Abstract
Left ventricular (LV) rotation occurs due to contraction of obliquely oriented myocardial fibres. Left ventricular twist (LVT) results from rotation of the apex and base in opposite directions. Although LVT is altered in various cardiac diseases, physiological factors that affect LVT remain incompletely understood. Isometric handgrip testing (IHGT), a well-established laboratory-based technique to increase LV afterload, was performed for 3 min at 40% maximum force generation in healthy human subjects (n = 18, mean age 29.7 ± 2.7 years). Speckle-tracking echocardiography was used to measure LV volumes, LV apical and basal rotation, peak systolic LVT and peak early diastolic untwisting rate (UTR) at rest and at peak IHGT. IHGT led to significant increase in systemic blood pressure (systolic, 120.6 ± 9.7 vs. 155.6 ± 14.5 mmHg, P < 0.001; diastolic, 67.5 ± 6.4 vs. 94.1 ± 21.1 mmHg, P < 0.001) and LV end-systolic volume (44.2 ± 7.8 vs. 50.5 ± 10.8 ml, P = 0.005), as well as a significant increase in heart rate (62.8 ± 11.7 vs. 84.7 ± 13.8 beats min−1; P < 0.001). IHGT produced a significant acute reduction in LV stroke volume (63.9 ± 12.0 vs. 49.4 ± 7.8 ml, P < 0.001). In this setting, there was a significant decrease in peak systolic apical rotation (11.9 ± 3.0 vs. 8.6 ± 2.2 deg, P < 0.001) and a resultant 25% decrease in peak systolic LVT (16.6 ± 2.8 vs. 12.5 ± 2.8 deg, P < 0.001). The magnitude of peak early diastolic UTR did not change (−114.5 ± 26.4 vs. −110.6 ± 39.8 deg s−1, P = 0.71). Peak systolic apical rotation and LVT decrease during IHGT in healthy humans. This impairment of LV twist mechanics may in part underlie the LV dysfunction that can occur in the clinical context of acute increase in afterload.
Key points
Left ventricular twist (LVT) results from rotation of the LV apex and base in opposite directions.
Although LVT is altered in various cardiac diseases, physiological factors that affect LVT remain incompletely understood.
In an experimental model involving healthy humans, isometric handgrip testing (IHGT) was performed to produce a clinically relevant form of LV afterload increase. The impact of IHGT on LV twist mechanics was assessed using speckle-tracking echocardiography.
IHGT produced significant increase in systemic blood pressure and acute reduction in LV stroke volume. In this setting, LV apical rotation and LVT significantly decreased.
Impairment of LV twist mechanics may in part underlie the LV dysfunction that can occur in the clinical context of acute increase in afterload.
Introduction
Left ventricular (LV) rotation occurs due to contraction of obliquely oriented myocardial fibres and is characterized by rotation of the apex and base in opposite directions (Streeter et al. 1969; Sengupta et al. 2008). When viewed from the apical perspective, there is counterclockwise rotation of the apex and clockwise rotation of the base around the LV long axis. The apex-to-base difference in LV rotation is LV twist (LVT) (Mor-Avi et al. 2011). Although the importance of LVT in the settings of human performance (exercise) and cardiovascular disease is recognized (Stuber et al. 1999; Takeuchi et al. 2007; Sade et al. 2008; Stohr et al. 2011), physiological factors that affect LVT remain incompletely understood (Russel et al. 2009). Speckle-tracking echocardiography (STE) has emerged as a technology to make the study of LVT more accessible and feasible, and compares favourably to the reference standard of cardiac magnetic resonance imaging (Helle-Valle et al. 2005; Notomi et al. 2005).
Using STE, we previously demonstrated that LVT is a preload-dependent phenomenon in healthy humans (Weiner et al. 2010a). In terms of afterload dependence, animal studies consistently demonstrate that an acute increase in afterload results in decreased LVT (Gibbons Kroeker et al. 1995; MacGowan et al. 1996; Dong et al. 1999). In contrast, limited human data derived from the study of transplanted hearts suggest that LVT may not be affected by acute afterload manipulations (Hansen et al. 1991; Moon et al. 1994). More recently, a human investigation showed that nitrosprusside infusion resulted in an increase in LVT (Park et al. 2010) and similar results were observed in children after interventional treatment of coarctation of the aorta (Laser et al. 2009).
To our knowledge, the impact of acute afterload increase on LVT in healthy human hearts has not been previously studied. Isometric handgrip testing (IHGT) produces an increase in mean arterial pressure (Hayashi et al. 1991) and thereby represents a safe and feasible method of imparting an acute increase in LV afterload. We hypothesized that the afterload challenge inherent in IHGT would result in a significant decrease in LVT. To assess this hypothesis, we utilized STE to study LV mechanics in healthy human subjects performing IHGT.
Methods
Ethical approval and study population
Healthy subjects were recruited from a hospital-wide email distribution advertisement at the Massachusetts General Hospital. Subjects were eligible if they were between ages 18 and 45 years and had a body mass index (BMI) of 18–29 kg m−2. Exclusion criteria included any condition thought to impact ventricular function including but not limited to a history of myocardial infarction, coronary heart disease, cardiomyopathy, hypertension, aortic stenosis, valvular regurgitation greater than mild in severity or significant prior exercise training. Ethical approval for this study conformed to the standards of the Declaration of Helsinki. The study protocol was approved by the Partners Healthcare Institutional Review Board before study initiation. Written informed consent was obtained from all subjects.
