Abstract
In healthy human beings, blood flow to dynamically contracting skeletal muscle is regulated primarily to match oxygen (O2) delivery closely with utilisation. This occurs across a wide range of exercise intensities, as well as when exercise is combined with conditions that modify blood O2 content. The red blood cells (RBCs), the primary O2 carriers in the blood, contribute to the regulation of the local processes matching O2 supply and demand. This is made possible by the ability of RBCs to release the vasoactive substance adenosine triphosphate (ATP) in response to reductions in erythrocyte and plasma O2, as well as to other adjuvant metabolic and mechanical stimuli. The regulatory role of RBCs in human beings is supported by the observations that, i) exercising skeletal muscle blood flow responds primarily to changes in the amount of O2 bound to the erythrocyte haemoglobin molecules, rather than the amount of O2 in plasma, and ii) exercising muscle blood flow can almost double (from 260 to 460 ml min−1 100 g−1) with alterations in blood O2 content, such that O2 delivery and  are kept constant. Besides falling blood O2 content, RBCs release ATP when exposed to increased temperature, reduced pH, hypercapnia, elevated shear stress and augmented mechanical deformation, i.e. conditions that exist in the microcirculation of active skeletal muscle. ATP is an attractive mediator signal for skeletal muscle blood flow regulation, not only because it can act as a potent vasodilator, but also because of its sympatholytic properties in the human limb circulations. These properties are essential to counteract the vasoconstrictor effects of concurrent increases in muscle sympathetic nerve activity and circulating vasoconstrictor substances during exercise. Comparison of the relative vasoactive potencies and sympatholytic properties of ATP, other nucleotides, and adenosine in human limbs, suggests that intravascular ATP exerts its vasodilator and sympatholytic effects directly, and not via its degradation compounds. In conclusion, current evidence clearly indicates that RBCs are involved directly in the regulation of O2 supply to human skeletal muscle during dynamic exercise. Further, intravascular ATP might be an important mediator in local metabolic sensing and signal transduction between the RBCs and the endothelial and smooth muscle cells in the vascular beds of skeletal muscle.
are kept constant. Besides falling blood O2 content, RBCs release ATP when exposed to increased temperature, reduced pH, hypercapnia, elevated shear stress and augmented mechanical deformation, i.e. conditions that exist in the microcirculation of active skeletal muscle. ATP is an attractive mediator signal for skeletal muscle blood flow regulation, not only because it can act as a potent vasodilator, but also because of its sympatholytic properties in the human limb circulations. These properties are essential to counteract the vasoconstrictor effects of concurrent increases in muscle sympathetic nerve activity and circulating vasoconstrictor substances during exercise. Comparison of the relative vasoactive potencies and sympatholytic properties of ATP, other nucleotides, and adenosine in human limbs, suggests that intravascular ATP exerts its vasodilator and sympatholytic effects directly, and not via its degradation compounds. In conclusion, current evidence clearly indicates that RBCs are involved directly in the regulation of O2 supply to human skeletal muscle during dynamic exercise. Further, intravascular ATP might be an important mediator in local metabolic sensing and signal transduction between the RBCs and the endothelial and smooth muscle cells in the vascular beds of skeletal muscle.

José González-Alonso, PhD (University of Texas at Austin) is an integrative physiologist with a particular interest in human cardiovascular regulation. He benefited tremendously from his extensive post-doctoral work at the Copenhagen Muscle Research Centre (University of Copenhagen). His research primarily focuses on the mechanisms underlying the circulatory limitations to exercise capacity and environmental stress in humans and the role of the erythrocytes and intravascular adenosine triphosphate (ATP) on the regulation of the skeletal muscle blood flow and oxygen delivery.]
Skeletal muscle blood flow during exercise
It has long been known that blood flow to working skeletal muscles increases with elevations in exercise intensity. The magnitude of increase in skeletal muscle perfusion from rest to maximal exercise in normal conditions can reach 100- to 160-fold in untrained human beings and in endurance-trained athletes performing single leg dynamic knee-extensor exercise, i.e. an increase from ∼2.5 to ∼250 and ∼400 ml min−1 (100 g)−1, respectively (assuming that all of the 2.5–3.0 kg of quadriceps muscles are activated at maximal exercise) (Andersen & Saltin 1985; Richardson et al. 1993; Saltin 2007). These estimates of maximal perfusion in human skeletal muscle are in agreement with values found in animal studies (Armstrong & Laughlin 1985; Munch et al. 1987; Laughlin et al. 2012) and demonstrate the enormous capacity of the skeletal muscle vasculature to dilate and increase blood flow, when metabolic demand increases. The delivery of O2 and nutrients to body tissues and organs, and the clearance of metabolic waste products and heat are a function of blood flow, the blood concentration of gases, substrates and metabolites, and the temperature of the blood. Under normal conditions, the concentration of O2 and nutrients in the arterial blood is normally maintained or changes only slightly during short duration exercise. Blood flow is therefore the primary determinant of O2 and fuel delivery to active skeletal muscle during dynamic exercise (Rowell 2004; Saltin 2007; Laughlin et al. 2012).
The rate of muscle O2 utilisation is generally thought to be a key factor that influences the magnitude of increase in blood flow to active skeletal muscle, termed exercise hyperaemia (Saltin 2007; Laughlin et al. 2012). Figure 1 illustrates this widely held principle by showing progressive increases in leg blood flow, leg O2 delivery, leg O2 extraction and leg 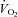 in healthy young men during incremental single leg dynamic knee-extension exercise. Notice the striking similarity in the rate of increase in leg O2 delivery and leg
in healthy young men during incremental single leg dynamic knee-extension exercise. Notice the striking similarity in the rate of increase in leg O2 delivery and leg 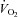 per unit of power (i.e. 16 and 13 ml min−1 W−1, respectively) (González-Alonso et al. 2008). These circulatory adjustments across the active leg tissues, primarily in the exercising quadriceps muscle in this model (Andersen et al. 1985; Richardson et al. 1998; González-Alonso et al. 2000), are for the most part accompanied by proportional increases in cardiac output and systemic O2 delivery, but a modest increase in mean arterial and central venous pressure (Fig. 1). Accordingly, a primary aim of the local and central control mechanisms involved in the regulation of cardiovascular function during dynamic steady-state exercise is to maintain a close match between O2 supply and demand in active muscle (Rowell 2004; Saltin 2007; Laughlin et al. 2012). However, close matching of O2 supply and utilisation is not a universal physiological phenomenon in active skeletal muscle across all exercise modes and intensities. For example, evidence in human models indicates that blood flow and O2 delivery to active locomotor skeletal muscles is restrained when engaging a large muscle mass, during intense whole-body exercise, e.g. cycling, skiing, rowing or running (Calbet et al. 2004; Mortensen et al. 2005, 2008). Figure 2 illustrates this concept by showing that leg perfusion (and thus its surrogate O2 delivery) is the same up to a power output of ∼100 W and is intimately related to leg
per unit of power (i.e. 16 and 13 ml min−1 W−1, respectively) (González-Alonso et al. 2008). These circulatory adjustments across the active leg tissues, primarily in the exercising quadriceps muscle in this model (Andersen et al. 1985; Richardson et al. 1998; González-Alonso et al. 2000), are for the most part accompanied by proportional increases in cardiac output and systemic O2 delivery, but a modest increase in mean arterial and central venous pressure (Fig. 1). Accordingly, a primary aim of the local and central control mechanisms involved in the regulation of cardiovascular function during dynamic steady-state exercise is to maintain a close match between O2 supply and demand in active muscle (Rowell 2004; Saltin 2007; Laughlin et al. 2012). However, close matching of O2 supply and utilisation is not a universal physiological phenomenon in active skeletal muscle across all exercise modes and intensities. For example, evidence in human models indicates that blood flow and O2 delivery to active locomotor skeletal muscles is restrained when engaging a large muscle mass, during intense whole-body exercise, e.g. cycling, skiing, rowing or running (Calbet et al. 2004; Mortensen et al. 2005, 2008). Figure 2 illustrates this concept by showing that leg perfusion (and thus its surrogate O2 delivery) is the same up to a power output of ∼100 W and is intimately related to leg 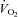 , irrespective of whether power output was generated only by the quadriceps muscles during knee-extensor exercise or spread among the different leg muscles during two leg cycling (Mortensen et al. 2008). These observations during single leg knee-extensor and two leg cycling exercise below 100 W are strong evidence of the intimate coupling between O2 delivery and metabolic demand for O2 discussed above in relation to the single leg knee-extensor exercise data presented in Fig. 1. However, during cycling exercise above a moderate intensity, the relationship between locomotor limb tissue perfusion/O2 supply and O2 utilisation is curvilinear. This indicates a mismatch between O2 supply and metabolic demand for O2 in the moderate to maximal intensity domain (Fig. 2) (Mortensen et al. 2008). As a consequence, muscle perfusion per unit of power is lower during maximal whole-body exercise than during maximal small-muscle exercise, thereby imposing a circulatory limitation to maximal aerobic capacity (Mortensen et al. 2008; Boushel et al. 2011). This blunting in skeletal muscle perfusion during intense whole-body exercise may reflect the interaction between an enhanced local vasoconstriction in the active muscle vasculature and the attainment of the limits in cardiac function (Calbet et al. 2004; Mortensen et al. 2005, 2008; Stöhr et al. 2011). However, the majority of human physical or exercise activities do not require near or maximal aerobic capacity; rather, they are performed within the range of submaximal exercise intensities, where muscle O2 supply and metabolic demand are matched closely. Thus, the consensus view reflected in the literature is that skeletal muscle perfusion reflects primarily the aerobic metabolic rate of the muscle and that factors and signals related to aerobic metabolism are heavily involved in the regulation of active skeletal muscle blood flow.
