Abstract
The alveolo-capillary barrier is effectively impermeable to large solutes such as proteins. A hallmark of acute lung injury/acute respiratory distress syndrome is the accumulation of protein-rich oedema fluid in the distal airspaces. Excess protein must be cleared from the alveolar space for recovery; however, the mechanisms of protein clearance remain incompletely understood. In intact rabbit lungs 29.8 ± 2.2% of the radio-labelled alveolar albumin was transported to the vascular compartment at 37°C within 120 min, as assessed by real-time measurement of 125I-albumin clearance from the alveolar space. At 4°C or 22°C significantly lower albumin clearance (3.7 ± 0.4 or 16.2 ± 1.1%, respectively) was observed. Deposition of a 1000-fold molar excess of unlabelled albumin into the alveolar space or inhibition of cytoskeletal rearrangement or clathrin-dependent endocytosis largely inhibited the transport of 125I-albumin to the vasculature, while administration of unlabelled albumin to the vascular space had no effect on albumin clearance. Furthermore, albumin uptake capacity was measured as about 0.37 mg ml−1 in cultured rat lung epithelial monolayers, further highlighting the (patho)physiological relevance of active alveolar epithelial protein transport. Moreover, gene silencing and pharmacological inhibition of the multi-ligand receptor megalin resulted in significantly decreased albumin binding and uptake in monolayers of primary alveolar type II and type I-like and cultured lung epithelial cells. Our data indicate that clearance of albumin from the distal air spaces is facilitated by an active, high-capacity, megalin-mediated transport process across the alveolar epithelium. Further understanding of this mechanism is of clinical importance, since an inability to clear excess protein from the alveolar space is associated with poor outcome in patients with acute lung injury/acute respiratory distress syndrome.
Key points
Under physiological conditions the lung alveoli are impermeable to protein.
In patients with acute lung injury/acute respiratory distress syndrome (ALI/ARDS) protein-rich oedema fluid accumulates in the distal airspaces and leads to a life-threatening impairment of alveolar gas exchange.
Albumin is a ligand of megalin, a member of the low-density lipoprotein (LDL)-receptor family.
We show that clearance of albumin from the distal air spaces is facilitated by active megalin-mediated transport across the alveolar epithelium.
Understanding of protein clearance mechanisms in the lung may ultimately lead to novel therapeutic approaches for the treatment of ALI/ARDS.
Introduction
Under physiological conditions, the alveolo-capillary barrier is effectively impermeable to large solutes such as proteins, primarily due to the tight epithelial monolayer which exhibits a reflection coefficient for proteins of ∼0.95 (Gorin & Stewart, 1979; Vadasz et al. 2007). In healthy individuals the protein concentration in the epithelial lining fluid (ELF), a thin fluid layer that covers the apical side of the alveolar epithelial monolayer, is approximately 8–10% of the plasma concentration (Kim & Malik, 2003). In contrast, during acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome (ARDS), which are characterized by disruption of the alveolo-capillary barrier and thus impaired epithelial permeability, protein-rich oedema accumulates in the alveolar space leading to markedly increased protein concentrations in the ELF (approximately 40–90% of the plasma level) (Hastings et al. 2004). In alveolar oedema fluid from non-survivors of ARDS, the concentration of ELF protein is three times higher than in survivors (Bachofen & Weibel, 1977). Therefore, the inability to remove excess protein from the alveolar space may play a major role in the poor outcome of patients with ARDS.
The mechanisms by which excess protein is cleared from the alveolar space are poorly understood although several processes have been implicated in these mechanisms. These include mucociliary clearance, phagocytosis by alveolar macrophages and degradation in the alveolar space by serine and metallo-proteases (Folkesson et al. 1996). Other studies have suggested that some proteins (such as albumin and insulin) may be internalized by receptor-mediated endocytosis and transported intact across the alveolo-capillary barrier by transcytosis (Berthiaume et al. 1989; Folkesson et al. 1990, 1992). Two major receptor-mediated endocytic pathways have been described termed the caveolae-mediated and clathrin-dependent endocytosis. While active transport of albumin across the endothelium is primarily facilitated by caveolae-mediated endocytosis (Mehta & Malik, 2006), the molecular mechanisms of protein uptake and its subsequent transport through the epithelial layer of the alveolo-capillary barrier remain largely unidentified.
Recent studies suggested that albumin may be taken up by cultured alveolar epithelial cells via clathrin-dependent endocytosis (Yumoto et al. 2006, 2012; Ikehata et al. 2008; Tagawa et al. 2008). This is in line with investigations showing a central role for the multiligand endocytic receptor megalin, a 600 kDa glycoprotein and member of the low-density lipoprotein (LDL)-receptor family (Saito et al. 1994) that is expressed at clathrin-coated pits, in renal tubular albumin reabsorption (Birn et al. 2000; Caruso-Neves et al. 2005; Birn & Christensen, 2006). Although megalin is expressed in primary alveolar epithelial cells (Kolleck et al. 2002b), involvement of this receptor in albumin transport across the alveolar epithelium has not yet been reported.
In the present study, by using intact isolated rabbit lungs as well as primary and cultured alveolar epithelial cell monolayers, we demonstrate that albumin transport across the alveolo-capillary barrier is an active, physiologically relevant, high-capacity endocytic process mediated by megalin. Determining the pathways of protein transport across alveolar epithelial cells and the alveolo-capillary barrier is crucial for developing novel therapeutic approaches for patients with ALI/ARDS.
Methods
Ethical approval
Animal experiments were approved by the local authorities (Regierungspräsidium, Giessen) in Germany.
Chemicals
Unless otherwise noted, chemicals were obtained from Sigma (Seelze, Germany).
Isolated and perfused rabbit lung
Lungs of healthy adult New Zealand White rabbits (3.0 ± 0.5 kg; Bauer, Neuenstein-Lohe, Germany) were isolated and handled as previously described (Seeger et al. 1994; Vadasz et al. 2005a, 2008b) (for further details please see also Supplementary methods data). The rabbits were anaesthetized with 0.5–0.7 ml of a mixture of xylazine (Rompun 20 mg ml−1; Bayer, Leverkusen, Germany) and ketamine (Ketavet 100 mg ml−1; Pfizer, Karlsruhe, Germany) in a ratio of 3:2. This mixture was also used during surgery in doses of 0.5–1 ml to ensure deep anaesthesia. Additionally, 8–10 ml lidocaine (Xylocain 2%, 20 mg ml−1; AstraZeneca, Wedel, Germany) was injected subcutaneously for local anaesthesia prior to performing the tracheotomy. The method of humane killing at the end of the experiment was giving an intravenous bolus of 2 ml ketamine–xylazine before an incision in the right ventricle was made and heart and lung were isolated and explanted.
Experimental protocol
The time-course of all experiments was 180 min. After the conclusion of a 20 min steady-state period, pharmacological agents diluted in DMSO, methanol or saline in the presence of the vehicles (for control experiments) were aerosolized into the lungs. For competitions studies bovine serum albumin (BSA) dissolved in PBS was applied either to the ELF (2 mg ml−1) or to the perfusate (20 mg ml−1). For inhibitions studies, the following compounds were nebulized and deposited into the ELF: (1) protease inhibitors: EDTA (ethylenediaminetetraacetic acid) in PBS (phosphate-buffered saline) (10 μg ml−1) and AEBSF in DMSO (dimethylsulfoxide; 0.25 mg ml−1); (2) clathrin inhibitors: chlorpromazine in PBS (5 μg ml−1) and phenylarsine oxide in DMSO (30 μg ml−1); and (3) inhibitors of cytoskeletal rearrangement/transcytosis: phalloidin in DMSO (1 μg ml−1) and monensin in methanol (15 μg ml−1). At the end of the experiments a bronchoalveolar lavage (BAL) was obtained as previously described (Ghofrani et al. 2001). Briefly, after the termination of perfusion, the lungs were lavaged with 50 ml of isosmolar unlabelled mannitol. The lavage fluid was then centrifuged to separate the cells from the supernatant. The fluorescein isothiocyanate (FITC)-albumin concentrations were obtained from BAL fluids (200 μl) in a Fusion microplate spectrofluorimeter (Packard; Dreieich, Germany) at an emission wavelength of 480 nm and an excitation wavelength of 520 nm, as described previously (Vadasz et al. 2005a).
