Abstract
NF-κB transcription factors are central regulators of inflammation and when dysregulated contribute to malignant transformation. IκB kinase ε (IKKε; IKKi, encoded by IKBKE) is a breast oncogene that is amplified in 30% of breast cancers and drives transformation in an NF-κB-dependent manner. Here we demonstrate that IKKε interacts with and phosphorylates tumor necrosis factor receptor-associated factor 2 (TRAF2) at Ser11 in vitro and in vivo. This activity promotes Lys63-linked TRAF2 ubiquitination and NF-κB activation and is essential for IKKε transformation. Breast cancer cells that depend on IKKε expression for survival are also dependent on TRAF2. This work defines TRAF2 phosphorylation to be one key effector of IKKε-induced mammary epithelial cell transformation.
INTRODUCTION
NF-κB activation plays an important role in innate immunity and inflammation. Several lines of evidence indicate that dysregulation of NF-κB signaling also contributes to malignant transformation through both cell autonomous and cell nonautonomous mechanisms. Although cancers associated with chronic inflammation are frequently dependent on aberrant NF-κB activity induced by the tumor microenvironment (11, 16, 17, 25), somatic mutations in components of the NF-κB pathway that lead to constitutive NF-κB activation also contribute directly to tumorigenicity (5, 8, 18, 20, 23).
Activation of the canonical NF-κB pathway is facilitated by the recruitment of receptor-associated adaptor molecules such as tumor necrosis factor (TNF) receptor-associated factors (TRAFs) (24). TRAF proteins mediate the formation of protein complexes that activate the classical IκB kinase (IKK) complex, consisting of the catalytic kinases IKKα and IKKβ and the regulatory subunit IKKγ/NEMO. IKK complex activation triggers proteasome-mediated degradation of the key inhibitory molecule IκBα that, in turn, permits nuclear translocation of NF-κB dimers. In addition to this canonical mode of activation in response to TNF, an array of inflammatory stimuli activates several signaling pathways that converge to activate NF-κB (10).
IκB kinase ε (IKKε; encoded by IKBKE) is a noncanonical IKK family member that activates both interferon and NF-κB signaling. In response to viruses, both IKKε and another noncanonical IKK, TBK1, form a complex to phosphorylate interferon regulatory factor 3 (IRF3) and IRF7 (6). This activity is essential for the nuclear translocation of IRF3 and IRF7 and transcriptional activation of type I interferon genes (9). In addition to its role in innate immunity, IKKε is also a breast oncogene that is amplified and overexpressed in up to 30% of breast cancers (4). Suppression of IKKε induces apoptosis in breast cancer cell lines that harbor increased IKKε copy number. IKKε overexpression induces malignant transformation in immortalized human and murine cells in an NF-κB-dependent manner (4).
We recently employed a scanning peptide library screen and Scansite bioinformatic analysis to identify an IKKε recognition motif and potential IKKε substrates. Using this combined proteomic and bioinformatic approach, we identified the familial tumor suppressor CYLD to be an IKKε substrate involved in cell transformation (15). As a deubiquitinase, CYLD acts as a negative regulator of NF-κB signaling. Phosphorylation of CYLD by IKKε at serine 418 inactivates CYLD function and leads to NF-κB activation. Although this phosphorylation event was necessary for IKKε-mediated transformation, cells rendered tumorigenic by IKKε expression were only partially dependent on CYLD for transformation. These observations suggested that IKKε regulates other effectors that participate in NF-κB pathway activation and transformation.
Here we identify TRAF2, an adaptor molecule that assembles active NF-κB signaling modules, as an IKKε substrate. IKKε phosphorylates TRAF2 at Ser11, and this activity is required for IKKε-induced NF-κB activation and transformation.
MATERIALS AND METHODS
Antibodies, plasmids, and reagents.
