Abstract
The regulation of blood flow to skeletal muscle involves a complex interaction between several locally formed vasodilators that are produced both in the skeletal muscle interstitium and intravascularly. The gas nitric oxide (NO) and the purines ATP and adenosine, are potent vasodilators that are formed by multiple cell types and released into the skeletal muscle interstitium and in plasma in response to muscle contraction. Cellular sources of ATP and NO in plasma are erythrocytes and endothelial cells, whereas interstitial sources are skeletal muscle cells and endothelial cells. Adenosine originates primarily from extracellular degradation of ATP. During exercise the concentrations of ATP and adenosine increase markedly in the interstitium with smaller increases occurring in plasma, and thus the interstitial concentration during exercise is severalfold higher than in plasma. The concentration of NO metabolites (NOx) in interstitium and plasma does not change during exercise and is similar in the two compartments. Adenosine and NO have been shown to contribute to exercise hyperaemia whereas the role of ATP remains unclear due to lack of specific purinergic receptor blockers. The relative role of intravascular versus interstitial vasodilators is not known but evidence suggests that both compartments are important. In cardiovascular disease, a reduced capacity to form adenosine in the muscle interstitium may be a contributing factor in increased peripheral vascular resistance.

Ylva Hellsten is head of the cardiovascular research group at the Department of Exercise and Sport Sciences, Section for Integrated Physiology, University of Copenhagen. The research group investigates the regulation of skeletal muscle blood flow and skeletal muscle angiogenesis in health and cardiovascular disease. Senior researcher Stefan P. Mortensen, DMSci, is leader of the cardiovascular group at the Centre of Inflammation and Metabolism at Rigshospitalet. He earned his master's degree from the University of Copenhagen and received post-doctoral training with Professor Bengt Saltin in Copenhagen. His main research interest is cardiovascular regulation during exercise and alterations in disease states.
Introduction
In skeletal muscle, several vasoactive compounds, including ATP, adenosine and nitric oxide (NO) contribute to the regulation of skeletal muscle blood flow at rest and during exercise. These vasoactive compounds can originate from several cellular sources and be present both in the intravascular and the extracellular spaces. The cellular origin of ATP, adenosine and NO in the intravascular space is likely to be endothelial cells and erythrocytes, whereas skeletal muscle cells and endothelial cells may be primary sources of these compounds in the muscle interstitial space. Both intravascular and extracellular vasoactive compounds are likely to be important for skeletal muscle blood flow regulation although their relative contributions remain unclear. This review focuses on formation and cellular sources of intravascular versus skeletal muscle interstitial ATP, adenosine and NO in humans. It also discusses evidence for their roles in exercise hyperaemia and how these systems may be altered in cardiovascular disease.
Origin of ATP and adenosine
ATP
Mechanical influence, such as shear stress and compression, can induce release of ATP from most cells, including endothelial cells, erythrocytes and skeletal muscle cells (Sprague et al. 1996; Burnstock, 1999; Buvinic et al. 2009; Mortensen et al. 2011). In the intravascular space, ATP released from erythrocytes in relation to off-loading of oxygen from the haemoglobin molecule has been suggested to contribute to blood flow regulation (Bergfeld & Forrester, 1992; Ellsworth et al. 1995) (Dietrich et al. 2000). The proposed model of release of ATP from erythrocytes is intriguing, in part because it fits the observation that blood flow is more closely related to the amount of O2 bound to haemoglobin than to the amount of O2 dissolved in plasma (i.e.  ) (Roach et al. 1999; González-Alonso et al. 2001, 2002). At rest, hypoxia increases leg blood flow and venous ATP levels, suggesting that this mechanism is operating (Mortensen et al. 2011), but the role of erythrocyte derived ATP during exercise remains uncertain. Inhibition of erythrocyte ATP release by CO (Jagger et al. 2001) does not lower venous plasma ATP levels but increases exercise hyperaemia (González-Alonso et al. 2002). Secondly, ATP release from erythrocytes is dependent on the cystic fibrosis transmembrane conductance regulator (Sprague et al. 1998), but cystic fibrosis patients who lack this regulator, have normal blood flow responses to exercise (Schrage et al. 2005).
) (Roach et al. 1999; González-Alonso et al. 2001, 2002). At rest, hypoxia increases leg blood flow and venous ATP levels, suggesting that this mechanism is operating (Mortensen et al. 2011), but the role of erythrocyte derived ATP during exercise remains uncertain. Inhibition of erythrocyte ATP release by CO (Jagger et al. 2001) does not lower venous plasma ATP levels but increases exercise hyperaemia (González-Alonso et al. 2002). Secondly, ATP release from erythrocytes is dependent on the cystic fibrosis transmembrane conductance regulator (Sprague et al. 1998), but cystic fibrosis patients who lack this regulator, have normal blood flow responses to exercise (Schrage et al. 2005).