Clinical assessment
The following clinical data were obtained on all subjects: age (years), height (m), weight (kg), and current/past medication use. Body surface area (BSA) was calculated using the Mosteller formula (Mosteller, 1987). Initial blood pressure was measured using a mercury column sphygmomanometer and an appropriately sized blood pressure cuff with the subject in the supine position after at least 10 min of quiet rest (at the completion of baseline echocardiography).
Experimental model for afterload increase
IHGT is a well-established laboratory-based technique to increase mean arterial pressure, heart rate, and muscle sympathetic nerve activity (Seals et al. 1991; Fu et al. 2002). IHGT was chosen as the haemodynamic perturbation in this study for logistical and scientific reasons. First, our inclusion of healthy humans imposes logistical limitations with regard to the invasiveness and manipulation that can be rightfully imposed on the subjects. IHGT is a safe, feasible, and validated method to produce acute afterload challenge and was therefore the perturbation used in this study. Second, IHGT is similar to the physiological afterload challenge that humans encounter clinically, in either exercise training (Lepley et al. 2010) or disease states (Rajagopal et al. 2007; Shingu et al. 2009).
In the current study, IHGT was performed using a commercially available digital hand-held dynamometer (North Coast Medical, Gilroy, CA, USA). To determine the maximum force generation, each subject performed three repetitive 1 s maximum-effort handgrip tests with the hand-held dynamometer in the right hand. The average value of the three maximum grip strength tests was computed and reported in newtons. In the situation where one of the three maximal efforts deviated from the peak effort by more than 25%, this value was excluded and the mean was derived from the remaining two values. Each subject then performed an endurance hand grip test at 40% maximum effort for a period of 3 min. This effort intensity was chosen in order to maximize fatigue but also to increase the likelihood that subjects would maintain the target effort level for the 3 min period and levels of sustained IHGT force above 40% maximum have not been shown to result in additional increases in blood pressure (Seals et al. 1993). The individualized target force and real-time measurement of instantaneous force generation were displayed on a computer monitor to guide the 3 min effort. Subjects were instructed to breath regularly during IHGT to prevent effort-associated Valsalva physiology. Blood pressure and heart rate were measured every 1 min during IHGT and for 3 min into the recovery period. Echocardiography, as described below, was performed at rest prior to IHGT and during the peak-IHGT period, which was defined as the final minute of IHGT.
Standard two-dimensional echocardiography
Transthoracic echocardiography was performed using a commercially available system (Vivid-I, GE Healthcare, Milwaukee, WI, USA) with a 1.9–3.8 mHz phased-array transducer. Subjects were studied after >5 min of quiet rest at baseline and again during the final 1 min of the 3 min IHGT. Two-dimensional (2D), pulsed-Doppler and colour tissue Doppler imaging were performed from standard parasternal and apical positions with 2D frame rates of 60–100 frames s−1 and tissue Doppler frame rates >100 frames s−1. For each subject we aimed to use the same frame rate for apical and basal 2D short-axis images to facilitate subsequent rotational analysis with STE. The peak-IHGT imaging protocol included the following: 2D parasternal short axis (base and apex), 2D apical four-chamber, pulsed-Doppler of mitral inflow and LV outflow tract, and tissue Doppler at the lateral and septal mitral annulus. All data were stored digitally, and off-line data analysis was performed (EchoPac, v. 7, GE Healthcare).
Cardiac parameters were measured in accordance with current guidelines (Lang et al. 2005). LV end-diastolic volume, LV end-systolic volume and LV ejection fraction were calculated from planar tracings of the LV endocardial border in the apical four-chamber view. LV length was measured in the apical four-chamber view and was defined as the end-diastolic length from the mitral valve hinge point plane to the most distal endocardium at the LV apex. Stroke volume was calculated as cross-sectional areaLVOT× VTILVOT, where cross-sectional area = π(radiusLVOT)2, LVOT is left ventricular outflow tract, and VTI is velocity–time integral. Cardiac output was calculated as the product of stroke volume and heart rate. Resting heart rates were obtained from the final loop of the resting echocardiogram and peak heart rate was defined as the highest heart rate during an image obtained during peak IHGT. Aortic valve opening/closure and mitral valve opening were measured from pulsed-wave Doppler images.
Peak systolic, early diastolic and late diastolic tissue velocities were obtained at the basal septal and lateral mitral annulus and were measured off-line from two-dimensional colour-coded tissue Doppler images and are reported as the average of three consecutive cardiac cycles. Longitudinal systolic strain measurements were made using speckle tracking analysis in the apical four chamber view, and the mean from six myocardial segments (basal septum, mid septum, apical septum, apical lateral, mid lateral and basal lateral) was reported.
Left ventricular rotation, twist and untwisting rate
For the purpose of LV rotation assessment, short-axis imaging standardization within and across subjects was maximized using the following criteria (Weiner et al. 2010a,b). The basal level was defined as the highest basal imaging plane at which uniform full thickness myocardium was observed surrounding the mitral valve at end-systole. As the location of apical imaging acquisition has been shown to confer significant variability in the measurement of apical rotation (van Dalen et al. 2008), we carefully standardized apical imaging. Specifically, from multiple apical acquisitions distal to the papillary muscles, the apical level was chosen as the imaging plane with no visible papillary muscles that most closely approximated an end-diastolic ratio of LV cavity diameter to total LV diameter (cavity + wall thickness) of 0.5 (Weiner et al. 2010a,b).