Radioactive tracers
125I-labelled bovine serum albumin (125I-albumin) (PerkinElmer, Rodgau, Germany) was used to monitor protein transport in the lung, as well as in cell culture experiments; 6 μCi of 125I-albumin were deposited into the alveolar space during a 10 min period. About 12 μCi of [3H] mannitol (PerkinElmer, Rodgau, Germany) was applied to the distal air space. In the case of isolated, ventilated and perfused rabbit lung experiments, tracers were deposited into the alveolar space by ultrasonic nebulization, while in cell culture experiments, radio-labelled substances were directly added to the cell culture medium.
Calculation of tracer kinetics
Counts of the gamma emitter 125I-albumin were recorded continuously by γ-detectors over the entire time-course of the experiment. The starting point of each tracer clearance measurement in the lung, as well as in the perfusate, was set at the end of the nebulization, and at this time point the amount of radiation in the lung was referenced to 100%. The clearance rate from the lungs, and transit into the perfusate, were calculated by measuring the area above the curve (AAC) as determined by the following equation:
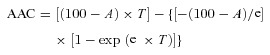 |
where A is the asymptote of the exponential curve, T is the time and e is the exponential function of the curve
Cell culture
Alveolar type II cells (ATII) were isolated from the lungs of Sprague–Dawley rats weighing 200–225 g, as previously described (Ridge et al. 2003; Vadasz et al. 2008a). The day of isolation and plating was designated culture day 0. In order to get ATI-like cells, cells were allowed to differentiate for 7 days (Dobbs, 1990; Danto et al. 1992). All experiments were conducted on days 4 (ATII) or 7 (ATI-like). The rat alveolar epithelial cell line RLE-6TN (ATCC, catalogue no. CRL-2300) was grown in DMEM supplemented with 10% fetal bovine serum, and 100 U ml−1 penicillin and 100 μg ml−1 streptomycin.
Cellular experiments
Primary rat alveolar epithelial cells were either used on day 4 as ATII cells or on day 7 as ATI-like cells. Cells were plated on permeable supports (BD Falcon, Heidelberg, Germany). Experiments were performed on monolayers, defined by transepithelial electrical resistance (TEER) of >1500 Ω cm2.
Binding, uptake and transport of 125I-albumin and FITC-labelled compounds
After media removal, plates and permeable supports were briefly rinsed with Dulbecco's phosphate-buffered saline containing 5 mm glucose (DPBS-G; PAN Biotech, Aidenbach, Germany), 0.1 mm CaCl2 dihydrate (Calbiochem, San Diego, CA, USA) and 0.5 mm MgCl2.6H2O. Cells were then pre-incubated for 10 min with 1.4 ml of DPBS-G followed by a pre-incubation with BSA in PBS (20 mg ml−1), receptor-associated protein (RAP) in H2O (1 μm) or vehicles. After treatment of the cells with these substances, 100 μl of DPBS-G containing 125I-albumin was applied to the cells. To assess transepithelial transport of 125I-albumin, buffer samples were taken from the basolateral side of the permeable support after 30 min. Experiments were terminated by aspiration of the medium and addition of ice-cold DPBS. The cell layers were washed thoroughly with ice-cold DPBS and were incubated with Solution X (DPBS-G, 0.5 mg ml−1 trypsin, 0.5 mg ml−1 proteinase K and 0.5 mm EDTA) in order to lift them from the permeable supports (EDTA-trypsin) and to dissolve labelled albumin bound to the cell surface (proteinase K) and spun down. Samples were quantified by γ-emission counting in a Packard γ-counter (Packard, Dreieich, Germany). Experiments with FITC-labelled compounds were performed as prescribed previously (Takano et al. 2002; Yumoto et al. 2006). Briefly, RLE-6TN cells grown on permeable support on 6-well plates were used. After removal of the culture medium, dish was washed and pre-incubated with DPBS-G for 20 min at experimental conditions. Then DPBS-G buffer containing FITC-albumin or FITC-dextran (70 kDa) was added to each dish and cells were incubated at 37°C or 4°C for 6 h At the end of the experiments cells were rinsed three times with ice-cold PBS and incubated with 0.5 ml of ice-cold Solution X before being scraped off the permeable support. After centrifugation at 9,8g for 5 min, samples were taken from the supernatant to assess the bound fraction. After the supernatant was aspirated, the pellet was solubilised in 0.5 ml 0.1% Triton X-100 (in PBS buffer without CaCl2 and MgCl2) for 30 min at room temperature and centrifuged for 5 min at 9,8g. Fluorescence was detected using an Infinite 200 (Tecan, San Jose, CA, USA) fluorescence spectrophotometer at an excitation wavelength of 500 nm and an emission wavelength of 520 nm.
Assessment of cell viability
Cell viability was assessed by the Trypan Blue exclusion dye method (Perry et al. 1997). Cells were plated on permeable supports as described before and viability of control cells and those exposed to drugs or their vehicles was assessed by adding 50 μl of Trypan Blue solution (0.4% (mass/vol) Trypan Blue in PBS) to the culture medium. After 1–2 min the ratio of nonviable cells (retaining the dye) to total number of cells was calculated to obtain the percentage of cell mortality.
Cell fractionation
The experiment was terminated by placing the cells on ice and washing them twice with ice-cold PBS. Cells were scraped into lysis buffer (50 mm Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mm NaCl, 1 mm EDTA, 1 mm phenylmethanesulfonyl fluoride, 1 mm sodium orthovanadate (Na3VO4), 0.1 mm dithiothreitol, 0.4 g ml−1 leupeptin and pepstatin) (Vadasz et al. 2008a).
Western blot analysis
Protein concentration was quantified by Bradford assay (Bio-Rad, Munich, Germany), and proteins were resolved in 10–15% polyacrylamide gels. Thereafter, proteins were transferred to nitrocellulose membranes (Optitran; Schleider & Schuell, Dassel, Germany) using a semi-dry transfer apparatus (Bio-Rad, Munich, Germany). Incubation with megalin (H-245) antibody 1:100 (sc-25470, Santa Cruz Biotechnology, Heidelberg, Germany) was performed overnight at 4°C. Blots were developed with a chemiluminescence detection kit (Thermo Fisher Scientific, Bonn, Germany), as recommended by the manufacturer.
Megalin mRNA expression
RNA was isolated form RLE-6TN and A549 cells using a Qiagen RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. cDNA was obtained using iScript cDNA Synthesis Kit (Bio-Rad, Munich, Germany). qPCR was performed with iTaq SYBRGreen Supermix with ROX (Bio-Rad) using a Mx300p cycler (Stratagen, Heidelberg, Germany). The following primer sequences were used: megalin (rn) sense ACGTAATGGCGTTTCTGGAC, megalin (rn) antisense CCCTGTCGGTTTTCACACTT, megalin (hs) sense GGCAAAAAGAGCCAGAGTTG, megalin (hs) antisense GCTGGTGAAGTTGGGTTTGT.
RNA interference of megalin
RLE-6TN cells were cultured until 40–60% confluence. Rat megalin siRNA (sc-108041, Santa Cruz Biotechnology) was transfected using lipofectamine RNAiMAX (Life Technologies Invitrogen, Darmstadt, Germany) according to the manufacturer's instructions. Scrambled sequence siRNA (Santa Cruz Biotechnology) and cy3-labelled negative control (Life Technologies) were used as controls. After 24 h cells were used to assess albumin uptake.
Fluorescence microscopy
Cells were plated on coverslips and used at day 4 (ATII) or day 7 (ATI-like). Medium was aspirated and replaced with DPBS-G; after 10 min of pre-incubation, pharmacological agents were applied and subsequently 50 μg ml−1 FITC-albumin was added to the cells. After 30 min, coverslips were washed with ice-cold PBS several times and cells were fixed with a 1:1 mixture of acetone–methanol for 10 min at −20°C. After washing the coverslips three times they were blocked in Tris-buffered saline containing (TBST) 0.1% Tween 20 (TBST) and 4% BSA for 30 min at room temperature. Coverslips were then incubated overnight at 4°C in rabbit polyclonal anti-rat megalin (sc-25470; Santa Cruz Biotechnology) in a 1:10 dilution in blocking solution. Coverslips were washed with 0.1% TBST and incubated in polyclonal donkey anti-rabbit cy3-conjugate secondary antibody (Millipore, Schwalbach, Germany) in a 1:100 dilution in blocking solution. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Life Technologies), a nucleic acid stain, and coverslips were plated on glass slides with fluorescent mounting medium (Dako, Glostrup, Denmark). Data were obtained and analysed with a fluorescence microscope (LeicaDMLA Q550/W; Leica Microsystems, Wetzlar, Germany), a digital camera (DC 300 FX; Leica Microsystems) and software (Q-Win; Leica Microsystems). Uptake of FITC-albumin was detected by density measurement via microscopic software (Q-Win).
