Abstract
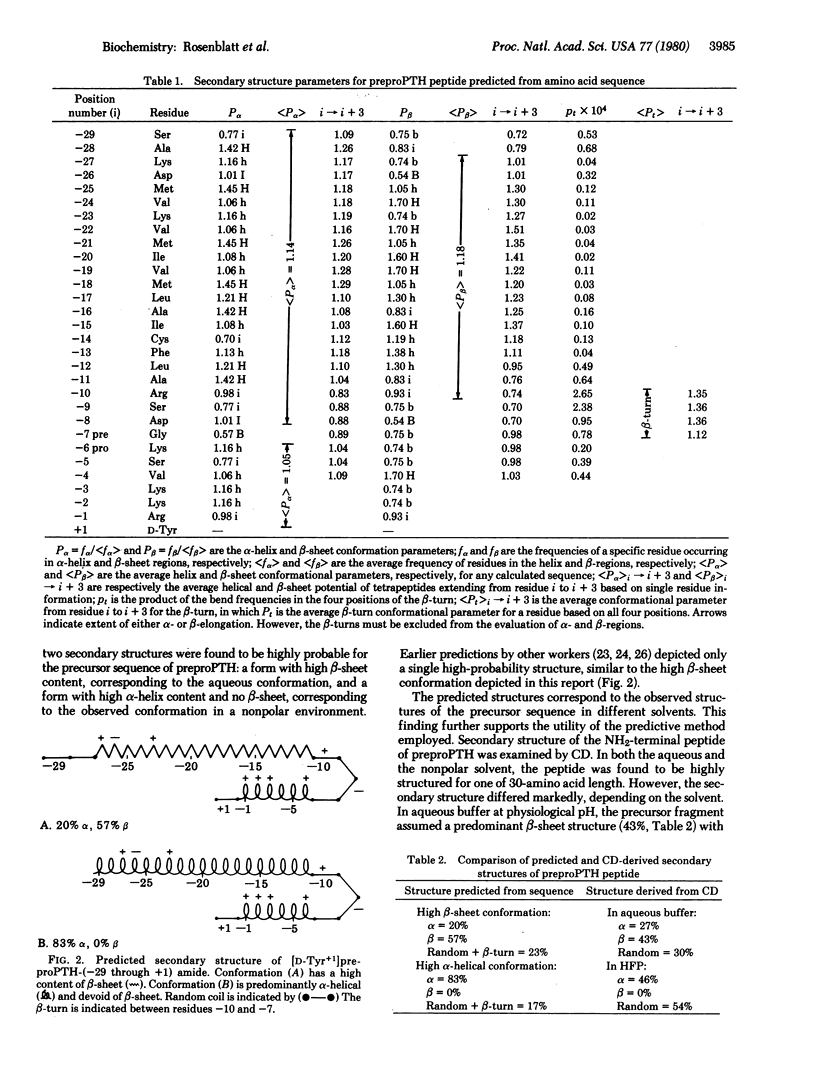
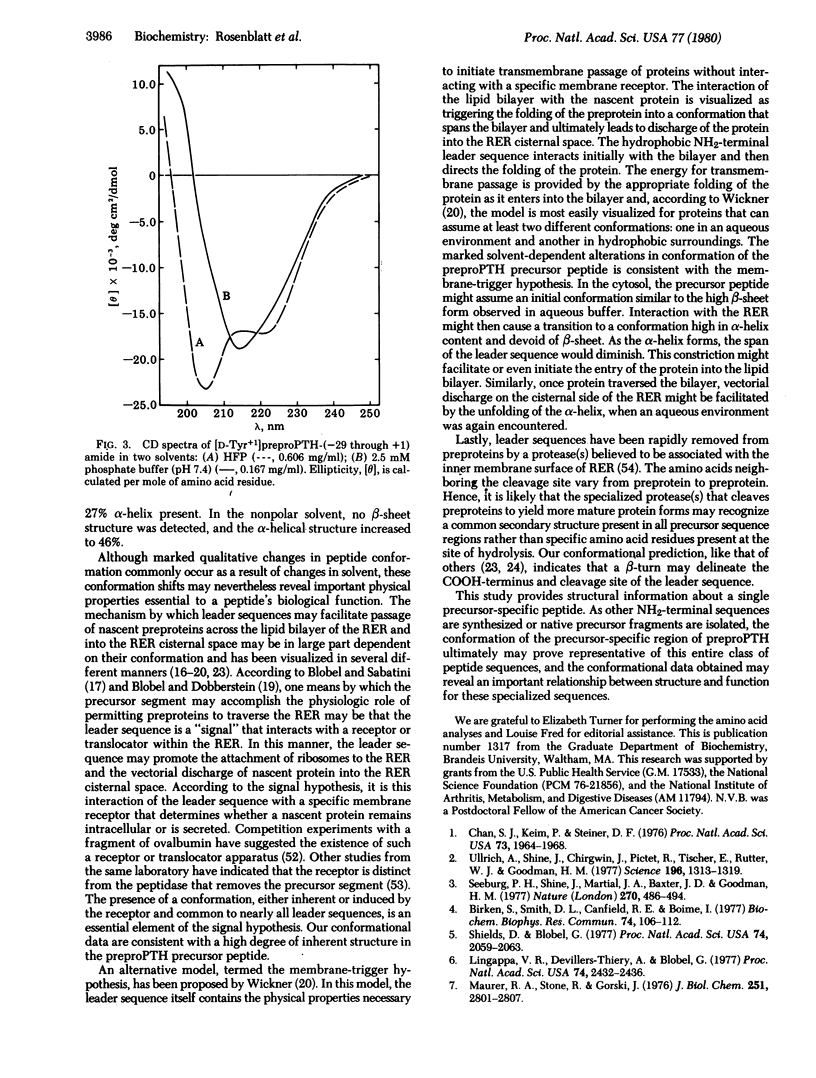
the secondary structure of a synthetic peptide representing the NH2-terminal, precursor-specific extension sequence of preproparathyroid hormone was studied. NH2-terminal extensions, or leader sequences, may serve a critical role in determining and facilitating the cellular secretion of proteins. These precursor regions, including the synthetic hormonal fragment studied, share common features of amino acid sequence and also may be similar in secondary structure. The secondary structure of the synthetic precursor peptide was predicted as described [Chou, P. Y. & Fasman, G. D. (1978) Adv. Enzymol. 47, 45-148]. The secondary structure was derived from circular dichroism spectra in both an aqueous buffer at physiological pH and in a nonpolar solvent selected to approximate the intramembranous environment. Two highly structured conformations were observed. In the aqueous buffer the secondary structure was 27% alpha-helix, 43% beta-sheet, and 30% random coil. In the nonpolar solvent the secondary structure was 46% alpha-helix, 0% beta-sheet, and 54% random coil. These findings correlated well with the two highest-probability structures predicted from the amino acid sequence. Both the relatively high content of secondary structure in a peptide of this size (30 amino acids) and the conformational transition observed in changing from aqueous to nonpolar environments may reflect structural properties critical to the physiological function of NH2-terminal extension sequences, and both are consistent with current theories regarding the role of precursor regions in the intracellular transport and secretion of proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A. J., Schaffhausen B., Langan T. A., Fasman G. D. Altered conformational effects of phosphorylated lysine-rich histone (f-1) in f-1--deoxyribonucleic acid complexes. Circular dichroism and immunological studies. Biochemistry. 1971 Mar 2;10(5):909–913. doi: 10.1021/bi00781a028. [DOI] [PubMed] [Google Scholar]
- Austen B. M. Predicted secondary structures of amino-terminal extension sequences of secreted proteins. FEBS Lett. 1979 Jul 15;103(2):308–313. doi: 10.1016/0014-5793(79)81351-4. [DOI] [PubMed] [Google Scholar]
- Birken S., Smith D. L., Canfield R. E., Boime I. Partial amino acid sequence of human placental lactogen precursor and its mature hormone form produced by membrane-associated enzyme activity. Biochem Biophys Res Commun. 1977 Jan 10;74(1):106–112. doi: 10.1016/0006-291x(77)91381-x. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boime I., Szczesna E., Smith D. Membrane-dependent cleavage of the human placental lactogen precursor to its native form in ascites cell-free extracts. Eur J Biochem. 1977 Mar 1;73(2):515–520. doi: 10.1111/j.1432-1033.1977.tb11345.x. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Zemell R., Kantor F., Schechter I. Independent expression of the gene coding for the constant domain of immunoglobulin light chain: evidence from sequence analyses of the precursor of the constant region polypeptide. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3157–3161. doi: 10.1073/pnas.74.8.