Speckle tracking analysis was used to measure LV rotation, LV twist (LVT) and untwisting rate (UTR). The highest-quality 2D basal and apical images that met the above pre-specified criteria were selected and the endocardium was traced. A full thickness myocardial region of interest (ROI) was selected. The software automatically segmented the LV short axis into six segments and selected suitable speckles for tracking. The reliability of tracking was confirmed by the reliability parameter of the system (V = valid tracking; X = unacceptable tracking). When the software signalled poor tracking efficiency, the observer readjusted the endocardial trace line and/or ROI width until an acceptable tracking score could be obtained. LV rotation at the basal and apical short-axis planes was determined as the average angular displacement of six myocardial segments. Curves of basal and apical LV rotation, LVT and UTR were automatically generated by the software (EchoPac, v. 7, GE Healthcare). Peak systolic LVT was calculated as the maximum instantaneous difference between peak systolic apical and basal rotation. The timing of peak systolic LVT was determined as a percentage of the systolic ejection period. Peak early diastolic UTR was defined as the peak untwisting velocity occurring in early diastole. The magnitude and timing of peak basal UTR and peak apical UTR were recorded from the untwisting curves. The time difference between peak basal UTR and peak apical UTR was calculated as: time peak basal UTR – time peak apical UTR. Therefore, a negative value indicates that peak apical UTR occurred after peak basal UTR and positive value that peak apical UTR occurred before peak basal UTR. LV rotation, LVT and UTR are reported as absolute values. Intra- and inter-observer variability in our laboratory for LV rotation, peak systolic LVT and peak early diastolic UTR have been previously examined and published (Weiner et al. 2010a,b).
Statistical analysis
Measurements are presented as means ± standard deviations. Differences in physiological variables during IHGT were assessed using repeated measures ANOVA. Baseline and peak-IHGT measurements were assessed for normality using the Shapiro–Wilk test and comparisons were then performed using Student's paired t test for continuous variables. A P value of <0.05 was considered significant.
Results
Subject characteristics and structural echocardiographic measurements
Twenty-two subjects completed the full protocol. Four subjects were excluded for technical factors that precluded complete LV rotation and LVT/UTR analysis: 2D image quality not adequate for STE at peak-IHGT (n = 2); frame rate differences between rest and peak-IHGT (n = 1); and dislodgement of ECG electrodes at peak-IHGT (n = 1). Therefore, 18 subjects (male = 15, female = 3) aged 29.7 ± 2.7 years were included in the final analysis. Baseline (resting) subject characteristics and echocardiographic measurements are shown in Table 1. All individuals had resting vital signs that were within normal limits and had structurally normal hearts. No subjects were taking prescription medications at the time of the study.
Table 1.
Baseline (resting) subject characteristics and echocardiographic measurements
| Height (m) | 1.7 ± 0.1 |
| Weight (kg) | 73.7 ± 9.3 |
| Body surface area (m2) | 1.9 ± 0.2 |
| Systolic blood pressure (mmHg) | 120.6 ± 9.7 |
| Diastolic blood pressure (mmHg) | 67.5 ± 6.4 |
| Heart rate (beats min−1) | 62.8 ± 11.7 |
| LV length (mm) | 79.6 ± 0.5 |
| LV end-diastolic volume (ml) | 110.8 ± 20.1 |
| LV end-systolic volume (ml) | 44.2 ± 7.8 |
| End-diastolic LV internal diameter (mm) | 48.1 ± 3.2 |
| Interventricular septum thickness (mm) | 8.3 ± 1.0 |
| Posterior wall thickness (mm) | 8.5 ± 0.9 |
| LV mass (g) | 147.3 ± 22.7 |
| Left atrial anterior-posterior dimension (mm) | 34.0 ± 2.3 |
Data presented as means ± SD.
Effects of isometric handgrip testing
Cardiac and haemodynamic parameters
One-second maximal force IHGT and 3 min of sustained IHGT at 40% maximum effort was successfully completed in all 18 subjects used in the analysis. The mean right hand maximum force generation was 397.7 ± 89.0 N. The blood pressure response to IHGT was similar in each subject. Specifically, systolic and diastolic blood pressures increased throughout the IHGT period (Fig. 1) with a significant increase in the mean values of systolic (120.6 ± 9.7 vs. 155.6 ± 14.5 mmHg, P < 0.001) and diastolic (67.5 ± 6.4 vs. 94.1 ± 21.1 mmHg, P < 0.001) blood pressure when resting and peak IHGT values were compared. After completion of IHGT, blood pressures returned to baseline values by 3 min. Heart rate also increased significantly with IHGT (62.8 ± 11.7 vs. 84.7 ± 13.8 beats min; P < 0.001). There was no change in LV length (8.0 ± 0.5 vs. 8.0 ± 0.4 cm; P = 0.95) and LV end-diastolic volume (110.8 ± 20.0 vs. 110.0 ± 18.1 ml; P = 0.79). Consistent with increased afterload, LV end-systolic volume increased (44.2 ± 7.8 vs. 50.5 ± 10.8; P = 0.005) at peak-IHGT. This led to a significant decrease in stroke volume (63.9 ± 12.0 vs. 49.4 ± 7.8; P < 0.001; Fig. 2). The decrease in stroke volume and concomitant increase in heart rate resulted in no significant change in cardiac output (3.9 ± 0.5 vs. 4.2 ± 0.7 l min−1; P = 0.27).