The cellular source of ATP in the muscle interstitial space is not fully elucidated. Erythrocytes, which may be considered primary sources of ATP in the blood vessels, are unlikely sources of interstitial ATP as the endothelium has been shown to be a solid barrier for nucleotides (Ballard et al. 1987). A lack of passage of nucleotides over the endothelium is also supported by the finding in humans that infusion of ATP in the femoral artery does not alter interstitial nucleotide and adenosine concentrations (Mortensen et al. 2009a). Instead, likely sources of interstitial ATP are skeletal muscle cells and/or endothelial cells (Fig. 1). Skeletal muscle cells have been shown to release ATP in response to muscle contraction, possibly through pannexin-1 hemichannels (Buvinic et al. 2009) and/or the cystic fibrosis transmembrane conductance regulator (CFTR) (Tu et al. 2010). Capillary endothelial cells may release ATP towards both the blood stream and the interstitium in response to shear stress (Burnstock, 1999), via a mechanism shown to be dependent on ATP synthase (Yamamoto et al. 2007). However, although these mechanisms have been documented in vitro, in vivo evidence is lacking. Sympathetic and motor nerves do not appear to greatly contribute to interstitial ATP during muscle contraction (Li et al. 2003).
Figure 1. Putative mechanisms by which intravascular and interstitial ATP, adenosine and NO contribute to regulation of skeletal muscle blood flow.
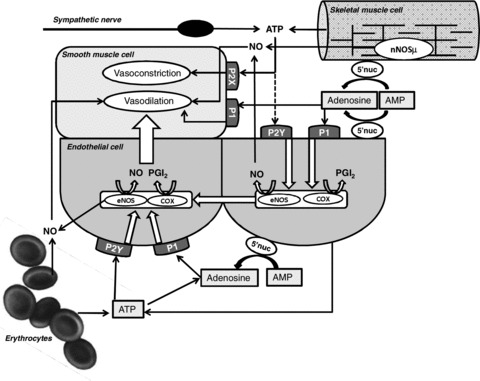
Intravascular ATP released from endothelial cells and red blood cells interact with P2Y receptors located on the luminal side of endothelial cells, which leads to the formation of NO and prostanoids that relaxes adjacent smooth muscle cells. Similarly, intravascular adenosine, generated from AMP via AMP 5′ nucleotidase, may act on P1 receptors to induce vasodilatation via endothelial formation of NO and prostacyclin. Interstitial ATP released from skeletal muscle cells and sympathetic nerves may interact with P2X receptors on smooth muscle cells to induce vasoconstriction but may also be degraded to adenosine and thereby induce vasodilatation. Furthermore, muscle interstitial ATP may potentially induce vasodilatation via action on endothelial P2Y receptors and consequent formation of NO and prostacyclin. Interstitial adenosine induces vasodilatation by interacting with P1 receptors on smooth muscle cells and/or by acting on P1 receptors on the abluminal side of capillary endothelial cells, which leads to dilatation upstream via formation of NO and prostacyclin. Formation of intravascular NO may occur via release from SNOHb in red blood cells, reduction of nitrite or formation in endothelial cells. In the skeletal muscle interstitium, sources of NO are skeletal muscle cells and endothelial cells. Abbbreviations: eNOS, endothelial NO synthase; COX, cyclooxygenase; PGI2, prostacyclin; 5′nuc, 5′-nucleotidase; P1, P2Y, P2X, purinergic receptors.
Adenosine
In the skeletal muscle interstitial space, adenosine originates from extracellular dephosphorylation of AMP via ecto AMP 5′-nucleotidase located on the skeletal muscle and endothelial cell membranes (Fig. 1) (Hellsten & Frandsen, 1997). The origin of interstitial AMP is probably ATP released from skeletal muscle and endothelial cells (Burnstock, 1999; Yamamoto et al. 2007; Buvinic et al. 2009). Intracellular degradation of ATP in the skeletal muscle does not appear to be a likely source as inhibition of nucleoside transport in contracting skeletal muscle cells in culture increases rather than decreases extracellular levels of adenosine, suggesting an uptake rather than release of adenosine during contraction (Lynge & Hellsten, 2000). The source of intravascular adenosine is probably mainly plasma ATP where soluble and ecto nucleotidases on the endothelium degrade ATP (Marcus et al. 2003; Heptinstall et al. 2005; Yegutkin et al. 2007).
