Abstract
Neonates respond to hypoxia initially by increasing ventilation, and then by markedly decreasing both ventilation (hypoxic ventilatory decline) and oxygen consumption (hypoxic hypometabolism). This latter process, which vanishes with age, reflects a tight coupling between ventilatory and thermogenic responses to hypoxia. The neurological substrate of hypoxic hypometabolism is unclear, but it is known to be centrally mediated, with a strong involvement of the 5-hydroxytryptamine (5-HT, serotonin) system. To clarify this issue, we investigated the possible role of VGLUT3, the third subtype of vesicular glutamate transporter. VGLUT3 contributes to glutamate signalling by 5-HT neurons, facilitates 5-HT transmission and is expressed in strategic regions for respiratory and thermogenic control. We therefore assumed that VGLUT3 might significantly contribute to the response to hypoxia. To test this possibility, we analysed this response in newborn mice lacking VGLUT3 using anatomical, biochemical, electrophysiological and integrative physiology approaches. We found that the lack of VGLUT3 did not affect the histological organization of brainstem respiratory networks or respiratory activity under basal conditions. However, it impaired respiratory responses to 5-HT and anoxia, showing a marked alteration of central respiratory control. These impairments were associated with altered 5-HT turnover at the brainstem level. Furthermore, under cold conditions, the lack of VGLUT3 disrupted the metabolic rate, body temperature, baseline breathing and the ventilatory response to hypoxia. We conclude that VGLUT3 expression is dispensable under basal conditions but is required for optimal response to hypoxic stress in neonates.
Key points
Hypoxic stress is an important cause of morbidity and mortality in neonates.
We examined the role of VGLUT3, an atypical transporter of glutamate present in serotonergic neurons involved in breathing and heat production, in the response to hypoxia.
The respiratory responses to chemical stimuli and the turnover of serotonin in the brainstem were impaired in newborn mice lacking VGLUT3.
Under cold conditions, metabolic rate, body temperature, baseline breathing and the ventilatory response to hypoxia were disrupted.
Thus, VGLUT3 expression is required for optimal response to hypoxic stress in neonates.
Introduction
Maintaining oxygen homeostasis through independent breathing is a critical ability throughout extrauterine life. Hypoxic stress, whether due to increased oxygen demands as in the cold, or to reduced oxygen delivery to tissues as in asphyxia, is a common cause of morbidity and mortality in neonates (Perrone et al. 2002). Neonates respond to hypoxia initially by increasing ventilation, and then by markedly decreasing ventilation (hypoxic ventilatory decline, HVD) and oxygen consumption (hypoxic hypometabolism), mainly through a centrally mediated inhibition of heat production (Saiki et al. 1994; Merazzi & Mortola, 1999; Bollen et al. 2009). This centrally mediated process is prominent in neonates but gradually vanishes with age (Massaro et al. 1995; Gautier, 1996; Mortola, 2004; Kamae et al. 2011). The neuronal substrate of hypoxic hypometabolism is unclear, but recent results support a strong involvement of the serotonin (5-hydroxytryptamine or 5-HT) system in the coupling of ventilatory and thermogenic responses to hypoxia (Alenina et al. 2009; Hodges et al. 2011; Osaka, 2011).
In this study, we examined the possible role of VGLUT3, the third subtype of vesicular glutamate transporter, in the ventilatory and thermoregulatory responses to neonatal hypoxic stress. Our interest in VGLUT3 originated in the intriguing observation that, contrary to VGLUT1 and VGLUT2, which are expressed by genuine glutamatergic neurons (Fremeau et al. 2004), VGLUT3 is present mostly in modulatory neurons (El Mestikawy et al. 2011). This is the case of cholinergic interneurons from the striatum, some GABAergic neurons from the cortex and the hippocampus, and most importantly with regards to oxygen homeostasis, in 5-HT neurons (El Mestikawy et al. 2011). In serotonergic neurons, VGLUT3 stimulates 5-HT transmission and provides the means to signal with glutamate (Amilhon et al. 2010; Varga et al. 2009). We anticipated that VGLUT3 may be important for the newborn's ability to generate the appropriate respiratory responses to hypoxic stress for two reasons. First, the two networks that compose the respiratory rhythm generator (RRG, Thoby-Brisson et al. 2009), the pre-Bötzinger complex (Smith et al. 1991) and the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG Onimaru & Homma, 2003), contain glutamatergic neurons and are both strongly modulated by 5-HT afferents. The RTN/pFRG, which is pivotal to central chemoreception (Guyenet et al. 2008), is densely innervated by VGLUT3 terminals from the raphe nuclei (Rosin et al. 2006). Second, thermoregulatory circuits contain VGLUT3 expressing cells (Morrison et al. 2008; Nakamura et al. 2005a; Schafer et al. 2002). In particular, VGLUT3 expressing cells in the raphe innervate the main neonatal thermogenic organ, the interscapular brown adipose tissue (Schafer et al. 2002; Nakamura et al. 2005a; Morrison et al. 2008).
To address the role of VGLUT3 in the response to hypoxic stress in neonates, we analysed mice lacking VGLUT3 (Vglut3−/−) using anatomical, biochemical, electrophysiological and integrative physiology approaches. Previous studies in Vglut3−/− mice showed that VGLUT3 is a resistance factor against psychological stress as early as infancy, but its possible role against physiological stress has never been investigated (Amilhon et al. 2010). We found that while the lack of VGLUT3 did not affect the histological organization of brainstem respiratory networks or respiratory activity in normal conditions, it disrupted respiratory and thermogenic responses to cold and hypoxia, and in particular, HVD and hypoxic hypometabolism. These results indicate that VGLUT3 expression is required for optimal response to hypoxic stress in neonates.
Methods
Ethical approval
Experimental protocols were approved by local committees, in accordance with the European Communities Council Directive of September 22, 2010 (2010/63/EU) for animal care, and were conducted in accordance with Canadian and French laws for animal care. The experiments also complied with the policies and regulations outlined by The Journal of Physiology (Drummond, 2009). All efforts were made to minimize animal suffering, especially by using fully non-invasive functional tests. Newborn mice used in in vivo experiments were killed by decapitation upon completion of physiological tests.
Animals
All experiments were performed in newborn (n = 474) wild-type, heterozygous and homozygous littermates obtained from crossing heterozygous Vglut3+/− mice (129/Sv × C57/BL6). Vglut3+/− parents (Gras et al. 2008) were housed at 24°C with a 12 h light–dark cycle and fed ad libitum.
Immunofluorescence
Six-day-old mice were anaesthetized deeply by an intraperitoneal injection of 1% sodium pentobarbital (60 mg (kg body weight)−1), and perfused transcardially with 150 ml warm 0.9% saline followed by 500 ml ice-cold 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4). The brains were dissected, conserving the pons, post-fixed by immersion in the same fixative, cryoprotected in phosphate-buffered saline (PBS) containing 10% sucrose and frozen in isopentane at −30°C. Ten-micrometre coronal sections were made with a cryostat at −20°C and thaw-mounted onto glass slides. Sections were hydrated with PBS and immersed in EDTA (10 mm). They were then washed with PBS containing gelatin (2 g l−1) and 0.25% Triton X-100 (buffer B) and incubated with buffer B supplemented with VGLUT3 guinea pig polyclonal antiserum (1/10000; Gras et al. 2008), PHOX2B rat monoclonal antiserum (1/800; kindly provided by Dr Christo Goridis, Ecole Normale Supérieure, Paris), 5-HT mouse monoclonal antiserum (1/50; Chemicon), VMAT2 rabbit monoclonal antiserum (1/5000; Chemicon) and NK1R rabbit monoclonal antiserum (1/5000; Sigma-Aldrich). VGLUT3, PHOX2B and 5-HT primary antibodies were detected with anti-guinea pig, anti-rat or anti-mouse IgG coupled to Alexa Fluor 488, Alexa Fluor 544 and Alexa Fluor 544 (1/2000; Molecular Probes, USA), respectively. VMAT2 and NK1R primary antibodies were detected using anti-rabbit IgG coupled to Alexa Fluor 544 (1/2000; Molecular Probes, USA). Sections were observed under a conventional fluorescence microscope (Zeiss, Germany) at 10× and 40× magnification. The Vglut3−/− mice is a constitutive knockout obtained by targeting exon 2 of the Vglut3 gene. This strategy results in a loss of this coding exon and a one base frame shift. Consequently, there is a complete loss of both mRNA and protein (Gras et al. 2008).
