) were prepared from [14C4]GA12 and [14C1]GA12, respectively, using a homogenate of developing pea cotyledons (Kamiya and Graebe, 1983). [14C4]GA25 (6.83 TBq mol−1) was prepared from [14C4]GA12 using a partially purified GA 20-oxidase from pumpkin endosperm (Lange et al., 1994). The open-lactone forms of GA15 and GA44 were prepared by heating the lactones in 0.5 m KOH at 90°C for 1 h in a sealed vial. An appropriate volume (3–5 μL) of the hydrolysate was then added directly to the incubation mixture.
Enzyme Assays
For incubations with different substrates, cell lysates (5 or 50 μL) were incubated for 1 h at 30°C with the substrate, which was added in 5 μL of methanol in the presence of 100 mm Tris-HCl, pH 7.5, and a cofactor mixture (5 μL, containing 80 mm 2-oxoglutarate, 80 mm ascorbate, 80 mm DTT, 10 mm FeSO4, 40 mg mL−1 BSA, and 20 mg mL−1 catalase in 100 mm Tris-HCl, pH 7.5) in a total volume of 0.1 mL. After the addition of acetic acid (10 μL) and water (140 μL), the incubation mixture was centrifuged at 3000 rpm for 10 min and then analyzed directly by HPLC with on-line radiomonitoring (MacMillan et al., 1997). Product identities were determined by full-scan GC-MS of methyl ester-trimethylsilyl ether derivatives by comparison with published data (Gaskin and MacMillan, 1991).
For kinetic studies [14C]GA9 added in 5 μL of methanol was incubated for 15 min at 30°C at different concentrations in the presence of cell lysate (equivalent to 0.22 μL containing 2 μg of protein) and 10 μL of the cofactor mixture in a total volume of 0.2 mL. [14C]GA20 was incubated with cell lysate (equivalent to 0.55 μL containing 5 μg of protein) under the same conditions except that 2.5 μL of the cofactor mixture was used and the total volume was 50 μL. The dependence of the GA-hydroxylation rate (determined by HPLC with on-line radiomonitoring as described above) on GA concentration was established by nonlinear regression analysis using Enzfitter (Elsevier, Cambridge, UK).
RESULTS AND DISCUSSION
Heterologous Expression of GA4 in E. coli
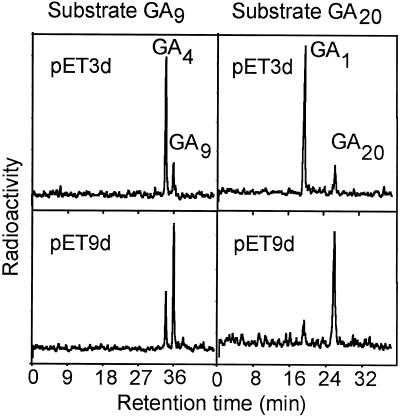
GA4 cDNA was inserted into pET3d and pET9d vectors, and expression was induced in E. coli with 5 mm IPTG. Cell lysates from cultures of bacteria containing either expression construct metabolized [14C]GA9 to [14C]GA4, and [14C]GA20 to [14C]GA1, with higher activity obtained with pET3d (Fig. 1). Lysates from bacteria containing vector with no insert did not metabolize either substrate (data not shown). Therefore, it is confirmed that GA4 encodes a GA 3β-hydroxylase. The LE gene of pea has now also been cloned and, after expression in E. coli, shown to encode a GA 3β-hydroxylase with 54% amino acid identity to the corrected (Chiang et al., 1997) GA4 sequence (Lester et al., 1997; Martin et al., 1997). Separation of proteins from total cell extracts of both E. coli cultures by SDS-PAGE revealed a band of the anticipated size (Mr approximately 40,000). Although this band was more intense in extracts from cells containing the pET9d construct, virtually all of the protein was present in the insoluble fraction in this case and was presumably present in inclusion bodies. Therefore, the pET3d vector was used to produce active protein for characterization of enzyme activity.
Figure 1.
HPLC-radiochromatograms from incubations of [14C]GA9 and [14C]GA20 with cell lysates of E. coli expressing GA4 in pET3d or pET9d. The equivalent of 0.05 or 0.12 μL of lysate was incubated in 100 μL total volume with GA9 or GA20, respectively, plus the necessary cofactors.