, irrespective of whether power output was generated only by the quadriceps muscles during knee-extensor exercise or spread among the different leg muscles during two leg cycling (Mortensen et al. 2008). These observations during single leg knee-extensor and two leg cycling exercise below 100 W are strong evidence of the intimate coupling between O2 delivery and metabolic demand for O2 discussed above in relation to the single leg knee-extensor exercise data presented in Fig. 1. However, during cycling exercise above a moderate intensity, the relationship between locomotor limb tissue perfusion/O2 supply and O2 utilisation is curvilinear. This indicates a mismatch between O2 supply and metabolic demand for O2 in the moderate to maximal intensity domain (Fig. 2) (Mortensen et al. 2008). As a consequence, muscle perfusion per unit of power is lower during maximal whole-body exercise than during maximal small-muscle exercise, thereby imposing a circulatory limitation to maximal aerobic capacity (Mortensen et al. 2008; Boushel et al. 2011). This blunting in skeletal muscle perfusion during intense whole-body exercise may reflect the interaction between an enhanced local vasoconstriction in the active muscle vasculature and the attainment of the limits in cardiac function (Calbet et al. 2004; Mortensen et al. 2005, 2008; Stöhr et al. 2011). However, the majority of human physical or exercise activities do not require near or maximal aerobic capacity; rather, they are performed within the range of submaximal exercise intensities, where muscle O2 supply and metabolic demand are matched closely. Thus, the consensus view reflected in the literature is that skeletal muscle perfusion reflects primarily the aerobic metabolic rate of the muscle and that factors and signals related to aerobic metabolism are heavily involved in the regulation of active skeletal muscle blood flow.
Figure 1. Leg and systemic haemodynamics during incremental single leg exercise.
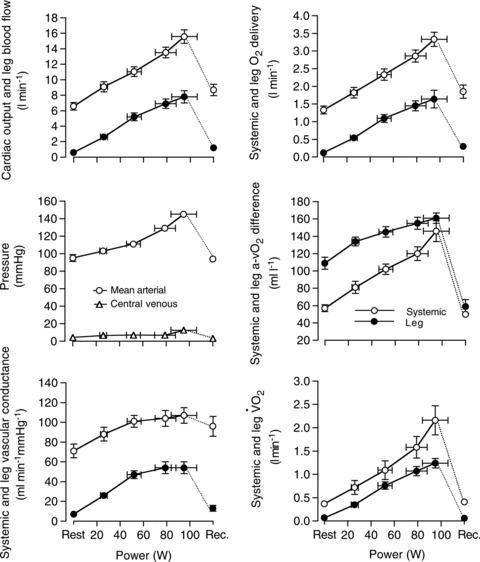
Cardiovascular variables were plotted against the increases in power output during single leg knee-extensor exercise. Note the similar rate of increase in leg O2 delivery and leg 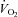 (16 and 13 ml min−1 W−1, respectively). In all graphs, open circles represent the systemic responses and the filled circles represent the leg responses. Central venous pressure is depicted in empty triangles. Reproduced from González-Alonso et al. (2008).
(16 and 13 ml min−1 W−1, respectively). In all graphs, open circles represent the systemic responses and the filled circles represent the leg responses. Central venous pressure is depicted in empty triangles. Reproduced from González-Alonso et al. (2008).
Figure 2. Leg haemodynamics during cycling and knee-extensor exercise.
Note the similar absolute values and rate of increase in leg blood flow and  until 100 W during both two leg cycling and single leg knee-extensor exercise, becoming attenuated thereafter during cycling in association with a blunting in leg blood flow and vascular conductance (A and B). Close relationship between leg O2 delivery and
until 100 W during both two leg cycling and single leg knee-extensor exercise, becoming attenuated thereafter during cycling in association with a blunting in leg blood flow and vascular conductance (A and B). Close relationship between leg O2 delivery and  responses during incremental exercise (C). Modified from Mortensen et al. (2008).
responses during incremental exercise (C). Modified from Mortensen et al. (2008).
Locally, skeletal muscle perfusion is determined by vascular conductance and perfusion pressure gradient, i.e. arteriovenous pressure difference. During dynamic incremental exercise, the increases in active limb muscle blood flow are primarily the result of increases in vascular conductance. The latter is indicative of vasodilatation of the vascular beds irrigating the active muscle, as the perfusion pressure gradient does not change during mild intensity exercise, or increases slightly, in comparison to flow during intense exercise (Fig. 1). In exercising limbs, the diameter of the large conduit vessels such as the femoral and brachial arteries, does not change significantly, or changes little, in comparison to the increases in arterial blood velocity (Rådegran 1997; Shoemaker et al. 1997). Hence, the enhanced vasodilatation, indicated by the global increases in limb vascular conductance during exercise, is attributable largely to increases in the diameter of small arteries and arterioles perfusing active muscle fibres (Segal 2005). A long-standing question is, what are the mechanisms underlying the apparently precise regulation of flow and O2 supply to active skeletal muscle during the majority of submaximal exercise conditions? Over more than a century of extensive investigation of this question, a number of local and central control mechanisms have been proposed to regulate active muscle blood flow. These include metabolic, myogenic, mechanical, humoral and neural mechanisms, which under in vivo conditions involve the interaction of multiple intravascular, interstitial and intracellular signalling pathways (Rowell 1993, 2004; Laughlin et al. 2012). Discussion of each of these mechanisms and their relative contributions to exercise hyperaemia in humans is beyond the scope of this symposium review and the reader is referred to the following excellent comprehensive review articles for a more extensive discourse on these topics: Shepherd et al. (1983), Rowell (1993), Saltin et al. (1998) and Laughlin et al. (2012). The current review will focus on recent evidence from human studies providing insight into the influence of red blood cell (RBC) signalling. In particular, the review will focus on the role of erythrocyte-derived ATP, as a local vascular control mechanism contributing to the matching in O2 supply and demand in skeletal muscle during dynamic exercise (Ellsworth et al. 1995; González-Alonso et al. 2002). Evidence supporting the involvement of erythrocytes in the control of O2 delivery to active skeletal muscle will be reviewed first.
Role of blood oxygen in the regulation of skeletal muscle perfusion and oxygen supply during exercise
Under normal exercise conditions, where arterial O2 content is stable, oxygen delivery to skeletal muscle is determined by blood flow. However, the magnitude of exercise hyperaemia is influenced greatly by large increases or decreases in blood O2 content, even when the metabolic demand for O2 remains unaltered. Accordingly, when human beings are exposed to alterations in arterial blood O2 content due to separate or combined manipulations of inspired O2 and carbon monoxide (CO), as well as anaemia, plasma volume expansion or polycythaemia, exercising skeletal muscle blood flow changes in relation to blood O2 such that O2 supply is well-preserved (Rowell et al. 1986; Richardson et al. 1995; Koskolou et al. 1997; Saltin et al. 1998; Roach et al. 1999; González-Alonso et al. 2002, 2006; Casey & Joyner 2011) (Fig. 3). Indeed, the capacity of the muscle circulation to respond to changes in blood O2 content appears to be substantial since perfusion to exercising quadriceps muscles almost doubles, with large alterations in blood O2 content during constant power submaximal knee-extensor exercise; consequently, O2 delivery and  remain constant (González-Alonso et al. 2006). For example, muscle perfusion increases from 260 ml min−1 (100 g)−1 during exercise with combined polycythaemia and hyperoxia to 460 ml min−1 (100 g)−1 during exercise with combined anaemia, plasma volume expansion and hypoxia, when total leg blood flow increases from 4.5 to 8 l min−1 (Fig. 3A). Notice that the difference in active limb tissue perfusion associated with changes in blood O2 content during moderate intensity small muscle mass exercise in trained individuals (i.e. 50% peak power) is 200 ml min−1 (100 g)−1, a value that is close to the reported maximal muscle perfusion values of untrained individuals (Andersen & Saltin 1985). Although these data from exercising human limb are unable to provide information about the precise site(s) of O2 sensing and signal transduction mechanisms, they nonetheless provide strong support for a critical role of blood O2 content in the regulation of active skeletal muscle blood flow.
remain constant (González-Alonso et al. 2006). For example, muscle perfusion increases from 260 ml min−1 (100 g)−1 during exercise with combined polycythaemia and hyperoxia to 460 ml min−1 (100 g)−1 during exercise with combined anaemia, plasma volume expansion and hypoxia, when total leg blood flow increases from 4.5 to 8 l min−1 (Fig. 3A). Notice that the difference in active limb tissue perfusion associated with changes in blood O2 content during moderate intensity small muscle mass exercise in trained individuals (i.e. 50% peak power) is 200 ml min−1 (100 g)−1, a value that is close to the reported maximal muscle perfusion values of untrained individuals (Andersen & Saltin 1985). Although these data from exercising human limb are unable to provide information about the precise site(s) of O2 sensing and signal transduction mechanisms, they nonetheless provide strong support for a critical role of blood O2 content in the regulation of active skeletal muscle blood flow.