Figure 1. Blood pressure response during isometric handgrip testing.
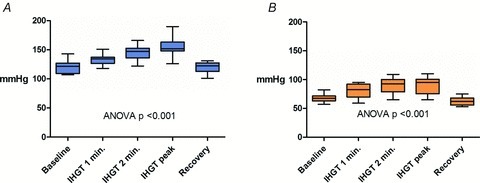
A, systolic blood pressure response during isometric handgrip testing. B, diastolic blood pressure response during isometric handgrip testing. IHGT: isometric handgrip testing. Box: mean ± 1 SD; whiskers: 5 and 95% confidence intervals.
Figure 2. Stroke volume decreased with isometric handgrip testing.
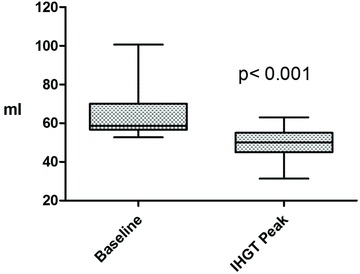
IHGT: isometric handgrip testing. Box: mean ± 1 SD; whiskers: 5 and 95% confidence intervals.
Systolic function
Baseline and peak-IHGT measures of systolic function are reported in Table 2. The increase in LV end-systolic volume with IHGT resulted in a decrease in LV ejection fraction, although the values at baseline and peak-IHGT are both considered within normal limits. Reductions in complementary measures of systolic function (peak systolic tissue velocity [Sm], longitudinal strain, and longitudinal strain rate) were also observed at peak-IHGT.
Table 2.
Comparison of LV functional parameters at baseline and at peak isometric handgrip testing
| Baseline | Peak-IHGT | P value | |
|---|---|---|---|
| Systolic function | |||
| LV ejection fraction (%) | 59.9 ± 3.1 | 54.3 ± 4.2 | <0.001 |
| LV peak lateral Sm (cm s−1) | 7.4 ± 1.6 | 6.1 ± 1.2 | <0.001 |
| Longitudinal strain (%) | −20.6 ± 2.9 | −18.2 ± 3.2 | 0.005 |
| Longitudinal strain rate (% s−1) | −1.31 ± 0.2 | −1.17 ± 0.2 | 0.01 |
| Diastolic function | |||
| Transmitral E-wave (cm s−1) | 80.6 ± 15.3 | 70.0 ± 15.4 | <0.001 |
| Transmitral A-wave (cm s−1) | 39.9 ± 8.0 | 55.2 ± 10.0 | <0.001 |
| E/A ratio | 2.1 ± 0.6 | 1.3 ± 0.3 | <0.001 |
| Em basal septum (cm s−1) | −9.7 ± 1.1 | −7.7 ± 1.4 | <0.001 |
| Am basal septum (cm s−1) | −5.2 ± 1.0 | −6.7 ± 1.2 | 0.001 |
| Em basal lateral LV (cm s−1) | −12.0 ± 1.7 | −8.7 ± 1.8 | <0.001 |
| Am basal lateral LV (cm s−1) | −3.6 ± 1.3 | −4.5 ± 1.6 | 0.03 |
Am: late diastolic peak tissue velocity; Em: early diastolic peak tissue velocity; IHGT: isometric handgrip testing; Sm: systolic peak tissue velocity.
Diastolic function
IHGT produced relative diastolic dysfunction as evidenced by consistent changes in numerous complementary parameters of diastolic function (Table 2). Specifically, we observed reductions in early trans-mitral filling (E-wave) and tissue relaxation (Em) velocities. This was accompanied by compensatory augmentation of late diastolic filling (A-wave) and tissue relaxation (Am) velocities.
Left ventricular rotation, twist, and untwisting rate
There was no significant change in LV length when rest was compared to peak-IHGT. Therefore, LV length correction did not change the findings and LV length-indexed values are not reported. Since correction for the increase in heart rate at peak-IHGT only intensified the results, raw values are reported due to the preference to report metrics that are familiar to the scientific community. Absolute values of LV rotation, LVT and untwisting parameters are detailed in Table 3. There was a significant 28% decrease in peak systolic apical rotation at peak-IHGT (Fig. 3A). In contrast, basal rotation was unchanged at peak-IHGT (Fig. 3B). This translated into a 25% reduction in peak systolic LVT (Fig. 4). Peak systolic LVT occurred earlier in the systolic ejection period due to peak systolic apical rotation occurring earlier at peak IHGT.
Table 3.