Statistical analysis of data
Numerical values are given as the mean ± standard error of mean (SEM). Comparisons between two groups were made using an unpaired, two-tailed Student's t test. Intergroup differences of three or more experimental groups were assessed by using a one-way analysis of variance (ANOVA) with post hoc Dunnett test or Newman–Keuls multiple comparison test, when values were only compared with controls. P values <0.05 were considered significant. GraphPad Prism 4 for Windows software (GraphPad Software, San Diego, CA, USA) was used for data plotting and statistical analysis.
Results
Clearance of albumin from the alveolar space of isolated rabbit lungs is an active process
At 37°C, 29.8 ± 2.2% of the 125I-labelled albumin deposited in the alveolar space was cleared from the lungs after 2 h (Fig. 1A). This movement of albumin was temperature dependent, as experiments conducted at 22°C and at 4°C resulted in a significantly lower clearance of the nebulized 125I-albumin (16.2 ± 1.1% and 3.7 ± 0.4%, respectively; Fig. 1A). Albumin clearance at 4°C is attributable exclusively to passive transport processes, because active processes are shut down at this temperature (Rutschman et al. 1993). Passive epithelial paracellular permeability for small solutes, as assessed by [3H] mannitol clearance from the lung, was not significantly affected by low temperature, when compared with control lungs at 37°C (Fig. 1B). Trichloracetic acid precipitation was employed to assess the intactness of 125I-albumin during the experiment. At baseline, 125I-albumin designed for aerosolization was found to be 95.4 ± 0.7% intact. After nebulization the bulk of radio-labelled albumin was intact in the alveolar space (87.6 ± 2.0%), with 86.9 ± 3.4% remaining unchanged throughout the experiment (Supplementary Fig. S1A).
Figure 1. Albumin transport across the alveolo-capillary barrier.
A, isolated, ventilated and perfused rabbit lungs were maintained either at 37°C (dark grey), 22°C (light grey) or 4°C (black). 125I-albumin was deposited into the alveolar space by nebulization. Counts were set at 100% immediately after nebulization. Each data point represents the mean of at least six independent experiments. For clarity, standard deviations have been omitted; however, they are incorporated into analyses of these data in B. B, 125I-albumin transport was quantified from the data presented in A. The [3H]mannitol flux (open bars) in lungs maintained at 37°C was set at 100%, while mannitol flux in lungs maintained at low temperature was expressed relative to this control value. Data represent the mean ± SD (n = 6 for all groups). C, control lungs (dark grey) were maintained at 37°C and sham-nebulized with normal saline after establishing steady-state equilibrium. A 1000-fold molar excess of native albumin was nebulized into the lungs (light grey). Alternatively, excess native albumin (20 mg ml−1) was applied to the perfusate of the lungs (black). 125I-albumin tracer was nebulized to the lungs, and elimination of this tracer from the lung was monitored. D, active 125I-albumin transport was set at 100% in control lungs and active transport of the tracer in excess native albumin-treated lungs was expressed relative to this control value. E, FITC-albumin was administered to the perfusate and quantified in the ELF from BAL samples. The FITC-albumin concentration in the perfusate was set to 100%, and the concentration of FITC in the BAL was calculated as a percentage of the FITC concentration in the perfusate. Bars represent the mean ± SEM (n = 6 for all groups); *P < 0.05, ***P < 0.001.
Furthermore, to rule out degradation of 125I-albumin in the alveolar space by proteases, EDTA or AEBSF, potent inhibitors of metallo-proteases and serine proteases, which represent the main source of protease activity in the alveolar space (Chiancone et al. 1986; Lunn & Sansone, 1994; Gross, 1995; Greenlee et al. 2007), were nebulized to the alveolar space. Of note, neither of the protease inhibitors affected 125I-albumin clearance suggesting that degradation of 125I-albumin in the alveolar space did not play a role in its removal (Supplementary Fig. S1B).
Albumin movement across the alveolo-capillary barrier is a saturable and unidirectional process
Isolated rabbit lungs were pre-nebulized with a 1000-fold molar excess of unlabelled albumin (compared with the radioactive tracer) prior to aerosolization of 125I-albumin to investigate whether transport of the radio-labelled protein can be inhibited by competition. Deposition of the excess native albumin into the alveolar space significantly decreased the active transport of 125I-albumin from the air space to the vascular compartment of the lung, since the rate of active transport of the tracer was inhibited by 31.6 ± 1.2% compared with control lungs that had been pre-nebulized with physiological saline (Fig. 1C and D).
Under normal conditions, the perfusate does not contain any protein, and thus an albumin gradient between the two sides of the alveolo-capillary barrier occurs. In additional studies, when excess unlabelled albumin was administered to the perfusate to eliminate this oncotic gradient between the alveolar and the vascular compartments, no effect on 125I-albumin transit was observed (Fig. 1C and D), further suggesting that passive movement of the radio-labelled protein was not a significant contributor to albumin clearance from the distal air space. These data indicate that active transport processes mediate the vast majority of the transepithelial albumin movement.
To further address the directionality of albumin transport across the alveolo-capillary barrier, FITC-albumin was administered to the vasculature, and the flux of FITC-albumin into the alveolar space was measured. Experiments were performed either at 37°C or at 4°C, and concentrations of FITC-albumin in the ELF and the perfusate were measured. While approximately 30% of the 125I-albumin deposited into the alveolar space was cleared over the time-course of the experiment (120 min) at 37°C (Fig. 1A), only 6.0 ± 1.1% of the FITC-albumin applied to the vasculature appeared in the alveolar space over the same time-course at 37°C (Fig. 1E). At 4°C a nearly identical transport rate of FITC-albumin from the vascular to the alveolar space was measured (5.3 ± 0.5%) (Fig. 1E), suggesting that the bulk of the FITC-albumin movement from vasculature to the epithelium was probably mediated by passive paracellular processes. These data collectively support the hypothesis that movement of albumin through the alveolo-capillary barrier was predominantly uni-directional, occurring from the alveolar to the vascular space.
Albumin transport is a specific and saturable process in vitro
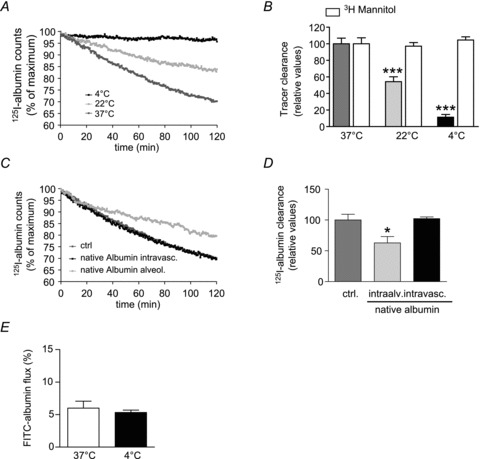
To further elucidate the mechanism of albumin transport across the alveolar epithelial barrier we employed primary rat alveolar type II and type I-like cells. We employed both ATII and ATI-like cells to investigate their contribution to albumin transport separately. We sought to investigate the effect of excess native albumin on labelled albumin cell surface binding, uptake by epithelial cells and transepithelial transport across the epithelial monolayer. By centrifugation, surface-bound albumin was separated from albumin, which was taken up by the cells. In the presence of a 100-fold molar excess of native albumin the binding of 125I-albumin to the epithelial cell surface was decreased by 71.4 ± 11.2% in ATII cells and by a similar amount in ATI-like cells (Fig. 2A and B). To ensure the same baseline conditions in primary epithelial cells, we used fluorescence microscopy to detect FITC-albumin uptake by density measurement. After viability of the epithelial cells was assessed by phase-contrast microscopy (data not shown), we detected fluorescence in the cell. Pre-incubation of the primary cells with native albumin resulted in a significant inhibition of FITC-albumin uptake in ATI-like cells (decreased by 74.0 ± 3.5%) (Fig. 2C) and ATII cells (decreased by 72.5 ± 4.6%) (Fig. 2D). Transepithelial transport of 125I-albumin, as assessed by taking samples from the basolateral side of the permeable supports, was significantly decreased after pre-incubation with an excess of unlabelled albumin. Transepithelial movement was reduced by 67.5 ± 15.2% in ATI-like cells and 77.6 ± 4.8% in ATII cells (Fig. 2E and F).