Figure 3. Limb blood flow and O2 delivery during exercise and altered blood O2 content.
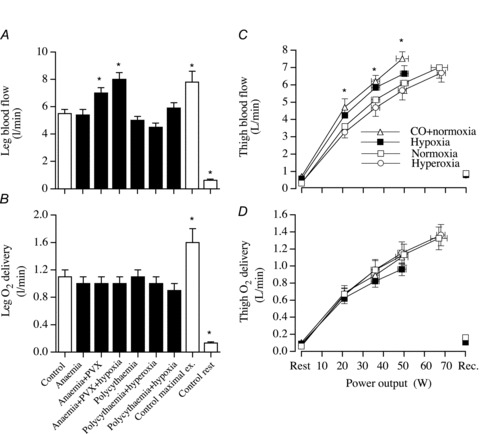
Leg blood flow (A) and O2 delivery (B) during submaximal one-legged knee-extensor exercise (∼50 W) in normocythaemia (control), anaemia, anaemia combined with plasma volume expansion (anaemia + PVX), anaemia combined with plasma volume expansion and hypoxia (anaemia + PVX + hypoxia), polycythaemia, polycythaemia combined with hyperoxia (polycythaemia + hyperoxia) and polycythaemia combined with hypoxia (polycythaemia + hypoxia). For comparison, haemodynamics and oxygenation data at rest and during peak one-legged knee-extensor exercise (95 ± 11 W) in the control condition are depicted. *Significantly different from exercise control, P < 0.05. Modified from González-Alonso et al. (2006). Thigh blood flow (C) and O2 delivery (D) at rest and during incremental knee-extensor exercise and after 10 min of recovery with exposure to normoxia, hypoxic hypoxia, hyperoxia and carbon monoxide hypoxia in normoxia. *Values during hypoxia and carbon monoxide in combination with normoxia are significantly different from normoxic control, P < 0.05. Reproduced from González-Alonso et al. (2002).
Blood O2 content is largely determined by O2 bound to the highly abundant haemoglobin molecules in the RBCs (250 million per RBC), but there is also a small amount of O2 dissolved within plasma (termed partial pressure of O2,  , and which accounts for 1.5% of the arterial blood O2 content). The
, and which accounts for 1.5% of the arterial blood O2 content). The 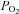 is widely thought to act as an important signal for the control of the cardiovascular system, particularly in the control of ventilation and heart rate in conditions that alter arterial blood O2 content (Jackson 1987; Baron et al. 1990; Pries et al. 1995). The question here is the role of
is widely thought to act as an important signal for the control of the cardiovascular system, particularly in the control of ventilation and heart rate in conditions that alter arterial blood O2 content (Jackson 1987; Baron et al. 1990; Pries et al. 1995). The question here is the role of 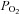 in local muscle blood flow regulation. Under conditions of systemic hypoxia or hyperoxia that are created by reducing or enriching the O2 content of inspiratory gases, respectively, arterial and venous levels of both oxyhaemoglobin and
in local muscle blood flow regulation. Under conditions of systemic hypoxia or hyperoxia that are created by reducing or enriching the O2 content of inspiratory gases, respectively, arterial and venous levels of both oxyhaemoglobin and 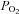 are affected. Accordingly, studies using systemic hypoxia and hyperoxia interventions cannot discriminate between the regulatory influences of O2 bound to haemoglobin and of
are affected. Accordingly, studies using systemic hypoxia and hyperoxia interventions cannot discriminate between the regulatory influences of O2 bound to haemoglobin and of  on muscle perfusion. In recent years, the theory has been advanced that vasoactive signals/substances released from the circulating erythrocytes during the offloading of O2 from the haemoglobin molecules (reflected by reductions in venous oxyhaemoglobin) contribute to the regulation of the supply of O2 and blood to vascular beds experiencing an augmented demand for O2 (Ellsworth et al. 1995; Stamler et al. 1997; Gladwin et al. 2000; González-Alonso et al. 2002). Accordingly, the level of oxyhaemoglobin in RBCs is thought to be a critical stimulus for the release of vasoactive substances underpinning metabolic vasodilatation in skeletal muscle.
on muscle perfusion. In recent years, the theory has been advanced that vasoactive signals/substances released from the circulating erythrocytes during the offloading of O2 from the haemoglobin molecules (reflected by reductions in venous oxyhaemoglobin) contribute to the regulation of the supply of O2 and blood to vascular beds experiencing an augmented demand for O2 (Ellsworth et al. 1995; Stamler et al. 1997; Gladwin et al. 2000; González-Alonso et al. 2002). Accordingly, the level of oxyhaemoglobin in RBCs is thought to be a critical stimulus for the release of vasoactive substances underpinning metabolic vasodilatation in skeletal muscle.
Important insight into the role of RBCs and O2 tension in the regulation of active limb muscle blood flow has emanated from experiments in human beings using CO inhalation in combination with the breathing of normoxic or hyperoxic gas mixtures (González-Alonso et al. 2001, 2002; Richardson et al. 2002; Hanada et al. 2003). CO inhalation offers the opportunity to independently manipulate arterial  and oxyhaemoglobin without altering RBC count. The affinity of haemoglobin for CO is some 200-fold greater than its affinity for O2 (Piantadosi 1987). Consequently, arterial oxyhaemoglobin and O2 content can be reduced with CO inhalation to the same extent as with systemic hypoxia exposure. Furthermore, arterial and venous
and oxyhaemoglobin without altering RBC count. The affinity of haemoglobin for CO is some 200-fold greater than its affinity for O2 (Piantadosi 1987). Consequently, arterial oxyhaemoglobin and O2 content can be reduced with CO inhalation to the same extent as with systemic hypoxia exposure. Furthermore, arterial and venous  can be manipulated independently of CO-induced changes in oxyhaemoglobin by breathing either normoxic or hyperoxic gas mixtures. Using this approach, we compared human exercising limb muscle blood flow, limb vascular conductance, circulating noradrenaline and muscle sympathetic nerve activity (MSNA) under two reduced O2 content conditions. Change in blood flow (relative to control) was similar during CO inhalation in combination with hyperoxia, and during hypoxic hypoxia, despite an 11-fold difference in arterial
can be manipulated independently of CO-induced changes in oxyhaemoglobin by breathing either normoxic or hyperoxic gas mixtures. Using this approach, we compared human exercising limb muscle blood flow, limb vascular conductance, circulating noradrenaline and muscle sympathetic nerve activity (MSNA) under two reduced O2 content conditions. Change in blood flow (relative to control) was similar during CO inhalation in combination with hyperoxia, and during hypoxic hypoxia, despite an 11-fold difference in arterial  between the two hypoxic conditions (550 vs. 40 mmHg; Fig. 4) (González-Alonso et al. 2001; Hanada et al. 2003). To put these findings in perspective, it is important to realise that the high arterial
between the two hypoxic conditions (550 vs. 40 mmHg; Fig. 4) (González-Alonso et al. 2001; Hanada et al. 2003). To put these findings in perspective, it is important to realise that the high arterial  (∼500–600 mmHg) and oxyhaemoglobin values produced with pure systemic hyperoxia are generally associated with a reduced MSNA and exercising limb blood flow (Welch et al. 1977; Seals et al. 1991; González-Alonso et al. 2002) (Fig. 3). We observed the opposite when inhalation of a hyperoxic gas mixture was combined with inhalation of CO. Furthermore, since sympathetic activation can diminish limb muscle perfusion (Joyner et al. 1992; Buckwalter et al. 1997), it is intriguing that the up to 2- to 4-fold elevations in MSNA and circulating noradrenaline fail to reduce resting limb blood flow, or to prevent a greater increase in exercising limb tissue blood flow in either hypoxic condition compared to normoxia (Hanada et al. 2003). These findings suggest the existence of compensatory vasodilator mechanisms linked to the oxygenation state of haemoglobin that are capable of overriding the elevated neural and humoral vasoconstrictor stimuli in resting and exercising limb muscles under hypoxic and submaximal exercise conditions.