Electrophysiology
Two- to three-day-old mice were chilled over crushed ice for several minutes until they were immobile and failed to respond to a tail pinch (Phifer & Terry, 1986). The brainstem and the first segment of cervical spinal cord were isolated and placed ventral side upward in a recording chamber (volume: 2 ml) as described (Suzue, 1984; Voituron et al. 2010). This en bloc preparation is devoid of peripheral chemoreceptors including the carotid bodies, O2 sensitive cells in the hypothalamus and peripheral thermoreceptors. Under basal conditions, the preparation was superfused at a rate of 4 ml min−1 at 27°C with artificial cerebrospinal fluid (aCSF; in mm: 129.0 NaCl, 3.35 KCl, 1.26 CaCl2, 1.15 MgCl2, 21.0 NaHCO3, 0.58 NaH2PO4 and 30.00 glucose) saturated with O2 and adjusted to pH 7.4 by bubbling with carbogen (95% O2 and 5% CO2). Recordings were performed as described (Voituron et al. 2010). Briefly, the electrical activity of a C4 ventral root was recorded using a suction electrode, filtered (100–3000 Hz), amplified (×5000), integrated (time constant 100 ms) and digitized through a Spike2 data analysis system (CED, Cambridge, UK; 5 kHz sampling frequency). Phrenic burst (PB) frequency (expressed in Hz) was defined as the frequency of spontaneous rhythmic C4 bursts. Integrated C4 activities were used to measure the duration of PBs (expressed in seconds) and the irregularity score (IS) of the PB cycle period. The IS was used to assess the variability of the respiratory cycle and was obtained by applying the formula: 100 × ABS (Pn–Pn−1)/Pn−1, with P being the period of the nth respiratory cycle (Viemari et al. 2005). After dissection, the preparations were superfused for at least 30 min with normal aCSF. The phrenic responses to 5-HT, acidosis and anoxia were examined on different preparations. To examine the PB response to 5-HT, normal aCSF was replaced with aCSF containing 5-HT (25 μm) for 5 min. The PB response to acidosis was examined by replacing normal aCSF (pH 7.4) with modified aCSF containing a reduced level of NaHCO3 (10.0 mm instead of 21 mm, pH 7.1; acidosis) (Voituron et al. 2010). The PB response to anoxia was examined by replacing normal aCSF by aCSF bubbled with 95% N2 and 5% CO2 for 5 min (anoxia). When subjecting preparations to modified aCSF, test-induced changes in PB frequency in a given preparation were expressed as a percentage of baseline levels determined during the 5 min period preceding the test.
Plethysmography
Breathing variables were measured noninvasively using a battery of four whole-body flow barometric plethysmographs, allowing the simultaneous measurement of breathing variables as previously described (Bollen et al. 2009). Each plethysmograph was composed of two 50 ml Plexiglas chambers, immersed in a thermoregulated water bath to maintain their temperature at given levels (26°C or 33°C). A 200 ml min−1 flow of dry air (Brooks airflow stabilizer, Urlo, Holland) was divided into two 100 ml min−1 flows through the chambers. The differential pressure between the chambers (GE Sensing transducer, Asnières, France, range: ±0.1 mbar) was converted into a digital signal at a sampling rate of 100 Hz, and processed (Labview, National Instruments, Austin, TX, USA).
The apparatus was calibrated before each session using a built-in pump incorporating a micro-syringe (Ito corporation, Fuji, Japan), which injected a sinusoidal airflow with a maximal amplitude of 2 μl and a frequency of 6 Hz into the animal chamber (Ramanantsoa et al. 2006). We measured respiratory frequency (Rf, Hz), tidal volume (VT, μl g−1), minute ventilation (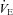 , μl s−1 g−1), and the number of apnoeas, defined as respiratory pauses longer than twice the preceding breath (Simakajornboon et al. 2004). The limitations of the plethysmographic method in newborn mice have been discussed elsewhere (Lofaso et al. 2007; Mortola & Frappell, 1998). Briefly, the absolute values of VT and
, μl s−1 g−1), and the number of apnoeas, defined as respiratory pauses longer than twice the preceding breath (Simakajornboon et al. 2004). The limitations of the plethysmographic method in newborn mice have been discussed elsewhere (Lofaso et al. 2007; Mortola & Frappell, 1998). Briefly, the absolute values of VT and 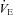 derived from the Drorbaugh and Fenn equation (Drorbaugh & Fenn, 1955; Epstein & Epstein, 1978) are purely indicative, whereas Rf and apnoea duration, as well as the relative change from baseline of VT, Rf and
derived from the Drorbaugh and Fenn equation (Drorbaugh & Fenn, 1955; Epstein & Epstein, 1978) are purely indicative, whereas Rf and apnoea duration, as well as the relative change from baseline of VT, Rf and 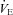 , are reliable measurements. O2 consumption (
, are reliable measurements. O2 consumption (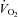 ) was measured in six-day-old pups in cold condition as previously described (Bollen et al. 2009). Baseline levels of breathing variables were calculated over the 3 min of air breathing prior to exposure to hypoxia (10% O2).
) was measured in six-day-old pups in cold condition as previously described (Bollen et al. 2009). Baseline levels of breathing variables were calculated over the 3 min of air breathing prior to exposure to hypoxia (10% O2).
Biochemical analysis of the brainstem serotonergic system
The medulla was quickly removed after decapitation on postnatal day 2 and kept at −80°C until analysis (Viemari et al. 2005). The medullary content of 5-HT, its precursor l-tryptophan (l-Trp) and its main metabolite from the monoamine oxidase A (MAOA) degradation pathway, 5-hydroxyindoleacetic acid (5-HIAA), were measured with high pressure liquid chromatography (HPLC) and electrochemical detection (Waters System: pump P510, electrochemical detector EC2465; Atlantis column DC18; mobile phase: citric acid, 50 mm; orthophosphoric acid, 50 mm; sodium octane sulfonic acid, 0.112 mm; EDTA, 0.06 mm; methanol, 5%; NaCl, 2 mm; pH 2.95). Contents were expressed in nanograms per gram of medulla.
Statistics
Lack of VGLUT3 was expected to impair 5-HT metabolism, respiratory control in vitro and physiological responses to hypoxic stress in vivo. We used Student's unpaired two-tailed t test for genotype group comparisons (mutant versus controls). Respiratory variables and 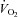 were firstly analysed in baseline conditions and in response to chemical stimuli using analyses of variance and Student's unpaired two-tailed t test. Litter had no significant effect, either as a main between factor or in interaction with genotype, and will not be mentioned further. All quantification was performed by investigators blind to the genotype, which was determined after all tests were completed. Statistical analyses were conducted using Statview 5 (Abacus Concepts, Berkeley, CA, USA).
were firstly analysed in baseline conditions and in response to chemical stimuli using analyses of variance and Student's unpaired two-tailed t test. Litter had no significant effect, either as a main between factor or in interaction with genotype, and will not be mentioned further. All quantification was performed by investigators blind to the genotype, which was determined after all tests were completed. Statistical analyses were conducted using Statview 5 (Abacus Concepts, Berkeley, CA, USA).
Results
As data from Vglut3+/+ and Vglut3+/− newborn mice were indistinguishable regardless of the variable considered, these two groups were pooled and thereafter designated as control pups.
Lack of VGLUT3 does not alter the histological organization and function of respiratory areas under basal conditions
We first compared coronal sections of the medulla of Vglut3−/− and control pups. No differences were seen in the organization of the characteristic NK1R signal of the pre-Bötzinger complex (Fig. 1A and B), or the medial bundle of NK1R-expressing fibres or the raphe pallidus (RPa), raphe magnus (RMg) and raphe obscurus (ROb) areas (Fig. 1A and B). Finally, we observed very ventral PHOX2B-expressing neurons in the RTN/pFRG area of control and Vglut3−/− pups, without any noticeable difference between these groups (Fig. 1A and B).
Figure 1. Lack of Vglut3 does not alter the histological organization and function of respiration-related areas under basal conditions.
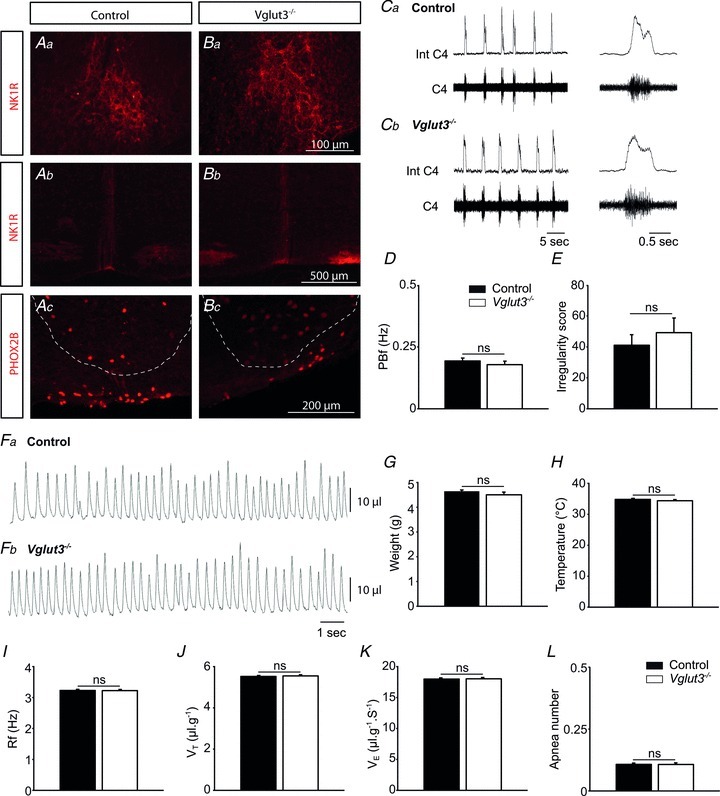
See Supplemental Fig. 1 for schematic representation of the brainstem sections. A and B, immunolabelling showing that NK1R (red) in the pre-Bötzinger complex (Aa and Ba) and at the midline raphe level (Ab and Bb) and Phox2B (red) at the RTN/pFRG level (Ac and Bc) have similar expression patterns in control and Vglut3−/− pups. Anatomical limits of the facial nucleus have been superimposed on Ac and Bc section (dotted line, Lazarenko et al. 2009). C, examples of phrenic bursts (PBs) recorded from the C4 root of in vitro brainstem preparations from control (a) and Vglut3−/− (b) pups. Int C4: integrated C4 activity. D and E, neither phrenic burst frequency (PBf) (D) nor the irregularity score (E) was significantly affected by the Vglut3 mutation under basal conditions (control n = 64; Vglut3−/−
n = 22). F, examples of recordings of respiratory variables using plethysmography in one control (a) and one Vglut3−/− (b) newborn mouse. G and H, Vglut3−/− pups (n = 53) had normal weights (G) and body temperatures (H) compared to control pups (n = 164). I–L, breathing patterns were not affected by the Vglut3 mutation under thermoneutral conditions, whatever the breathing variable considered: mean respiratory frequency (Rf) (I), tidal volume (VT) (J), ventilation (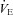 ) (K), or number of apnoeas per 30 s period (L). ns: non significant (P > 0.05); Student's unpaired t test. Values shown are means ± SEM. See Tables 1 and 2 for full statistical analyses.