Figure 2. In vitro 125I-albumin transport in the presence of excess unlabelled albumin.
Primary rat alveolar epithelial type I-like (ATI) and type II (ATII) cells were used for experiments on day 3 (ATII) or day 7 (ATI). ATI (A) and ATII (B) were pre-incubated in the absence or presence of 1000-fold excess native albumin and subsequently incubated with 125I-albumin; binding of 125I-albumin to the cell surface was measured in the presence and absence of 100-fold excess of bovine serum albumin (BSA), n = 4. In ATI (C) and ATII (D) cells were pre-incubated in absence (open bars) or presence (grey bars) of a 1000-fold molar excess of native albumin prior to incubation with 50 μg ml−1 of FITC-albumin. FITC-albumin uptake was assessed via density measurement, n = 3. The amount of 125I-albumin that was transported across the epithelial monolayer of ATI (F) and ATII (G) cells in absence or presence of an excess amount of native albumin was assessed by measurement of γ-radiation, n = 4. Data represent the mean ± SEM ***P < 0.001; **P < 0.01.
These data demonstrate that albumin binding and uptake by alveolar epithelial cells as well as transepithelial transport across the alveolar epithelium is an active process, which takes place at comparable rates in both alveolar epithelial cell types.
Albumin binding and uptake are concentration dependent
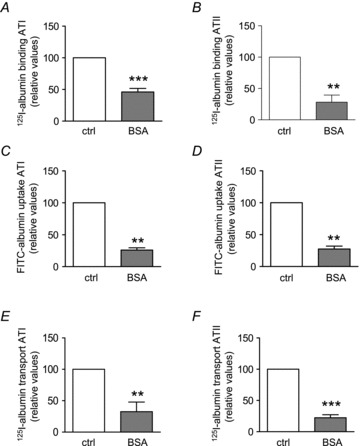
The effect of various concentrations of FITC-labelled albumin (0.02–100 mg ml−1) on binding and uptake was studied using RLE-6TN cell monolayers. At 37°C binding and uptake of FITC-albumin is a concentration-dependent process (Fig. 3A and B) and demonstrates saturation at concentrations higher than 10 mg ml−1 with a Km of 0.37 mg ml−1 for binding. In contrast to FITC-albumin, FITC-labelled dextran of a comparable size (70 kDa) displayed no concentration dependency at 37°C. As an additional control we conducted binding and uptake studies at 4°C and them to be reduced by 40% and 20%, respectively. Furthermore, there was no difference in binding and uptake between FITC-albumin and FITC-dextran at 4°C (Fig. 3A and B), suggesting that the process at 4°C is passive and non-specific.
Figure 3. FITC-albumin binding and uptake.
RLE-TN6 monolayers (transepithelial electrical resistance > 1500 Ω cm2) were used in all experiments. FITC-albumin and FITC-dextran (70 kDa) binding (A) and uptake (B) were measured after 6 h at 37°C and 4°C, n = 4. FITC-albumin and -dextran concentrations were determined by fluorescence. Data represent the mean ± SEM.
Albumin clearance from the alveolar space is primarily mediated by clathrin-dependent endocytosis
To further investigate the mechanisms of endocytosis that mediate the transport of 125I-albumin from the alveolar compartment to the vasculature, we set out to explore the role of clathrin-dependent endocytosis. When the clathrin-mediated endocytosis inhibitor, phenylarsine oxide (Visser et al. 2004), was nebulized into the alveolar space, a significant decrease in active 125I-albumin transit from the alveolar epithelium to the vascular compartment of the lung was evident, since the rate of 125I-albumin active transport was inhibited by 30.2 ± 1.2% (compared with control lungs that had been pre-nebulized with physiological saline) (Fig. 4A and B). Additionally, in further experiments, another clathrin-dependent endocytosis inhibitor, chlorpromazine (Wang et al. 1993), was administered to the air spaces prior to aerosolization of 125I-albumin to confirm the findings with phenylarsine oxide. Application of chlorpromazine, similar to the findings with phenylarsine oxide, resulted in a clear decrease (33.1 ± 2.2% compared with control lungs treated with vehicle only) (Fig. 4A and B) in the transport rate of aerosolized 125I-albumin from the alveolar compartment to the vasculature. These data are concordant with former studies, which had shown that FITC-albumin uptake requires clathrin-dependent endocytosis in RLE-6TN cells (Yumoto et al. 2006). In further experiments, phalloidin oleate, an inhibitor of actin cytoskeletal rearrangement (Stenbeck & Horton, 2004) was used to study the effect of transcytosis on alveolar albumin transport. Deposition of phalloidin oleate into the ELF significantly decreased the active transport of the radio-labelled protein from the alveolar space to the vasculature of the lung (Fig. 4C and D), since the active transport rate of 125I-albumin was inhibited by 51.4 ± 1.2% compared with control lungs. Additionally, monensin, another potent inhibitor of transcytosis (Sakagami et al. 2002), significantly decreased the rate of active radio-labelled albumin transit by 34.0 ± 1.2% (Fig. 4C and D). These data collectively suggest that transcytosis plays an important role in movement of albumin from the distal air spaces to the vasculature.
Figure 4. Albumin transport across the alveolar epithelium with inhibition of clathrin and transcytosis.
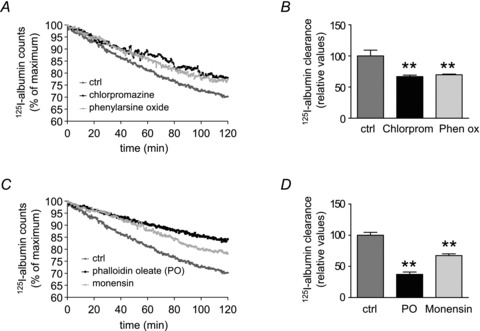
A and B, lungs were either aerosolized sham-treated (dark grey) or with chlorpromazine (black) or phenylarsine oxide (light grey). C and D, control lungs (dark grey) were sham-nebulized or treated with phalloidin oleate (PO, black) or monensin (light grey). Each data point represents the mean of six independent experiments (A and C). 125I-albumin transport was set at 100% in control lungs and active transport of the tracer was expressed relative to this control value (B and D). Bars represent the mean ± SEM (n = 6 for all groups), **P < 0.01.
Albumin uptake in the alveolar epithelium is mediated by megalin via clathrin-dependent endocytosis
Clathrin-dependent endocytosis is a receptor-mediated process. The two endocytic receptors megalin and cubilin emerged to be most important for the binding and uptake of albumin in kidney epithelial cells of the proximal tubules (Birn et al. 2000; Birn & Christensen, 2006). In contrast to cubilin, megalin has a transmembrane domain and therefore seems to be the partner directly interacting with albumin (Verroust et al. 2002). It has already been proposed that the function of megalin is required for FITC-albumin uptake in RLE-6TN cells (Yumoto et al. 2006). We were using primary ATII and ATI-like cells to investigate the involvement of megalin in the binding, uptake and transepithelial transport of albumin. Megalin mRNA is expressed in lung alveolar epithelial cells lines RLE-6TN and A549 (Kolleck et al. 2002a,b) (Fig. 5A) and is known to be expressed in embryonic lung tissue (Fisher & Howie, 2006). Receptor-associated protein (RAP) is one of megalin's many ligands which binds megalin with high affinity and therefore, by competition, partially blocks albumin binding (Verroust et al. 2002; Verroust & Christensen, 2002).
Figure 5. Uptake of FITC-albumin by primary rat alveolar epithelial type I-like (ATI) cells after incubation with receptor-associated protein (RAP).