Origin of NO
Intravascular NO may originate from endothelial NO synthase (eNOS) present in endothelial cells, via S-nitrosohaemoglobin (SNOHb; Pawloski et al. 2001) and from reduction of nitrite by deoxyhaemoglobin (Hon et al. 2010). In the skeletal muscle interstitium, the sources of NO are likely to be neuronal NOS (nNOS), present primarily in skeletal muscle cells, and eNOS in endothelial cells (Frandsen et al. 1996). The endothelial cell contribution to interstitial NO concentrations would likely originate from the capillary level. Intraarterially infused adenosine has been shown to increase the concentration of NO in the muscle interstitium, suggesting release of NO towards the interstitium from capillary endothelial cells, as NO is unlikely to traverse smooth muscle cells (Nyberg et al. 2010) (Fig. 1). At the arteriolar level, a directional release towards the interstitium would allow for the most localized effect through direct action on the smooth muscle cells, whereas NO released at the capillary level could affect smooth muscle of the upstream terminal arteriole or a nearby arteriole. Release of NO towards the circulation from the capillary level would appear to be less effective considering the short half-life of this molecule unless NO is carried as for example SNOHb or nitrite, which allows for a circulating source of NO.
The other suggested source of NO in the muscle interstitium, the skeletal muscle cell, contains large amounts of nNOS (Bredt et al. 1990) where the predominant isoform is nNOSμ located both in the cytosol and bound to the sarcolemma via the dystrophin complex (Brenman et al. 1996). Isolated skeletal muscle (Balon & Nadler, 1994) and skeletal muscle cells in culture (Silveira et al. 2003) produce and release NO in response to contraction, and thus nNOS in skeletal muscle is likely to be a source of muscle interstitial NO. This notion is further supported by the demonstration that NO derived from nNOSμ is important for functional sympatholysis and is consequently likely to be involved in blood flow regulation (Thomas et al. 2003).
Intravascular and interstitial ATP and adenosine levels
Determinations of plasma levels of adenosine and ATP are crucial for the understanding of the role of these purines in the regulation of skeletal muscle blood flow and to elucidate which physiological stimuli that can induce their release. ATP and adenosine are short-lived substances, which continuously are released, taken up by cells (Forrester, 1972; Milner et al. 1990, 1996; Wang et al. 2005) or degraded by soluble (Yegutkin et al. 2007) and membrane bound (Marcus et al. 2003; Heptinstall et al. 2005) enzymes. The short half-life of ATP and adenosine is essential as it allows for a precise regulation of vascular tone within the muscle, but it imposes a challenge for accurate measurements of these purines. The actual ATP and adenosine levels in the vascular beds, supplying and draining skeletal muscles in humans, therefore remain controversial with resting venous plasma ATP concentrations ranging from 30 to 1000 nm depending on the method and sampling site (Gorman et al. 2007). Similarly, data on the ATP response during exercise are conflicting with some studies reporting unchanged (Rosenmeier et al. 2004; González-Alonso et al. 2006; Dufour et al. 2010) and others increased (González-Alonso et al. 2002; Mortensen et al. 2011) plasma concentrations of ATP with exercise. To overcome the problems with a continuous release and degradation of nucelotides, intravascular microdialysis probes (Fig. 2) have been used to determine ATP and adenosine levels in arterial (Mortensen et al. 2009b, 2011) and venous blood (Riksen et al. 2005; Mortensen et al. 2009b, 2011). Intravascular microdialysis probes allow ATP and adenosine to be separated from cells and degrading enzymes in vivo. With this method, plasma ATP levels of 100–150 nm at rest and of 360–560 nm in arterial and venous blood during exercise (Mortensen et al. 2011) have been reported (Fig. 3). The similar elevation in arterial and venous concentration of ATP during contraction, in combination with the estimated half-life of ATP of <1 s (Mortensen et al. 2011), provides evidence that ATP is continuously released from the arterial to the venous side in the vasculature. Although release of ATP in response to haemoglobin deoxygenation may contribute to capillary and early venous ATP concentrations, other signals for ATP release may be more important at the arterial level. Arterial ATP concentration increase only in the exercising leg and not the resting leg (Mortensen et al. 2011), and thus mechanical compression, which has been shown to increase arterial ATP concentrations (Mortensen et al. 2011), is an important stimulus, whereas shear stress, induced by either passive exercise or arterial infusion of acetylcholine, appears to be less important (Mortensen et al. 2011).
Figure 2. Schematic drawing of a microdialysis probe.

The microdialysis probe consists of thin tubing containing a circular dialysis membrane. The cut-off of the membrane can vary and is selected according to the molecular mass of the compound of interest. The microdialysis probe is positioned in a skeletal muscle or, when positioned in large blood vessels, advanced through catheters into the intraluminal space. The tubing is perfused with a buffer (perfusate) by use of a pump, generally at rates of 0.1–5 μl min−1. For the intravascular probes, low-molecular heparin is added to the perfusate to avoid blood clotting. At the site of the dialysis membrane, exchange occurs between compounds in the interstitial fluid and the perfusate. The outlet buffer, dialysate, is collected and analysed for compounds of interest.