) (K), or number of apnoeas per 30 s period (L). ns: non significant (P > 0.05); Student's unpaired t test. Values shown are means ± SEM. See Tables 1 and 2 for full statistical analyses.
We next examined whether the lack of VGLUT3 affected neonatal RRG function. We compared the activity produced by isolated RRGs from Vglut3−/− and control pups. Under basal conditions, phrenic bursts (PBs) had similar shapes, amplitudes, frequencies and irregularity scores in Vglut3−/− and control preparations (Fig. 1C and D, and Table 1 for statistical analyses).
Table 1.
RRG responses to chemical stimuli
| Control | Vglut3−/− | |
|---|---|---|
| Baseline | n = 64 | n = 22 |
| PB frequency (cycles min−1) | 11.6 ± 0.6 | 10.7 ± 1.0 |
| PB duration (s) | 0.9 ± 0.1 | 0.9 ± 0.1 |
| PB irregularity score (AU) | 41.1 ± 6.8 | 49.2 ± 9.3 |
| Response to 5-HT | n = 14 | n = 11 |
| PB frequency (%) at 1 min | 130.9 ± 9.3†† | 118.5 ± 8.0†,* |
| Response to acidosis | n = 12 | n = 4 |
| PB frequency (%) at 5 min | 132 ± 4††† | 153 ± 13††,* |
| Response to anoxia | n = 18 | n = 8 |
| PB frequency (%) | ||
| at 1 min | 87.2 ± 6.3††† | 94.7 ± 5.7 |
| at 5 min | 57.8 ± 5.5†††† | 97.4 ± 14.5*** |
PB: Phrenic burst; %: percentage of pre-stimulus value; AU: arbitrary units. *, **, ***: significant difference between Vglut3−/− and control groups (P < 0.05, P < 0.01 and P < 0.001, respectively, Student's unpaired t test.). †, ††, †††, ††††: significant difference between post-stimulus and pre-stimulus levels (P < 0.05, P < 0.01, P < 0.001 and P < 0.0001, respectively, Student's paired t test). Values are means ± SEM.
As these in vitro results were obtained using completely deafferented medullary RRGs, we decided to examine breathing variables in intact, unrestrained pups using plethysmography under thermoneutral conditions (33°C, Fig. 1F). Statistical comparisons between Vglut3−/− and control pups are summarized in Table 2. Vglut3−/− and control pups had similar weights (Fig. 1G), temperatures (Fig. 1H) and breathing patterns under normoxia, regardless of the breathing variable considered (Fig. 1I–L).
Table 2.
Baseline breathing variables under thermoneutral (33°C) and cold conditions (26°C) in normoxia
| Thermoneutrality (33°C) | Cold (26°C) | |||
|---|---|---|---|---|
| Control n = 164 | Vglut3−/− n = 53 | Control n = 200 | Vglut3−/− n = 63 | |
| Weight (g) | 4.6 ± 0.9 | 4.5 ± 0.9 | 3.6 ± 0.8 | 3.4 ± 0.8 |
| T (°C) | 34.8 ± 0.1 | 34.4 ± 0.2 | 34.3 ± 1.1 | 34.1 ± 0.1 |
| ΔT (°C) | 0.5 ± 0.1 | 0.8 ± 0.2 | −4.5 ± 0.1 | −5.2 ± 0.1*** |
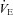 (μl g−1 s−1) (μl g−1 s−1) |
16.0 ± 0.2 | 16.3 ± 0.4 | 18.1 ± 0.6 | 11.5 ± 0.6*** |
| Rf (Hz) | 3.23 ± 0.54 | 3.22 ± 0.57 | 2.83 ± 0.93 | 2.19 ± 0.61*** |
| VT (μl g−1) | 5.52 ± 1.14 | 5.55 ± 1.11 | 6.27 ± 2.02 | 5.18 ± 1.92*** |
| AI (apnoea min−1) | 0.42 ± 1.2 | 0.42 ± 1.14 | 1.22 ± 2.84 | 3.68 ± 3.78*** |
| n = 29 | n = 7 | |||
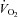 (ml min−1 g−1) (ml min−1 g−1) |
— | — | 0.084 ± 0.02 | 0.069 ± 0.02*** |
T (°C): body temperature before plethysmographic recordings (and before cold exposure in cold experiments); ΔT (°C): variation in body temperature after plethysmographic recordings (duration: 30 min). 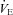 : ventilation; Rf: breathing frequency; VT: tidal volume. AI: apnoea index, number of apnoeas per minute;
: ventilation; Rf: breathing frequency; VT: tidal volume. AI: apnoea index, number of apnoeas per minute; 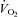 : oxygen consumption. **, ***: significant difference between Vglut3−/− and control pups (P < 0.01 and P < 0.001 respectively, Student's unpaired t test). Values are means ± SEM.
: oxygen consumption. **, ***: significant difference between Vglut3−/− and control pups (P < 0.01 and P < 0.001 respectively, Student's unpaired t test). Values are means ± SEM.
Lack of VGLUT3 affects the 5-HT modulation of respiratory activity and 5-HT metabolism
VGLUT3 is expressed in 5-HT neurons from the dorsal and medial raphe (Amilhon et al. 2010). Because raphe neurons have a profound influence on respiratory activity (Hilaire et al. 2010; Hodges & Richerson, 2010a), we examined the distribution of VGLUT3 in the rostral raphe nuclei, especially those involved in the control of breathing. We performed double immunolabelling for VGLUT3 and vesicular monoamine transporter 2 (VMAT2, Fig. 2A) on the one hand and for VGLUT3 and 5-HT on the other (Fig. 2B and C). As shown previously in adult mice (Amilhon et al. 2010), VGLUT3 colocalized poorly with VMAT2-positive somata and terminals in the dorsal raphe (DR), raphe pontis (RPn), RMg and RPa (Fig. 2A). Double-labelling for VGLUT3 and 5-HT confirmed the presence of VGLUT3-expressing neurons within the ROb and RMg (Fig. 2C). The colocalization of VGLUT3 and 5-HT signals in the somata of cells was observed in the ROb (Fig. 2B) and the RMg (Fig. 2C). No VGLUT3-expressing neurons were observed either in the pre-Bötzinger complex or in the RTN/pFRG areas of control pups, identified using anatomical landmarks and NK1R and PHOX2b expression (Rosin et al. 2006).
Figure 2. Lack of Vglut3 impairs 5-HT metabolism in the brainstem, and the RRG response to 5-HT.
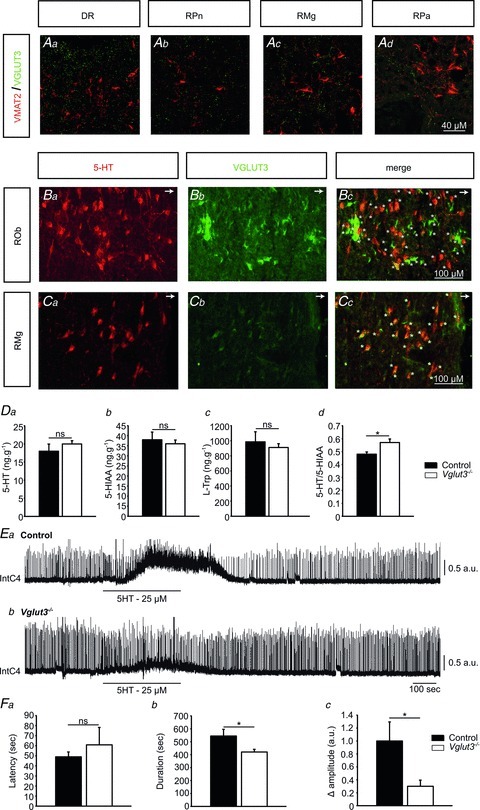
See Supplemental Fig. 1 for schematic representation of the brainstem sections. A, immunolabelling showing that Vglut3 (green) colocalizes poorly with VMAT2 (red) in control mice at the level of the dorsal raphe (DR) (a), raphe pontis (RPn) (b), raphe magnus (RMg) (c) and raphe pallidus nuclei (Rpa) (d). B and C, immunodetection of 5-HT (red) and VGLUT3 (green) expression in coronal sections of control mice at the level of the raphe obscurus nucleus (ROb). Arrows in the upper right corner of panels B and C point toward the ventral medullary surface (B) and RMg (C). VGLUT3 was found in soma of cells at the ROb (Bb) and RMg (Cc) levels, and is co-expressed with 5-HT in cells of ROb and at RMg level (stars, yellow cells in Bc and Cc, respectively). D, 5-HT turnover in Vglut3−/− pups (n = 12) differs from that in controls (n = 6). Endogenous levels of 5-HT (a), its main metabolite 5-hydroxyindoleacetic acid (5-HIAA) (b) and its precursor l-tryptophan (l-Trp) (c) in the brainstem of Vglut3−/− and control pups, measured by HPLC. The 5-HT/5-HIAA ratio was significantly higher in Vglut3−/− than in control pups (d). E and F, the spinal response to exogenous 5-HT is impaired in Vglut3−/− pups. E, examples of phrenic bursts recorded from the C4 root of in vitro brainstem preparations from control (a) and Vglut3−/− (b) pups, superfused with normal aCSF and aCSF containing 5-HT (25 μm, 5 min). Int C4: integrated C4 activity. F, tonic discharges in response to 5-HT appeared with the same latency in Vglut3−/− (n = 11) and control (n = 14) preparations (a) but were shorter (b) and of smaller amplitude (c) in Vglut3−/− preparations compared to control preparations (*P < 0.05; Student's unpaired t test, ns: non significant). a.u.: arbitrary units. Values given are means ± SEM. See Table 1 for full statistical analyses.
