Abstract
Objective
To compare the time to urinary tract infection (UTI) and the rates of asymptomatic bacteriuria and urinary P-fimbriated Escherichia coli during a 6-month period in women ingesting cranberry vs placebo juice daily.
Patients and Methods
Premenopausal women with a history of recent UTI were enrolled from November 16, 2005, through December 31, 2008, at 2 centers and randomized to 1 of 3 arms: 4 oz of cranberry juice daily, 8 oz of cranberry juice daily, or placebo juice. Time to UTI (symptoms plus pyuria) was the main outcome. Asymptomatic bacteriuria, adherence, and adverse effects were assessed at monthly visits.
Results
A total of 176 participants were randomized (120 to cranberry juice and 56 to placebo) and followed up for a median of 168 days. The cumulative rate of UTI was 0.29 in the cranberry juice group and 0.37 in the placebo group (P=.82). The adjusted hazard ratio for UTI in the cranberry juice group vs the placebo group was 0.68 (95% confidence interval, 0.33-1.39; P=.29). The proportion of women with P-fimbriated urinary E coli isolates during the intervention phase was 10 of 23 (43.5%) in the cranberry juice group and 8 of 10 (80.0%) in the placebo group (P=.07). The mean dose adherence was 91.8% and 90.3% in the cranberry juice group vs the placebo group. Minor adverse effects were reported by 24.2% of those in the cranberry juice group and 12.5% in the placebo group (P=.07).
Conclusion
Cranberry juice did not significantly reduce UTI risk compared with placebo. The potential protective effect we observed is consistent with previous studies and warrants confirmation in larger, well-powered studies of women with recurrent UTI. The concurrent reduction in urinary P-fimbriated E coli strains supports the biological plausibility of cranberry activity.
Trial Registration
clinicaltrials.gov Identifier: NCT00128128
Urinary tract infection (UTI) is an exceedingly common outpatient problem among young, healthy women, resulting in considerable morbidity and health care costs.1,2 In addition to the cost burden, increasing antibiotic resistance is making treatment of these infections more problematic.3,4 Thus, safe and effective nonantimicrobial prevention strategies are needed. One preventive approach that has been used for generations is ingestion of cranberry products.5,6 In vitro data have shown that cranberry inhibits primarily P-fimbriated uropathogenic strains of Escherichia coli from adhering to uroepithelial cells, which is a critical step in the development of UTI.7-9 However, these findings have not been correlated directly with clinical outcomes or verified in clinical studies.
Cranberry products have been evaluated as a UTI-preventive agent in several patient populations, including children and adults with neuropathic bladders, elderly men and women, pregnant women, and healthy, nonpregnant women.10-17 The results from some, but not all, of these trials suggest that cranberry may reduce the incidence of bacteriuria and UTI, particularly in healthy women with acute, uncomplicated cystitis.6
In 2003, the National Center for Complementary and Alternative Medicine provided a standardized, well-characterized cranberry juice cocktail and matching placebo juice for conduct of studies that evaluated potential health benefits of cranberry products. The aim of this current study was to conduct a phase 2, proof-of-principle clinical trial assessing the ability of cranberry to reduce the rates of UTI, asymptomatic bacteriuria, and urinary P-fimbriated E coli in premenopausal women with a history of recent UTI.
Patients and Methods
Study Population
This was a multicenter study, with simultaneous enrollment at the Hall Health Primary Care Center at the University of Washington, Seattle, and at the Yale–New Haven Hospital General Clinical Research Center, New Haven, CT. The study began on November 16, 2005, and ended on December 31, 2008. Premenopausal women 18 to 45 years of age with a history of one or more clinician-diagnosed UTIs in the past 12 months were eligible to participate. In addition, women had to agree to avoid all foods containing cranberries or other Vaccinium species (eg, blueberries) other than the study product for the 6-month duration of the trial. Women were not eligible if they had prediagnosed anatomical abnormalities of the urinary tract, a history of kidney stones, diabetes mellitus, malignant neoplasm other than skin disease, known allergy or intolerance to cranberry products, symptoms of vaginitis or cystitis, or asymptomatic bacteriuria (≥105 colony-forming units (CFU)/mL of the same uropathogen on 2 consecutive cultures on 2 separate days); were pregnant, lactating, not regularly using contraceptives, using prophylactic antimicrobials, or taking warfarin; or had used antibiotics in the past 7 days or investigational drugs in the past 30 days. All study procedures were approved by the local institutional review board at each site.