Figure 3. Change in leg blood flow and plasma ATP levels during passive exercise, rhythmic thigh compressions and dynamic exercise.

The change in leg blood flow and arterial and venous ATP concentrations with passive movement, rhythmic thigh compressions and exercise are depicted from baseline conditions. The ATP concentrations were obtained by use of microdialysis placed in the femoral artery and vein. Data are means ± SEM for 6 subjects. *Different from baseline conditions, P < 0.05. Figure modified from Mortensen et al. (2011).
The plasma adenosine concentration at rest are similar to that of ATP, e.g. around 20–50 nm; however, in contrast to ATP, the adenosine levels do not increase in concentration with exercise (Mortensen et al. 2009b), indicating that plasma adenosine is not a major contributor to exercise hyperaemia. Nevertheless, intravascular adenosine formation appears to be increased during exercise as the adenosine concentration is maintained despite the large increases in blood flow that occur during exercise (Mortensen et al. 2009b). Compared to plasma concentrations of purines, interstitial purine levels during exercise are many-fold higher, further supporting a lack of diffusion of ATP and adenosine across the endothelium. Muscle interstitial ATP concentrations are approximately 200–400 nm at rest and increase in relation to increasing exercise intensity and levels of several micromolar have been observed (Fig. 4) (Hellsten et al. 1998; Mortensen et al. 2009b; Hansen et al. 2011). The concentration of adenosine in the muscle interstitium increases from approximately 200 nm at rest, and the concentration increases at a low rate with increasing exercise intensity up to ∼2 μm during intense exercise (Fig. 4) (Hellsten et al. 1998). The fact that muscle interstitial concentrations are so relatively high is intriguing and underscores a potential role for extracellular purines as signalling compounds for various physiological events such as blood flow regulation and angiogenesis (Høier et al. 2010).
Figure 4. Muscle interstitial concentrations of ATP and adenosine and leg blood flow during exercise.

Interstitial concentrations of ATP (open squares) and adenosine (filled circles) were determined in skeletal muscle of seven healthy male subjects by use of microdialysis technique. Leg blood flow was assessed by ultrasound Doppler. Measurements were performed at rest and during bouts of knee-extensor exercise at varying intensities performed in random order. Figure modified from Hellsten et al. (1998).
The concentration of NO in humans in vivo has only indirectly been assessed, primarily by measurements of the stable NO products nitrite and nitrate (NOx), either separately or combined. As concentrations of nitrite and nitrate are the result of formation of NO and reduction of nitrite to NO, the levels are associated with some uncertainties and only provide indirect evidence for NO formation. Resting levels of plasma NOx have been reported to be in the range of 50 to 80 μm (Nyberg et al. 2012) and the levels have been reported to either remain unaltered (Fig. 5) (Nyberg et al. 2012) or to be extracted from plasma by skeletal muscle during exercise (Kingwell et al. 1997; Lewis et al. 1999). Nitrite concentrations, which may provide a more sensitive measure of changes in NO, have been shown to remain unaltered in femoral arterial and venous plasma during exercise (Dufour et al. 2010). As in vitro studies clearly show release of NO by muscle cells in response to contraction (Silveira et al. 2003), the unaltered interstitial NOx levels suggest that the rate of NOx removal is similar to that of NOx formation from NO. NOx removal from the interstitium can occur either through release to the blood stream, cellular uptake, or formation of NO from nitrite within the interstitium. However, based on the measurements of a-v differences of NOx during exercise (Nyberg et al. 2012), release of NOx to the blood stream appears unlikely.
Figure 5. Skeletal muscle interstitial and plasma concentrations of NOx at rest and during exercise.
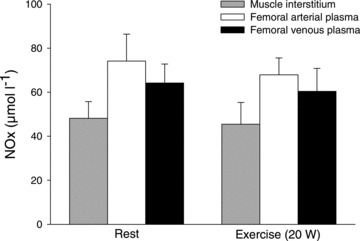
Muscle interstitial fluid, obtained by microdialysis, and plasma samples obtained from the femoral artery and vein were collected from 8 middle aged subjects at rest and during one leg knee extensor exercise. Figure modified from Nyberg et al. (2012).
Vasodilator effects of ATP, adenosine and NO
Infusion of ATP or adenosine into the femoral artery leads to a dose–response increase in femoral arterial blood flow with a maximum level of 8–9 litres min−1, which is similar to what is observed during maximal exercise (Rådegran & Calbet, 2001; Rosenmeier et al. 2004) although a higher dose of adenosine than ATP is required to reach this level (Rosenmeier et al. 2004). Infusion of the NO donor sodium nitroprusside (SNP) into the femoral artery appears to elicit a similar level of vascular conductance as ATP and adenosine although the increase in blood flow with SNP is smaller, approximately 3 litres min−1, due to the parallel large drop in perfusion pressure (Rådegran & Saltin, 1999). The role of physical activity for the vasodilator response to these substances has not been well documented, but inactivity appears to increase the vasodilator response to ATP despite a reduction in muscle P2Y2 receptor content (Mortensen et al. 2012). It therefore appears likely that nucleotidases and/or receptor sensitivity are altered with physical activity and play a key role in purinergic signalling.