The antibodies used included Myc (clone 4A6; Millipore), cIAP1, TBK1, TANK, TRAF2, and Lys63- and Lys48-linkage-specific polyubiquitin (Cell Signaling Technologies), V5-horeseradish peroxidase (Invitrogen), ubiquitin (Ub; Santa Cruz Biotechnology), IKKε and β-actin (Sigma-Aldrich), hemagglutinin (HA; clone 12C5; Boehringer Mannheim), and a mouse monoclonal TRAF2 antibody (Imgenex). The IKKε phosphosubstrate antibody was previously described (15). The phospho-TRAF2 (Ser11) antibody was previously described (3). The phospho-TRAF2 (Ser 11) antibody used in Fig. 4D was generated in collaboration with Cell Signaling Technologies. Anti-V5 affinity gel agarose was obtained from Sigma-Aldrich. Primary breast cancer tumor lysates were obtained from Origene or generated by lysing primary breast tumor specimens in radioimmunoprecipitation assay buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM Na2EDTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin).
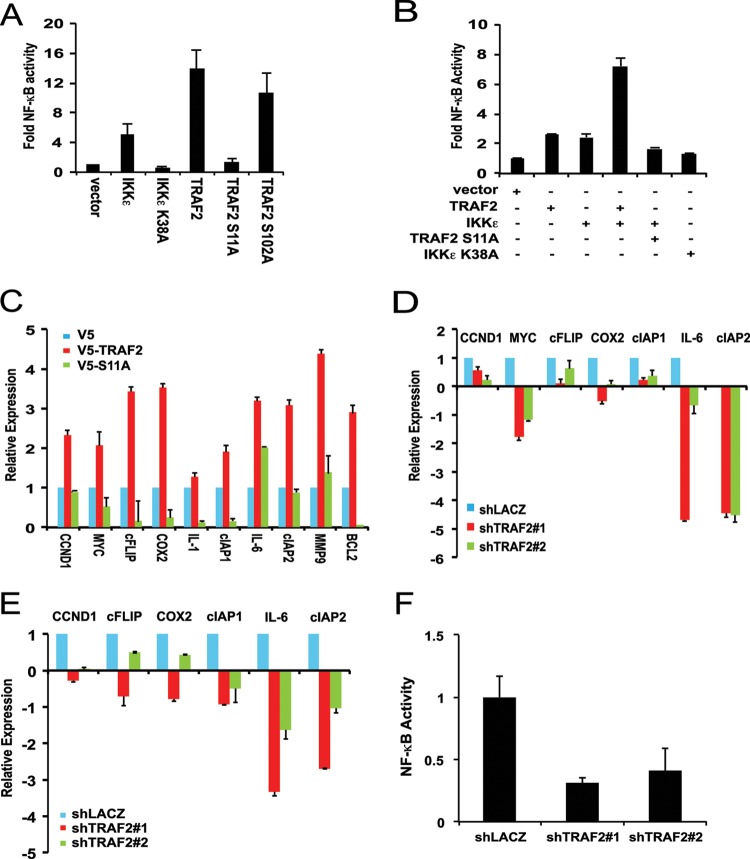
Fig 4.