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. J., Keim P., Steiner D. F. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1964–1968. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Beta-turns in proteins. J Mol Biol. 1977 Sep 15;115(2):135–175. doi: 10.1016/0022-2836(77)90094-8. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of beta-turns. Biophys J. 1979 Jun;26(3):367–383. doi: 10.1016/S0006-3495(79)85259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. The conformation of glucagon: predictions and consequences. Biochemistry. 1975 Jun 3;14(11):2536–2541. doi: 10.1021/bi00682a037. [DOI] [PubMed] [Google Scholar]
- Cohn D. V., Smardo F. L., Jr, Morrissey J. J. Evidence for internal homology in bovine preproparathyroid hormone. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1469–1471. doi: 10.1073/pnas.76.3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasman G. D., Chou P. Y., Adler A. J. Prediction of the conformation of the histones. Biophys J. 1976 Oct;16(10):1201–1238. doi: 10.1016/S0006-3495(76)85768-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisow M. J. Polypeptide secondary structure may direct the specificity of prohormone conversion. FEBS Lett. 1978 Mar 1;87(1):111–114. doi: 10.1016/0014-5793(78)80146-x. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Kemper B., Potts J. T., Jr, Rich A. Parathyroid mRNA directs the synthesis of pre-proparathyroid hormone and proparathyroid hormone in the Krebs ascites cell-free system. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1114–1121. doi: 10.1016/0006-291x(75)90789-5. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Potts J. T., Jr Biosynthesis of parathyroid hormone (second of two parts). N Engl J Med. 1978 Sep 21;299(12):635–644. doi: 10.1056/NEJM197809212991205. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Rosenblatt M., Dee P. C., Potts J. T., Jr Cellular processing of pre-proparathyroid hormone involves rapid hydrolysis of the leader sequence. J Biol Chem. 1979 Nov 10;254(21):10596–10599. [PubMed] [Google Scholar]
- Habener J. F., Rosenblatt M., Kemper B., Kronenberg H. M., Rich A., Potts J. T., Jr Pre-proparathyroid hormone; amino acid sequence, chemical synthesis, and some biological studies of the precursor region. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2616–2620. doi: 10.1073/pnas.75.6.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. C., Blobel G. Post-translational cleavage of presecretory proteins with an extract of rough microsomes from dog pancreas containing signal peptidase activity. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5598–5602. doi: 10.1073/pnas.74.12.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Mulligan R. C., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone: a direct translation product of parathyroid messenger RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3731–3735. doi: 10.1073/pnas.71.9.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Devillers-Thiery A., Blobel G. Nascent prehormones are intermediates in the biosynthesis of authentic bovine pituitary growth hormone and prolactin. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2432–2436. doi: 10.1073/pnas.74.