Characteristics of Recombinant GA4
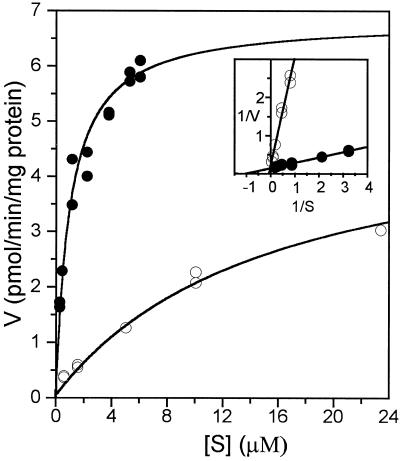
[14C]GA9 and [14C]GA20 were compared as the substrates for recombinant GA4 in cell lysates. Plots of reaction rate against substrate concentration, produced by nonlinear regression analysis (Fig. 2), yielded Km values of 1.0 μm (Vmax 6.8 pmol min−1 mg−1) and 15 μm (Vmax 2.8 pmol min−1 mg−1) for GA9 and GA20, respectively. The preference for GA9 as a substrate is consistent with the predominance of non-13-hydroxylated GAs in Arabidopsis; the major C19-GA identified from entire shoots is GA4 (Talon et al., 1990). Furthermore, the Arabidopsis GA 20-oxidases convert non-13-hydroxylated substrates, e.g. GA12, more effectively than their 13-hydroxylated equivalents, e.g. GA53 (Phillips et al., 1995). Therefore, the preferred biosynthetic pathway in Arabidopsis appears to involve conversion of GA12 to GA9, which is then 3β-hydroxylated to GA4. The Km values are very similar to those determined recently for the GA 3β-hydroxylase from pea, obtained by expression of the LE cDNA in E. coli (Martin et al., 1997). In pea shoots, which produce mainly 13-hydroxylated GAs, the preference of the enzyme for GA9 was unexpected.
Figure 2.
Michaelis-Menten and Lineweaver-Burk (inset) plots for the 3β-hydroxylation of GA9 to GA4 (•) and of GA20 to GA1 (○) by cell lysates from recombinant E. coli expressing GA4 in pET3d.
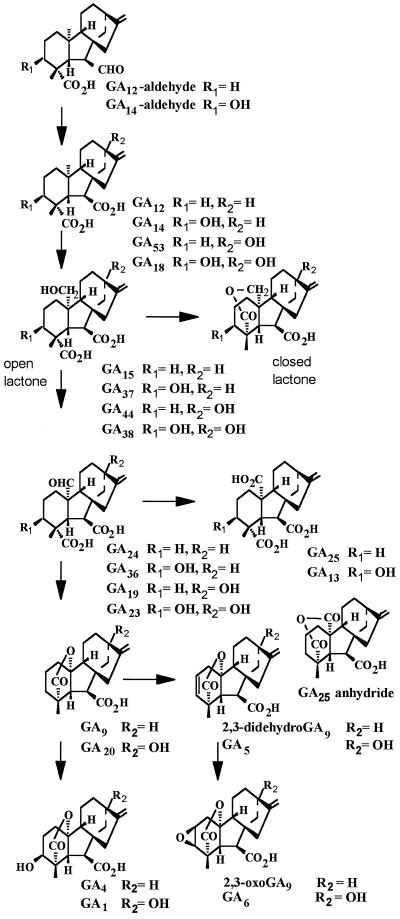
The substrate specificity of GA4 was further tested by incubating cell lysate with a number of nonhydroxylated and 13-hydroxylated C19- and C20-GAs at similar concentrations (1.8 μm; Table I); products, when formed, were identified by full-scan GC-MS. The structures of the tested potential substrates and products are shown in Figure 3, where they are arranged in their proposed biosynthetic relationship. In most cases the substrates were labeled with 14C, enabling their degree of conversion to be quantified by radiomonitoring after HPLC. Therefore, their relative effectiveness as substrates could be readily compared. To compare the broad range of hydroxylation efficiencies encountered with these substrates, incubations were performed with both 5- and 50-μL cell lysate volumes, when appropriate. In general, C19-GAs were much better substrates than C20-GAs, and the presence of a 13-hydroxy group reduced the efficiency of conversion. No conversion was observed with GA12-aldehyde, GA12, GA53, GA19, or GA25, whereas GA15, GA44, and GA24 were hydroxylated to GA37, GA38, and GA36, respectively. GA15, as the lactone, was hydroxylated almost as efficiently as GA20. In contrast, the open-lactone forms of GA15 and GA44 were poor substrates.