Role of interstitial versus intravascular purines in skeletal muscle blood flow
Although indirect evidence for a role for intravascular ATP has been provided through arterial infusion of ATP and measurements of plasma ATP during various conditions (González-Alonso et al. 2002; Rosenmeier et al. 2004), the actual contribution of intravascular ATP to skeletal muscle blood flow regulation in vivo remains unclear. The suggested role of ATP is further supported by the observation that ATP can override sympathetic vasoconstrictor activity (functional sympatholysis) similarly to exercise, although a dissociation between the ability of ATP and exercise to override sympathetic nerve activity has also been reported (Kirby et al. 2011; Thaning et al. 2011). Selective P2 receptor antagonists for use in humans are needed to determine the precise role of ATP in blood flow regulation.
In the muscle interstitium, ATP has mainly been attributed a role in vasoconstriction. During muscle contraction, ATP is released into the extracellular space in proportion to the intensity of contraction (Hellsten et al. 1998; Li et al. 2003). Once released, interstitial ATP may contribute to the exercise pressor reflex via activation of P2X receptors, which leads to sensitization of thin fibre muscle afferents (Li & Sinoway, 2002; Hanna & Kaufman, 2003). This mechanism of action is in accordance with findings from contracting human skeletal muscle demonstrating an intensity-dependent increase in both interstitial ATP and noradrenaline (Mortensen et al. 2009a). As the pressor reflex opposes the blood pressure reducing effect of the profound vasodilatation that occurs with exercise, the reflex activation induced by interstitial ATP is likely to be important for maintaining an adequate perfusion pressure across the contracting muscle.
It should also be emphasized that, although mainly a vasoconstrictor role of interstitial ATP has been described so far, a vasodilator effect of extraluminal ATP, primarily via degradation to adenosine, has been shown in the cheek pouch of hamsters (Duza & Sarelius, 2003). This dilatory effect could be mediated through formation of NO and other vasodilators (Nyberg et al. 2010). Furthermore, the potential role of interstitial ATP as a mediator of functional sympatholysis remains unexplored. It is an attractive possibility that a single substance could stimulate afferent fibres to increase sympathetic nerve activity, and at the same time locally impair sympathetic vasoconstrictor activity and stimulate the formation of vasodilator substances within the contracting muscle.
Inhibition of adenosine receptors has been shown to reduce exercise hyperaemia in the leg by 15–20% (Rådegran & Calbet, 2001; Mortensen et al. 2009b), suggesting that adenosine is essential for regulation of skeletal muscle blood flow. The observation that adenosine increases in the muscle interstitial fluid at a rate associated with the intensity of contraction (Hellsten et al. 1998; Costa et al. 2000), whereas exercise does not influence plasma adenosine concentrations (Mortensen et al. 2009b), does indicate that it is mainly interstitial adenosine that is responsible for the adenosine component of exercise hyperaemia. The vasodilator effect of interstitial adenosine could occur through direct activation of adenosine receptors on smooth muscle cells (Herlihy et al. 1976; Marshall, 2007) and/or by activation of receptors on the abluminal side of endothelial cells (Ray et al. 2002; Nyberg et al. 2010) and on skeletal muscle cells (Nyberg et al. 2010), as these cell types are likely to be responsible for the formation of NO and prostacyclin induced by infusion of adenosine into the interstitium (Nyberg et al. 2010). In human skeletal muscle, the A1, the A2A and the A2B receptors have been found present on endothelial and smooth muscle cells (Lynge & Hellsten, 2000), but their respective roles for adenosine induced vasodilatation in human skeletal muscle have not been elucidated.
Studies in which NO synthase has been inhibited in parallel with at least one other vasodilator system have clearly shown that NO contributes to exercise hyperaemia in humans, although it does not appear to be an essential vasodilator (Hillig et al. 2003; Mortensen et al. 2007). The finding that NOx in plasma and the muscle interstitium remain unaltered during exercise should probably not be interpreted to contradict a role for NO in exercise hyperaemia. The direct measurements of NO formation in vitro that show that NO is released upon muscle contraction (Silveira et al. 2003) suggest that a more direct method for determination of NO in vivo could reveal increased production also in humans.