(∼500–600 mmHg) and oxyhaemoglobin values produced with pure systemic hyperoxia are generally associated with a reduced MSNA and exercising limb blood flow (Welch et al. 1977; Seals et al. 1991; González-Alonso et al. 2002) (Fig. 3). We observed the opposite when inhalation of a hyperoxic gas mixture was combined with inhalation of CO. Furthermore, since sympathetic activation can diminish limb muscle perfusion (Joyner et al. 1992; Buckwalter et al. 1997), it is intriguing that the up to 2- to 4-fold elevations in MSNA and circulating noradrenaline fail to reduce resting limb blood flow, or to prevent a greater increase in exercising limb tissue blood flow in either hypoxic condition compared to normoxia (Hanada et al. 2003). These findings suggest the existence of compensatory vasodilator mechanisms linked to the oxygenation state of haemoglobin that are capable of overriding the elevated neural and humoral vasoconstrictor stimuli in resting and exercising limb muscles under hypoxic and submaximal exercise conditions.
Figure 4. Influence of blood O2 on leg perfusion and sympathetic nerve activity.
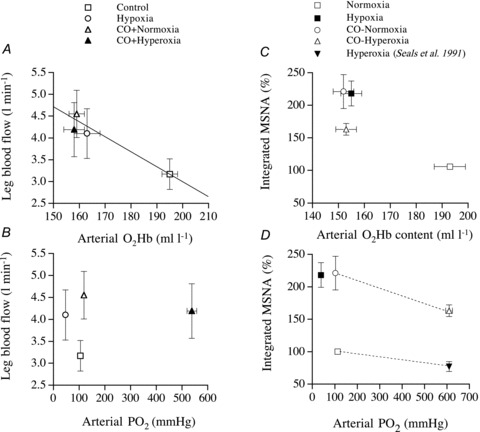
Relationship between leg blood flow or total muscle sympathetic nerve activity versus arterial oxyhaemoglobin and  in healthy humans exposed to normoxia, hypoxia, carbon monoxide inhalation in normoxia, and carbon monoxide inhalation in hyperoxia. Modified from González-Alonso et al. (2001) and Hanada et al. (2003). Note that the integrated MSNA vs. arterial
in healthy humans exposed to normoxia, hypoxia, carbon monoxide inhalation in normoxia, and carbon monoxide inhalation in hyperoxia. Modified from González-Alonso et al. (2001) and Hanada et al. (2003). Note that the integrated MSNA vs. arterial 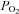 graph also depicts data from a published study during hyperoxia (Seals et al. 1991).
graph also depicts data from a published study during hyperoxia (Seals et al. 1991).
Systemic hypoxia not only reduces blood O2 content, but also intracellular O2 markers (Richardson et al. 1995). Thus, it is still possible that the greater increases in skeletal muscle blood flow with hypoxic hypoxia and CO-hypoxia could also be due to reductions in intracellular 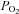 . However, intracellular O2 markers such as
. However, intracellular O2 markers such as 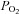 and MbO2 saturation cannot explain the elevation in exercising limb muscle blood flow with CO inhalation-mediated hypoxia compared to normoxia since quadriceps muscle
and MbO2 saturation cannot explain the elevation in exercising limb muscle blood flow with CO inhalation-mediated hypoxia compared to normoxia since quadriceps muscle  and MbO2 saturation are the same during exercise in normoxia and the CO trials in line with a normal venous
and MbO2 saturation are the same during exercise in normoxia and the CO trials in line with a normal venous 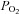 (Richardson et al. 2002). Thus, exercising leg blood flow responses in these experimental conditions are unrelated to arterial, venous or muscle
(Richardson et al. 2002). Thus, exercising leg blood flow responses in these experimental conditions are unrelated to arterial, venous or muscle 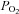 (González-Alonso et al. 2001, 2002; Richardson et al. 2002; Hanada et al. 2003). Collectively, these observations suggest that the main vascular O2 sensor locus for the control of blood flow lies in the erythrocyte itself, rather than in the
(González-Alonso et al. 2001, 2002; Richardson et al. 2002; Hanada et al. 2003). Collectively, these observations suggest that the main vascular O2 sensor locus for the control of blood flow lies in the erythrocyte itself, rather than in the 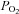 -sensitive areas of the vascular endothelium, vascular smooth muscle or skeletal muscle. A fundamental question is then how do the RBCs signal to the vascular endothelium and smooth muscle to increase or decrease skeletal muscle blood flow in relation to the changes in oxyhaemoglobin. To date, three O2-dependent signalling mechanisms have been proposed: (1) the release of ATP from erythrocytes (Ellsworth et al. 1995), (2) the formation of S-nitrosohaemoglobin (Jia et al. 1996; Stamler et al. 1997), and (3) the reduction of nitrite to vasoactive NO by deoxygenation (Gladwin et al. 2000). Evidence in humans of the role of erythrocyte-derived ATP in the regulation of limb muscle blood flow is discussed below. The cellular and molecular mechanisms of erythrocyte-derived ATP release, its role in blood distribution in skeletal muscle, and the role of intravascular NO and nitrite in the control of circulation are discussed in companion reviews (Ellsworth & Sprague 2012; Hellsten et al. 2012; Owusu et al. 2012).
-sensitive areas of the vascular endothelium, vascular smooth muscle or skeletal muscle. A fundamental question is then how do the RBCs signal to the vascular endothelium and smooth muscle to increase or decrease skeletal muscle blood flow in relation to the changes in oxyhaemoglobin. To date, three O2-dependent signalling mechanisms have been proposed: (1) the release of ATP from erythrocytes (Ellsworth et al. 1995), (2) the formation of S-nitrosohaemoglobin (Jia et al. 1996; Stamler et al. 1997), and (3) the reduction of nitrite to vasoactive NO by deoxygenation (Gladwin et al. 2000). Evidence in humans of the role of erythrocyte-derived ATP in the regulation of limb muscle blood flow is discussed below. The cellular and molecular mechanisms of erythrocyte-derived ATP release, its role in blood distribution in skeletal muscle, and the role of intravascular NO and nitrite in the control of circulation are discussed in companion reviews (Ellsworth & Sprague 2012; Hellsten et al. 2012; Owusu et al. 2012).
The role of erythrocyte-derived ATP in the control of skeletal muscle perfusion
Four decades ago, Forrester & Lind (1969) and Forrester (1972) showed that venous plasma [ATP] increases during human forearm exercise compared to rest. The ATP source, however, was not identified. Twenty years later, Bergfeld & Forrester (1992) demonstrated that human RBCs release ATP when exposed to hypoxia and hypercapnia in vitro. This original finding highlighted that RBCs could be an important source of ATP in the vasculature of active tissues. In 1995, Ellsworth et al. demonstrated that low 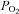 and low pH are strong stimuli for ATP release from RBCs and that ATP placed into an arteriole causes a significant conducted arteriolar vasodilatation (which is really important as a local change in diameter has little impact on flow) and that ATP applied to capillaries results in an increase in red blood cell supply rate in the capillaries (Ellsworth et al. 1995). In this classic study, Ellsworth and co-workers first proposed the hypothesis that ‘RBC is not only the major O2 carrier but also serves as an O2 sensor and affecter of changes in O2 delivery via its release of ATP, which subsequently binds to P2Y receptors on the vascular endothelium, altering vessel calibre’. We tested this hypothesis a few years later in healthy human beings, by measuring femoral venous and arterial plasma [ATP], blood gases and haemodynamics during incremental single leg knee-extensor exercise under conditions of normoxia, hypoxia and hyperoxia, and during CO inhalation in combination with normoxia (González-Alonso et al. 2002). These interventions produced reciprocal alterations in arterial O2 content and thigh blood flow, but equal thigh O2 delivery and
and low pH are strong stimuli for ATP release from RBCs and that ATP placed into an arteriole causes a significant conducted arteriolar vasodilatation (which is really important as a local change in diameter has little impact on flow) and that ATP applied to capillaries results in an increase in red blood cell supply rate in the capillaries (Ellsworth et al. 1995). In this classic study, Ellsworth and co-workers first proposed the hypothesis that ‘RBC is not only the major O2 carrier but also serves as an O2 sensor and affecter of changes in O2 delivery via its release of ATP, which subsequently binds to P2Y receptors on the vascular endothelium, altering vessel calibre’. We tested this hypothesis a few years later in healthy human beings, by measuring femoral venous and arterial plasma [ATP], blood gases and haemodynamics during incremental single leg knee-extensor exercise under conditions of normoxia, hypoxia and hyperoxia, and during CO inhalation in combination with normoxia (González-Alonso et al. 2002). These interventions produced reciprocal alterations in arterial O2 content and thigh blood flow, but equal thigh O2 delivery and 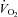 (Fig. 3). In support of Ellsworth and co-workers’ hypothesis, plasma [ATP] in the effluent blood from the exercising thigh increased in all conditions; however, the magnitude of increase was greater during intense hypoxic exercise than during the corresponding normoxic exercise. Plasma [ATP] also tended to be attenuated during hyperoxic exercise and during exercise with CO inhalation combined with normoxia (Fig. 5). Interestingly, venous plasma [ATP] from the non-exercising limb remained low, but was closely related to changes in (O2+ CO)Hb fraction, as in the exercising leg. The observation that exercising limb blood flow was similarly elevated during the CO and hypoxic hypoxia trials suggests that CO and/or other vasodilator substances mitigated the effects of a reduced plasma ATP during the CO compared to hypoxia trial.