Table I.
Efficiency of 3β-hydroxylation of potential GA substrates by cell lysates from E. coli expressing GA4
| Potential Substrate | Potential Product | Conversion in 1
h
|
Mass Spectrum of Product | |
|---|---|---|---|---|
| 5 μL of Lysate | 50 μL of Lysate | |||
| % | m/z (% relative abundance) | |||
| GA12-aldehydea | GA14-aldehyde | –b | 0 | |
| GA12a | GA14 | – | 0 | |
| GA53a | GA18 | – | 0 | |
| GA15a | GA37 | 75 | 100 | M+ 440(33), 438(19), 432(25), 425(14), 408(25), 350(34), 348(12), 342(10), 318(100), 316(32), 310(50), 292(43), 290(21), 288(46), |
| GA44c | GA38 | – | 60 | M+ 528(43), 526(25), 520(22), 513(3), 497(3), 467(4), 438(11), 377(9), 282(13), 240(16), 209(100), 207(49) |
| GA15 open lactonea | GA37 | 0 | 25 | |
| GA44 open lactonec | GA38 | – | 0 | |
| GA24c | GA36 | 19 | 67 | M+ 464(5), 449(5), 432(39), 404(29), 376(15), 375(14), 342(26), 314(48), 286(100), 255(22), 227(41), 211(51), 173(48), 129(82) |
| GA19c | GA23 | – | 0 | |
| GA25a | GA13 | – | 0 | |
| GA25-anhydrided | GA13 | – | 40e | M+ 492(0), 477(2), 436(7), 400(19), 342(8), 282(17), 251(8), 223(11), 160(13), 129(100) |
| GA9c | GA4 | 100 | – | M+ 420(19), 402(9), 392(12), 388(23), 360(12), 345(6), 330(22), 302(15), 298(22), 291(63), 286(100), 263(32), 234(46), 230(34), 227(90), 226(82), 203(37), 175(35), 129(63) |
| GA20c | GA1 | 73 | – | M+ 508(100), 493(9), 450(21), 378(19), 377(15), 359(7), 315(12), 237(11), 209(41), 195(18), 182(13) |
| 2,3-Didehydro-GA9c | 2,3-Oxido-GA9 | 43 | 100 | M+ 346(6), 314(100), 286(6), 267(8), 242(95), 225(43), 224(51), 214(18), 181(26), 155(57) |
| GA5f | GA6 | – | 1e | M+ 433(100), 417(8), 374(15), 304(76), 236(44), 208(61) |
Products were identified by comparison of their mass spectra with published data for unlabeled compounds (Gaskin and MacMillan, 1991).
[1, 7, 11, 18-14C4];
–, Not determined.
[17-14C].
Unlabeled.
Determined by GC-MS.
[17-13C, 3H2].
Figure 3.
Structures of potential substrates and products arranged in their proposed biosynthetic relationship.
It seems likely that these GAs in the lactone form mimic the C19-GA substrates, which contain a γ-lactone. The low level of hydroxylation of the open-lactone form of GA15 may be due to some lactone formation during incubation. In solution, GA24 is likely to exist partially as a lactol between the C-20 aldehyde and the C-19 carboxylic acid group and would therefore also mimic the C19-GA structure. Whereas the tricarboxylic acid GA25 was not metabolized by GA4, its 19–20 anhydride (Fig. 3) was hydroxylated to give GA13. The incubation was conducted with unlabeled GA25 anhydride and, therefore, it was not possible to determine the conversion efficiency accurately. However, on the basis of GC-MS on the total extracted products, about 40% of the 1.4 nmol of substrate was converted by 120 μL of cell lysate (140 μL total incubation volume) in 1 h, indicating relatively efficient conversion. The expected product, GA13 anhydride, would be converted to GA13 on acidification of the incubation mixture prior to extraction.