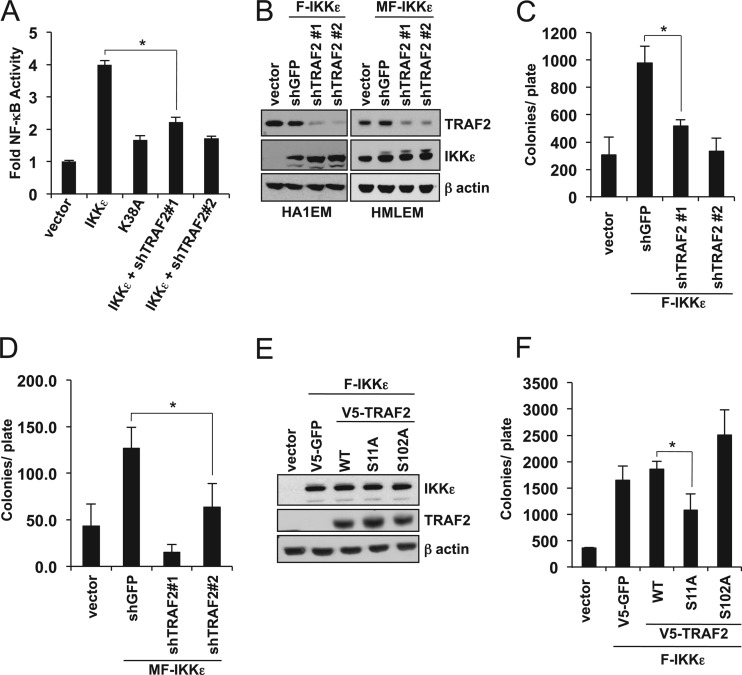
IKKε promotes TRAF2-induced NF-κB activation. (A) IKKε- and TRAF2-induced NF-κB activation. Stable NF-κB reporter HEK293T cells were transfected as indicated, and raw light unit (RLU) activity was measured and normalized to the activity observed with the control vector. (B) IKKε-induced NF-κB activation in the presence of wild-type TRAF2 and TRAF2 S11A. HA1EM cells were cotransfected as indicated with SV40-Renilla luciferase and an NF-κB luciferase. Raw light unit activity was normalized to the activity observed with the control vector. Results reported as means ± SDs of three experiments. (C to E) Change in expression of NF-κB target genes in HMLEM cells transduced with V5-TRAF2 (C), V5-TRAF2 Ser11A IKKε-transformed HMLEM cells expressing TRAF2-specfic shRNA (shTRAF2#1 and shTRAF2#2) (D), and ZR-75-1 cells following TRAF2 suppression (E). Relative expression was quantified by RT-PCR using ΔΔCT (where CT is the threshold cycle) and normalized to the levels observed with control (V5 and shLacZ) cells. (F) NF-κB activity in ZR-75-1 cells following TRAF2 suppression. Stable NF-κB reporter ZR-75-1 cells were transduced as indicated, and raw light unit activity was measured and normalized to the activity observed with the control (shLacZ).
Myc-TRAF2 was created by PCR cloning into the BamHI site of the 3× Myc (pEBB) vector. Glutathione S-transferase (GST)–IKKε and GST-K38A have been described previously (15). Flag-IKKε and Myr-Flag-IKKε were used as described previously (4). Myc-TRAF2 S11A, Myc-TRAF2 S102A, Myc-TRAF2 S274A, Myc-TRAF2 S327A, and Myc-TRAF2 S408A were created using the QuikChange site-directed mutagenesis protocol (Stratagene). pWZL-TRAF2 wild type (WT) and mutants with site-directed mutations were created by subcloning into the BamHI site of the pWZL-BLAST retroviral expression vector(s). V5-green fluorescent protein (GFP), V5-TRAF2, V5-TRAF2 S11A, and V5-TRAF2 S102A were generated by gateway cloning into the pLEX-V5-BLAST lentiviral vector. HA-ubiquitin, HA–Ub K63-only, and HA–Ub K48-only were used as described previously (1, 4). Short hairpin RNA (shRNA) against IKKε (shIKKε) and shCYLD lentiviral constructs have been described previously (4, 15). Additional shRNA constructs were obtained from the RNAi Consortium (Broad Institute). Clone identification numbers for the shRNA constructs are pLKO-shTRAF2#1 (TRCN0000010891), pLKO-shTRAF#2 (TRCN0000004571), pLKO-shGFP (TRCN0000072181), pLKO-shLacZ (TRCN0000072231), pLKO-shcIAP1#1 (TRC0000003780), pLKO-shcIAP1#2 (TRC0000003782), pLKO-shcIAP2#1 (TRC0000003778), pLKO-shcIAP2#2 (TRC0000003776), and pLKO-shLuc (TRCN0000072243). We note that the shRNAs called pLKO-shcIAP2#1 and pLKO-shcIAP2#2 target sequences found in both cIAP1 and cIAP2 and thus target both cIAP1 and cIAP2.