Study Procedures
Recruitment methods included advertisements in the local area and clinician referral at the time of a UTI visit to one of the clinics. After written informed consent, women provided a urine sample for testing for asymptomatic bacteriuria and pregnancy. Women with a positive test result for pregnancy or asymptomatic bacteriuria were not eligible for enrollment and randomization. Participants complying with all inclusion and exclusion criteria and consenting to study participation were randomized to 1 of the 3 groups (4 oz of cranberry juice, 8 oz of cranberry juice, or placebo) in a 1:1:1 ratio. The biostatistician (S.C.) produced the randomization code and scheme through a computer-generated process. Randomization was stratified by site. Identification numbers were randomly preassigned to study participants, and the corresponding treatment group was stored and assigned from our Web-based system to ensure concealment of intervention allocation. At enrollment, women completed a brief questionnaire, provided a midstream clean catch specimen for urinalysis and culture, and self-collected vaginal and rectal swabs.
The active and placebo juices were manufactured and provided by Ocean Spray Cranberries Inc under National Center for Complementary and Alternative Medicine contract NOTCA-02-014 and approved for investigational use under Food and Drug Administration Investigational New Drug No. 67,799. The active juice contained 27% cranberry juice and sucralose (Splenda), similar in composition to the commercially available low-calorie Ocean Spray cranberry juice cocktail. The placebo juice was of similar color and taste but did not contain cranberry juice. Women returned for follow-up on a monthly basis for 6 months and whenever they experienced symptoms of a UTI.
Primary Outcome Assessment
The primary outcome was time to a symptomatic UTI event that met criteria for clinical or culture-confirmed UTI. Individuals with acute onset of urinary symptoms were assessed by study staff for dysuria plus one or more of the symptoms of frequency, urgency, suprapubic pain, hematuria, and pyuria (white blood cell count >10/μL in unspun urine by hemocytometer or a positive leukocyte esterase dipstick result). Women who met both the symptom and pyuria criteria were diagnosed as having symptomatic UTI. If the urine culture result was positive (≥103 CFU/mL of a uropathogen), the episode was categorized as culture-confirmed UTI. If the urine culture result was negative, alternate diagnoses were excluded (detailed below), and if symptoms resolved with therapy, the episode was categorized as a clinical UTI. Women with symptoms of UTI but no pyuria and a negative urine culture result were not treated and were evaluated via examination and urine nucleic acid testing for alternate diagnoses, including vaginitis or cervicitis caused by Chlamydia trachomatis or Neisseria gonorrhoeae.18 Women with symptomatic UTI during the study period continued to drink the juice and remained in the study for the full 6 months unless they were lost to follow-up or elected to discontinue.
Secondary Outcomes
Asymptomatic bacteriuria was defined as 105 CFU/mL or more of a uropathogen in a woman without symptoms of UTI and was assessed at the monthly follow-up visits. E coli isolates from baseline vaginal and rectal cultures and from symptomatic UTI and asymptomatic bacteriuria episodes occurring during the study were further evaluated in the laboratory for the presence of functional P fimbriation, as described below. Adherence and adverse effects were captured by a structured questionnaire administrated by direct interview at every follow-up visit. Adherence to the study product dosing regimen was calculated by dividing the reported number of ingested doses by the expected number of doses per month. Weighted monthly means were calculated for each treatment group.