Comparison of left ventricular rotation, twist, and untwisting at rest and at peak isometric handgrip testing
| Baseline | Peak-IHGT | P Value | |
|---|---|---|---|
| Systolic parameter | |||
| Peak apical rotation (deg) | 11.9 ± 3.0 | 8.6 ± 2.2 | <0.001 |
| Time to peak apical rotation (% SEP) | 98.4 ± 2.7 | 93.8 ± 6.3 | 0.007 |
| Peak basal rotation (deg) | −5.3 ± 2.3 | −4.5 ± 2.6 | 0.19 |
| Peak LVT (deg) | 16.6 ± 2.8 | 12.5 ± 2.8 | <0.001 |
| Time to peak LVT (% SEP) | 97.4 ± 2.7 | 93.9 ± 6.2 | 0.03 |
| Diastolic parameter | |||
| Peak apical UTR (deg s−) | −85.8 ± 30.0 | −81.9 ± 37.7 | 0.71 |
| Peak basal UTR (deg s−) | 47.5 ± 25.2 | 55 ± 27.1 | 0.36 |
| Time difference between peak apical and basal UTR (ms) | −37.6 ± 41.9 | 25.9 ± 28.9 | <0.001 |
| Peak UTR (deg s−) | −114.5 ± 26.4 | −110.6 ± 39.8 | 0.71 |
IHGT: isometric handgrip test; LVT: left ventricular twist; SEP: systolic ejection period; UTR: untwisting rate.
Figure 3. Left ventricular rotation and isometric handgrip testing.
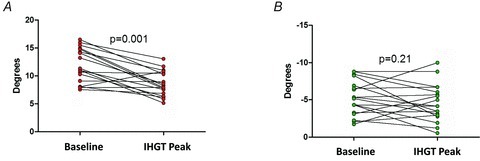
A, apical rotation decreased with isometric handgrip testing. B, there was no significant change in basal rotation with isometric handgrip testing. IHGT: isometric handgrip testing.
Figure 4. Representative example of left ventricular rotation and twist curves.
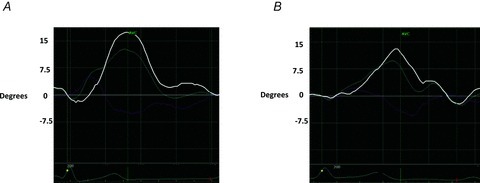
A, representative example of left ventricular twist (LVT) versus time curve at rest. B, LVT versus time curve from the same study subject at peak isometric handgrip testing, demonstrating reduced apical rotation (light blue curve) and LVT (white curve). Peak systolic apical rotation and LVT both occur earlier in the systolic ejection period at peak afterload. Light blue curve: apical rotation; pink curve: basal rotation; white curve: twist; AVC: aortic valve closure.
Unlike peak systolic LVT, peak early diastolic UTR did not significantly decrease at peak-IHGT. However, the relative timing of peak apical UTR and peak basal UTR changed with the haemodynamic perturbation. Specifically, apical UTR under resting conditions occurred after basal UTR (a negative value in Table 3) while at peak-IHGT apical UTR occurred before basal UTR (a positive value in Table 3; Fig. 5).
Figure 5. Representative example of untwisting rate (UTR) curves.
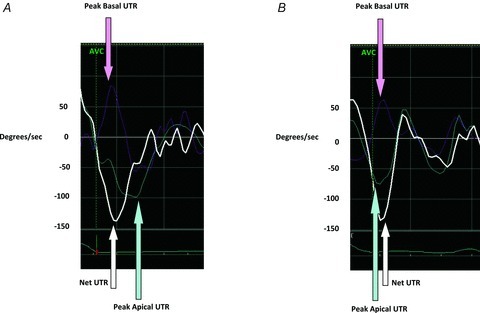
A, at rest, peak apical UTR (light blue arrow) occurs after peak basal UTR (pink arrow). B, at peak isometric handgrip testing, peak apical UTR has shifted to the left (light blue arrow) and now occurs before peak basal UTR (pink arrow). This ‘left-shift’ of peak apical UTR, presumably the result of earlier termination of peak systolic apical rotation, leads to more synchronized apical and basal UTR with resultant preservation of net UTR. AVC: aortic valve closure; UTR: untwisting rate.
Discussion
We examined the impact of isometric handgrip testing on LV mechanics including LV rotation, LV twist (LVT) and LV untwisting rate (UTR). IHGT produced acute LV afterload increase as evidenced by increases in systemic blood pressure and LV end-systolic volume. In this context, peak systolic apical rotation occurred earlier in the systolic ejection period and was decreased in magnitude. This resulted in a significant decrease in peak systolic LVT thus confirming the hypothesis that LVT is decreased in the setting of IHGT-mediated acute LV afterload increase.
Prior animal studies have demonstrated that manipulation of loading conditions influences the twisting motion of the heart (Gibbons Kroeker et al. 1995; MacGowan et al. 1996; Dong et al. 1999). Specifically, the afterload dependence of LVT was studied by magnetic resonance imaging in 10 isolated, blood perfused, ejecting canine hearts while preload was kept constant. Between low and high afterloads, there was a significant reduction in LVT (MacGowan et al. 1996). Similar results, namely a reduction in LVT with acute increase in LV afterload, were obtained from other elegant animal work (Dong et al. 1999).
Initial data characterizing the relationship between changes in acute loading conditions and LVT in human hearts suggest that LVT may not be afterload dependent. These studies included humans in whom radio-opaque beads had been inserted at the time of cardiac transplantation and no effect of methoxamine-induced afterload changes on LVT was found (Hansen et al. 1991; Moon et al. 1994). The study of heart transplant patients introduced several potential complicating factors including the use of fluoroscopic imaging to measure rotation/twist, altered innervation of transplanted hearts, and the lack of an intact pericardium (Tanaka et al. 2008). In contrast, more recent human studies have investigated the impact of a reduction in LV afterload, either with nitroprusside infusion (Park et al. 2010) or catheterization-based intervention for aortic coarctation (Laser et al. 2009), and document an increase in LVT with the decrease in afterload.