These results indicate that a presumably polar bridge between C atoms 4 and 10 (lactone, lactol, or anhydride) is necessary for substrate binding to the 3β-hydroxylase. This requirement is in contrast to that of a GA 2β,3β-dihydroxylase, which was recently cloned from pumpkin endosperm and shown to utilize C20-GAs, particularly GA25, more effectively than C19-GAs (Lange et al., 1997). The high substrate specificity of the Arabidopsis 3β-hydroxylase for bridged, non-13-hydroxylated C20-GAs may account for the occurrence of the 3β-hydroxylated C20-GAs GA37, GA36, and GA13 in shoots of this species (Talon et al., 1990). The first two compounds would be formed by 3β-hydroxylation of the relatively abundant intermediates GA15 and GA24, whereas GA13 may be a minor product of 20-oxidase activity on GA36. The major product of this activity is likely to be GA4.
The 2,3-didehydro-GAs GA5 and 2,3-didehydroGA9 were converted by GA4 to the corresponding epoxides, GA6 and 2,3-oxidoGA9, but these conversions were less efficient than those of their saturated analogs, GA20 and GA9. Epoxidation of GA5 has also been observed with a GA 3β-hydroxylase from immature seeds of Phaseolus vulgaris (Kobayashi et al., 1991) and contrasts the conversion of GA5 to GA3 in seeds of Marah macrocarpus, a reaction that is initiated by oxidation at C-1 (Albone et al., 1990). The 3β-hydroxylation of GA9 and GA20 by GA4 is regiospecific, with no indication of 2,3-desaturation or 2β-hydroxylation, as was found for the enzyme from P. vulgaris (Smith et al., 1990).
ACKNOWLEDGMENTS
We thank Dr. I. Yamaguchi (University of Tokyo), Professor J. MacMillan (IACR-Long Ashton Research Station), and Professor L.N. Mander (Australian National University, Canberra), for gifts of radiolabeled GAs, and Dr. C.L. Willis (School of Chemistry, University of Bristol, UK) for providing the GA25 anhydride.
Abbreviation:
- IPTG
isopropyl-β-thiogalactoside
Footnotes
IACR receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the United Kingdom.
LITERATURE CITED
- Albone KS, Gaskin P, MacMillan J, Phinney BO, Willis CL. Biosynthetic origin of gibberellins A3 and A7 in cell-free preparations from seeds of Marah macrocarpus and Malus domestica. Plant Physiol. 1990;94:132–142. doi: 10.1104/pp.94.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang H-H, Hwang I, Goodman HM. Isolation of the Arabidopsis GA4 locus. Plant Cell. 1995;7:195–201. doi: 10.1105/tpc.7.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang H-H, Hwang I, Goodman HM. Correction to: Isolation of the Arabidopsis GA4 locus (Plant Cell 7: 195–201) Plant Cell. 1997;9:979–980. doi: 10.1105/tpc.7.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Martínez JL, López-Diaz I, Sánchez-Beltrán MJ, Phillips AL, Ward DA, Gaskin P, Hedden P. Isolation and transcript analysis of gibberellin 20-oxidase genes in pea and bean in relation to fruit development. Plant Mol Biol. 1997;33:1073–1084. doi: 10.1023/a:1005715722193. [DOI] [PubMed] [Google Scholar]
- Gaskin P, MacMillan J (1991) GC-MS of the Gibberellins and Related Compounds: Methodology and a Library of Spectra. Cantock's Enterprises, Bristol, UK
- Graebe JE, Hedden P, Gaskin P, MacMillan J. Biosynthesis of gibberellins Al2, Al5, A24, A36 and A37 by a cell-free system from Cucurbita maxima. Phytochemistry. 1974;13:1433–1440. [Google Scholar]
- Hedden P, Kamiya Y. Gibberellin biosynthesis: enzymes, genes and their regulation. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:431–460. doi: 10.1146/annurev.arplant.48.1.431. [DOI] [PubMed] [Google Scholar]
- Kamiya Y, Graebe JE. The biosynthesis of all major pea gibberellins in a cell-free system from Pisum sativum L. Phytochemistry. 1983;22:681–689. [Google Scholar]