VGLUT3 and glutamate have been found to modify 5-HT vesicular filling by a mechanism named vesicular synergy (Amilhon et al. 2010). Thus, the lack of VGLUT3 may alter tissue concentrations of 5-HT and its metabolites in the brainstem. To address this issue, we measured endogenous levels of 5-HT, its precursor l-tryptophan (l-Trp) and its main metabolite 5-hydroxyindoleacetic acid (5-HIAA) using HPLC separation and electrochemical detection (Fig. 2D). The inverse (although non-significant) differences in 5-HT and 5-HIAA content between Vglut3−/− and control brainstems led to a significantly higher 5-HT/5-HIAA ratio in Vglut3−/− than in control brainstems (∼19%, see Fig. 2D for statistical analyses). This difference revealed an alteration in 5-HT turnover in Vglut3−/− pups, as previously reported in the hippocampus of adult Vglut3−/− mice (Amilhon et al. 2010).
Since alterations in 5-HT metabolism during development modify the formation of brain circuits (Deneris & Wyler, 2012), including the respiratory networks (Hilaire et al. 2010), we next examined whether the abnormal 5-HT/5-HIAA ratio in the brainstem of Vglut3−/− mice was associated with abnormal 5-HT modulation of respiratory activity. To this end, we applied exogenous 5-HT to the artificial cerebrospinal fluid (aCSF) of Vglut3−/− and control preparations for 5 min. Biphasic responses were observed in both preparations, but with quantitative differences (Fig. 2E, and Table 1 for statistical analyses). The initial component of this response corresponds to the medullary response and the late component to a spinal response with a tonic discharge of phrenic motoneurons (Bou-Flores et al. 2000). Although response latencies were not significantly different between groups, the response was shorter lived and of smaller amplitude in Vglut3−/− than in control preparations (see Fig. 2F for statistical analyses).
Lack of VGLUT3 disrupts respiratory control
We tested whether the lack of VGLUT3 affected the RRG responses to acidosis and anoxia, both of which are strongly modulated by 5-HT (Caubit et al. 2010). We measured the PB frequency response to central acidosis by replacing normal aCSF with acidified aCSF for 5 min (Fig 3A). This stimulus markedly stimulates PB frequency in normal preparations (Voituron et al. 2010). Acidosis increased PB frequency in both Vglut3−/− and control preparations (Fig. 3A). However, PB frequency reached higher values in Vglut3−/− than in control preparations (Fig. 3A, and Table 1 for statistical analyses).
Figure 3. Lack of Vglut3 disrupts respiratory control and thermogenesis in the cold.
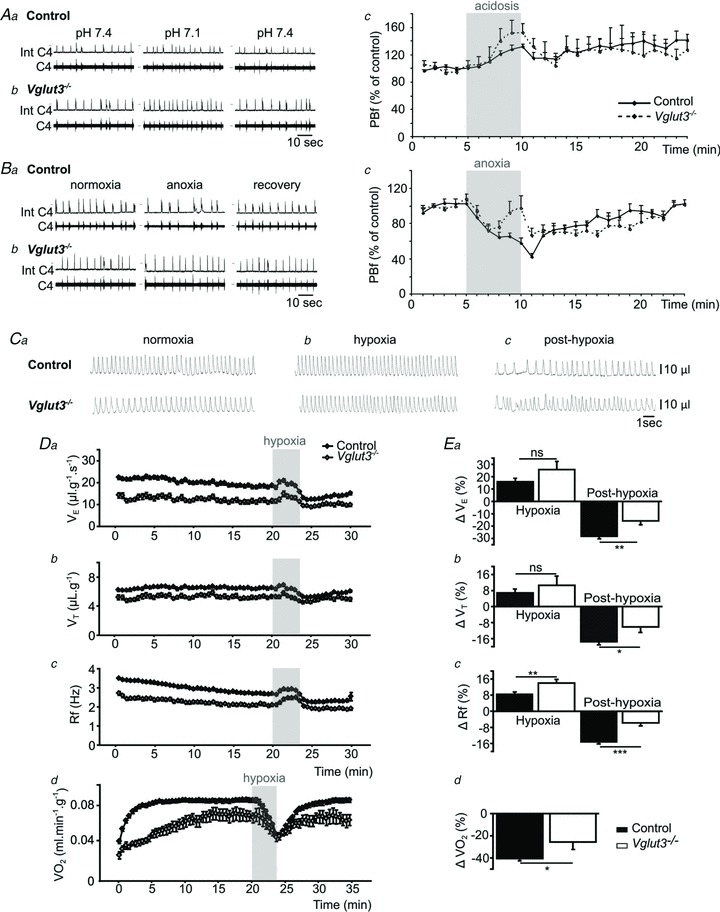
Aa and b, examples of changes in central respiratory drive induced by acidosis in Control (a) and Vglut3−/− pups (b) in vitro. Int C4: integrated C4 activity (C4). Ac, the effect of acidosis (shaded) on mean phrenic burst frequency (PBf) was significantly increased in Vglut3−/− pups (n = 4) when compared to control pups (n = 12, P < 0.05; Student's unpaired t test). Ba and b, examples of changes in central respiratory drive induced by anoxia in control (a) and Vglut3−/− pups (b) in vitro. Bc, the effect of anoxia (shaded) was significantly increased in Vglut3−/− pups (n = 8) when compared to controls (n = 18, P < 0.001). C, examples of plethysmographic recordings in 6-day-old Vglut3−/− and control pups during normoxia (a), hypoxia (10% O2) (b) and post-hypoxia (Cc). Da–c, breathing variables: ventilation (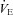 ), tidal volume (VT) and breathing frequency (Rf). Vglut3−/−pups (n = 63) displayed decreased ventilation during both normoxia and hypoxia, compared to control pups (n = 200). Both groups displayed a biphasic response to hypoxia (shaded), with the initial increase in
), tidal volume (VT) and breathing frequency (Rf). Vglut3−/−pups (n = 63) displayed decreased ventilation during both normoxia and hypoxia, compared to control pups (n = 200). Both groups displayed a biphasic response to hypoxia (shaded), with the initial increase in 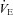 being followed by a marked decrease (under the control of VT and Rf). Ea–c, percentage change in breathing variables in response to hypoxia, relative to pre-hypoxic levels (hyperpnoeic response and hypoxic decline); HVD was significantly lower in Vglut3−/− pups. Dd, oxygen consumption (
being followed by a marked decrease (under the control of VT and Rf). Ea–c, percentage change in breathing variables in response to hypoxia, relative to pre-hypoxic levels (hyperpnoeic response and hypoxic decline); HVD was significantly lower in Vglut3−/− pups. Dd, oxygen consumption (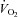 ) at 26°C under normoxia and in response to hypoxia (shaded) in 6-day-old Vglut3−/− (n = 7) and control pups (n = 29). The smaller
) at 26°C under normoxia and in response to hypoxia (shaded) in 6-day-old Vglut3−/− (n = 7) and control pups (n = 29). The smaller 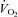 in Vglut3−/− pups (P < 0.001) reflected their impaired thermogenesis. Ed, percentage change in
in Vglut3−/− pups (P < 0.001) reflected their impaired thermogenesis. Ed, percentage change in 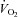 relative to pre-hypoxic levels was smaller in Vglut3−/− pups. *P < 0.05; **P < 0.01; ***P < 0.001, Student's unpaired t test. Values shown are group means ± SEM. See Tables 1 and 2 for full statistical analyses.
relative to pre-hypoxic levels was smaller in Vglut3−/− pups. *P < 0.05; **P < 0.01; ***P < 0.001, Student's unpaired t test. Values shown are group means ± SEM. See Tables 1 and 2 for full statistical analyses.