To our knowledge, our study is the first to examine the effect of IHGT-mediated acute afterload increase on LVT in healthy native human hearts. We have demonstrated a decrease in the magnitude of LV apical rotation and LVT with IHGT. Factors such as changes in LV geometry and intrinsic muscle fibre function may provide explanations for the mechanisms underlying the decreased LVT observed with IHGT. First, it has been shown that LVT is related to volume changes (as estimated by radial shortening) during ejection in canine hearts and that LVT is linearly related to volume during ejection (Beyar et al. 1989). There was an increase in end-systolic volume and a decrease in LV stroke volume at peak-IGHT in our study, thereby explaining some of the observed decrease in LVT. Second, fundamental muscle fibre physiology must be considered. It is well established that muscle fibres operate optimally when close to an ideal length (often their resting length) and that when stretched or shortened, generate less shortening and contractile force (Gordon et al. 1966). It is possible that IHGT altered myocardial fibre length sufficiently to impair function.
Our finding that IHGT did not significantly reduce peak early diastolic UTR is somewhat unexpected. This finding suggests that IHGT produces an ‘uncoupling’ of systolic rotation/twist and diastolic untwisting. Previous investigations by our group (Weiner et al. 2010a,b), have demonstrated significant correlations between changes in LVT and UTR in response to haemodynamic stressors. Although precise mechanisms explaining this uncoupling observed in response to IHGT remain speculative, changes in the relative timing of peak apical and basal UTR may be explanatory. At rest, peak apical UTR occurs after peak basal UTR. In contrast, at peak-IHGT, peak apical UTR occurs earlier in diastole such that it occurs before peak basal UTR (Fig. 5). This ‘left-shift’ of peak apical UTR, presumably the result of pressure mediated, earlier termination of peak systolic apical rotation, leads to more synchronized apical and basal UTR with resultant preservation of net UTR. Untwisting may therefore serve as a relatively volume-independent index of relaxation since peak untwisting is normalized by the increased intraventricular pressure produced by IHGT. The fact that IHGT produced an increase in heart rate warrants mention in the context of interpreting a time-dependent parameter such as UTR. However, when corrected of heart rate (i.e. UTR/heart rate), the findings did not change.
There are several distinct implications of our findings. First, the present study is the initial in vivo assessment of LV twist mechanics in response to IHGT in healthy human hearts. Our results, consistent with previous animal work, demonstrate that IHGT-mediated acute LV afterload increase reduces apical rotation and LVT. This observation may provide an important mechanistic explanation for the clinically relevant LV dysfunction that can occur in the context of acute increases in LV afterload (Vlcek et al. 2008). Further studies examining the human myocardial response to IHGT in cardiomyopathic disease states are warranted and ongoing by our group. Second, our study provides an example of uncoupling of LVT and UTR characterized by decreased LVT but relative preservation of early diastolic UTR. We demonstrate that changes in the relative timings of apical and basal twist mechanics are explanatory. This may represent a compensatory mechanism by which the myocardium is able to preserve untwisting properties in the face of acute haemodynamic stress. Third, the current study highlights a potential important difference between the effects of acute and chronic LV afterload increase. Prior studies of LV twist mechanics indicate that LVT is increased in chronic high afterload conditions (Stuber et al. 1999; Nagel et al. 2000; Sengupta et al. 2008). Thus, it appears that initial pressure-mediated decline in LVT may ultimately transition to an adaptive increase in LVT if elevated afterload is sustained.
Study limitations
We evaluated young, healthy subjects. This limits the generalizability of our findings to specific cardiac disease states and to older subjects (Notomi et al. 2006). Second, we did not use invasive techniques to measure LV afterload. However, the observed increase in systemic blood pressure and LV end-systolic volume is consistent with IHGT serving as an effective method of augmenting LV afterload. Third, we acknowledge that IHGT does not produce an isolated increase in LV afterload given that heart rate increase and heightened sympathetic nervous system activity are also part of the integrated physiological response. However, both increases in heart rate and contractility (result of increased sympathetic tone) are known to increase the magnitude of LVT (Dong et al. 1999). Thus, our finding of decreased LVT despite increased heart rate only emphasizes the negative impact of acute afterload on LV apical rotation and LVT. Fourth, we recognize that normalizing LV twist by the ratio of the distance between basal and apical imaging planes to the radius of the LV may assist in comparing LV twist measurements across studies. However, variability produced when attempting to identify the same LV plane in the apical four-chamber and short-axis views did not allow us to normalize our data in this fashion.
Conclusions
Our results indicate that peak systolic apical rotation and LVT are decreased in response to IHGT-mediated LV afterload challenge in the healthy human heart. Defining the impact of IHGT on myocardial twist mechanics in the healthy heart serves as the foundation for future studies examining its impact in the settings of human exercise performance and cardiomyopathic disease states.
Acknowledgments
This work was supported by Career Development Award grant number 10-G-16 from the American Society of Echocardiography (R.B.W.) and grant number 09FTF2220328 from the American Heart Association (A.L.B). The authors have no conflicts of interest to report.