Adenosine and NO formation in essential hypertension
We have recently shown that blood flow to contracting muscles in the leg is lower in humans with essential hypertension (Nyberg et al. 2012). This attenuated response to exercise was associated with a lower increase in interstitial adenosine, but not a reduced vasodilator response to arterially infused adenosine nor to NO mediated vasodilatation (Hellsten et al. 2012). Interestingly, in the same studies, 8 weeks of aerobic high-intensity training normalized the exercise induced increase in interstitial adenosine but did not affect eNOS levels (Nyberg et al. 2012). These observations, combined with a report of a reduced interstitial concentration of ATP during muscle contraction after a period of exercise training in individuals with mild hypertension (Hansen et al. 2011), suggest that alterations in the formation of interstitial adenosine and ATP may contribute to the impaired leg exercise hyperaemia observed in essential hypertension.
Conclusion
The marked increase in blood flow to the muscle which occurs when the muscle tissue is activated, is associated with an increase in both intravascular and muscle interstitial ATP and adenosine concentrations. A role for adenosine in exercise hyperaemia has been demonstrated, but the importance of ATP remains uncertain because of the lack of selective antagonists for human use. Thus, although the increase in these purines in the muscle interstitium is many-fold greater than in plasma, the precise contribution and role of intravascular versus interstitial ATP and adenosine to the regulation of skeletal muscle blood flow remains unclear. Although skeletal muscle cells and erythrocytes clearly release NO during conditions of muscle activity, the concentration of NO metabolites remains unaltered in the human muscle interstitium as well as in plasma during exercise. This suggests that removal of NO metabolites is similar to NO formation or that measurements of NO metabolites may not be a sufficiently sensitive method for the detection of small changes in NO production. Sensitive methodology for direct measurements of NO in humans in vivo is thus warranted. Cardiovascular disease appears to be associated with a reduced increase in interstitial adenosine with exercise but not in the vasodilator response to infused adenosine, suggesting that poor capacity to form adenosine rather than a decreased sensitivity to adenosine may contribute to the increased peripheral resistance in these individuals.
References
- Ballard HJ, Cotterrell D, Karim F. Appearance of adenosine in venous blood from the contracting gracilis muscle and its role in vasodilatation in the dog. J Physiol. 1987;387:401–413. doi: 10.1113/jphysiol.1987.sp016580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;77:2519–2521. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvinic S, Almarza G, Bustamante M, Casas M, López J, Riquelme M, Sáez JC, Huidobro-Toro JP, Jaimovich E. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J Biol Chem. 2009;284:34490–34505. doi: 10.1074/jbc.M109.057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F, Heusinkveld J, Ballog R, Davis S, Biaggioni I. Estimation of skeletal muscle interstitial adenosine during forearm dynamic exercise in humans. Hypertension. 2000;35:1124–1128. doi: 10.1161/01.hyp.35.5.1124. [DOI] [PubMed] [Google Scholar]
- Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG., Jr Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol. 2000;278:H1294–H1298. doi: 10.1152/ajpheart.2000.278.4.H1294. [DOI] [PubMed] [Google Scholar]
- Dufour SP, Patel RP, Brandon A, Teng X, Pearson J, Barker H, Ali L, Yuen AH, Smolenski RT, Gonzalez-Alonso J. Erythrocyte dependent regulation of human skeletal muscle blood flow: role of varied oxyhemoglobin and exercise on nitrite, S-nitrosohemoglobin and ATP. Am J Physiol Heart Circ Physiol. 2010;299:H1936–1946. doi: 10.1152/ajpheart.00389.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duza T, Sarelius IH. Conducted dilations initiated by purines in arterioles are endothelium dependent and require endothelial Ca2+ Am J Physiol Heart Circ Physiol. 2003;285:H26–H37. doi: 10.1152/ajpheart.00788.2002. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- Forrester T. An estimate of adenosine triphosphate release into the venous effluent from exercising human forearm muscle. J Physiol. 1972;224:611–628. doi: 10.1113/jphysiol.1972.sp009915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen U, Lopez-Figueroa M, Hellsten Y. Localization of nitric oxide synthase in human skeletal muscle. Biochem Biophys Res Commun. 1996;227:88–93. doi: 10.1006/bbrc.1996.1472. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol. 2006;572:295–305. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol. 2001;530:331–341. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MW, Feigl EO, Buffington CW. Human plasma ATP concentration. Clin Chem. 2007;53:318–325. doi: 10.1373/clinchem.2006.076364. [DOI] [PubMed] [Google Scholar]
- Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol. 2003;94:1437–1445. doi: 10.1152/japplphysiol.01011.2002. [DOI] [PubMed] [Google Scholar]
- Hansen AH, Nyberg M, Bangsbo J, Saltin B, Hellsten Y. Exercise training alters the balance between vasoactive compounds in skeletal muscle of individuals with essential hypertension. Hypertension. 2011;58:943–949. doi: 10.1161/HYPERTENSIONAHA.111.176529. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Frandsen U. Adenosine formation in contracting primary rat skeletal muscle cells and endothelial cells in culture. J Physiol. 1997;504:695–704. doi: 10.1111/j.1469-7793.1997.695bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y, Jensen L, Thaning P, Nyberg M, Mortensen SP. Impaired formation of vasodilators in peripheral tissue in essential hypertension is normalized by exercise traiing, role of adenosine and prostanoids. J Hypertension. 2012 doi: 10.1097/HJH.0b013e328356dd57. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Maclean D, Rådegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98:6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- Heptinstall S, Johnson A, Glenn J, White A. Adenine nucleotide metabolism in human blood – important roles for leukocytes and erythrocytes. J Thromb Haemost. 2005;3:2331–2339. doi: 10.1111/j.1538-7836.2005.01489.x. [DOI] [PubMed] [Google Scholar]
- Herlihy JT, Bockman EL, Berne RM, Rubio R. Adenosine relaxation of isolated vascular smooth muscle. Am J Physiol. 1976;230:1239–1243. doi: 10.1152/ajplegacy.1976.230.5.1239. [DOI] [PubMed] [Google Scholar]
- Hillig T, Krustrup P, Fleming I, Osada T, Saltin B, Hellsten Y. Cytochrome P450 2C9 plays an important role in the regulation of exercise-induced skeletal muscle blood flow and oxygen uptake in humans. J Physiol. 2003;546:307–314. doi: 10.1113/jphysiol.2002.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høier B, Olsen K, Nyberg M, Bangsbo J, Hellsten Y. Contraction-induced secretion of VEGF from skeletal muscle cells is mediated by adenosine. Am J Physiol Heart Circ Physiol. 2010;299:H857–H862. doi: 10.1152/ajpheart.00082.2010. [DOI] [PubMed] [Google Scholar]
- Hon YY, Sun H, Dejam A, Gladwin MT. Characterization of erythrocytic uptake and release and disposition pathways of nitrite, nitrate, methemoglobin, and iron-nitrosyl hemoglobin in the human circulation. Drug Metab Dispos. 2010;38:1707–1713. doi: 10.1124/dmd.110.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol. 2001;280:H2833–H2839. doi: 10.1152/ajpheart.2001.280.6.H2833. [DOI] [PubMed] [Google Scholar]
- Kingwell BA, Sherrard B, Jennings GL, Dart AM. Four weeks of cycle training increases basal production of nitric oxide from the forearm. Am J Physiol Heart Circ Physiol. 1997;272:H1070–H1077. doi: 10.1152/ajpheart.1997.272.3.H1070. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Modulation of postjunctional α-adrenergic vasoconstriction during exercise and exogenous ATP infusions in ageing humans. J Physiol. 2011;589:2641–2653. doi: 10.1113/jphysiol.2010.204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TV, Dart AM, Chin-Dusting JPF, Kingwell BA. Exercise training increases basal nitric oxide production from the forearm in hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 1999;19:2782–2787. doi: 10.1161/01.atv.19.11.2782. [DOI] [PubMed] [Google Scholar]
- Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol. 2002;283:H2636–H2643. doi: 10.1152/ajpheart.00395.2002. [DOI] [PubMed] [Google Scholar]
- Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol. 2003;95:577–583. doi: 10.1152/japplphysiol.00185.2003. [DOI] [PubMed] [Google Scholar]
- Lynge J, Hellsten Y. Distribution of adenosine A1, A2A and A2B receptors in human skeletal muscle. Acta Physiol Scand. 2000;169:283–290. doi: 10.1046/j.1365-201x.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- Marcus AJ, Broekman MJ, Drosopoulos JHF, Islam N, Pinsky DJ, Sesti C, Levi R. Heterologous cell-cell interactions: thromboregulation, cerebroprotection and cardioprotection by CD39 (NTPDase-1) J Thromb Haemost. 2003;1:2497–2509. doi: 10.1111/j.1538-7836.2003.00479.x. [DOI] [PubMed] [Google Scholar]
- Marshall JM. The roles of adenosine and related substances in exercise hyperaemia. J Physiol. 2007;583:835–845. doi: 10.1113/jphysiol.2007.136416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner P, Bodin P, Burnstock G. Long-term guanethidine sympathectomy suppresses flow-induced release of ATP and endothelin from endothelial cells isolated from adult rat aorta. J Vasc Res. 1996;33:139–145. doi: 10.1159/000159142. [DOI] [PubMed] [Google Scholar]