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Katz F. N., Lodish H. F., Blobel G. A signal sequence for the insertion of a transmembrane glycoprotein. Similarities to the signals of secretory proteins in primary structure and function. J Biol Chem. 1978 Dec 25;253(24):8667–8670. [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Blobel G. Chicken ovalbumin contains an internal signal sequence. Nature. 1979 Sep 13;281(5727):117–121. doi: 10.1038/281117a0. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Shields D., Woo S. L., Blobel G. Nascent chicken ovalbumin contains the functional equivalent of a signal sequence. J Cell Biol. 1978 Nov;79(2 Pt 1):567–572. doi: 10.1083/jcb.79.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A., Stone R., Gorski J. Cell-free synthesis of a large translation product of prolactin messenger RNA. J Biol Chem. 1976 May 10;251(9):2801–2807. [PubMed] [Google Scholar]
- Merrifield R. B. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Ericsson L. H., Walsh K. A. Precursor of egg white lysozyme. Amino acid sequence of an NH2-terminal extension. J Biol Chem. 1977 Sep 25;252(18):6386–6393. [PubMed] [Google Scholar]
- Rosenblatt M., Habener J. F., Tyler G. A., Shepard G. L., Potts J. T., Jr Chemical synthesis of the precursor-specific region of pre-proparathyroid hormone. J Biol Chem. 1979 Feb 25;254(4):1414–1421. [PubMed] [Google Scholar]
- Schmeckpeper B. J., Adams J. M., Harris A. W. Detection of a possible precursor of immunoglobulin light chain in MOPC 41 A plasmacytoma cells. FEBS Lett. 1975 Apr 15;53(1):95–98. doi: 10.1016/0014-5793(75)80691-0. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Shine J., Martial J. A., Baxter J. D., Goodman H. M. Nucleotide sequence and amplification in bacteria of structural gene for rat growth hormone. Nature. 1977 Dec 8;270(5637):486–494. doi: 10.1038/270486a0. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss A. W., Bennett C. D., Donohue A. M., Rodkey J. A., Alberts A. W. Rat liver pre-proalbumin: complete amino acid sequence of the pre-piece. Analysis of the direct translation product of albumin messenger RNA. J Biol Chem. 1977 Oct 10;252(19):6846–6855. [PubMed] [Google Scholar]
- Suchanek G., Kreil G., Hermodson M. A. Amino acid sequence of honeybee prepromelittin synthesized in vitro. Proc Natl Acad Sci U S A. 1978 Feb;75(2):701–704. doi: 10.1073/pnas.75.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman P. M., Tushinski R. J., Bancroft F. C. Pregrowth hormone: product of the translation in vitro of messenger RNA coding for growth hormone. Proc Natl Acad Sci U S A. 1976 Jan;73(1):29–33. doi: 10.1073/pnas.73.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan D., Aviv H., Leder P. Purification and properties of biologically active messenger RNA for a myeloma light chain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1967–1971. doi: 10.1073/pnas.69.7.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Walter P., Jackson R. C., Marcus M. M., Lingappa V. R., Blobel G. Tryptic dissection and reconstitution of translocation activity for nascent presecretory proteins across microsomal membranes. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1795–1799. doi: 10.1073/pnas.76.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]