Cell culture, transfection, immunoprecipitation, subcellular fractionation, and immunoblotting.
HEK293T, MDA-MB-453, and MCF-7 cells were obtained from ATCC and were grown in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS). NIH 3T3 cells were obtained from ATCC and grown in DMEM containing 10% bovine calf serum. HA1EM and HMLEM cells have been described previously (4). BT549, BT474, MDA-MB-453, T47D, and ZR-75-1 cells were obtained from ATCC and maintained in RPMI 1640 containing 10% FBS. Transfection experiments were performed using Fugene (Roche). Immunoprecipitations and immunoblotting experiments were conducted as described previously (14, 22). For immunoprecipitation experiments assessing ubiquitination, lysates were boiled in 0.5% SDS to disrupt noncovalent interactions. Densitometry was performed using ImageJ software.
In vitro kinase assays.
Recombinant GST-IKKε (Invitrogen) and recombinant GST-TRAF2 (Novus Biologicals) were used for in vitro kinase assays. Kinase buffer contained 50 mM Tris (pH 7.5), 12 mM MgCl2, 1 mM β-glycerophosphate, 100 μM ATP, and 10 μCi [γ-32P]ATP/reaction mixture. Reaction mixtures were incubated at 30°C for 1 h, resolved by SDS-PAGE, and directly exposed to radiography film.
NF-κB reporter assays and quantitative RT-PCR.
HA1EM and HEK293T cells were transfected with a pTRH1-NF-κB-luciferase reporter (System Biosciences) in parallel with pRL-SV40-Renilla luciferase (Promega). NF-κB activity was measured using a Dual-Glo luciferase assay (Promega) at 36 h posttransfection. Alternatively, GloResponse NF-κB-RE-luc2P HEK293T cells (Promega) were transfected as indicated and NF-κB activity was measured at 36 h posttransfection according to the protocol for the One-Glo luciferase assay (Promega). Luciferase values were normalized to Renilla luciferase values to yield relative light units. Quantitative reverse transcription-PCR (RT-PCR) for NF-κB target genes was conducted as described previously (4).
In vitro cell transformation assay.
Growth of NIH 3T3, HA1EM, or HMLE cells in soft agar was determined by plating 5 × 104 cells in triplicate in 0.4% Noble agar. Microscopic (diameter, greater than 100 μm) or macroscopic (diameter, greater than 1,500 μm) colonies were counted 28 days after plating using ImageJ software.
In vitro ubiquitination assay.
TRAF2 was isolated by Myc immunoprecipitation from 3× Myc-TRAF2-transfected HEK293T cells. cIAP1 and cIAP2 were isolated by Flag immunoprecipitation from Flag-cIAP1- and Flag-cIAP2-transfected HEK293T cells. Recombinant E1 ubiquitin-activating enzyme (E-304), Ubc13 E2 enzyme (E2-664), and ubiquitin (U-100H) were purchased from Boston Biochem. Reactions were carried out at 35°C for 2 h in 50 nM HEPES (pH 7.8), 10 mM MgCl2, and 4 mM ATP, and the reaction mixtures contained 50 nmol E1, 150 nmol E2, 10 μg ubiquitin, immunopurified cIAP1 and cIAP2, and TRAF2-protein G-Sepharose (40 μg each). Reactions were terminated with the addition of SDS loading dye and analyzed by immunoblotting.
Viability and proliferation measurements.
Proliferation assays were performed in duplicate using a Vi-Cell counter every 7 days for 14 days, and the results were plotted as a function of population doubling (PD) versus time. PD was defined as [log2(number of cells counted/number of cells plated)].
RESULTS
IKKε phosphorylates and binds to TRAF2.