Laboratory Methods
All laboratory procedures were performed with the investigators masked to the treatment group. Urinalyses and cultures were performed using standard methods, as previously described.19 Vaginal and rectal swabs from both study sites were transported in Amies swabs to the University of Washington Research Laboratory. E coli were identified using standard microbiological procedures20, 21 and were frozen at −80°C for later determination of P fimbriation. P-fimbriated E coli isolates were defined as demonstrating a multiplex polymerase chain reaction positive for papA, papC, papEF, and one or more of the papG alleles, as well as being capable of mediating mannose-resistant hemagglutination of human red blood cells.20,22
Sample Size
On the basis of previous studies from similar populations, we expected 35% of women with a history of UTI to have a symptomatic UTI recurrence within 6 months.18 Originally, we planned enrollment of 350 women to give a sample size of 210 in the cranberry group (105 taking 4 oz and 105 taking 8 oz) and 105 in the placebo group, allowing for a 10% dropout rate, to provide 80% power to detect an absolute reduction of 15% in the rate of symptomatic UTI recurrence.
Statistical Analyses
Data management and analyses were performed at Yale University School of Medicine. As part of the a priori analysis plan to control type I error, a hierarchical testing procedure was used to first examine differences between the cranberry and placebo groups (combined 4- and 8-oz doses in each group) at the 2-sided .05 significance level and, if significant, test for differences between the doses. The latter was not performed because the combined dose comparison was not significant. The analysis was performed on the modified intent-to-treat population, which included any individual with at least one postrandomization assessment. Continuous variables were presented as mean ± SD. Categorical variables were presented as number (percentage). Continuous variables were compared using the t test. Categorical variables were compared using the χ2 test. Time to first UTI was assessed using the Kaplan-Meier method. The treatment arms were compared using the log-rank test. Cox proportional hazards regression was adjusted for age, frequency of sexual activity, race, site, history of kidney infection, and number of bladder infections. All analyses were performed using SAS statistical software, version 9.2 (SAS Institute Inc, Cary, NC).
Results
Among 345 women screened, 136 were ineligible and 23 declined participation (Figure 1). The study was terminated in December 2008 before the target sample size could be achieved because of administrative and budget issues. Thus, 186 women (100 from Seattle and 86 from New Haven) were enrolled and randomized. After enrollment, 10 women dropped out before the first follow-up visit (Figure 1), leaving 176 women (120 in the cranberry juice group and 56 in the placebo group) in the modified intent-to-treat population. The median follow-up time for each participant was 168 days. Reasons for early withdrawal included being too busy, moving away from the area, wanting antibiotic prophylaxis, becoming pregnant, and being diagnosed as having a kidney stone (1 individual in the placebo arm).
FIGURE 1.
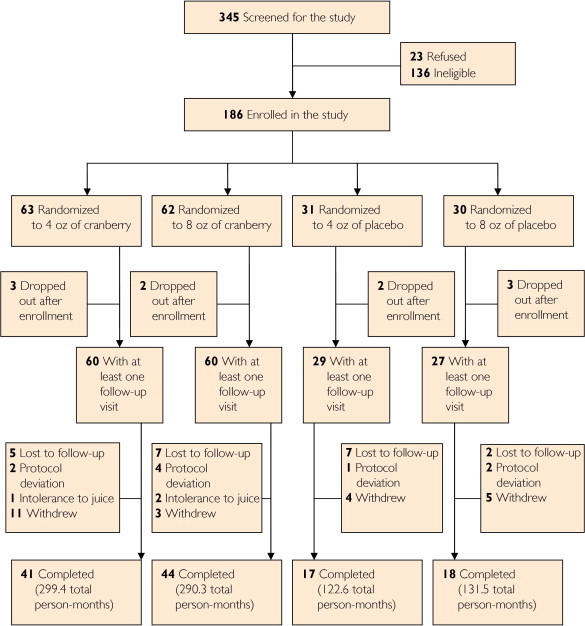
Study flow chart.
The demographics and baseline characteristics of the 176 randomized women were similar across treatment groups and sites (Table 1). The mean age was 25 years, 133 (75.6%) were white, and almost 100% were sexually active. The mean number of UTIs in the past year was 1.8 in the placebo group and 2.1 in the cranberry group (P=.37). A history of pyelonephritis was more frequent in the placebo group and was further evaluated in the regression models.