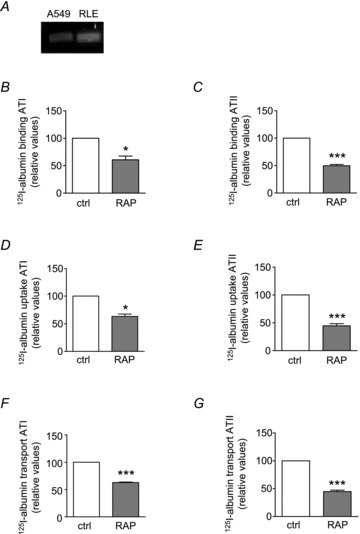
A, mRNA expression of megalin in A549 and RLE-TN6 cells. Binding (B and C), uptake (D and E) and transport (F and G) of 125I-albumin in ATI (B, D and F) and ATII (C, E and G) cells is partially blocked by RAP, n = 4. Data represent the mean ± SEM, *P < 0.05, ***P < 0.001.
We show that RAP inhibited 125I-albumin binding by 39.2 ± 11.8% in ATI-like cells and 50.1 ± 4.5% in ATII cells (Fig. 5B and C). The uptake of 125I-albumin was also assessed by conducting cellular transport studies. After pre-treatment with RAP, the cell monolayers were incubated with 125I-albumin for 30 min. Application of RAP inhibited the uptake of 125I-albumin by 36.8 ± 7.5% in ATI-like cells and by 55.4 ± 7.7% in ATII cells (Fig. 5D and E). Pre-incubation of the cells with 1 μm RAP prior to the application of 125I-albumin resulted in decreased transepithelial transport rates. Transepithelial movement were slowed by 37.2 ± 2% and by 55 ± 5% in ATII cells (Fig. 5F and G). Together these data suggest that albumin uptake by alveolar epithelial cells as well as transepithelial transport across the alveolar epithelium is a receptor-mediated process which involves the multi-ligand endocytic receptor megalin.
To further elucidate the role of megalin as a potential receptor for albumin endocytosis in the alveolar epithelium, we evaluated the effect of silencing the megalin gene. We employed a cell line with high transfection efficiency, compared with that of primary alveolar epithelial type II cells, the RLE-6TN cell line. This cultured cell line has already beenevaluated as an in vitro model system for alveolar epithelial cells (Yumoto et al. 2006). To ensure similar base-line conditions in RLE-6TN cells compared with primary alveolar epithelial cells, we evaluated the effect of an excess amount of native albumin on the binding, uptake and transport of labelled albumin (Supplementary Fig. S2). Since their behaviour was similar to that of ATII cells, we can assume the same underlying mechanisms for albumin clathrin-mediated endocytosis and transcytosis. This was further confirmed by additional studies in which inhibitors of caveolae-mediated endocytosis, N-ethylmalaimide (NEM) (Schnitzer et al. 1995) or filipin (Schnitzer et al. 1994), were applied to RLE monolayers. Of note, neither application of NEM nor filipin affected binding, uptake and transport of FITC-labelled albumin across the epithelium (Supplementary Fig. S3).
RLE-6TN cells were transfected then with rat megalin siRNA consisting of three target-specific 19- to 25-nt siRNAs; as a control we used a scrambled control siRNA. To establish transfection efficiency we determined megalin protein expression after transfection with siRNA (Fig. 6A) and by immunofluorescence compared with scrambled RNA (Fig. 6B). Megalin was silenced in RLE-6TN cells, which were plated on permeable supports to conduct cellular transport studies assessing binding, uptake and transepithelial transport of 125I-albumin. Cells were transfected with rat megalin siRNA on day 6 and experiments were performed on day 7. We found that binding of 125I-albumin was blocked by 85.7 ± 2.8% after gene silencing of megalin (Fig. 6C). 125I-albumin uptake by RLE-6TN cells was impaired by 88.9 ± 3.2% after RNA interference of megalin (Fig. 6D). Transepithelial transport across the RLE-6TN monolayer was decreased by 85.4 ± 2.7% when megalin gene expression was silenced by RNA interference (Fig. 6E). In conclusion, cellular transport studies confirmed that binding, uptake and transepithelial transport of 125I-albumin were strongly reduced when megalin expression was suppressed by siRNA. Altogether these data suggest that the multi-ligand endocytic receptor megalin facilitates albumin transport across the alveolar epithelium.
Figure 6. Albumin transport across the alveolar epithelium in RLE-TN6 cells treated with siRNA megalin.
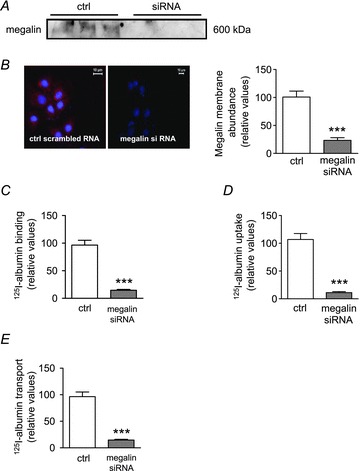
A, megalin protein expression and suppression with megalin siRNA in RLE-6TN cells, n = 3. B, megalin expression in RLE-6TN cells 24 h after gene silencing with megalin siRNA. Megalin expression was detected by immunofluorescence: left, control transfected with 50 pm scrambled siRNA; right, transfected with 50 pm rat megalin siRNA. C, binding of 125I-albumin to RLE-6TN cells was blocked by gene silencing of megalin (megalin siRNA) compared with control with scrambled RNA (ctrl). Binding of 125I-albumin to the cell surface was assessed by measuring γ-radiation, 30 min after administration, n = 3. D, 125I-albumin uptake by RLE-6TN cells 24 h after transfection with megalin siRNA. n = 3. E, transepithelial transport of 125I-albumin across monolayers of RLE-6TN cells after transfection of megalin siRNA. Thirty minutes after administration of 125I-albumin samples were taken from the basolateral side of the monolayer and the amount of 125I-albumin was determined by measurement of γ-radiation, n = 3; data represent the mean ± SEM, ***P < 0.001.
Discussion
Mechanisms of protein clearance in the lung are of great clinical significance as excess alveolar albumin, in particular, has been implicated in the pathogenesis of ARDS and has been identified as a prognostic factor for this syndrome (Bachofen & Weibel, 1977). Levels of plasma proteins are elevated in the alveolar fluids from patients with ARDS, contributing to the persistence of pulmonary oedema and thereby impeding healing, and thus clearance, of the excess alveolar protein, which is a key step in the resolution of acute lung injury (Hastings et al. 2004). Therefore, identifying the mechanisms that drive removal of excess protein content from the alveolar space is of high clinical importance. Recent in vitro studies have shown that A549 and RLE-6TN cells, two cell culture models for type II alveolar epithelial cells, absorb albumin via clathrin-mediated endocytosis (Kim & Malik, 2003; Kim et al. 2003; Yumoto et al. 2006). Very little is known about the mechanisms of protein clearance in the lungs.
We addressed this question using cell cultures systems and an isolated, ventilated and perfused rabbit lung model. Employing an intact, but isolated organ model has several advantages: (1) being able to discern the (patho)physiology of the lung from the remainder of the organism, (2) the capability to monitor and control haemodynamics and measure relevant physiological parameters (Seeger et al. 1994) and (3) ability to monitor transport rates in real-time. Previous reports agree that mucociliary clearance and phagocytosis by macrophages contribute marginally to the clearance of excess protein from the air spaces, whereas cleavage of proteins, and passive or active transport across the epithelium might be of importance in trafficking of proteins across the alveolar epithelium (Hastings et al. 2004). Furthermore, it remains controversial whether albumin transport across the alveolo-capillary barrier is an active process (Matthay et al. 1985; Berthiaume et al. 1989; Hastings et al. 1994). Earlier studies suggested that protein transport across the alveolo-capillary barrier was mainly a passive process. However, there are several possible explanations for these discrepant results. First, instillation of a significant amount of fluid in the airspace of unanaesthetized animals could cause atelectasis and hence decreasing the lung surface area available for protein reabsorption. In our model we applied positive end-expiratory pressure and therefore prevented the formation of atelectasis. Second, by instilling proteinaceus fluid intratracheally only a relatively small amount of protein might reach the distal airspaces, whereas we used nebulization as a method of tracer delivery which resulted in about 50% of the tracer being deposited into the lungs which is comparable with previously published results (Vadasz et al. 2005b).