We previously demonstrated that IKKε phosphorylates and inactivates the tumor suppressor CYLD using an integrated proteomic and bioinformatic approach (15). Although CYLD is one IKKε substrate that contributes to cell transformation, we found that suppression of CYLD did not fully inhibit IKKε-mediated tumorigenicity, suggesting that other IKKε effectors contribute to cell transformation. When we reexamined the list of candidate IKKε substrates, we recognized that TRAF2, a molecule that plays an essential role in TNF-induced NF-κB activation, harbors several potential IKKε substrate recognition motifs. To test whether IKKε phosphorylates TRAF2, we performed an in vitro kinase assay using recombinant GST-tagged IKKε and recombinant GST-tagged TRAF2. We observed robust autophosphorylation of IKKε and also verified that TRAF2 was strongly phosphorylated by IKKε (Fig. 1A). To confirm that IKKε phosphorylates TRAF2 in vivo, we first coexpressed a Myc epitope-tagged TRAF2 and either GST-tagged IKKε or kinase-inactive IKKε K38A in transformed human embryonic kidney epithelial (HEK293T) cells. We then isolated TRAF2 immune complexes and found that TRAF2 was phosphorylated by wild-type IKKε in a kinase-dependent manner using an IKKε phosphosubstrate antibody (Fig. 1B). These observations demonstrate that IKKε phosphorylates TRAF2 both in vitro and in vivo.
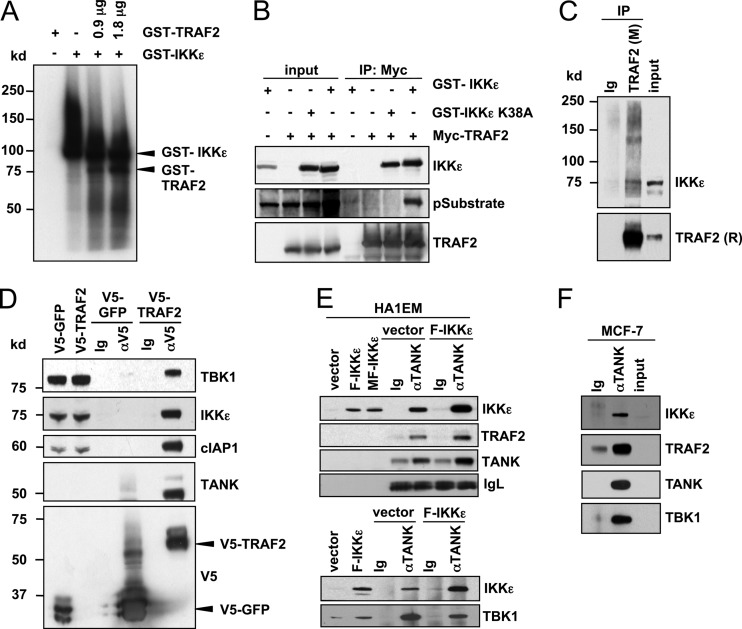
Fig 1.
IKKε phosphorylates TRAF2 and forms a complex with TRAF2, TANK, TBK1, and cIAP1. (A) In vitro phosphorylation of TRAF2 by IKKε. Kinase assay using recombinant GST-IKKε and GST-TRAF2. GST-TRAF2 (0.9 μg and 1.8 μg) was used as a substrate for GST-IKKε. (B) Phosphorylation of TRAF2 by IKKε in HEK293T cells. Myc-TRAF2 immune complexes were isolated from HEK293T cells expressing the indicated proteins and analyzed by immunoblotting with antibodies specific for IKKε, IKKε phosphosubstrates, or TRAF2. IP, immunoprecipitation. (C) Endogenous interactions between IKKε and TRAF2 in IKKε-transformed cells. TRAF2 immune complexes were isolated from HA1EM cells using a murine (M) monoclonal antibody and analyzed by immunoblotting with IKKε and rabbit (R) TRAF2 antibodies. (D) TRAF2, IKKε, TANK, cIAP1, and TBK1 form a complex. V5 immune complexes were isolated from HA1EM cells transduced with V5-GFP or V5-TRAF2 and analyzed by immunoblotting with antibodies specific for V5, TANK, cIAP1, IKKε, or TBK1. Murine immunoglobulin (Ig) was used for control immunoprecipitations. (E) Endogenous TANK, TRAF2, and IKKε form a complex. TANK immune complexes were isolated from HA1EM cells transduced with control vector or F-IKKε and analyzed by immunoblotting with antibodies specific for IKKε, TRAF2, and TANK. Rabbit Ig was used for control immunoprecipitations, and Ig light chain (IgL) is shown as a loading control. (F) Endogenous interaction of IKKε, TRAF2, TANK, and TBK1 in MCF-7 cells. TANK immune complexes were isolated from MCF-7 cells and analyzed by immunoblotting with the indicated antibodies.