(Fig. 3). In support of Ellsworth and co-workers’ hypothesis, plasma [ATP] in the effluent blood from the exercising thigh increased in all conditions; however, the magnitude of increase was greater during intense hypoxic exercise than during the corresponding normoxic exercise. Plasma [ATP] also tended to be attenuated during hyperoxic exercise and during exercise with CO inhalation combined with normoxia (Fig. 5). Interestingly, venous plasma [ATP] from the non-exercising limb remained low, but was closely related to changes in (O2+ CO)Hb fraction, as in the exercising leg. The observation that exercising limb blood flow was similarly elevated during the CO and hypoxic hypoxia trials suggests that CO and/or other vasodilator substances mitigated the effects of a reduced plasma ATP during the CO compared to hypoxia trial.
Figure 5. Plasma ATP and erythrocyte oxygenation.
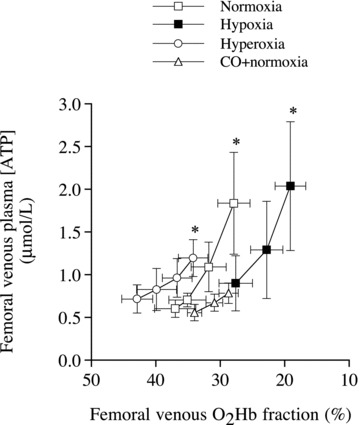
Relationship between femoral venous plasma ATP concentration and O2Hb fraction during incremental knee-extensor exercise with exposure to normoxia, hypoxic hypoxia, hyperoxia and carbon monoxide (CO) inhalation in normoxia. *Significantly higher than mild exercise within a given condition, P < 0.05. Modified from González-Alonso et al. (2002).
Surprisingly, plasma [ATP] in the femoral artery increased with the rise in exercise intensity. Although there is still controversy about the absolute values (Gorman et al. 2007), the increases in venous and arterial plasma [ATP] during incremental and constant high intensity exercise have been confirmed in several studies (González-Alonso et al. 2004; Rosenmeier et al. 2004; Yegutkin et al. 2007; Mortensen et al. 2007), including a recent report using novel intravascular microdialysis probes inserted proximal to the exercising muscles (Mortensen et al. 2011). Increases in venous and arterial plasma [ATP] during exercise have been shown in the coronary circulation (Farias et al. 2005) and across the human brain during maximal constant load cycling exercise (González-Alonso et al. 2004). However, studies using mild constant load single leg exercise have failed to demonstrate significant changes in plasma [ATP] despite significant alterations in blood O2 content and limb blood flow (González-Alonso et al. 2006; Dufour et al. 2010). These findings point out some inconsistencies in the literature, which might be due at least in part to differences in the procedures employed to assess plasma ATP. Methodological issues such as the position of the catheter, the use of stop solutions during blood sample collection, the time lapse before ATP measurement and the correction of plasma [ATP] values for haemolysis can all influence the absolute plasma [ATP] (Gorman et al. 2007). Placement of the catheter in the direction of the exercising muscles appears to be the most critical issue for detecting small changes in plasma [ATP] occurring during low intensity exercise (Mortensen et al. 2011).
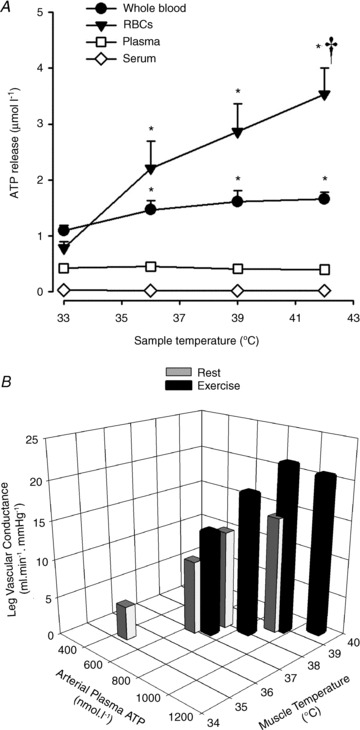
Compelling evidence in vitro demonstrates that the reductions in haemoglobin O2 saturation and 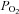 are potent stimuli for erythrocyte ATP release (Jagger et al. 2001; Ellsworth 2004; Ellsworth et al. 2009). In vivo, however, there is some evidence that plasma [ATP] increases in the arterial inflow during incremental exercise, but does so in the presence of unchanged blood O2 content (González-Alonso et al. 2002, 2004; Yegutkin et al. 2007; Mortensen et al. 2011). Likewise, plasma [ATP] increases markedly in the venous and arterial blood of humans with mitochondrial myopathy, who do not display an increase in muscle O2 extraction during exercise (Jeppesen et al. 2012). These observations indicate that during exercise, stimuli other than reductions in blood oxygenation must also increase ATP release from erythrocytes and/or other vascular or interstitial sources. There is evidence that RBCs release ATP in vitro when exposed to hypercapnia (Bergfeld & Forrester 1992), reduced pH (Ellsworth et al. 1995), augmented mechanical deformation (Sprague et al. 1998), elevated shear stress (Wan et al. 2008) and increased temperature (Kalsi & González-Alonso 2012), conditions that are all present in the microcirculation of active skeletal muscle. For instance, temperature has recently been shown to be a potent stimulus for erythrocyte ATP release in vitro (Kalsi & González-Alonso 2012) (Fig. 6). This finding provides insight into a potential ATP source accounting for the rise in plasma ATP in humans exposed to passive heat stress and heat stress combined with exercise (González-Alonso et al. 2004; Pearson et al. 2011). Heat may also provide a possible mechanism underpinning the significant increase in skeletal muscle blood flow with heat stress (Keller et al. 2010) and combined heat stress and exercise, both conditions being associated with the rise in arterial plasma [ATP] and muscle temperature (Pearson et al. 2011) (Fig. 6). Therefore, the rise in plasma [ATP] in conditions where oxyhaemoglobin and
are potent stimuli for erythrocyte ATP release (Jagger et al. 2001; Ellsworth 2004; Ellsworth et al. 2009). In vivo, however, there is some evidence that plasma [ATP] increases in the arterial inflow during incremental exercise, but does so in the presence of unchanged blood O2 content (González-Alonso et al. 2002, 2004; Yegutkin et al. 2007; Mortensen et al. 2011). Likewise, plasma [ATP] increases markedly in the venous and arterial blood of humans with mitochondrial myopathy, who do not display an increase in muscle O2 extraction during exercise (Jeppesen et al. 2012). These observations indicate that during exercise, stimuli other than reductions in blood oxygenation must also increase ATP release from erythrocytes and/or other vascular or interstitial sources. There is evidence that RBCs release ATP in vitro when exposed to hypercapnia (Bergfeld & Forrester 1992), reduced pH (Ellsworth et al. 1995), augmented mechanical deformation (Sprague et al. 1998), elevated shear stress (Wan et al. 2008) and increased temperature (Kalsi & González-Alonso 2012), conditions that are all present in the microcirculation of active skeletal muscle. For instance, temperature has recently been shown to be a potent stimulus for erythrocyte ATP release in vitro (Kalsi & González-Alonso 2012) (Fig. 6). This finding provides insight into a potential ATP source accounting for the rise in plasma ATP in humans exposed to passive heat stress and heat stress combined with exercise (González-Alonso et al. 2004; Pearson et al. 2011). Heat may also provide a possible mechanism underpinning the significant increase in skeletal muscle blood flow with heat stress (Keller et al. 2010) and combined heat stress and exercise, both conditions being associated with the rise in arterial plasma [ATP] and muscle temperature (Pearson et al. 2011) (Fig. 6). Therefore, the rise in plasma [ATP] in conditions where oxyhaemoglobin and 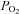 do not decline indicates that increases in erythrocyte-derived ATP release in vivo are most likely an integrative response involving several stimuli.
do not decline indicates that increases in erythrocyte-derived ATP release in vivo are most likely an integrative response involving several stimuli.