We then measured the RRG response to central anoxia by replacing normal aCSF with anoxic aCSF for 5 min (Fig. 3B). As expected (Hilaire et al. 2010), in control preparations, PB frequency was depressed throughout the 5 min of exposure to anoxia, and recovered slowly over 10–15 min after the resumption of normal aCSF (Fig. 3Bb). In contrast, in Vglut3−/− preparations, the anoxia-induced decrease in PB frequency was short lived, and PB frequency returned to control levels after 2–3 min despite the maintenance of central anoxia (Fig. 3Bc, and Table 1 for statistical analyses). However, resumption of normal aCSF produced an immediate decrease in frequency, which was greatly similar to that of control preparations. Taken together, these results suggest that the mechanisms controlling the depression of PB frequencies during anoxia and post-anoxia may be different, as previously proposed for HVD and post-HVD in vivo (Renolleau et al. 2001), and that only the depression of PB frequency during anoxia was affected by the lack of VGLUT3.
Next, we examined the breathing pattern of 6-day-old pups in air or hypoxia, in cold condition (Fig. 3C). Cold condition increases thermogenesis and oxygen demand, which, combined with hypoxia, result in strong hypoxic stress. Under normoxia, the ventilation (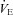 ) of Vglut3−/− pups was significantly lower (about 37%) than in controls, mostly due to their decreased respiratory frequency, Rf (Fig. 3D, and Table 2 for statistical analyses). Hypoxia elicited a biphasic ventilatory response in both groups with an initial
) of Vglut3−/− pups was significantly lower (about 37%) than in controls, mostly due to their decreased respiratory frequency, Rf (Fig. 3D, and Table 2 for statistical analyses). Hypoxia elicited a biphasic ventilatory response in both groups with an initial 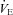 increase reflecting the RRG response to carotid body chemoreceptor activation by peripheral hypoxia, and a subsequent HVD reflecting the RRG response to central hypoxia (Fig. 3D). The increase in
increase reflecting the RRG response to carotid body chemoreceptor activation by peripheral hypoxia, and a subsequent HVD reflecting the RRG response to central hypoxia (Fig. 3D). The increase in 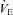 during the augmentation phase of the hypoxic response resulted from a combination of the rise in VT and in Rf, as commonly reported (Bissonnette, 2000), although an Rf-based increase under similar conditions has also been reported (Bollen et al. 2009). Because of the delay in the wash-out of the hypoxic mixture (about 90 s), maximal ventilatory depression was reached after the switch to normoxia (Fig. 3Da–c). The depressive phase of
during the augmentation phase of the hypoxic response resulted from a combination of the rise in VT and in Rf, as commonly reported (Bissonnette, 2000), although an Rf-based increase under similar conditions has also been reported (Bollen et al. 2009). Because of the delay in the wash-out of the hypoxic mixture (about 90 s), maximal ventilatory depression was reached after the switch to normoxia (Fig. 3Da–c). The depressive phase of 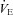 resulted from a decrease in VT and Rf, as previously reported (Bissonnette, 2000; Bollen et al. 2009). When expressed as the percentage change relative to average pre-hypoxic baseline values, the initial
resulted from a decrease in VT and Rf, as previously reported (Bissonnette, 2000; Bollen et al. 2009). When expressed as the percentage change relative to average pre-hypoxic baseline values, the initial 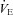 increase in response to hypoxia was not significantly different between groups, although the Rf increase in response to hypoxia was significantly higher in Vglut3−/− than in control pups (Fig. 3E). However, HVD, expressed as the percentage decrease from pre-hypoxic levels, was significantly attenuated in Vglut3−/− pups as compared to controls (Fig. 3E). Thus, both in vivo and in vitro data consistently showed that the lack of VGLUT3 altered the inhibitory effect of hypoxia on respiratory activity.
increase in response to hypoxia was not significantly different between groups, although the Rf increase in response to hypoxia was significantly higher in Vglut3−/− than in control pups (Fig. 3E). However, HVD, expressed as the percentage decrease from pre-hypoxic levels, was significantly attenuated in Vglut3−/− pups as compared to controls (Fig. 3E). Thus, both in vivo and in vitro data consistently showed that the lack of VGLUT3 altered the inhibitory effect of hypoxia on respiratory activity.
Lack of VGLUT3 disrupts the thermogenic response to cold
The cold induced a greater decrease in body temperature in Vglut3−/− than in control pups (see statistics in Table 2). We assessed thermogenesis by measuring O2 consumption (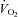 ) as a function of body weight during cold exposure. Both groups increased their
) as a function of body weight during cold exposure. Both groups increased their 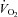 in an attempt to generate heat and maintain body temperature until a plateau was reached (Fig. 3Dd). However, Vglut3−/− pups exhibited a much slower rate of increase during the first 12–13 min of normoxia (Fig. 3Dd). Furthermore, the plateau level of
in an attempt to generate heat and maintain body temperature until a plateau was reached (Fig. 3Dd). However, Vglut3−/− pups exhibited a much slower rate of increase during the first 12–13 min of normoxia (Fig. 3Dd). Furthermore, the plateau level of 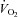 (steady state) was significantly lower in Vglut3−/− pups as compared to controls (Fig. 3Dd; see statistics in Table 2), revealing a weaker thermogenic response to cold. The difference in
(steady state) was significantly lower in Vglut3−/− pups as compared to controls (Fig. 3Dd; see statistics in Table 2), revealing a weaker thermogenic response to cold. The difference in 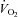 between groups was small compared to the difference in
between groups was small compared to the difference in 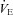 (18%vs. 36%, respectively), indicating that blunted thermogenesis in Vglut3−/− pups did not fully account for their lower
(18%vs. 36%, respectively), indicating that blunted thermogenesis in Vglut3−/− pups did not fully account for their lower 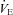 levels. Thus, the lack of VGLUT3 altered both the time constant and the steady state of the thermogenic response to cold, suggesting that the activation of brown adipose tissue was diminished in Vglut3−/− compared to control pups.
levels. Thus, the lack of VGLUT3 altered both the time constant and the steady state of the thermogenic response to cold, suggesting that the activation of brown adipose tissue was diminished in Vglut3−/− compared to control pups.
In both groups, hypoxia sharply depressed 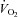 (hypometabolic response to hypoxia, Fig. 3Dd), a centrally mediated defensive response to hypoxia in newborns (Mortola, 2004) that reflects the inhibition of thermogenesis (Mortola & Gautier, 1995). The
(hypometabolic response to hypoxia, Fig. 3Dd), a centrally mediated defensive response to hypoxia in newborns (Mortola, 2004) that reflects the inhibition of thermogenesis (Mortola & Gautier, 1995). The 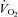 decrease relative to pre-hypoxic levels was smaller in Vglut3−/− than in control pups (see statistics in Fig. 3Ed), indicating a blunted hypometabolic response to hypoxia. Furthermore, unlike controls, Vglut3−/− pups failed to restore
decrease relative to pre-hypoxic levels was smaller in Vglut3−/− than in control pups (see statistics in Fig. 3Ed), indicating a blunted hypometabolic response to hypoxia. Furthermore, unlike controls, Vglut3−/− pups failed to restore 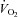 to pre-hypoxic levels upon the return to normoxia (Fig. 3Dd). Thus, Vglut3−/− pups displayed a decreased ability to increase
to pre-hypoxic levels upon the return to normoxia (Fig. 3Dd). Thus, Vglut3−/− pups displayed a decreased ability to increase 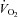 and produce heat in response to cold, as well as a decreased ability to depress
and produce heat in response to cold, as well as a decreased ability to depress 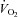 in response to hypoxia.
in response to hypoxia.
Discussion
Whereas the lack of VGLUT3 did not significantly alter respiratory activity under basal conditions, it did so in response to chemical stimuli in vitro, revealing a dysfunction of the metabolic control of breathing. Furthermore, in vivo, the lack of VGLUT3 impaired respiratory and thermogenic responses to cold and hypoxia, and in particular, two main components of the defensive strategy to hypoxic stress: HVD and hypometabolism.
The histological analysis of brainstem respiration-related areas did not reveal any significant differences between Vglut3−/− and control mice, in line with functional results in vitro and in vivo under basal conditions. However, we cannot disregard the possibility that the lack of VGLUT3 led to subtle differences in the structure or the number of neurons in some populations under scrutiny.
Brainstem–spinal cord preparations produce periodic activity that corresponds to the respiratory rhythm generated in the brainstem of intact animals, although at a much lower rate (Suzue, 1984). This preparation is commonly regarded as a valuable tool for the analysis of central mechanisms of respiratory control (Johnson et al. 2012). However, these preparations present specific characteristics that may hamper extrapolation to in vivo conditions. In particular, under normal conditions, superficial medullary tissue layers, where respiratory rhythm is generated, are hyperoxic (Brockhaus et al. 1993; Okada et al. 1993a), whereas deep medullary layers are hypoxic (Okada et al. 1993a,b). Bath application of an anoxic mixture, as in the present study, may produce large and unpredictable gradients of oxygen levels in the preparation. However, we may preclude the possibility that anoxia caused notable damage to respiratory networks because the changes induced in PB frequency are reversible, as previously reported (Okada et al. 1998, 2007; Voituron et al. 2006, 2010, 2011).
As in most studies using brainstem–spinal cord preparations, we found that bath acidification led to an increase in the respiratory frequency rather than to an increase in the amplitude of phrenic nerve bursting (Ballanyi et al. 1999). This is in contrast with previous data in newborn mice showing that hypercapnia primarily causes an increase in VT, with a less prominent increase in respiratory frequency (Ramanantsoa et al. 2011). It is generally agreed that the attenuated in vitro effects of acidification on the amplitude of phrenic nerve bursting might be related to the removal of afferent sensory inputs in brainstem–spinal cord preparations (Ballanyi et al. 1999). Thus, while brainstem–spinal cord preparations are suitable for the analysis of the effects of acidification on the central respiratory network, direct extrapolation to in vivo conditions must be done cautiously. In fact, in vivo and in vitro approaches are complementary in this respect.