Our results show that under physiological conditions (37°C) approximately 30% of the albumin deposited into the airspace via nebulization was cleared within a 2 h experimental period. The clearance of 125I-labelled albumin is almost completely abated at 4°C compared with control conditions whereas passive paracellular permeability is unaffected by temperature. In our isolated rabbit lung model an albumin gradient between the alveolar compartment and vascular space exists (as the perfusate is protein-free), which might promote passive movement of albumin from the distal air space to the vasculature. However, in the presence of excess albumin in the vascular compartment, therefore eliminating that gradient, no change in the clearance of 125I-albumin from the airspaces occurred.
It was also found that movement of albumin through the alveolo-capillary barrier was unidirectional. When FITC-labelled albumin was administered to the vascular compartment of the lung, the transport rate from the vasculature to the alveolar compartment was less dependent on temperature and was similar to the movement rate measured at 4°C from the air space to the perfusate. Thus, movement of albumin through the air–blood barrier of the lung is facilitated mainly via active transport in a uni-directional manner. The high albumin clearance rate observed in our isolated ventilated lung preparation was not due to albumin tracer dissociation or fragmentation of the protein. Thus, our findings suggest that albumin transport across the alveolo-capillary barrier is an active, temperature-dependent and uni-directional process that is independent of albumin degradation by proteases in the ELF. Therefore we concluded that intact albumin was probably taken up by the alveolar epithelium. To determine the route through which 125I-albumin was taken up by the alveolar epithelium we measured 125I-albumin clearance from the alveolar airspaces in the presence of a 1000-fold molar excess of unlabelled albumin. Deposition of the excess native albumin into the alveolar space significantly decreased the active transport of 125I-albumin out of the air spaces; however, only about 30% of the active albumin transport was inhibited. In contrast, when similar experiments were performed in cultured alveolar epithelial cells, a 100-fold molar excess of unlabelled albumin resulted in an almost complete block of radio-labelled albumin uptake. We believe that the somewhat less marked effects of excess albumin in our intact lung preparation were a result of inability of our aerosol delivery system to deposit the complete amount of albumin to the alveoli. Albumin absorption across the alveolar epithelium could occur by two pathways: (1) non-specific endocytosis (macropinocytosis) or (2) receptor-mediated endocytosis. However, it is well established that macropinocytosis occurs at a much lower rate than estimated for albumin transport (John et al. 2001; Kim & Malik, 2003). Furthermore, in contrast to our findings, pinocytosis is not a saturable process nor can it be competitively inhibited (Conner & Schmid, 2003). Clathrin-mediated endocytosis occurs in all mammalian cells and carries out the continuous uptake of nutrients, including several proteins, after binding of the molecules to specific receptors (Conner & Schmid, 2003). Coated pits are formed by the assembly of cytosolic coat proteins, the main assembly unit being clathrin (Owen, 2004). In a cell culture-based model RLE-6TN, an alveolar type II-like cell line, it has been shown that albumin is absorbed via clathrin-mediated endocytosis (Yumoto et al. 2006). The effect of clathrin inhibitors, chlorpromazine and phenylarsine oxide, were first examined on albumin clearance from the distal air space. Chlorpromazine inhibits the process by causing the loss of coated pits from the cell surface, probably by preventing AP-2 binding to membranes (Wang et al. 1993). Phenylarsine oxide inhibits clathrin-mediated endocytosis by reacting with sulfhydryls to form stable ring structures thereby preventing formation of the clathrin-coated vesicle (Visser et al. 2004). When either of these inhibitors was deposited into the alveolar space of lungs by aerosolization, a significant decrease in 125I-albumin transport was evident, suggesting an important role for clathrin function in the uptake of albumin by the alveolar epithelium. Inhibition of caveolae-mediated endocytosis did not affect albumin clearance, further emphasizing the pivotal role of clathrin-mediated endocytosis in alveolar protein transport. These findings are in line with a recent report by Yumoto et al. who found no inhibition of albumin uptake in alveolar epithelial cells after blocking caveolae-mediated endocytosis with nystatine, indomethacin or methyl-β-cyclodextrin (Yumoto et al. 2006). After completion of endocytosis internalized proteins could follow two basic pathways: (1) transport of proteins through the epithelium mediated by transcytosis (Tuma & Hubbard, 2003) or (2) transport to lysosomes and/or proteasomes for degradation (Rivett, 1990). The application of the actin stabilizer PO, a potent inhibitor of endo- and transcytosis, resulted in a marked block of 125I-albumin clearance. When monensin, an inhibitor of cellular vesicular transport, was administered albumin clearance from the lung was significantly inhibited, confirming the notion that transcytosis is required for albumin removal from the alveolar space. However, the degree of inhibition with monensin was not as pronounced as the effect of PO on albumin transport. This corroborates our hypothesis that endo- and/or transcytotic events are mediating protein movement across the alveolo-capillary barrier.
The complexity of the isolated lung model makes it difficult to ascribe physiological and biochemical events to specific cell types. Therefore, we employed primary cells and culture models and the alveolar cell culture line RLE-6TN for additional molecular studies. We primarily isolated ATII cells and secondarily differentiated them into ATI-like cells, which is a well established model system for ATI cells (Ridge et al. 2003). We assessed binding of labelled albumin to the cell surface of ATII and ATI-like cells, as well as uptake by the cells. Since cells were plated on permeable supports, measurement of transepithelial transport was also possible. Binding, uptake and transepithelial transport of labelled albumin were almost completely blocked by competition with unlabelled albumin, in both alveolar epithelial cell lines. These data indicate that albumin uptake by primary alveolar epithelial cells and its subsequent transcytosis are facilitated by an active transport process, which can be competitively inhibited. Interestingly, ATI-like and ATII cells displayed similar behaviour during those experiments. ATI cells cover over 90% of the alveolar surface; however, as the number of ATII and ATI cells is approximately identical, we assume that both cells types play an equal role in protein clearance. We also demonstrated that albumin binding and uptake at 37°C is a concentration-dependent and saturable process, whereas uptake and binding at 4°C occurs at a much lower and linear rate and is therefore thought to be passive. As an additional control we used FITC-labelled dextran (with 70 kDa of comparable size) and found its kinetic also to be linear and temperature independent, therefore again supporting the hypothesis of an active specific albumin transport. To study the capacity of protein transport we used clinically relevant concentrations of FITC-labelled albumin ranging from physiological protein concentrations in the ELF to pathological concentrations. We found the capacity of our system well within the range of proteinaceous exudates found in patients with ARDS (40–90% of the plasma protein concentration) (Hastings et al. 2004) and thus highly clinically relevant. Several receptors for albumin have been identified including the multiligand, endocytic receptors megalin and cubilin. In epithelia co-expressing megalin and cubilin the two receptors act in concert, mediating endocytosis of the same ligands (Birn & Christensen, 2006). Importantly, megalin and cubilin were recently shown to be essential for albumin reabsorption from the proximal tubule of the kidney (Birn et al. 2000). Megalin and cubilin have been recently reported to be expressed in the lung, where they appear to mediate high-density lipoprotein uptake by the alveolar epithelium (Kolleck et al. 2002b); however, involvement of the receptors in albumin uptake by ATII and ATI-like cells has not yet been reported. To investigate whether megalin mediates albumin uptake, receptor-associated protein (RAP) (Verroust et al. 2002; Verroust & Christensen, 2002), which binds to megalin with high affinity, was used.
We treated rat primary ATII and ATI-like cells with RAP (Williams et al. 1992), which binds to megalin, thereby competitively inhibiting the binding of albumin. Binding, uptake and transepithelial transport of albumin were significantly decreased when cells were treated with RAP in both type II and type I-like cells, suggesting a central role for the megalin/cubilin receptor complex in alveolar epithelial albumin endocytosis. However, pharmacological inhibitors may have non-specific effects on unrelated proteins, receptors and signalling pathways. RAP, for instance, is also known to modulate ligand interactions with LDL receptor-related protein (LRP), another multi-ligand receptor in the low-density lipoprotein receptor family (Herz et al. 1991). Thus, to further elucidate the specific role of megalin in albumin uptake by alveolar epithelial cells, RNA interference via the siRNA approach to specifically suppress megalin was used. In megalin knocked down RLE-6TN cells the uptake of 125I-albumin and FITC-albumin was almost completely blocked, as well as the binding and transepithelial transport of 125I-albumin. Collectively, these data indicate that megalin facilitates albumin endocytosis in the alveolar epithelium of the lung. This finding is in line with the observation that homozygous megalin knock-out mice die almost immediately after birth due to respiratory failure (Willnow et al. 1996). Heterozygous mice live to adulthood; however, under normal circumstances the protein concentration in the ELF is quite low. Further studies are required to investigate how those mice would behave when challenged in a model of acute lung injury.