TABLE 1.
Demographics and Characteristics of Women Randomized to Cranberry vs Placebo Juicea
| Variable | Placebo (n=56) | Cranberry juiceb (n=120) | P value |
|---|---|---|---|
| Site | .98 | ||
| Yale | 26 (46.4) | 56 (46.7) | |
| Washington | 30 (53.6) | 64 (53.3) | |
| Mean (SD) age (y) | 26.4 (6.5) | 25.3 (6.6) | .33 |
| Race | .43 | ||
| White | 40 (71.4) | 93 (77.5) | |
| Black | 5 (8.9) | 4 (3.3) | |
| Asian | 9 (16.1) | 17 (14.2) | |
| Other | 2 (3.6) | 6 (5.0) | |
| Lifetime UTIs | .10 | ||
| ≤2 | 12 (22.2) | 43 (35.8) | |
| 3-5 | 21 (38.9) | 30 (25.0) | |
| ≥6 | 21 (38.9) | 47 (39.2) | |
| Mean (SD) No. of bladder infections last year | 1.8 (1.2) | 2.1 (1.4) | .37 |
| Previous kidney infection | 14 (26.9) | 15 (12.7) | .02 |
| Ever sexually active | 51 (94.4)c | 116 (96.7) | .49 |
| Mean (SD) sexual partners in past year | 1.5 (1.1) | 1.4 (0.9) | .54 |
| Sexual frequencyd | .11 | ||
| Weekly or more | 32 (65.3) | 85 (78.0) | |
| Less than weekly | 17 (34.7) | 24 (22.0) | |
| Using birth control pill | 18 (35.3) | 61 (52.1) | .04 |
| Using spermicidal product | 2 (3.9) | 13 (11.1) | .15 |
Data are presented as No. (percentage) of study participants unless indicated otherwise. UTI = urinary tract infection.
Comparison of total cranberry and placebo groups as per a priori analysis plan.
Denominator for this variable is 54.
Denominator for this variable is 49 for the placebo group and 109 for the cranberry group.
Proportions, Cumulative Rates, and Time to First UTI
There were 72 UTIs (18 clinical and 54 culture confirmed) in follow-up, occurring in 50 women. No differences were found in the proportions of women having a clinical or culture-confirmed UTI in the cranberry group (33/120, 27.5%) or placebo group (17/56, 30.4%) (P=.70). The proportions of women having more than one UTI during the study period were also similar: 10 women (8.3%) in the cranberry group and 4 women (7.1%) in the placebo group.
The cumulative rate of women with a clinical or culture-confirmed UTI at 6 months was 0.29 (95% confidence interval [CI], 0.21-0.38) in the cranberry group and 0.37 (95% CI, 0.25-0.54) in the placebo group (P=.82). The time to first UTI was not significantly different between the 2 groups (Figure 2). The unadjusted hazard ratio for UTI in the cranberry vs placebo group was 0.78 (95% CI, 0.43-1.41; P=.41). After adjusting for age, baseline sexual frequency, race, study site, pyelonephritis history, and number of bladder infections, the hazard ratio for UTI in the cranberry vs placebo group was 0.68 (95% CI, 0.33-1.39; P=.29). As per the a priori analysis plan, dose-specific analyses were not performed because the combined dose comparisons were not significant.
FIGURE 2.
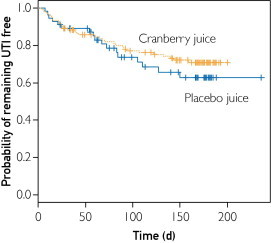
Kaplan-Meier curve for time to symptomatic urinary tract infection (UTI) in women ingesting cranberry juice (orange line) vs placebo juice (blue line). No significant difference was found in time to UTI between the groups (P=.41; log-rank test).