- Kobayashi M, Gaskin P, Spray CR, Phinney BO, MacMillan J. The metabolism of gibberellin A20 to gibberellin A1 by tall and dwarf mutants of Oryza sativa and Arabidopsis thaliana. Plant Physiol. 1994;106:1367–1372. doi: 10.1104/pp.106.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Kwak S-S, Kamiya Y, Yamane H, Takahashi N, Sakurai A. Conversion of GA5 to GA6 and GA3 in cell-free systems from Phaseolus vulgaris and Oryza sativa. Agric Biol Chem. 1991;55:249–251. [Google Scholar]
- Koornneef M, van der Veen JH. Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) Heynh. Theor Appl Genet. 1980;58:257–263. doi: 10.1007/BF00265176. [DOI] [PubMed] [Google Scholar]
- Lange T, Robatzek S, Frisse A. Cloning and expression of a gibberellin 2β,3β-hydroxylase cDNA from pumpkin endosperm. Plant Cell. 1997;9:1459–1467. doi: 10.1105/tpc.9.8.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange T, Schweimer A, Ward DA, Hedden P, Graebe JE. Separation and characterization of three 2-oxoglutarate-dependent dioxygenases from Cucurbita maxima L. endosperm involved in gibberellin biosynthesis. Planta. 1994;195:98–107. [Google Scholar]
- Lester DR, Ross JJ, Davies PJ, Reid JB. Mendel's stem length gene (Le) encodes a gibberellin 3β-hydroxylase. Plant Cell. 1997;9:1435–1443. doi: 10.1105/tpc.9.8.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan J. Biosynthesis of the gibberellin plant hormones. Nat Prod Rep. 1997;14:221–243. [Google Scholar]
- MacMillan J, Ward DA, Phillips AL, Sánchez-Beltrán MJ, Gaskin P, Lange T, Hedden P. Gibberellin biosynthesis from gibberellin A12-aldehyde in endosperm and embryos of Marah macrocarpus. Plant Physiol. 1997;113:1369–1377. doi: 10.1104/pp.113.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DN, Proebsting WM, Hedden P. Mendel's dwarfing gene: cDNAs from the Le alleles and the function of the expressed proteins. Proc Natl Acad Sci USA. 1997;94:8907–8911. doi: 10.1073/pnas.94.16.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Ward DA, Uknes S, Appleford NEJ, Lange T, Huttly AK, Gaskin P, Graebe JE, Hedden P. Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol. 1995;108:1049–1057. doi: 10.1104/pp.108.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JJ. Recent advances in the study of gibberellin mutants. Plant Growth Regul. 1994;15:193–206. [Google Scholar]
- Smith VA, Gaskin P, MacMillan J. Partial purification and characterization of the gibberellin A20 3β-hydroxylase from seeds of Phaseolus vulgaris. Plant Physiol. 1990;94:1390–1401. doi: 10.1104/pp.94.3.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponsel VM, Schmidt FW, Porter SG, Nakayama M, Kohlstruk S, Estelle M. Characterization of new gibberellin-responsive semidwarf mutants of Arabidopsis. Plant Physiol. 1997;115:1009–1020. doi: 10.1104/pp.115.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TP, Goodman HM, Ausubel FM. Cloning the Arabidopsis GA1 locus by genomic subtraction. Plant Cell. 1992;4:119–128. doi: 10.1105/tpc.4.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TP, Kamiya Y. The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthetase A of gibberellin biosynthesis. Plant Cell. 1994;6:1509–1518. doi: 10.1105/tpc.6.10.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon M, Koornneef M, Zeevaart JAD. Endogenous gibberellins in Arabidopsis thaliana and possible steps blocked in the biosynthetic pathways of the semidwarf ga4 and ga5 mutants. Proc Natl Acad Sci USA. 1990;87:7983–7987. doi: 10.1073/pnas.87.20.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YL, Li L, Wu K, Peeters AJM, Gage D, Zeevaart JAD. The GA5 locus of Arabidopsis thaliana encodes a multifunctional gibberellin 20-oxidase: molecular cloning and functional expression. Proc Natl Acad Sci USA. 1995;92:6640–6644. doi: 10.1073/pnas.92.14.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]