In summary, we provide evidence that under physiological conditions, albumin transport in the alveolar epithelium is facilitated by an active, megalin-mediated transport process. Our data suggest that alveolar cells do possess a system to actively clear albumin from the alveolar space with a capacity sufficient to clear proteinacoeus exudates in concentrations seen in ARDS. We demonstrated this by using a physiological isolated rabbit lung model as well as in primary alveolar type I and type II cells. Further studies are warranted to elucidate the regulatory mechanisms underlying alveolar epithelial transport in health and disease. Better understanding of these mechanisms may ultimately lead to novel therapeutic approaches for the treatment of ARDS.
Acknowledgments
The authors thank Miriam Wessendorf for excellent technical assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG/IRTG1062), the Excellence Cluster ‘Cardio Pulmonary System’ (ECCPS), the German Center for Lung Research (DZL), the Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz (LOEWE) of the Hessen State Ministry of Higher Education, Research and the Arts (for K.M., S.H., R.E.M., W.S. and I.V.) and the University Medical Center Giessen and Marburg (grant 62589064; for I.V.). I.V. was supported by the Else Kröner Memorial Award.
Glossary
- AEC
alveolar epithelial cells
- ALI
acute lung injury
- ARDS
adult respiratory distress syndrome
- ATI
alveolar type I-like cell
- ATII
alveolar type II cell
- BAL
bronchoalveolar lavage
- ELF
epithelial lining fluid
- FITC
fluorescein isothiocyanate
- PO
phalloidin oleate
- RAP
receptor-associated protein
- RLE
rat alveolar epithelial cell
Author contributions
All laboratory experiments were performed at the Department of Internal Medicine at the Justus Liebig University, Universities of Giessen and Marburg Lung Center. The authors contributed to the following aspects of the study: conception and design of the experiments: I.V., W.S., R.E.M. and C.U.V.; collection, analysis and interpretation of data: Y.B., S.R., C.U.V., N.M.G., B.A.G., K.M. and S.H.; drafting the article or revising it critically for important intellectual content: C.U.V., S.R., Y.B., B.A.G. and I.V. All authors approved the final version of the manuscript.
Supplementary material
Supplementary methods
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
References
- Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977;116:589–615. doi: 10.1164/arrd.1977.116.4.589. [DOI] [PubMed] [Google Scholar]
- Berthiaume Y, Albertine KH, Grady M, Fick G, Matthay MA. Protein clearance from the air spaces and lungs of unanesthetized sheep over 144 h. J Appl Physiol. 1989;67:1887–1897. doi: 10.1152/jappl.1989.67.5.1887. [DOI] [PubMed] [Google Scholar]
- Birn H, Christensen EI. Renal albumin absorption in physiology and pathology. Kidney Int. 2006;69:440–449. doi: 10.1038/sj.ki.5000141. [DOI] [PubMed] [Google Scholar]
- Birn H, Fyfe JC, Jacobsen C, Mounier F, Verroust PJ, Orskov H, Willnow TE, Moestrup SK, Christensen EI. Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J Clin Invest. 2000;105:1353–1361. doi: 10.1172/JCI8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso-Neves C, Kwon SH, Guggino WB. Albumin endocytosis in proximal tubule cells is modulated by angiotensin II through an AT2 receptor-mediated protein kinase B activation. Proc Natl Acad Sci U S A. 2005;102:17513–17518. doi: 10.1073/pnas.0507255102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiancone E, Thulin E, Boffi A, Forsen S, Brunori M. Evidence for the interaction between the calcium indicator 1,2-bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid and calcium-binding proteins. J Biol Chem. 1986;261:16306–16308. [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Danto SI, Zabski SM, Crandall ED. Reactivity of alveolar epithelial cells in primary culture with type I cell monoclonal antibodies. Am J Respir Cell Mol Biol. 1992;6:296–306. doi: 10.1165/ajrcmb/6.3.296. [DOI] [PubMed] [Google Scholar]
- Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 1990;258:L134–L147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- Fisher CE, Howie SE. The role of megalin (LRP-2/Gp330) during development. Dev Biol. 2006;296:279–297. doi: 10.1016/j.ydbio.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Folkesson HG, Matthay MA, Westrom BR, Kim KJ, Karlsson BW, Hastings RH. Alveolar epithelial clearance of protein. J Appl Physiol. 1996;80:1431–1445. doi: 10.1152/jappl.1996.80.5.1431. [DOI] [PubMed] [Google Scholar]
- Folkesson HG, Westrom BR, Dahlback M, Lundin S, Karlsson BW. Passage of aerosolized BSA and the nona-peptide dDAVP via the respiratory tract in young and adult rats. Exp Lung Res. 1992;18:595–614. doi: 10.3109/01902149209031697. [DOI] [PubMed] [Google Scholar]
- Folkesson HG, Westrom BR, Karlsson BW. Permeability of the respiratory tract to different-sized macromolecules after intratracheal instillation in young and adult rats. Acta Physiol Scand. 1990;139:347–354. doi: 10.1111/j.1748-1716.1990.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Ghofrani HA, Kohstall MG, Weissmann N, Schmehl T, Schermuly RT, Seeger W, Grimminger F. Alveolar epithelial barrier functions in ventilated perfused rabbit lungs. Am J Physiol Lung Cell Mol Physiol. 2001;280:L896–L904. doi: 10.1152/ajplung.2001.280.5.L896. [DOI] [PubMed] [Google Scholar]
- Gorin AB, Stewart PA. Differential permeability of endothelial and epithelial barriers to albumin flux. J Appl Physiol. 1979;47:1315–1324. doi: 10.1152/jappl.1979.47.6.1315. [DOI] [PubMed] [Google Scholar]
- Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross NJ. Extracellular metabolism of pulmonary surfactant: the role of a new serine protease. Annu Rev Physiol. 1995;57:135–150. doi: 10.1146/annurev.ph.57.030195.001031. [DOI] [PubMed] [Google Scholar]
- Hastings RH, Folkesson HG, Matthay MA. Mechanisms of alveolar protein clearance in the intact lung. Am J Physiol Lung Cell Mol Physiol. 2004;286:L679–L689. doi: 10.1152/ajplung.00205.2003. [DOI] [PubMed] [Google Scholar]
- Hastings RH, Folkesson HG, Petersen V, Ciriales R, Matthay MA. Cellular uptake of albumin from lungs of anesthetized rabbits. Am J Physiol Lung Cell Mol Physiol. 1995;269:L453–L462. doi: 10.1152/ajplung.1995.269.4.L453. [DOI] [PubMed] [Google Scholar]
- Herz J, Goldstein JL, Strickland DK, Ho YK, Brown MS. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/α2-macroglobulin receptor. J Biol Chem. 1991;266:21232–21238. [PubMed] [Google Scholar]
- Ikehata M, Yumoto R, Nakamura K, Nagai J, Takano M. Comparison of albumin uptake in rat alveolar type II and type I-like epithelial cells in primary culture. Pharm Res. 2008;25:913–922. doi: 10.1007/s11095-007-9426-x. [DOI] [PubMed] [Google Scholar]
- John TA, Vogel SM, Minshall RD, Ridge K, Tiruppathi C, Malik AB. Evidence for the role of alveolar epithelial gp60 in active transalveolar albumin transport in the rat lung. J Physiol. 2001;533:547–559. doi: 10.1111/j.1469-7793.2001.0547a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Malik AB. Protein transport across the lung epithelial barrier. Am J Physiol Lung Cell Mol Physiol. 2003;284:L247–L259. doi: 10.1152/ajplung.00235.2002. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Matsukawa Y, Yamahara H, Kalra VK, Lee VH, Crandall ED. Absorption of intact albumin across rat alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2003;284:L458–L465. doi: 10.1152/ajplung.00237.2002. [DOI] [PubMed] [Google Scholar]