Asymptomatic Bacteriuria and Culture-Confirmed E coli UTI
Asymptomatic bacteriuria was identified at least once during the study period in 101 of 176 women (57.4%), including 75 of 120 women (62.5%) in the cranberry juice group and 26 of 56 women (46.4%) in the placebo group (P=.04). E coli was the causative organism of asymptomatic bacteriuria in 29 of 75 women (38.7%) women in the cranberry juice group and 11 of 26 women (42.3%) in the placebo group.
Symptomatic E coli UTI was diagnosed in 33 of 176 women (18.8%): 24 of 120 (20.0%) in the cranberry juice group and 9 of 56 (16.1%) in the placebo group (P=.53). Among the 38 women with a culture-confirmed UTI during the study period, E coli was the causative pathogen in 33 (86.8%).
P-Fimbriated E coli
Both treatment groups started out at baseline with no urinary E coli as per study inclusion criteria. E coli strains from 33 (23 in the cranberry juice group and 10 in the placebo group) of the 73 women with E coli asymptomatic bacteriuria or symptomatic UTI events during the intervention period were available for testing for P fimbriae. The proportions of urinary E coli that were P fimbriated were 10 of 23 (43.5%) in the cranberry juice group and 8 of 10 (80.0%) in the placebo group (P=.07). Overall, the odds of having P-fimbriated E coli in the urine during the intervention period in the cranberry group were one-fifth the odds in the placebo group (odds ratio, 0.19; 95% CI, 0.03-1.11).
At baseline, vaginal colonization with P-fimbriated E coli was identified in 13 of 46 women (28.3%) in the cranberry group and 11 of 23 (47.8%) in the placebo group (P=.11). P-fimbriated rectal E coli from the baseline visit were isolated in 24 of 84 women (29%) and 17 of 45 women (37.8%) in the cranberry juice and placebo groups, respectively (P=.20).
Adverse Effects
No serious adverse events occurred in either of the study groups. Minor adverse effects included primarily gastrointestinal (constipation, heartburn, loose stool), vaginal (itching, dryness), and other (migraine) symptoms. The proportions of women who reported minor adverse effects were 29 of 120 (24.2%) in the cranberry juice group and 7 of 56 (12.5%) in the placebo group (P=.07). Three women in the cranberry group stopped taking the study product because of gastrointestinal symptoms thought to be related to the study product.
Adherence With the Study Products
The proportion of women who reported missing one dose or more of their study product was not different between treatment groups at any of the monthly follow-up assessments. The mean number of doses missed each month was 1 to 3, resulting in a mean monthly dose adherence of 27.1 of 30 (90.3%) and 27.5 of 30 (91.8%) in the placebo and cranberry groups, respectively. Adherence to use of the study product was not independently associated with the time to first UTI (data not shown).
Discussion
Regular ingestion of cranberry products is a popular strategy for preventing UTI. Our study used a standardized cranberry product and matching placebo and demonstrated an overall reduced hazard ratio of 0.68 for the incidence of UTI in women ingesting cranberry juice vs those ingesting placebo, a difference that was not statistically significant but in concordance with findings of previous studies.6 The reduced proportion of urinary P-fimbriated E coli strains in women ingesting cranberry juice vs those ingesting placebo demonstrated in our study provides, for the first time to our knowledge, an in vitro correlate to the clinical outcome of symptomatic UTI.