Glossary
- IHGT
isometric handgrip testing
- LVT
left ventricular twist
- STE
speckle-tracking echocardiography
- UTR
untwisting rate
Author contributions
The isometric handgrip testing protocol and echocardiography were performed in the laboratory of Dr Aaron Baggish. Individual author contributions were as follows: conception and design of the experiments (R.B.W. and A.L.B.); collection, analysis and interpretation of data (R.B.W., A.E.W., J.H.K., T.J.W., M.H.P and A.L.B.); drafting the article and revising it critically for important intellectual content (R.B.W., M.H.P. and A.L.B.). All authors approved the final version.
References
- Beyar R, Yin FC, Hausknecht M, Weisfeldt ML, Kass DA. Dependence of left ventricular twist-radial shortening relations on cardiac cycle phase. Am J Physiol Heart Circ Physiol. 1989;257:H1119–H1126. doi: 10.1152/ajpheart.1989.257.4.H1119. [DOI] [PubMed] [Google Scholar]
- Dong SJ, Hees PS, Huang WM, Buffer SA, Jr, Weiss JL, Shapiro EP. Independent effects of preload, afterload, and contractility on left ventricular torsion. Am J Physiol Heart Circ Physiol. 1999;277:H1053–H1060. doi: 10.1152/ajpheart.1999.277.3.H1053. [DOI] [PubMed] [Google Scholar]
- Fu Q, Levine BD, Pawelczyk JA, Ertl AC, Diedrich A, Cox JF, Zuckerman JH, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC, Jr, Cooke WH, Robertson RM, Baisch FJ, Blomqvist CG, Eckberg DL, Robertson D, Biaggioni I. Cardiovascular and sympathetic neural responses to handgrip and cold pressor stimuli in humans before, during and after spaceflight. J Physiol. 2002;544:653–664. doi: 10.1113/jphysiol.2002.025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons Kroeker CA, Tyberg JV, Beyar R. Effects of load manipulations, heart rate, and contractility on left ventricular apical rotation. An experimental study in anesthetized dogs. Circulation. 1995;92:130–141. doi: 10.1161/01.cir.92.1.130. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DE, Daughters GT, 2nd, Alderman EL, Ingels NB, Stinson EB, Miller DC. Effect of volume loading, pressure loading, and inotropic stimulation on left ventricular torsion in humans. Circulation. 1991;83:1315–1326. doi: 10.1161/01.cir.83.4.1315. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Dote K, Sunaga Y, Sugiura T, Iwasaka T, Inada M. Evaluation of preload reserve during isometric exercise testing in patients with old myocardial infarction: Doppler echocardiographic study. J Am Coll Cardiol. 1991;17:106–111. doi: 10.1016/0735-1097(91)90711-h. [DOI] [PubMed] [Google Scholar]
- Helle-Valle T, Crosby J, Edvardsen T, Lyseggen E, Amundsen BH, Smith HJ, Rosen BD, Lima JA, Torp H, Ihlen H, Smiseth OA. New noninvasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation. 2005;112:3149–3156. doi: 10.1161/CIRCULATIONAHA.104.531558. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Laser KT, Haas NA, Jansen N, Schäffler R, Palacios Argueta JR, Zittermann A, Peters B, Körperich H, Kececioglu D. Is torsion a suitable echocardiographic parameter to detect acute changes in left ventricular afterload in children. J Am Soc Echocardiogr. 2009;22:1121–1128. doi: 10.1016/j.echo.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Lepley AS, Hatzel BM. Effects of weightlifting and breathing technique on blood pressure and heart rate. J Strength Cond Res. 2010;24:2179–2183. doi: 10.1519/JSC.0b013e3181e2741d. [DOI] [PubMed] [Google Scholar]
- MacGowan GA, Burkhoff D, Rogers WJ, Salvador D, Azhari H, Hees PS, Zweier JL, Halperin HR, Siu CO, Lima JA, Weiss JL, Shapiro EP. Effects of afterload on regional left ventricular torsion. Cardiovasc Res. 1996;31:917–925. [PubMed] [Google Scholar]
- Moon MR, Ingels NB, Jr, Daughters GT, 2nd, Stinson EB, Hansen DE, Miller DC. Alterations in left ventricular twist mechanics with inotropic stimulation and volume loading in human subjects. Circulation. 1994;89:142–150. doi: 10.1161/01.cir.89.1.142. [DOI] [PubMed] [Google Scholar]
- Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. doi: 10.1016/j.echo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- Nagel E, Stuber M, Burkhard B, Fischer SE, Scheidegger MB, Boesiger P, Hess OM. Cardiac rotation and relaxation in patients with aortic valve stenosis. Eur Heart J. 2000;21:582–589. doi: 10.1053/euhj.1999.1736. [DOI] [PubMed] [Google Scholar]
- Notomi Y, Lysyansky P, Setser RM, Shiota T, Popović ZB, Martin-Miklovic MG, Weaver JA, Oryszak SJ, Greenberg NL, White RD, Thomas JD. Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging. J Am Coll Cardiol. 2005;45:2034–2041. doi: 10.1016/j.jacc.2005.02.082. [DOI] [PubMed] [Google Scholar]