The increase in the 5-HT/5-HIAA ratio in the brainstem of Vglut3−/− pups is in line with previous observations in the hippocampus of adult Vglut3−/− mice (Amilhon et al. 2010). This increase may reflect decreased turnover of 5-HT in Vglut3−/− pups (El Mestikawy et al. 2011). The link between respiratory control and 5-HT metabolism in mice has been previously established by comparing two strains of inbred mice (C57BL/6J vs. FVB/N) that display differences in 5-HT metabolism in the medulla and in respiratory control (Menuet et al. 2011). In newborn mice, large differences in l-Trp and 5-HIAA content were observed between the two strains, leading to a 3-fold higher 5-HT/5-HIAA ratio in FVB/N mice. These differences in 5-HT metabolism were associated with greater respiratory irregularity in C57BL/6J mice, both in vitro and in vivo (Menuet et al. 2011). In the present study, l-Trp and 5-HIAA content was not significantly different between Vglut3−/− and control brainstems, and the 5-HT/5-HIAA ratio was 19% higher in Vglut3−/− mice when compared to controls. This relatively small difference in 5-HT metabolism was not associated with differences in respiratory activity under basal conditions, but might have been sufficient to impair this activity under highly demanding hypoxic conditions.
Both the initial (medullary) and the late (spinal) responses to 5-HT were reduced in Vglut3−/− preparations. The reduced response to 5-HT in Vglut3−/− preparations is consistent with its role in potentiating 5-HT vesicular accumulation and the exocytotic secretion of 5-HT. However, the present results do not indicate whether these abnormal responses reflect changes in 5-HT storage and release, or developmental changes in 5-HT receptor expression, although previous results do not indicate any major modification of 5-HT receptors (Amilhon et al. 2010). Previous studies have shown that 5-HT acts at the medullary level and that its effects are due to its specificity for the rostro-ventral respiratory column and not due to a diffuse action on all medullary respiratory centres (see Hilaire et al. (2010) and Hodges & Richerson (2010b) for recent reviews on the respiratory effects of 5-HT). Therefore, the putative impairment of 5-HT signalling due to Vglut3 inactivation may account for the abnormal response to 5-HT. However, we cannot fully discount the influence of 5-HT induced depolarization of larger cell populations in the brainstem (e.g. the A5 area of the caudal pons; Di Pasquale et al. 1992) and spinal cord, and the subsequent release of a large variety of inhibitory and excitatory neurotransmitters, on a part of the response of Vglut3−/− preparations in the present study. With these limitations in mind, considering that 5-HT is pivotal to respiratory function (Hilaire et al. 2010) and thermoregulation (Morrison et al. 2008), the present results support the view that the abnormal respiratory and thermogenic responses to cold in Vglut3−/− pups may be caused by abnormal 5-HT metabolism. Abnormal 5-HT signalling may also account for the abnormal RRG response to acidosis, which is closely dependent on 5-HT (Voituron et al. 2010) and mediated by pH-sensitive RTN/pFRG neurons (Guyenet et al. 2008), which receive dense 5-HT inputs from VGLUT3-positive terminals (Nakamura et al. 2005b; Stornetta et al. 2005; Rosin et al. 2006).
The fact that the lack of VGLUT3 increased the response of isolated RRG to acidosis is intriguing. It suggests that glutamate release by 5-HT neurons might inhibit breathing through its action on specific populations of respiratory neurons (Monnier et al. 2003). Furthermore, we cannot disregard the possibility that this effect is caused by non-5HT neurons with a potential impact on respiratory activity, as detailed below, or by compensatory mechanisms occurring during perinatal development.
Both our in vitro and in vivo results argue for the involvement of Vglut3 in HVD. In vitro, the decrease in PB frequency in response to anoxia in control preparations confirmed the results of previous studies using similar preparations (Ballanyi et al. 1999), as well as transverse slice preparations (Ramirez et al. 1997). In vivo, hypoxia depressed ventilation, as consistently observed in newborn mammals (Teppema & Dahan, 2010). Thus, both in vitro and in vivo experiments confirmed that hypoxia decreased respiratory frequency, and showed that the lack of VGLUT3 disrupted this response. Since the RRG in in vitro preparations is isolated and lacks excitatory input from peripheral carotid body chemoreceptors, the abnormal RRG response of Vglut3−/− pups to anoxia can be ascribed to the inhibitory drive from hypoxia-sensing neurons of the ventral medulla (Voituron et al. 2006). In keeping with our in vitro data, HVD in vivo was blunted in Vglut3−/− pups, leading to greater 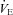 and Rf. Previous studies have shown that blocking 5-HT2 receptors in adult mice suppresses HVD (Kanamaru & Homma, 2009). Taken together, these results suggest that the lack of VGLUT3 disrupts a common mechanism accounting for both in vitro and in vivo findings. However, the decrease in PB frequency in vitro (frequently referred to as ‘short-term depression’ under these experimental conditions; Dick & Coles, 2000; Powell et al. 1998) and the decrease in ventilation in vivo had markedly different time courses, which may at least partially reflect different mechanisms. Therefore, caution must be used in attempting to integrate both results within a unifying framework, as previously noted (Bissonnette, 2000).
and Rf. Previous studies have shown that blocking 5-HT2 receptors in adult mice suppresses HVD (Kanamaru & Homma, 2009). Taken together, these results suggest that the lack of VGLUT3 disrupts a common mechanism accounting for both in vitro and in vivo findings. However, the decrease in PB frequency in vitro (frequently referred to as ‘short-term depression’ under these experimental conditions; Dick & Coles, 2000; Powell et al. 1998) and the decrease in ventilation in vivo had markedly different time courses, which may at least partially reflect different mechanisms. Therefore, caution must be used in attempting to integrate both results within a unifying framework, as previously noted (Bissonnette, 2000).
The weaker thermogenic response of Vglut3−/− pups to cold indicates that the range of phenotypic defects extends beyond respiratory control disorders. This defect is probably related to the fact that a brainstem network that includes raphe neurons that express VGLUT3 through postganglionic sympathetic neurons controls brown adipose cells (Schafer et al. 2002; Nakamura et al. 2005a; Morrison et al. 2008). Furthermore, the hypometabolic response to hypoxia (i.e. the decrease in thermogenesis and 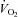 consistently observed in newborn mammals during hypoxia; Mortola, 2004) was blunted in Vglut3−/− pups. The mechanisms underlying hypoxic hypometabolism are complex and, to date, elusive, but they may involve a decrease in central thermosensitivity at the level of the hypothalamic medial preoptic area (Tattersall & Milsom, 2009), a target for VGLUT3-expressing non-5-HT neurons of the midbrain raphe nuclei (Hioki et al. 2010). The drop in arterial
consistently observed in newborn mammals during hypoxia; Mortola, 2004) was blunted in Vglut3−/− pups. The mechanisms underlying hypoxic hypometabolism are complex and, to date, elusive, but they may involve a decrease in central thermosensitivity at the level of the hypothalamic medial preoptic area (Tattersall & Milsom, 2009), a target for VGLUT3-expressing non-5-HT neurons of the midbrain raphe nuclei (Hioki et al. 2010). The drop in arterial 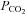 that normally accompanies hypoxic hypometabolism during and after hypoxia may not have been present in Vglut3−/−, thus counteracting HVD and accounting for the present ventilatory data.
that normally accompanies hypoxic hypometabolism during and after hypoxia may not have been present in Vglut3−/−, thus counteracting HVD and accounting for the present ventilatory data.
The implication of 5-HT signalling defects in the Vglut3−/− phenotype is supported by the similarities between Vglut3−/− pups and those lacking central 5-HT neurons (Lmx1bf/f/p pups), which display decreased ventilation and body temperatures when exposed to cold (Hodges et al. 2009). Furthermore, newborn mice lacking 60–70% of their 5-HT neurons (Pet-1−/−) display reduced thermogenic and ventilatory responses to cold (Cummings et al. 2011b) and reduced ability to survive episodic severe hypoxia (Cummings et al. 2011a). The critical role of 5-HT neurons in respiratory and thermoregulatory functions was recently confirmed in conscious mice using the inducible and reversible suppression of neuronal excitability, which attenuated the ventilatory response to CO2 and the thermogenic response to cold (Ray et al. 2011).
While the role of VGLUT3 in 5-HT neuronal activity may account for the present results, further studies are necessary to substantiate this interpretation, and other mechanisms may also be involved. Firstly, in addition to 5-HT neurons, the raphe contains a population of VGLUT3-positive cells that are not serotonergic (Commons, 2009; Jackson et al. 2009). Both subclasses of VGLUT3-positive neurons from the raphe could exert modulatory effects on 5-HT transmission and contribute to the response to hypoxic stress in Vglut3−/− mice. Also, VGLUT3 expression is prominent in nitroxidergic neurons and glial processes of the dorsolateral, medial and commissural subnuclei of the nucleus tractus solitarii (NTS; Lin, 2009), all regions that receive afferents from carotid body chemoreceptors (Finley & Katz, 1992). The lack of VGLUT3 may also affect the response to hypoxic stress through its effects on these structures. Furthermore, as previously noted, VGLUT3 is expressed in various neuronal populations including cholinergic interneurons of the dorsal and ventral striatum, and subpopulations of cortical and hippocampal basket cells (Fremeau et al. 2002). VGLUT3 expression has also been reported in non-neuronal cells such as insulin secreting β-cells of the pancreas (Gammelsaeter et al. 2011), chromaffin cells of the adrenal medulla (Olivan et al. 2011), and in the liver (Gras et al. 2002). Some of these populations might be indirectly implicated in some aspects of breathing control and metabolism. Previous analyses have shown that adult mice lacking VGLUT3 have a number of diverse disorders such as pain, deafness and non-convulsive seizures (Ruel et al. 2008; Seal et al. 2008, 2009), suggesting that Vglut3 may interfere with a large variety of neuromodulatory and behavioural phenotypes beyond the 5-HT system (El Mestikawy et al. 2011).