Our study differs in both methods and results from that of a recently published trial using the same products and a similar population.23 Barbosa-Cesnik et al23 used a more frequent dosing regimen and studied a larger sample of predominantly college-age women. The major difference was in the definition of the primary outcome, symptomatic UTI, which in the study by Barbosa-Cesnik et al required culture confirmation. Diagnosis of acute cystitis without a urine culture in women who present with dysuria in the absence of vaginal discharge is consistent with the definition used in the studies forming the basis of the sample size estimates for both trials and with definitions used by international clinical practice guidelines.18,24,25 The evidence for not requiring culture confirmation for this population is derived from well-designed studies that demonstrate that up to 15% to 20% of women have negative culture results or low-count bacteriuria but still respond to a short course of UTI-directed treatment and have no other diagnosis.3,24 Requiring positive culture results could partly explain the lower-than-expected event rates. Our study did not suggest any activity of the placebo juice, as evidenced by a recurrent UTI rate exactly as predicted by previous studies.18,25
Despite these differences, the overall conclusion that cranberry juice did not demonstrate a statistically significantly reduction in the rate of recurrent UTI compared with placebo in women with recent acute cystitis is similar in both studies. Neither study was adequately powered to evaluate a 50% reduction, given the smaller sample sizes and lower event rates than those assumed in the original power calculations.26 Interestingly, a recent Cochrane meta-analysis found a significantly reduced incidence of UTIs at 12 months with a relative risk of 0.66 (95% CI, 0.46-0.90) in cranberry juice vs placebo/control groups, similar to the hazard ratio of 0.68 that we report here; albeit, the 95% CI in our study is wider and spans a nonsignificant difference.6
The most intriguing and novel finding in our study is the strong, although not statistically significant, trend toward lower rates of urinary P-fimbriated E coli isolated from women who received cranberry juice compared with those who received placebo juice. Our findings are strengthened by the fact that both groups started out without any urinary E coli at baseline because inclusion criteria required the absence of asymptomatic bacteriuria. The prevalence rates of P fimbriation among rectal and vaginal E coli, the most likely sources of urinary isolates, were lower in women ingesting cranberry juice vs placebo by 10% to 20% at the baseline visit and were not measured during the intervention phase. These differences were not statistically significant and were of smaller magnitude compared with the differences among the urinary isolates but could have contributed in part to the lower rate of urinary E coli in the cranberry arm during the intervention phase. Although the mechanism of cranberry activity in the prevention of UTI is not fully elucidated, one leading hypothesis is that a cranberry component, or a direct metabolite, inhibits P-fimbriated E coli from attaching to the bladder epithelium.27 In vitro studies have demonstrated this adherence inhibition with proanthocyanidin extract from cranberries, human urine collected after cranberry ingestion, and growth of E coli on cranberry-containing media.9,27,28 None of these previous in vitro studies longitudinally evaluated clinical outcomes. Similarly, none of the clinical studies correlated observed patient outcomes with in vitro findings. Our data are the first, to our knowledge, to report on both clinical and in vitro outcomes and suggest a concurrent but nonsignificant reduction in the cumulative rate of symptomatic UTI and urinary P-fimbriated E coli in women ingesting cranberry juice vs placebo juice. The lack of statistical significance in the results warrants caution in making definitive conclusions, and future well-powered studies will be needed to further evaluate the correlations between clinical and in vitro effects.
Tolerability of cranberry juice has been problematic in previous reports. Even though we used a lower volume of juice than most previous studies, women in the cranberry juice arm had a higher rate of minor adverse events compared with women in the placebo arm. Despite the adverse effects, adherence to ingestion of the cranberry and placebo juices was high compared with what previous studies have found, likely related to the once-daily dosing regimen. The optimal balance between a high cranberry juice concentration and tolerability may be best achieved with cranberry solids, which have shown promising effects in previous studies and are probably a more viable option for continuous daily prophylaxis.11
The main limitation of our study is that we did not achieve our desired sample size and thus were not powered to show a significant effect of cranberry on the cumulative rate of UTI. Adherence was based on self-report rather than measurement of an active urinary metabolite because the latter was not available during the trial. Overall, the limitations of our study highlight some of the limitations of real-life use of cranberry juice for prevention of UTI. Consistent daily ingestion of a glass or more of cranberry juice cocktail may not be feasible or tolerated for prolonged periods.
Conclusion
The strengths of this double-blinded, randomized, controlled study include the use of well-characterized and standardized cranberry and placebo products, multicenter enrollment, and evaluation of clinical and bacterial virulence outcomes. Our data demonstrated a potentially protective effect of cranberry with a hazard ratio for UTI of 0.68 compared with placebo, but it was not statistically significant, and the wide CIs do not exclude the possibility of a lack of risk reduction. However, the observation that this therapy reduced infection with P-fimbriated E coli strains suggests a cohort that may benefit from cranberry juice therapy. This observation should be pursued with additional mechanistic and perhaps clinical study in patients infected with this organism type.