- Notomi Y, Srinath G, Shiota T, Martin-Miklovic MG, Beachler L, Howell K, Oryszak SJ, Deserranno DG, Freed AD, Greenberg NL, Younoszai A, Thomas JD. Maturational and adaptive modulation of left ventricular torsional biomechanics: Doppler tissue imaging observation from infancy to adulthood. Circulation. 2006;113:2534–2541. doi: 10.1161/CIRCULATIONAHA.105.537639. [DOI] [PubMed] [Google Scholar]
- Park SJ, Nishimura RA, Borlaug BA, Sorajja P, Oh JK. The effect of loading alterations on left ventricular torsion: a simultaneous catheterization and two-dimensional speckle tracking echocardiographic study. Eur J Echocardiogr. 2010;11:770–777. doi: 10.1093/ejechocard/jeq064. [DOI] [PubMed] [Google Scholar]
- Rajagopal K, Bridges C, Rojagopal KR. Towards an understanding of the mechanics underlying aortic dissection. Biomech Model Mechanobiol. 2007;6:345–359. doi: 10.1007/s10237-006-0069-3. [DOI] [PubMed] [Google Scholar]
- Russel IK, Gotte MJ, Bronzwaer JG, Knaapen P, Paulus WJ, van Rossum AC. Left ventricular torsion: an expanding role in the analysis of myocardial dysfunction. JACC Cardiovasc Imaging. 2009;2:648–655. doi: 10.1016/j.jcmg.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Sade LE, Demir O, Atar I, Muderrisoglu H, Ozin B. Effect of mechanical dyssynchrony and cardiac resynchronization therapy on left ventricular rotational mechanics. Am J Cardiol. 2008;101:1163–1169. doi: 10.1016/j.amjcard.2007.11.069. [DOI] [PubMed] [Google Scholar]
- Seals DR, Victor RG. Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc Sport Sci Rev. 1991;19:313–349. [PubMed] [Google Scholar]
- Seals DR. Influence of force on muscle and skin sympathetic nerve activity during sustained isometric contractions in humans. J Physiol. 1993;462:147–159. doi: 10.1113/jphysiol.1993.sp019548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging. 2008;1:366–376. doi: 10.1016/j.jcmg.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Shingu Y, Shiiya N, Ooka T, Tachibana T, Kubota S, Morita S, Matsui Y. Augmentation index is elevated in aortic aneurysm and dissection. Ann Thorac Surg. 2009;87:1373–1377. doi: 10.1016/j.athoracsur.2009.02.049. [DOI] [PubMed] [Google Scholar]
- Stohr EJ, Gonzalez-Alonso J, Pearson J, Low DA, Ali L, Barker H, Shave R. Effects of graded heat stress on global left ventricular function and twist mechanics at rest and during exercise in healthy humans. Exp Physiol. 2011;96:114–124. doi: 10.1113/expphysiol.2010.055137. [DOI] [PubMed] [Google Scholar]
- Streeter DD, Jr, Spotnitz HM, Patel DP, Ross J, Jr, Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circ Res. 1969;24:339–347. doi: 10.1161/01.res.24.3.339. [DOI] [PubMed] [Google Scholar]
- Stuber M, Scheidegger MB, Fischer SE, Nagel E, Steinemann F, Hess OM, Boesiger P. Alterations in the local myocardial motion pattern in patients suffering from pressure overload due to aortic stenosis. Circulation. 1999;100:361–368. doi: 10.1161/01.cir.100.4.361. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Nishikage T, Nakai H, Kokumai M, Otani S, Lang RM. The assessment of left ventricular twist in anterior wall myocardial infarction using two-dimensional speckle tracking imaging. J Am Soc Echocardiogr. 2007;20:36–44. doi: 10.1016/j.echo.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Oishi Y, Mizuguchi Y, Miyoshi H, Ishimoto T, Nagase N, Yamada H, Oki T. Contribution of the pericardium to left ventricular torsion and regional myocardial function in patients with total absence of the left pericardium. J Am Soc Echocardiogr. 2008;21:268–274. doi: 10.1016/j.echo.2007.05.035. [DOI] [PubMed] [Google Scholar]
- van Dalen BM, Vletter WB, Soliman OI, ten Cate FJ, Geleijnse ML. Importance of transducer position in the assessment of apical rotation by speckle tracking echocardiography. J Am Soc Echocardiogr. 2008;21:895–898. doi: 10.1016/j.echo.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Vlcek M, Bur A, Woisetschlager C, Herkner H, Laggner AN, Hirschl MM. Association between hypertensive urgencies and subsequent cardiovascular events in patients with hypertension. J Hypertens. 2008;26:657–662. doi: 10.1097/HJH.0b013e3282f4e8b6. [DOI] [PubMed] [Google Scholar]
- Weiner RB, Weyman AE, Khan AM, Reingold JS, Chen-Tournoux AA, Scherrer-Crosbie M, Picard MH, Wang TJ, Baggish AL. Preload dependency of left ventricular torsion: the impact of normal saline infusion. Circ Cardiovasc Imaging. 2010a;3:672–678. doi: 10.1161/CIRCIMAGING.109.932921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner RB, Hutter AM, Jr, Wang F, Kim J, Weyman AE, Wood MJ, Picard MH, Baggish AL. The impact of endurance exercise training on left ventricular torsion. JACC Cardiovasc Imaging. 2010b;3:1001–1009. doi: 10.1016/j.jcmg.2010.08.003. [DOI] [PubMed] [Google Scholar]