Figure 6. Influence of temperature on ATP release and limb tissue vascular conductance regulation.
A, heating whole blood and RBC fraction resulted in significant increases in ATP release, but not in plasma or serum. *Significantly higher than values at 33° C, P < 0.05. †Significantly higher than values at 36° C, P < 0.05. Modified from Kalsi & González-Alonso (2012). B, net leg vasodilatation with passive heat stress and heat stress exercise in vivo in human subjects is strongly related to increases in arterial plasma ATP and muscle temperature (r2 = 0.87; P = 0.001). Reproduced from Pearson et al. (2011).
Despite the uncertainty about the precise stimuli for erythrocyte ATP release in vivo, it is clear that the observed increases in plasma [ATP] during incremental exercise and high intensity constant power exercise can induce profound local increases in limb muscle blood flow and proportional elevations in cardiac output. These changes occur independently of the myriad other vasoactive compounds that also rise during exercise. A dose-dependent increase in limb blood flow has been shown repeatedly with infusion of ATP in the femoral and brachial arteries (Duff et al. 1954; Rogen et al. 1994; González-Alonso et al. 2002; Rosenmeier et al. 2004; Kirby et al. 2008; Mortensen et al. 2011), but not with infusion in the femoral vein (González-Alonso et al. 2008). The ATP infusion-mediated limb tissue vasodilatation occurs without increases in venous plasma or interstitial [ATP] (González-Alonso et al. 2002; Rosenmeier et al. 2004; Mortensen et al. 2009b, 2011), indicating that ATP acts intraluminally via vascular endothelial pathways. The released ATP binds to P2Y purinergic receptors in the vascular endothelium, thereby stimulating the production of endothelial NO, prostaglandins and/or endothelial-derived hyperpolarization factors (EDHFs), which in turn act upon the surrounding vascular smooth muscle cells to cause local and conducted vasodilatation (Ellsworth et al. 2009; Mortensen et al. 2009a). Blockade of NO and prostaglandins formation with combined inhibition of NO synthase and cyclooxygenase has been shown to only partially blunt the increase in limb blood flow and vascular conductance evoked by intra-arterial infusion of ATP (Mortensen et al. 2009a; Crecelius et al. 2011). The lack of complete blockade suggests that other, yet to be discovered endothelial factors might also be involved in ATP induced vasodilatation. Importantly, maximal ATP induced vasodilatation in the leg reaches 8 litres min−1, a value similar to that measured during maximal exercise (Rosenmeier et al. 2004; González-Alonso et al. 2008). Moreover, the vasodilator potency of ATP is much greater than that of its degradative compounds ADP, AMP and adenosine, but similar to UTP (i.e. ATP (100) = UTP (100) ≫ adenosine (5.8) > ADP (2.7) > AMP (1.7)) (Rosenmeier et al. 2008). Both ATP and UTP act via the same purinergic P2Y2 receptors, which are expressed abundantly in the endothelium of microvessels and smooth muscle cells of human limbs (Mortensen et al. 2009b). This supports the notion that smaller increases in plasma [ATP] than plasma [ADP], [AMP] or [adenosine] would be necessary to produce the same increase in limb muscle blood flow and suggests that intravascular ATP exerts its vasodilator effect directly and not via its degradation compounds.
In understanding the role of erythrocyte-derived ATP on local blood flow regulation, it is important to bear in mind that exercise hyperaemia is the net result of the interplay among multiple changes in vasodilator and vasoconstrictor signals/substances in the vascular beds of active muscles (Clifford & Hellsten 2004), the relative contributions of which vary with increases in the amount of muscle mass engaged during exercise (Fig. 2). In this context, intravascular ATP is an attractive mediator signal for the local regulation of skeletal muscle blood flow because not only can it act as a potent vasodilator at rest and when superimposed during exercise, but it also possesses sympatholytic properties in human limb muscle circulations (Rosenmeier et al. 2004, 2008; Kirby et al. 2008). The latter is essential to counteract the concurrent local increases in α-adrenergic vasoconstriction during exercise. Using intra-arterial administration of the drug tyramine to evoke endogenous noradrenaline release from sympathetic nerve endings, we provided the first evidence of the sympatholytic effects of circulating ATP (Rosenmeier et al. 2004); in contrast to adenosine-mediated hyperaemia, ATP infusion-induced leg hyperaemia persisted during co-infusion of tyramine in a similar manner to that observed during exercise. In a subsequent study, Kirby et al. (2008) demonstrated that intravascular ATP modulates both postjunctional α1- and α2-adrenergic vasoconstriction and that this response is graded with the rate of ATP infusion, and thus the magnitude of ATP-induced hyperaemia. In addition, Rosenmeier et al. (2008) showed that neither ADP, nor AMP nor adenosine infusion abolishes tyramine-mediated increases in vasoconstrictor drive. This contrasted with the total blunting of sympathetic vasoconstriction produced by ATP and UTP infusion. Therefore, it also appears that the sympatholytic effects of ATP in the skeletal muscle vasculature are largely mediated via ATP itself, which modulates both postjunctional α1- and α2-adrenergic vasoconstriction depending upon the magnitude of hyperaemia.
Whether erythrocyte-derived ATP plays a role as a controller of total blood flow to the exercising muscle, a local distributor of blood flow within the active muscle or both remain unknown. However, it is clear that the increase in total blood flow drives most of the rise in 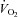 in active muscle and that increases in O2 extraction due to redistribution of blood flow within muscle only accounts for a small part of the haemodynamic response to exercise. This notion is illustrated in Fig. 1 showing that the rise in leg
in active muscle and that increases in O2 extraction due to redistribution of blood flow within muscle only accounts for a small part of the haemodynamic response to exercise. This notion is illustrated in Fig. 1 showing that the rise in leg 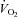 during incremental dynamic knee-extensor exercise to volitional exhaustion is associated with an up to 20-fold elevation in total leg blood flow whereas leg a-v O 2 difference increases by up to 60% compared to resting conditions. ATP infusion into a major human artery increases total limb blood flow during submaximal and maximal in human subjects, yet limb
during incremental dynamic knee-extensor exercise to volitional exhaustion is associated with an up to 20-fold elevation in total leg blood flow whereas leg a-v O 2 difference increases by up to 60% compared to resting conditions. ATP infusion into a major human artery increases total limb blood flow during submaximal and maximal in human subjects, yet limb 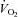 remains unaltered because of a parallel reduction in O2 extraction (Rosenmeier et al. 2004; Calbet et al. 2006). The fact that intra-femoral artery ATP infusion results in a reduction in O2 extraction across the maximally exercising limb (i.e. in a condition where metabolic demand for O2 is unmet; see Fig. 2) suggests an effect of exogenous ATP on less-active muscle fibres and other non-contracting tissues (Calbet et al. 2006). Taken together, these findings indicate that ATP has to act locally at the level of the active muscle microcirculation to be effective in increasing and/or distributing blood flow where it is needed.
remains unaltered because of a parallel reduction in O2 extraction (Rosenmeier et al. 2004; Calbet et al. 2006). The fact that intra-femoral artery ATP infusion results in a reduction in O2 extraction across the maximally exercising limb (i.e. in a condition where metabolic demand for O2 is unmet; see Fig. 2) suggests an effect of exogenous ATP on less-active muscle fibres and other non-contracting tissues (Calbet et al. 2006). Taken together, these findings indicate that ATP has to act locally at the level of the active muscle microcirculation to be effective in increasing and/or distributing blood flow where it is needed.
Summary
Evidence discussed in this review provides strong support for a pivotal role of circulating RBCs in the regulation of the local vascular processes matching O2 supply and demand in active skeletal muscle in humans. In humans, RBCs are proposed to contribute to these local regulatory processes, in part, by releasing the vasodilator and sympatholytic substance ATP into the vascular lumen in response to several metabolic and mechanical stimuli (Fig. 7). The released ATP binds to vascular endothelial P2Y purinergic receptors expressed in the endothelium of microvessels and smooth muscle cells of human limbs. In so doing, it induces profound local and conducted vasodilatation by stimulating endothelial NO, prostaglandins, EDHF production and possibly other endothelial vasoactive signals. In human limbs, ATP appears to exert it local vasodilator and sympatholytic effects directly, and not via its degradation compounds ADP, AMP or adenosine. Local heating is suggested as a therapeutic means of increasing blood flow and oxygen and fuel delivery to human limb tissues by stimulating erythrocyte ATP release and other temperature sensitive vasodilator pathways.
Figure 7. Schematic diagram illustrating how erythrocyte-derived ATP contributes to the local regulation of O2 supply to active skeletal muscle.