Acknowledgments
We are greatly indebted to Amy Howell, PhD, (Rutgers University, Chatsworth, NJ) for contributions to the study concept and design; D.C. Dugdale, MD, medical director, and the staff at Hall Health Primary Care Center (University of Washington, Seattle), and Michael Kozal, MD, and the staff at the General Clinical Research Center (Yale University, New Haven, CT) for assistance with study execution; Ellen Cassen, ARNP, and Natalie DeShaw, BS, (Hall Health Center) and Laurie Feldman, MHSA, and Lauren Perley, MS, (Yale University) for assistance in the development of study protocols, data collection, project coordination, and data management; Cynthia Brandt, MD, (Yale University) for database development; Yanhong Deng, MPH, (Yale University) for data analyses; Sheila Manuguid, BS, Cheryl Wobbe, BS, and Alex Chang, BS, (University of Washington Urinary Tract Infection Research Laboratory) for laboratory assistance; and to the study participants.
Footnotes
Grant Support: This work was funded by grant RO1 AT002105 (K.G.) from the National Center for Complementary and Alternative Medicine, and this publication was made possible by a Clinical and Translational Science Award grant UL1 RR024139 from the National Center for Research Resources, a component of the National Institutes of Health, and the National Institutes of Health Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or the National Institutes of Health.
References
- 1.Dielubanza E.J., Schaeffer A.J. Urinary tract infections in women. Med Clin North Am. 2011;95(1):27–41. doi: 10.1016/j.mcna.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Griebling T.L. Urologic diseases in America project: trends in resource use for urinary tract infections in women. J Urol. 2005;173(4):1281–1287. doi: 10.1097/01.ju.0000155596.98780.82. [DOI] [PubMed] [Google Scholar]
- 3.Gupta K., Hooton T.M., Naber K.G. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 4.Zalmanovici Trestioreanu A., Green H., Paul M., Yaphe J., Leibovici L. Antimicrobial agents for treating uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2010;10 doi: 10.1002/14651858.CD007182.pub2. CD007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raz R., Chazan B., Dan M. Cranberry juice and urinary tract infection. Clin Infect Dis. 2004;38(10):1413–1419. doi: 10.1086/386328. [DOI] [PubMed] [Google Scholar]
- 6.Jepson R.G., Craig J.C. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;1 doi: 10.1002/14651858.CD001321.pub4. CD001321. [DOI] [PubMed] [Google Scholar]
- 7.Tempera G., Corsello S., Genovese C., Caruso F.E., Nicolosi D. Inhibitory activity of cranberry extract on the bacterial adhesiveness in the urine of women: an ex-vivo study. Int J Immunopathol Pharmacol. 2010;23(2):611–618. doi: 10.1177/039463201002300223. [DOI] [PubMed] [Google Scholar]
- 8.Howell A.B., Botto H., Combescure C. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect Dis. 2010;10:94. doi: 10.1186/1471-2334-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta K., Chou M.Y., Howell A., Wobbe C., Grady R., Stapleton A.E. Cranberry products inhibit adherence of p-fimbriated Escherichia coli to primary cultured bladder and vaginal epithelial cells. J Urol. 2007;177(6):2357–2360. doi: 10.1016/j.juro.2007.01.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kontiokari T., Sundqvist K., Nuutinen M., Pokka T., Koskela M., Uhari M. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ. 2001;322(7302):1571. doi: 10.1136/bmj.322.7302.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9(3):1558–1562. [PubMed] [Google Scholar]
- 12.Schlager T.A., Anderson S., Trudell J., Hendley J.O. Effect of cranberry juice on bacteriuria in children with neurogenic bladder receiving intermittent catheterization. J Pediatr. 1999;135(6):698–702. doi: 10.1016/s0022-3476(99)70087-9. [DOI] [PubMed] [Google Scholar]