- Kolleck I, Sinha P, Rustow B. Vitamin E as an antioxidant of the lung: mechanisms of vitamin E delivery to alveolar type II cells. Am J Respir Crit Care Med. 2002a;166:S62–S66. doi: 10.1164/rccm.2206019. [DOI] [PubMed] [Google Scholar]
- Kolleck I, Wissel H, Guthmann F, Schlame M, Sinha P, Rustow B. HDL-holoparticle uptake by alveolar type II cells: effect of vitamin E status. Am J Respir Cell Mol Biol. 2002b;27:57–63. doi: 10.1165/ajrcmb.27.1.4774. [DOI] [PubMed] [Google Scholar]
- Lunn G, Sansone EB. Degradation and disposal of some enzyme inhibitors. Scientific note. Appl Biochem Biotechnol. 1994;48:57–59. doi: 10.1007/BF02796162. [DOI] [PubMed] [Google Scholar]
- Matthay MA, Berthiaume Y, Staub NC. Long-term clearance of liquid and protein from the lungs of unanesthetized sheep. J Appl Physiol. 1985;59:928–934. doi: 10.1152/jappl.1985.59.3.928. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Owen DJ. Linking endocytic cargo to clathrin: structural and functional insights into coated vesicle formation. Biochem Soc Trans. 2004;32:1–14. doi: 10.1042/bst0320001. [DOI] [PubMed] [Google Scholar]
- Perry SW, Epstein LG, Gelbard HA. Simultaneous in situ detection of apoptosis and necrosis in monolayer cultures by TUNEL and trypan blue staining. Biotechniques. 1997;22:1102–1106. doi: 10.2144/97226st01. [DOI] [PubMed] [Google Scholar]
- Ridge KM, Olivera WG, Saldias F, Azzam Z, Horowitz S, Rutschman DH, Dumasius V, Factor P, Sznajder JI. Alveolar type 1 cells express the α2 Na,K-ATPase, which contributes to lung liquid clearance. Circ Res. 2003;92:453–460. doi: 10.1161/01.RES.0000059414.10360.F2. [DOI] [PubMed] [Google Scholar]
- Rivett AJ. Eukaryotic protein degradation. Curr Opin Cell Biol. 1990;2:1143–1149. doi: 10.1016/0955-0674(90)90168-e. [DOI] [PubMed] [Google Scholar]
- Rutschman DH, Olivera W, Sznajder JI. Active transport and passive liquid movement in isolated perfused rat lungs. J Appl Physiol. 1993;75:1574–1580. doi: 10.1152/jappl.1993.75.4.1574. [DOI] [PubMed] [Google Scholar]
- Saito A, Pietromonaco S, Loo AK, Farquhar MG. Complete cloning and sequencing of rat gp330/“megalin,” a distinctive member of the low density lipoprotein receptor gene family. Proc Natl Acad Sci U S A. 1994;91:9725–9729. doi: 10.1073/pnas.91.21.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M, Byron PR, Rypacek F. Biochemical evidence for transcytotic absorption of polyaspartamide from the rat lung: effects of temperature and metabolic inhibitors. J Pharm Sci. 2002;91:1958–1968. doi: 10.1002/jps.10188. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Allard J, Oh P. NEM inhibits transcytosis, endocytosis, and capillary permeability: implication of caveolae fusion in endothelia. Am J Physiol Heart Circ Physiol. 1995;268:H48–H55. doi: 10.1152/ajpheart.1995.268.1.H48. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger W, Walmrath D, Grimminger F, Rosseau S, Schutte H, Kramer HJ, Ermert L, Kiss L. Adult respiratory distress syndrome: model systems using isolated perfused rabbit lungs. Methods Enzymol. 1994;233:549–584. doi: 10.1016/s0076-6879(94)33060-3. [DOI] [PubMed] [Google Scholar]
- Stenbeck G, Horton MA. Endocytic trafficking in actively resorbing osteoclasts. J Cell Sci. 2004;117:827–836. doi: 10.1242/jcs.00935. [DOI] [PubMed] [Google Scholar]
- Tagawa M, Yumoto R, Oda K, Nagai J, Takano M. Low-affinity transport of FITC-albumin in alveolar type II epithelial cell line RLE-6TN. Drug Metab Pharmacokinet. 2008;23:318–327. doi: 10.2133/dmpk.23.318. [DOI] [PubMed] [Google Scholar]
- Takano M, Nakanishi N, Kitahara Y, Sasaki Y, Murakami T, Nagai J. Cisplatin-induced inhibition of receptor-mediated endocytosis of protein in the kidney. Kidney Int. 2002;62:1707–1717. doi: 10.1046/j.1523-1755.2002.00623.x. [DOI] [PubMed] [Google Scholar]
- Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- Vadasz I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Toth PT, Lecuona E, Witters LA, Schumacker PT, Chandel NS, Seeger W, Sznajder JI. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J Clin Invest. 2008a;118:752–762. doi: 10.1172/JCI29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadasz I, Morty RE, Kohstall MG, Olschewski A, Grimminger F, Seeger W, Ghofrani HA. Oleic acid inhibits alveolar fluid reabsorption: a role in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005a;171:469–479. doi: 10.1164/rccm.200407-954OC. [DOI] [PubMed] [Google Scholar]
- Vadasz I, Morty RE, Olschewski A, Konigshoff M, Kohstall MG, Ghofrani HA, Grimminger F, Seeger W. Thrombin impairs alveolar fluid clearance by promoting endocytosis of Na+,K+-ATPase. Am J Respir Cell Mol Biol. 2005b;33:343–354. doi: 10.1165/rcmb.2004-0407OC. [DOI] [PubMed] [Google Scholar]
- Vadasz I, Raviv S, Sznajder JI. Alveolar epithelium and Na,K-ATPase in acute lung injury. Intensive Care Med. 2007;33:1243–1251. doi: 10.1007/s00134-007-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadasz I, Schermuly RT, Ghofrani HA, Rummel S, Wehner S, Muhldorfer I, Schafer KP, Seeger W, Morty RE, Grimminger F, Weissmann N. The lectin-like domain of tumor necrosis factor-alpha improves alveolar fluid balance in injured isolated rabbit lungs. Crit Care Med. 2008b;36:1543–1550. doi: 10.1097/CCM.0b013e31816f485e. [DOI] [PubMed] [Google Scholar]
- Verroust PJ, Birn H, Nielsen R, Kozyraki R, Christensen EI. The tandem endocytic receptors megalin and cubilin are important proteins in renal pathology. Kidney Int. 2002;62:745–756. doi: 10.1046/j.1523-1755.2002.00501.x. [DOI] [PubMed] [Google Scholar]
- Verroust PJ, Christensen EI. Megalin and cubilin – the story of two multipurpose receptors unfolds. Nephrol Dial Transplant. 2002;17:1867–1871. doi: 10.1093/ndt/17.11.1867. [DOI] [PubMed] [Google Scholar]
- Visser CC, Stevanovic S, Heleen Voorwinden L, Gaillard PJ, Crommelin DJ, Danhof M, De Boer AG. Validation of the transferrin receptor for drug targeting to brain capillary endothelial cells in vitro. J Drug Target. 2004;12:145–150. doi: 10.1080/10611860410001701706. [DOI] [PubMed] [Google Scholar]
- Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Ashcom JD, Argraves WS, Strickland DK. A novel mechanism for controlling the activity of alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein. Multiple regulatory sites for 39-kDa receptor-associated protein. J Biol Chem. 1992;267:9035–9040. [PubMed] [Google Scholar]
- Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, Herz J. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci U S A. 1996;93:8460–8464. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumoto R, Nishikawa H, Okamoto M, Katayama H, Nagai J, Takano M. Clathrin-mediated endocytosis of FITC-albumin in alveolar type II epithelial cell line RLE-6TN. Am J Physiol Lung Cell Mol Physiol. 2006;290:L946–L955. doi: 10.1152/ajplung.00173.2005. [DOI] [PubMed] [Google Scholar]
- Yumoto R, Suzuka S, Oda K, Nagai J, Takano M. Endocytic uptake of FITC-albumin by human alveolar epithelial cell line A549. Drug Metab Pharmacokinet. 2012;27:336–343. doi: 10.2133/dmpk.dmpk-11-rg-127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