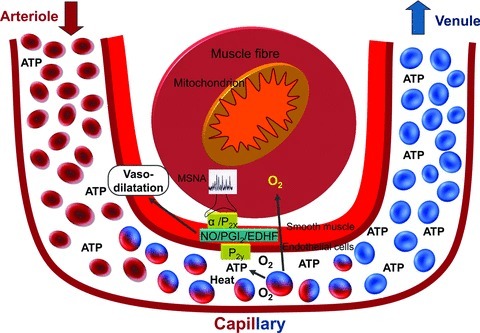
The red blood cells contribute to the regulation of the local processes matching O2 supply and demand, in part by releasing the vasodilator and sympatholytic substance ATP into the vascular lumen. In vivo in exercising limb muscle, ATP is released from red blood cells in response to several metabolic and mechanical stimuli, including reduced oxyhaemoglobin,  , and pH and augmented blood temperature, mechanical deformation of RBCs and elevated shear stress. The released ATP binds to vascular endothelial P2Y purinergic receptors expressed in the endothelium of microvessels and smooth muscle cells of human limbs. In so doing, it induces profound local and conducted vasodilatation by stimulating endothelial NO, prostaglandins, EDHF production and possibly other endothelial vasoactive signals, while also modulating α1- and α2-adrenergic vasoconstrictor influences of increases in muscle sympathetic nerve activity (MSNA) during exercise.
, and pH and augmented blood temperature, mechanical deformation of RBCs and elevated shear stress. The released ATP binds to vascular endothelial P2Y purinergic receptors expressed in the endothelium of microvessels and smooth muscle cells of human limbs. In so doing, it induces profound local and conducted vasodilatation by stimulating endothelial NO, prostaglandins, EDHF production and possibly other endothelial vasoactive signals, while also modulating α1- and α2-adrenergic vasoconstrictor influences of increases in muscle sympathetic nerve activity (MSNA) during exercise.
Future directions
Whilst scientific and circumstantial evidence in humans exposed to CO inhalation and other interventions that drastically alter blood O2 content supports a pivotal role of the erythrocyte in O2 transport, it is as yet unknown whether erythrocyte-derived ATP signalling is essential for the local regulation of total blood flow and its distribution within exercising muscle. Future research should address: (1) the precise time course of the plasma [ATP] responses in relation to the well-established immediate increases in local muscle blood flow during exercise, (2) the relative contribution of different stimuli such as O2 and temperature on erythrocyte derived ATP during exercise, and (3) the precise signalling mechanisms involved in ATP-induced vasodilatation and sympatholysis and their implications for blood flow regulation in contracting muscle across the full range of exercise hyperaemia. Answering these questions requires the development of new methods to measure ATP in real time in humans as well as the development of specific erythrocyte ATP channels/transporters blockers and downstream purinergic receptor antagonists to block ATP-mediated dilatation and examine whether exercise hyperaemia is compromised.
Acknowledgments
I am grateful to all the co-investigators at the Copenhagen Muscle Research Centre (CMRC) (University of Copenhagen) and Centre for Sports Medicine and Human Performance (Brunel University) who made it possible, and fun, to complete some of the studies discussed in this review. The studies conducted at the CMRC were supported by the Danish National Research Foundation, the Copenhagen Hospital System, Novo Nordisk and Lundbeck Foundation. Recent studies at Brunel University were supported by the Gatorade Sport Science Institute.
Glossary
- EDHF
endothelial-derived hyperpolarization factor
- MSNA
muscle sympathetic nerve activity
- RBC
red blood cell
References
- Andersen P, Adams RP, Sjogaard G, Thorboe A, Saltin B. Dynamic knee-extensor as model for study of isolated exercising muscle in humans. J Appl Physiol. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RB, Laughlin MH. Rat muscle blood flows during high-speed locomotion. J Appl Physiol. 1985;59:1322–1328. doi: 10.1152/jappl.1985.59.4.1322. [DOI] [PubMed] [Google Scholar]
- Baron JF, Vicaut E, How X, Develleroy M. Independent role of arterial O2 tension in local control of coronary blood flow. Am J Physiol Heart Circ Physiol. 1990;258:H1388–1394. doi: 10.1152/ajpheart.1990.258.5.H1388. [DOI] [PubMed] [Google Scholar]
- Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovascular Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- Boushel R, Gnaiger E, Calbet JA, González-Alonso J, Wright-Paradis C, Søndergaard H, Ara I, Helge JW, Saltin B. Muscle mitochondrial capacity exceeds maximal oxygen delivery in humans. Mitochondrion. 2011;11:303–307. doi: 10.1016/j.mito.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Mueller PJ, Clifford PS. Sympathetic vasoconstriction in active skeletal muscles during dynamic exercise. J Appl Physiol. 1997;83:1575–1580. doi: 10.1152/jappl.1997.83.5.1575. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Lundby C, Sander M, Robach P, Saltin B, Boushel R. Effects of ATP-induced leg vasodilation on VO2 peak and leg O2 extraction during maximal exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R447–453. doi: 10.1152/ajpregu.00746.2005. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Jensen-Urstad M, van Hall G, Holmberg HC, Rosdahl H, Saltin B. Maximal vascular conductances during whole body upright exercise in humans. J Physiol. 2004;558:319–331. doi: 10.1113/jphysiol.2003.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, Joyner MJ. Local control of skeletal muscle blood flow during exercise: influence of available oxygen. J Appl Physiol. 2011;111:1527–1538. doi: 10.1152/japplphysiol.00895.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Richards JC, Garcia LJ, Woyles WF, Larson DG, Luckasen GJ, Dinenno FA. Mechanisms of ATP-mediated vasodilation in humans: modest role for nitric oxide and vasodilating prostaglandins. Am J Physiol Heart Circ Physiol. 2011;301:H1310–H1310. doi: 10.1152/ajpheart.00469.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff F, Patterson GC, Shepherd JT. A quantitative study of the response to adenosine triphosphate of the blood vessels of the human hand and forearm. J Physiol. 1954;125:581–589. doi: 10.1113/jphysiol.1954.sp005182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour SP, Patel RP, Brandon A, Teng X, Pearson J, Barker H, Ali L, Yuen AH, Smolenski RT, González-Alonso J. Erythrocyte-dependent regulation of human skeletal muscle blood flow: role of varied oxyhemoglobin and exercise on nitrite, S-nitrosohemoglobin, and ATP. Am J Physiol Heart Circ Physiol. 2010;299:H1936–1946. doi: 10.1152/ajpheart.00389.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc. 2004;36:35–41. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology. 2009;24:107–116. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Sprague RS. Regulation of blood flow distribution in skeletal muscle: role of erythrocyte-released ATP. J Physiol. 2012;590:4985–4991. doi: 10.1113/jphysiol.2012.233106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias M, III, Gorman WM, Savage M, Feigl EO. Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol. 2005;288:H1586–H1590. doi: 10.1152/ajpheart.00983.2004. [DOI] [PubMed] [Google Scholar]
- Forrester T. An estimate of adenosine triphosphate release into the venous effluent from exercising human forearm muscle. J Physiol. 1972;224:611–628. doi: 10.1113/jphysiol.1972.sp009915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T, Lind AR. Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J Physiol. 1969;204:347–364. doi: 10.1113/jphysiol.1969.sp008917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, Ognibene FP, Cannon RO., 3rd Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci U S A. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Dalsgaard MK, Osada T, Volianitis S, Dawson EA, Yoshiga CC, Secher NH. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol. 2004;557:331–342. doi: 10.1113/jphysiol.2004.060574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol. 2006;572:295–305. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dufour SP. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol. 2008;586:2405–2417. doi: 10.1113/jphysiol.2008.152058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Olsen B, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B. Heat production in human skeletal muscle at onset of intense dynamic exercise. J Physiol. 2000;524:603–615. doi: 10.1111/j.1469-7793.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol. 2001;530:331–341. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MW, Feigl EO, Buffington CW. Human plasma ATP concentration. Clin Chem. 2007;53:318–325. doi: 10.1373/clinchem.2006.076364. [DOI] [PubMed] [Google Scholar]
- Hanada A, Sander M, González-Alonso J. Human skeletal muscle sympathetic nerve activity, heart rate and limb haemodynamics with reduced blood oxygenation and exercise. J Physiol. 2003;551:635–647. doi: 10.1113/jphysiol.2003.044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y, Nyberg M, Mortensen SP. Contribution of intravascular versus interstitial purines and nitric oxide in the regulation of exercise hyperaemia in humans. J Physiol. 2012;590:5015–5023. doi: 10.1113/jphysiol.2012.234963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WF. Arteriolar oxygen reactivity: where is the sensor? Am J Physiol Heart Circ Physiol. 1987;253:H1120–H1126. doi: 10.1152/ajpheart.1987.253.5.H1120. [DOI] [PubMed] [Google Scholar]
- Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of the erythrocyte in regulating local O2 delivery mediated by haemoglobin oxygenation. Am J Physiol Heart Circ Physiol. 2001;280:H2833–H2839. doi: 10.1152/ajpheart.2001.280.6.H2833. [DOI] [PubMed] [Google Scholar]
- Jeppesen TD, Vissing J, González-Alonso J. Influence of erythrocyte oxygenation and intravascular ATP on resting and exercising skeletal muscle blood flow in humans with mitochondrial myopathy. Mitochondrion. 2012;12:414–422. doi: 10.1016/j.mito.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Jia L, Bonaventura C, Bonaventura J, Stamler J. S-Nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Nauss LA, Warner MA, Warner DO. Sympathetic modulation of blood flow and oxygen uptake in rhythmically contracting human foream muscles. Am J Physiol Heart Circ Physiol. 1992;263:H1073–H1083. doi: 10.1152/ajpheart.1992.263.4.H1078. [DOI] [PubMed] [Google Scholar]
- Kalsi KK, González-Alonso J. Temperature-dependent release of ATP from human erythrocytes: Mechanism for the control of local tissue perfusion. Exp Physiol. 2012;97:419–432. doi: 10.1113/expphysiol.2011.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Sander M, Stallknecht B, Crandall CG. α-Adrenergic vasoconstrictor responsiveness is preserved in the heated human leg. J Physiol. 2010;588:3799–3808. doi: 10.1113/jphysiol.2010.194506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Carlson CL, Dinenno FA. Graded sympatholytic effect of ATP on postjunctional α-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol. 2008;586:4305–4316. doi: 10.1113/jphysiol.2008.154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskolou MD, Roach RC, Calbet JAL, Rådegran G, Saltin B. Cardiovascular responses to dynamic exercise with acute anaemia in humans. Am J Physiol Heart Circ Physiol. 1997;273:H1787–H1793. doi: 10.1152/ajpheart.1997.273.4.H1787. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol. 2012;2:321–447. doi: 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
-
Mortensen SP, Damsgaard R, Dawson EA, Secher NH, González-Alonso J. Restrictions in systemic and locomotor skeletal muscle perfusion, oxygen supply and
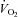 during high-intensity whole-body exercise in humans. J Physiol. 2008;586:2621–2635. doi: 10.1113/jphysiol.2007.149401. [DOI] [PMC free article] [PubMed] [Google Scholar]
during high-intensity whole-body exercise in humans. J Physiol. 2008;586:2621–2635. doi: 10.1113/jphysiol.2007.149401. [DOI] [PMC free article] [PubMed] [Google Scholar] - Mortensen SP, Dawson EA, Yoshiga CC, Dalsgaard MK, Damsgaard R, Secher NH, González-Alonso J. Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. J Physiol. 2005;566:273–285. doi: 10.1113/jphysiol.2005.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, González-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol. 2009a;296:R1140–R1148. doi: 10.1152/ajpregu.90822.2008. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, González-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol. 2007;581:853–861. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, González-Alonso J, Nielsen JJ, Saltin B, Hellsten Y. Muscle interstitial ATP and norepinephrine concentrations in the human leg during exercise and ATP infusion. J Appl Physiol. 2009b;107:1757–1762. doi: 10.1152/japplphysiol.00638.2009. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Thaning P, Nyberg M, Saltin B, Hellsten Y. Local release of ATP into the arterial inflow and venous drainage of human skeletal muscle: insight from ATP determination with the intravascular microdialysis technique. J Physiol. 2011;589:1847–1857. doi: 10.1113/jphysiol.2010.203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch TI, Haidet GC, Orway GA, Longhurst JC, Mitchell JH. Training effects on regional blood flow response to maximal exercise in foxhounds. J Appl Physiol. 1987;62:1724–1732. doi: 10.1152/jappl.1987.62.4.1724. [DOI] [PubMed] [Google Scholar]
- Owusu BY, Stapley R, Patel RP. Nitric oxide formation versus scavenging: the red blood cell balancing act. J Physiol. 2012;590:4993–5000. doi: 10.1113/jphysiol.2012.234906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi CA. Carbon monoxide, oxygen transport and oxygen metabolism. J Hyperbaric Med. 1987;2:27–44. [Google Scholar]
- Pearson J, Low DA, Stöhr E, Kalsi K, Ali L, Barker H, González-Alonso J. Hemodynamic responses to heat stress in the resting and exercising human leg: insight into the effect of temperature on skeletal muscle blood flow. Am J Physiol Regul Integr Comp Physiol. 2011;300:R663–673. doi: 10.1152/ajpregu.00662.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries AR, Heide J, Ley K, Klotz KF, Gaehtgens P. Effect of oxygen tension on regulation of arteriolar diameter in skeletal muscle in situ. Microvasc Res. 1995;49:289–299. doi: 10.1006/mvre.1995.1025. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Frank LR, Haseler LJ. Dynamic knee-extensor and cycle exercise: functional MRI of muscular activity. Int J Sports Med. 1998;19:182–187. doi: 10.1055/s-2007-971901. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise: evidence of limited O2 transport. J Clin Invest. 1995;96:1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Saltin B, González-Alonso Effect of mild carboxy-hemoglobin on exercising skeletal muscle: intravascular and intracellular evidence. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1131–R1139. doi: 10.1152/ajpregu.00226.2002. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol. 1993;75:1911–1926. doi: 10.1152/jappl.1993.75.4.1911. [DOI] [PubMed] [Google Scholar]
- Roach RC, Koskolou MD, Calbet JAL, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol Heart Circ Physiol. 1999;276:H438–H445. doi: 10.1152/ajpheart.1999.276.2.H438. [DOI] [PubMed] [Google Scholar]
- Rogen GA, Smits P, Thien T. Characterisation of ATP-induced vasodilation in the human forearm vascular bed. Circulation. 1994;90:1891–1898. doi: 10.1161/01.cir.90.4.1891. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, González-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmeier JB, Yegutkin GG, González-Alonso J. Activation of ATP/UTP-selective receptors increases blood flow and blunts sympathetic vasoconstriction in human skeletal muscle. J Physiol. 2008;586:4993–5002. doi: 10.1113/jphysiol.2008.155432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876–2003): cycles of revision and new vision. J Appl Physiol. 2004;97:384–392. doi: 10.1152/japplphysiol.01220.2003. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. [Google Scholar]
- Rowell LB, Saltin B, Kiens B, Christiansen NJ. Is peak quadriceps blood flow in humans even higher during exercise in hypoxemia? Am J Physiol Heart Circ Physiol. 1986;251:H1038–H1044. doi: 10.1152/ajpheart.1986.251.5.H1038. [DOI] [PubMed] [Google Scholar]
- Rådegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee-extensor exercise in humans. J Appl Physiol. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Saltin B. Exercise hyperaemia: magnitude and aspects on regulation in humans. J Physiol. 2007;583:819–823. doi: 10.1113/jphysiol.2007.136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Rådegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, MacDonald MJ, Hughson RL. Time course of brachial artery diameter responses to rhythmic handgrip exercise in humans. Cardiovascular Res. 1997;35:125–131. doi: 10.1016/s0008-6363(97)00100-4. [DOI] [PubMed] [Google Scholar]
- Stöhr E, González-Alonso J, Shave R. Left ventricular mechanical limitations to stroke volume in healthy humans during incremental exercise. Am J Physiol Heart Circ Physiol. 2011;301:H478–H487. doi: 10.1152/ajpheart.00314.2011. [DOI] [PubMed] [Google Scholar]
- Seals DR, Johnson DG, Fregosi RF. Hyperoxia lowers sympathetic activity at rest but not during exercise in humans. Am J Physiol Regul Integr Comp Physiol. 1991;260:R873–878. doi: 10.1152/ajpregu.1991.260.5.R873. [DOI] [PubMed] [Google Scholar]
- Shepherd JT. Circulation to skeletal muscle. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, The Cardiovascular System, vol. III, Peripheral Circulation and Organ Blood Flow. Bethesda: American Physiological Society; 1983. pp. 319–370. [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires cystic fibrosis transmembrane conductance regulator activity. Am J Physiol Heart Circ Physiol. 1998;275:H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohaemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- Wan J, Ristenpart WD, Stone HA. Dynamics of shear-induced ATP release from red blood cells. Proc Natl Acad Sci U S A. 2008;86:1662–1666. doi: 10.1073/pnas.0805779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch HG, Bonde-Petersen F, Graham T, Klausen K, Secher N. Effects of hyperoxia on leg blood flow and metabolism during exercise. J Appl Physiol. 1977;42:385–390. doi: 10.1152/jappl.1977.42.3.385. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG, Samburski SS, Mortensen SP, Jalkanen S, González-Alonso J. Intravascular ADP and soluble nucleotidases contribute to acute prothrombotic state during vigorous exercise in humans. J Physiol. 2007;579:553–564. doi: 10.1113/jphysiol.2006.119453. [DOI] [PMC free article] [PubMed] [Google Scholar]