We next examined whether IKKε interacts with TRAF2. In HEK293T cells coexpressing Myc-TRAF2 and GST-IKKε or the kinase-inactive mutant GST-IKKε K38A, we found that both WT and K38A IKKε and TRAF2 formed a readily detectable complex (Fig. 1B). To confirm that this interaction occurs under conditions where IKKε expression induces cell transformation, we determined whether IKKε interacts with endogenous TRAF2 in immortal but nontumorigenic human embryonic kidney (HEK) cells expressing human TERT (hTERT), simian virus 40 (SV40) large T and small t oncoproteins, and a constitutively active MEKDD allele (HA1EM cells) (4). Using these cells, we isolated endogenous TRAF2 immune complexes and verified that IKKε and TRAF2 interact (Fig. 1C).
TRAF2 has been shown to form a complex with the ubiquitin ligase cIAP1, the TRAF family member-associated NF-κB activator (TANK), and TBK1, a noncanonical IKK closely related to IKKε (26, 33). This complex acts as a scaffold that facilitates the activation of NF-κB signaling. To determine whether cIAP1 and TANK were also present in the complex formed by IKKε and TRAF2, we isolated TRAF2 immune complexes from HA1EM cells expressing V5-tagged TRAF2 and detected endogenous IKKε, TBK1, cIAP1, and TANK (Fig. 1D). We also assessed whether this complex forms in IKKε-transformed HA1EM cells. Specifically, we isolated TANK immune complexes from HA1EM cells and HA1EM cells stably expressing Flag epitope-tagged IKKε (HA1EM-F-IKKε) and found a complex composed of endogenous TANK, TRAF2, cIAP1, TBK1, and IKKε (Fig. 1E). Overexpression of IKKε increased the abundance of the complex, suggesting that IKKε drives the recruitment of these components. To verify these observations, we examined whether this complex is present in breast cancer cells that depend on IKKε for survival. Specifically, we confirmed that the IKKε-TANK-TRAF2-cIAP1-TBK1 complex is formed in MCF-7 cells (Fig. 1F). These findings provide evidence that IKKε forms a complex with TRAF2, TANK, TBK1, and cIAP1.
IKKε phosphorylates TRAF2 on Ser11 to recruit the canonical IKK complex and RIP1.
To identify the TRAF2 residue(s) that is phosphorylated by IKKε, we surveyed the TRAF2 protein sequence for serine residues that correspond to the IKKε kinase recognition motif (15). This motif consists of a central serine phosphorylation site neighbored by a leucine residue at the +1 position and hydrophobic residues at the −2 and +3 positions (Fig. 2A). We found five TRAF2 serine residues (positions 11, 102, 274, 327, and 408) that matched this motif (Fig. 2A).