In conclusion, the present results identify VGLUT3 as a protective factor against hypoxic stress in newborns, thus extending its known role in the defence against psychological stress to autonomic functions. The use of temporal and spatial conditional mutants in future studies should improve our understanding of the molecular mechanisms underlying these defensive functions.
Acknowledgments
This work was supported by grants from Institut National de la Santé et de la Recherche Médicale (INSERM), Agence Nationale pour la Recherche (ANR). B.A. was supported by a fellowship from Fondation pour la Recherche Médicale (FRM), and E.L. by Fédération pour la Recherche sur le Cerveau (FRC) and France Alzheimer. We are indebted to Dr Christo Goridis (Ecole Normale Supérieure, Paris) for providing PHOX2B rat monoclonal antiserum.
Translational perspective
Hypoxia–ischaemia is a major cause of acute brain injury in neonates. Hypoxic stress may be aggravated by cold, especially in preterm infants (Miller et al. 2011). While keeping in mind the limitations of extrapolating data from mice to humans, the present results in 6-day-old mice might shed some light on the response to hypoxic stress in preterm human infants of 32–35 weeks of gestational age (Hagberg et al. 2002). These results suggest that the lack of VGLUT3 compromises the ability of preterms to produce two components of the defence response to hypoxia: hypoxic hypometabolism and hypoxic ventilatory decline. The present results consolidate the concept that major components of the response to hypoxic stress (ventilatory, thermoregulatory, behavioural) in newborns are tightly coupled at the central level, and that the newborn's inability to produce this integrated response may originate in its central organization rather that in its components. The involvement of Vglut3 in the response to hypoxic stress in newborns suggests that Vglut3 could be a potential genetic marker of vulnerability to hypoxic damage. However, further investigations are required to better understand the role of Vglut3 in this process and to identify it as a potential pharmacological target against hypoxic stress in neonates.
Glossary
- AI
apnoea index
- AU
arbitrary unit
- pFRG
parafacial respiratory group
- 5-HIAA
5-hydroxyindoleacetic acid
- HVD
hypoxic ventilatory decline
- IS
irregularity score
- l-Trp
l-tryptophan
- MAOA
monoamine oxidase A
- PB
phrenic burst
- RMg
raphe magnus
- ROb
raphe obscurus
- RPa
raphe pallidus
- RPn
raphe pontis
- RRG
respiratory rhythm generator
- RTN
retrotrapezoid nucleus
- VGLUT3
vesicular glutamate transporter type 3
Author contributions
S.E.M., G.H. and J.G. designed the experiments; S.M., N.V., A.S., E.V., L.M., N.R., B.A., O.P., E.L., B.M., S.M., N.V., A.S., S.E.M., G.H. and J.G. collected and analysed data; S.M., N.V., S.E.M., G.H. and J.G. analysed data and wrote the manuscript. All authors approved the final version for publication.
Supplementary material
Supplemental Fig. 1
References
- Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci U S A. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, Miot S, Gras C, Gardier AM, Gallego J, Hamon M, Lanfumey L, Gasnier B, Giros B, El Mestikawy S. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci. 2010;30:2198–2210. doi: 10.1523/JNEUROSCI.5196-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1391–1400. doi: 10.1152/ajpregu.2000.278.6.R1391. [DOI] [PubMed] [Google Scholar]
- Bollen B, Bouslama M, Matrot B, Rotrou Y, Vardon G, Lofaso F, Van den Bergh O, D’Hooge R, Gallego J. Cold stimulates the behavioral response to hypoxia in newborn mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1503–1511. doi: 10.1152/ajpregu.90582.2008. [DOI] [PubMed] [Google Scholar]
- Bou-Flores C, Lajard AM, Monteau R, De Maeyer E, Seif I, Lanoir J, Hilaire G. Abnormal phrenic motoneuron activity and morphology in neonatal monoamine oxidase A-deficient transgenic mice: possible role of a serotonin excess. J Neurosci. 2000;20:4646–4656. doi: 10.1523/JNEUROSCI.20-12-04646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K, Smith JC, Richter DW. Microenvironment of respiratory neurons in the in vitro brainstem–spinal cord of neonatal rats. J Physiol. 1993;462:421–445. doi: 10.1113/jphysiol.1993.sp019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caubit X, Thoby-Brisson M, Voituron N, Filippi P, Bevengut M, Faralli H, Zanella S, Fortin G, Hilaire G, Fasano L. Teashirt 3 regulates development of neurons involved in both respiratory rhythm and airflow control. J Neurosci. 2010;30:9465–9476. doi: 10.1523/JNEUROSCI.1765-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG. Locally collateralizing glutamate neurons in the dorsal raphe nucleus responsive to substance P contain vesicular glutamate transporter 3 (VGLUT3) J Chem Neuroanat. 2009;38:273–281. doi: 10.1016/j.jchemneu.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Hewitt JC, Li A, Daubenspeck JA, Nattie EE. Postnatal loss of brainstem serotonin neurones compromises the ability of neonatal rats to survive episodic severe hypoxia. J Physiol. 2011a;589:5247–5256. doi: 10.1113/jphysiol.2011.214445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Li A, Nattie EE. Brainstem serotonin deficiency in the neonatal period: autonomic dysregulation during mild cold stress. J Physiol. 2011b;589:2055–2064. doi: 10.1113/jphysiol.2010.203679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneris ES, Wyler SC. Serotonergic transcriptional networks and potential importance to mental health. Nat Neurosci. 2012;15:519–527. doi: 10.1038/nn.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale E, Morin D, Monteau R, Hilaire G. Serotonergic modulation of the respiratory rhythm generator at birth: an in vitro study in the rat. Neurosci Lett. 1992;143:91–95. doi: 10.1016/0304-3940(92)90240-8. [DOI] [PubMed] [Google Scholar]
- Dick TE, Coles SK. Ventrolateral pons mediates short-term depression of respiratory frequency after brief hypoxia. Respir Physiol. 2000;121:87–100. doi: 10.1016/s0034-5687(00)00121-3. [DOI] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mestikawy S, Wallen-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci. 2011;12:204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- Epstein MA, Epstein RA. A theoretical analysis of the barometric method for measurement of tidal volume. Respir Physiol. 1978;32:105–120. doi: 10.1016/0034-5687(78)90103-2. [DOI] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signalling by glutamate. Proc Natl Acad Sci U S A. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Gammelsaeter R, Coppola T, Marcaggi P, Storm-Mathisen J, Chaudhry FA, Attwell D, Regazzi R, Gundersen V. A role for glutamate transporters in the regulation of insulin secretion. PloS One. 2011;6:e22960. doi: 10.1371/journal.pone.0022960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier H. Interactions among metabolic rate, hypoxia, and control of breathing. J Appl Physiol. 1996;81:521–527. doi: 10.1152/jappl.1996.81.2.521. [DOI] [PubMed] [Google Scholar]
- Gras C, Amilhon B, Lepicard EM, Poirel O, Vinatier J, Herbin M, Dumas S, Tzavara ET, Wade MR, Nomikos GG, Hanoun N, Saurini F, Kemel ML, Gasnier B, Giros B, El Mestikawy S. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat Neurosci. 2008;11:292–300. doi: 10.1038/nn2052. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol. 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8:30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Voituron N, Menuet C, Ichiyama RM, Subramanian HH, Dutschmann M. The role of serotonin in respiratory function and dysfunction. Respir Physiol Neurobiol. 2010;174:76–88. doi: 10.1016/j.resp.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hioki H, Nakamura H, Ma YF, Konno M, Hayakawa T, Nakamura KC, Fujiyama F, Kaneko T. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Comp Neurol. 2010;518:668–686. doi: 10.1002/cne.22237. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Best S, Richerson GB. Altered ventilatory and thermoregulatory control in male and female adult Pet-1 null mice. Respir Physiol Neurobiol. 2011;177:133–140. doi: 10.1016/j.resp.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir Physiol Neurobiol. 2010a;173:256–263. doi: 10.1016/j.resp.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J Appl Physiol. 2010b;108:1425–1432. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci. 2009;29:10341–10349. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Bland BH, Antle MC. Nonserotonergic projection neurons in the midbrain raphe nuclei contain the vesicular glutamate transporter VGLUT3. Synapse. 2009;63:31–41. doi: 10.1002/syn.20581. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Turner SM, Huxtable AG, Ben-Mabrouk F. Isolated in vitro brainstem-spinal cord preparations remain important tools in respiratory neurobiology. Respir Physiol Neurobiol. 2012;180:1–7. doi: 10.1016/j.resp.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamae T, Kiyomizu K, Nakazawa T, Tadokoro S, Kashiwagi H, Honda S, Kanakura Y, Tomiyama Y. Bleeding tendency and impaired platelet function in a patient carrying a heterozygous mutation in the thromboxane A2 receptor. J Thromb Haemost. 2011;9:1040–1048. doi: 10.1111/j.1538-7836.2011.04245.x. [DOI] [PubMed] [Google Scholar]