- Milner P, Kirkpatrick KA, Ralevic V, Toothill V, Pearson J, Burnstock G. Endothelial cells cultured from human umbilical vein release ATP, substance P and acetylcholine in response to increased flow. Proc Biol Sci. 1990;241:245–248. doi: 10.1098/rspb.1990.0092. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, González-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol. 2007;581:853–861. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez-Alonso J, Nielsen JJ, Saltin B, Hellsten Y. Muscle interstitial ATP and norepinephrine concentrations in the human leg during exercise and ATP infusion. J Appl Physiol. 2009a;107:1757–1762. doi: 10.1152/japplphysiol.00638.2009. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Mørkeberg J, Thaning P, Hellsten Y, Saltin B. Two weeks of muscle immobilization impairs functional sympatholysis, but increases exercise hyperemia and the vasodilatory responsiveness to infused ATP. Am J Physiol Heart Circ Physiol. 2012;302:H2074–H2082. doi: 10.1152/ajpheart.01204.2011. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension. 2009b;53:993–999. doi: 10.1161/HYPERTENSIONAHA.109.130880. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Thaning P, Nyberg M, Saltin B, Hellsten Y. Local release of ATP into the arterial inflow and venous drainage of human skeletal muscle: insight from ATP determination with the intravascular microdialysis technique. J Physiol. 2011;589:1847–1857. doi: 10.1113/jphysiol.2010.203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Jensen LG, Thaning P, Hellsten Y, Mortensen SP. Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: effect of high-intensity aerobic training. J Physiol. 2012;590:1481–1494. doi: 10.1113/jphysiol.2011.225136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Mortensen SP, Thaning P, Saltin B, Hellsten Y. Interstitial and plasma adenosine stimulate nitric oxide and prostacyclin formation in human skeletal muscle. Hypertension. 2010;56:1102–1108. doi: 10.1161/HYPERTENSIONAHA.110.161521. [DOI] [PubMed] [Google Scholar]
- Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- Rådegran G, Calbet JA. Role of adenosine in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand. 2001;171:177–185. doi: 10.1046/j.1365-201x.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- Rådegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol. 1999;276:H1951–H1960. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- Ray CJ, Abbas MR, Coney AM, Marshall JM. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol. 2002;544:195–209. doi: 10.1113/jphysiol.2002.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riksen NP, van Ginneken EE, van den Broek PH, Smits P, Rongen GA. In vivo evidence against a role for adenosine in the exercise pressor reflex in humans. J Appl Physiol. 2005;99:522–527. doi: 10.1152/japplphysiol.00108.2005. [DOI] [PubMed] [Google Scholar]
- Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol Heart Circ Physiol. 1999;276:H438–H445. doi: 10.1152/ajpheart.1999.276.2.H438. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, González-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Wilkins BW, Dean VL, Scott JP, Henry NK, Wylam ME, Joyner MJ. Exercise hyperemia and vasoconstrictor responses in humans with cystic fibrosis. J Appl Physiol. 2005;99:1866–1871. doi: 10.1152/japplphysiol.00616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira LR, Pereira-Da-Silva L, Juel C, Hellsten Y. Formation of hydrogen peroxide and nitric oxide in rat skeletal muscle cells during contractions. Free Radic Biol Med. 2003;35:455–464. doi: 10.1016/s0891-5849(03)00271-5. [DOI] [PubMed] [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol Heart Circ Physiol. 1996;271:H2717–H2722. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol. 1998;275:H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- Thaning P, Bune LT, Zaar M, Saltin B, Rosenmeier JB. Functional sympatholysis during exercise in patients with type 2 diabetes with intact response to acetylcholine. Diabetes Care. 2011;34:1186–1191. doi: 10.2337/dc10-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Shaul PW, Yuhanna IS, Froehner SC, Adams ME. Vasomodulation by skeletal muscle-derived nitric oxide requires α-syntrophin-mediated sarcolemmal localization of neuronal nitric oxide synthase. Circ Res. 2003;92:554–560. doi: 10.1161/01.RES.0000061570.83105.52. [DOI] [PubMed] [Google Scholar]
- Tu J, Le G, Ballard HJ. Involvement of the cystic fibrosis transmembrane conductance regulator in the acidosis-induced efflux of ATP from rat skeletal muscle. J Physiol. 2010;588:4563–4578. doi: 10.1113/jphysiol.2010.195255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Olivecrona G, Gotberg M, Olsson ML, Winzell MS, Erlinge D. ADP acting on P2Y13 receptors is a negative feedback pathway for ATP release from human red blood cells. Circ Res. 2005;96:189–196. doi: 10.1161/01.RES.0000153670.07559.E4. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Shimizu N, Obi S, Kumagaya S, Taketani Y, Kamiya A, Ando J. Involvement of cell surface ATP synthase in flow-induced ATP release by vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1646–H1653. doi: 10.1152/ajpheart.01385.2006. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG, Samburski SS, Mortensen SP, Jalkanen S, Gonzalez-Alonso J. Intravascular ADP and soluble nucleotidases contribute to acute prothrombotic state during vigorous exercise in humans. J Physiol. 2007;579:553–564. doi: 10.1113/jphysiol.2006.119453. [DOI] [PMC free article] [PubMed] [Google Scholar]