- 13.Avorn J., Monane M., Gurwitz J.H., Glynn R.J., Choodnovskiy I., Lipsitz L.A. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271(10):751–754. doi: 10.1001/jama.1994.03510340041031. [DOI] [PubMed] [Google Scholar]
- 14.Haverkorn M.J., Mandigers J. Reduction of bacteriuria and pyuria using cranberry juice. JAMA. 1994;272(8):590. doi: 10.1001/jama.272.8.590a. [DOI] [PubMed] [Google Scholar]
- 15.McMurdo M.E., Argo I., Phillips G., Daly F., Davey P. Cranberry or trimethoprim for the prevention of recurrent urinary tract infections?: a randomized controlled trial in older women. J Antimicrob Chemother. 2009;63(2):389–395. doi: 10.1093/jac/dkn489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wing D.A., Rumney P.J., Preslicka C.W., Chung J.H. Daily cranberry juice for the prevention of asymptomatic bacteriuria in pregnancy: a randomized, controlled pilot study. J Urol. 2008;180(4):1367–1372. doi: 10.1016/j.juro.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess M.J., Hess P.E., Sullivan M.R., Nee M., Yalla S.V. Evaluation of cranberry tablets for the prevention of urinary tract infections in spinal cord injured patients with neurogenic bladder. Spinal Cord. 2008;46(9):622–626. doi: 10.1038/sc.2008.25. [DOI] [PubMed] [Google Scholar]
- 18.Gupta K., Hooton T.M., Roberts P.L., Stamm W.E. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135(1):9–16. doi: 10.7326/0003-4819-135-1-200107030-00004. [DOI] [PubMed] [Google Scholar]
- 19.Czaja C.A., Stamm W.E., Stapleton A.E. Prospective cohort study of microbial and inflammatory events immediately preceding Escherichia coli recurrent urinary tract infection in women. J Infect Dis. 2009;200(4):528–536. doi: 10.1086/600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stapleton A., Moseley S., Stamm W.E. Urovirulence determinants in Escherichia coli isolates causing first-episode and recurrent cystitis in women. J Infect Dis. 1991;163(4):773–779. doi: 10.1093/infdis/163.4.773. [DOI] [PubMed] [Google Scholar]
- 21.Murray P. Manual of Clinical Microbiology. 9th ed. ASM Press; Herdon, VA: 2007. [Google Scholar]
- 22.Johnson J.R., Stell A.L. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis. 2000;181(1):261–272. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- 23.Barbosa-Cesnik C., Brown M.B., Buxton M., Zhang L., Debusscher J., Foxman B. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52(1):23–30. doi: 10.1093/cid/ciq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bent S., Nallamothu B.K., Simel D.L., Fihn S.D., Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287(20):2701–2710. doi: 10.1001/jama.287.20.2701. [DOI] [PubMed] [Google Scholar]
- 25.Foxman B., Gillespie B., Koopman J. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151(12):1194–1205. doi: 10.1093/oxfordjournals.aje.a010170. [DOI] [PubMed] [Google Scholar]
- 26.Eells S.J., McKinnell J.A., Miller L.G. Daily cranberry prophylaxis to prevent recurrent urinary tract infections may be beneficial in some populations of women. Clin Infect Dis. 2011;52(11):1393–1394. doi: 10.1093/cid/cir190. author reply 1394-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howell A.B., Vorsa N., Der Marderosian A., Foo L.Y. Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial-cell surfaces by proanthocyanidin extracts from cranberries. N Engl J Med. 1998;339(15):1085–1086. doi: 10.1056/NEJM199810083391516. [DOI] [PubMed] [Google Scholar]
- 28.Lavigne J.P., Bourg G., Combescure C., Botto H., Sotto A. In-vitro and in-vivo evidence of dose-dependent decrease of uropathogenic Escherichia coli virulence after consumption of commercial Vaccinium macrocarpon (cranberry) capsules. Clin Microbiol Infect. 2008;14(4):350–355. doi: 10.1111/j.1469-0691.2007.01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