- Kanamaru M, Homma I. Dorsomedial medullary 5-HT2 receptors mediate immediate onset of initial hyperventilation, airway dilation, and ventilatory decline during hypoxia in mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R34–41. doi: 10.1152/ajpregu.90802.2008. [DOI] [PubMed] [Google Scholar]
- Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol. 2009;517:69–86. doi: 10.1002/cne.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LH. Glutamatergic neurons say NO in the nucleus tractus solitarii. J Chem Neuroanat. 2009;38:154–165. doi: 10.1016/j.jchemneu.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofaso F, Dauger S, Matrot B, Vardon G, Gaultier C, Gallego J. Inhibitory effects of repeated hyperoxia on breathing in newborn mice. Eur Respir J. 2007;29:18–24. doi: 10.1183/09031936.00111705. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Mortola JP, Massaro D. Sexual dimorphism in the architecture of the lung's gas-exchange region. Proc Natl Acad Sci U S A. 1995;92:1105–1107. doi: 10.1073/pnas.92.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuet C, Kourdougli N, Hilaire G, Voituron N. Differences in serotoninergic metabolism possibly contribute to differences in breathing phenotype of FVB/N and C57BL/6J mice. J Appl Physiol. 2011;110:1572–1581. doi: 10.1152/japplphysiol.00117.2011. [DOI] [PubMed] [Google Scholar]
- Merazzi D, Mortola JP. Effects of changes in ambient temperature on the Hering-Breuer reflex of the conscious newborn rat. Pediatr Res. 1999;45:370–376. doi: 10.1203/00006450-199903000-00014. [DOI] [PubMed] [Google Scholar]
- Miller SS, Lee HC, Gould JB. Hypothermia in very low birth weight infants: distribution, risk factors and outcomes. J Perinatol. 2011;31(Suppl 1):S49–56. doi: 10.1038/jp.2010.177. [DOI] [PubMed] [Google Scholar]
- Monnier A, Alheid GF, McCrimmon DR. Defining ventral medullary respiratory compartments with a glutamate receptor agonist in the rat. J Physiol. 2003;548:859–874. doi: 10.1113/jphysiol.2002.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola J, Gautier H. Interaction between metabolism and ventilation: effects of respiratory gases and temperature. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. New York: Marcel Dekker; 1995. pp. 1011–1064. [Google Scholar]
- Mortola JP. Implications of hypoxic hypometabolism during mammalian ontogenesis. Respir Physiol Neurobiol. 2004;141:345–356. doi: 10.1016/j.resp.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Frappell PB. On the barometric method for measurements of ventilation, and its use in small animals. Can J Physiol Pharmacol. 1998;76:937–944. doi: 10.1139/cjpp-76-10-11-937. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kobayashi S, Kaneko T. Sympathetic premotor neurons mediating thermoregulatory functions. Neurosci Res. 2005a;51:1–8. doi: 10.1016/j.neures.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005b;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Kawai A, Muckenhoff K, Scheid P. Role of the pons in hypoxic respiratory depression in the neonatal rat. Respir Physiol. 1998;111:55–63. doi: 10.1016/s0034-5687(97)00105-9. [DOI] [PubMed] [Google Scholar]
- Okada Y, Masumiya H, Tamura Y, Oku Y. Respiratory and metabolic acidosis differentially affect the respiratory neuronal network in the ventral medulla of neonatal rats. Eur J Neurosci. 2007;26:2834–2843. doi: 10.1111/j.1460-9568.2007.05891.x. [DOI] [PubMed] [Google Scholar]
- Okada Y, Muckenhoff K, Holtermann G, Acker H, Scheid P. Depth profiles of pH and PO2 in the isolated brain stem-spinal cord of the neonatal rat. Respir Physiol. 1993a;93:315–326. doi: 10.1016/0034-5687(93)90077-n. [DOI] [PubMed] [Google Scholar]
- Okada Y, Muckenhoff K, Scheid P. Hypercapnia and medullary neurons in the isolated brain stem-spinal cord of the rat. Respir Physiol. 1993b;93:327–336. doi: 10.1016/0034-5687(93)90078-o. [DOI] [PubMed] [Google Scholar]
- Olivan AM, Perez-Rodriguez R, Roncero C, Arce C, Gonzalez MP, Oset-Gasque MJ. Plasma membrane and vesicular glutamate transporter expression in chromaffin cells of bovine adrenal medulla. J Neurosci Res. 2011;89:44–57. doi: 10.1002/jnr.22529. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka T. Hypoxia-induced hypothermia mediated by noradrenaline and nitric oxide in the rostromedial preoptic area. Neuroscience. 2011;179:170–178. doi: 10.1016/j.neuroscience.2011.01.056. [DOI] [PubMed] [Google Scholar]
- Perrone S, Bracci R, Buonocore G. New biomarkers of fetal-neonatal hypoxic stress. Acta Paediatr. 2002;91:135–138. doi: 10.1111/j.1651-2227.2002.tb02919.x. [DOI] [PubMed] [Google Scholar]
- Phifer CB, Terry LM. Use of hypothermia for general anaesthesia in preweanling rodents. Physiol Behav. 1986;38:887–890. doi: 10.1016/0031-9384(86)90058-2. [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Ramanantsoa N, Hirsch MR, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault PL, Matrot B, Fortin G, Brunet JF, Gallego J, Goridis C. Breathing without CO2 chemosensitivity in conditional Phox2b mutants. J Neurosci. 2011;31:12880–12888. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanantsoa N, Vaubourg V, Dauger S, Matrot B, Vardon G, Chettouh Z, Gaultier C, Goridis C, Gallego J. Ventilatory response to hyperoxia in newborn mice heterozygous for the transcription factor Phox2b. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1691–1696. doi: 10.1152/ajpregu.00875.2005. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJ, Wilken B. Developmental changes in the hypoxic response of the hypoglossus respiratory motor output in vitro. J Neurophysiol. 1997;78:383–392. doi: 10.1152/jn.1997.78.1.383. [DOI] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renolleau S, Dauger S, Autret F, Vardon G, Gaultier C, Gallego J. Maturation of baseline breathing and of hypercapnic and hypoxic ventilatory responses in newborn mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1746–1753. doi: 10.1152/ajpregu.2001.281.5.R1746. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Ruel J, Emery S, Nouvian R, Bersot T, Amilhon B, Van Rybroek JM, Rebillard G, Lenoir M, Eybalin M, Delprat B, Sivakumaran TA, Giros B, El Mestikawy S, Moser T, Smith RJ, Lesperance MM, Puel JL. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am J Hum Genet. 2008;83:278–292. doi: 10.1016/j.ajhg.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki C, Matsuoka T, Mortola JP. Metabolic-ventilatory interaction in conscious rats: effect of hypoxia and ambient temperature. J Appl Physiol. 1994;76:1594–1599. doi: 10.1152/jappl.1994.76.4.1594. [DOI] [PubMed] [Google Scholar]
- Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, Lustig LR, Edwards RH. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simakajornboon N, Vlasic V, Li H, Sawnani H. Effect of prenatal nicotine exposure on biphasic hypoxic ventilatory response and protein kinase C expression in caudal brain stem of developing rats. J Appl Physiol. 2004;96:2213–2219. doi: 10.1152/japplphysiol.00935.2003. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Simmons JR, McQuiston TJ, Vujovic N, Weston MC, Guyenet PG. Coexpression of vesicular glutamate transporter-3 and γ-aminobutyric acidergic markers in rat rostral medullary raphe and intermediolateral cell column. J Comp Neurol. 2005;492:477–494. doi: 10.1002/cne.20742. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem–spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall GJ, Milsom WK. Hypoxia reduces the hypothalamic thermogenic threshold and thermosensitivity. J Physiol. 2009;587:5259–5274. doi: 10.1113/jphysiol.2009.175828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev. 2010;90:675–754. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat Neurosci. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, Hangya B, Holderith N, Magee JC, Freund TF. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–453. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Pena F, Zanella S, Bevengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voituron N, Frugiere A, Champagnat J, Bodineau L. Hypoxia-sensing properties of the newborn rat ventral medullary surface in vitro. J Physiol. 2006;577:55–68. doi: 10.1113/jphysiol.2006.111765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voituron N, Frugiere A, Mc Kay LC, Romero-Granados R, Dominguez-Del-Toro E, Saadani-Makki F, Champagnat J, Bodineau L. The kreisler mutation leads to the loss of intrinsically hypoxia-activated spots in the region of the retrotrapezoid nucleus/parafacial respiratory group. Neuroscience. 2011;194:95–111. doi: 10.1016/j.neuroscience.2011.07.062. [DOI] [PubMed] [Google Scholar]
- Voituron N, Shvarev Y, Menuet C, Bevengut M, Fasano C, Vigneault E, El Mestikawy S, Hilaire G. Fluoxetine treatment abolishes the in vitro respiratory response to acidosis in neonatal mice. PLoS One. 2010;5:e13644. doi: 10.1371/journal.pone.0013644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