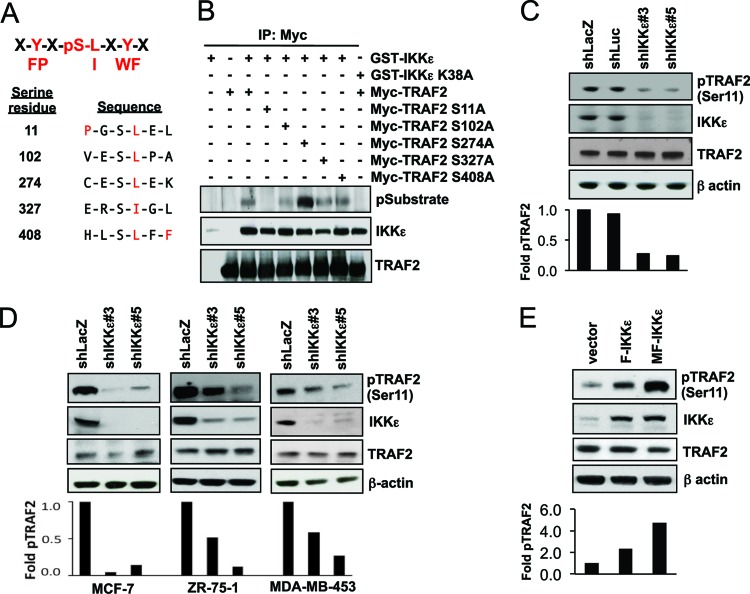
Fig 2.
IKKε phosphorylates TRAF2 at Ser11. (A) IKKε recognition motif and candidate TRAF2 sequences that match the motif. Highlighted residues (red) indicate a match to the recognition motif. Amino acid residues indicated as one-letter symbols (X, degenerate amino acid). (B) IKKε phosphorylates TRAF2 at Ser11. HEK293T cells were transfected as indicated. Myc-TRAF2 immune complexes were isolated and analyzed with antibodies specific for TRAF2, IKKε, and IKKε phosphosubstrates. (C and D) Effects of suppressing IKKε on TRAF2 Ser11 phosphorylation. Immunoblot of phospho-TRAF2 (Ser11) in HA1EM cells (C) or breast cancer cell lines (D) transduced with shLacZ or shLuc controls or IKKε-specific shRNAs (shIKKε#3 and shIKKε#5). Total IKKε, TRAF2, and β-actin expression is shown. The results of densitometry analysis are shown in the lower panel. (E) TRAF2 Ser11 phosphorylation in IKKε-transformed HA1EM cells. Immunoblotting was performed as described for panels C and D on HA1EM cells expressing a control vector, F-IKKε, or MF-IKKε. The results of densitometry analysis are shown in the lower panel.
We then interrogated whether these sites are phosphorylated by IKKε by generating serine-to-alanine substitutions for each putative target serine residue. After coexpressing GST-IKKε with wild type or each of these TRAF2 mutants in HEK293T cells, we isolated TRAF2 immune complexes and found that each of the TRAF2 mutants retained the ability to bind IKKε (Fig. 2B). However, when we examined the phosphorylation status of wild-type and mutant TRAF2, we found that IKKε phosphorylated TRAF2 S102A, TRAF2 S274A, TRAF2 S327A, and TRAF2 S408A comparably to wild-type TRAF2. In contrast, we found that IKKε failed to phosphorylate TRAF2 S11A (Fig. 2B), indicating that IKKε targets TRAF2 Ser11 for phosphorylation.
To examine whether IKKε phosphorylates endogenous TRAF2, we investigated the consequences of manipulating IKKε expression on the phosphorylation of TRAF2 at Ser11. First, we suppressed IKKε in HA1EM cells and several breast cancer cell lines that depend on IKKε for tumorigenic growth (MCF-7, ZR-75-1, and MDA-MB-453 cells). We introduced two distinct IKKε-specific shRNAs in these cells and measured TRAF2 phosphorylation at Ser11 using a phospho-TRAF2 (Ser11)-specific antibody (3). Suppression of IKKε in HA1EM cells with these two IKKε-specific shRNAs resulted in 3.6- and 4.2-fold decreases in the levels of TRAF2 Ser11 phosphorylation, respectively (Fig. 2C). We also observed a decrease in TRAF2 Ser11 phosphorylation after IKKε suppression in MCF-7, ZR-75-1, and MDA-MB-453 cells (Fig. 2D). To corroborate these observations, we determined whether expression of IKKε promoted an increase in TRAF2 phosphorylation at Ser11. We previously showed that the introduction of F-IKKε or myristoylated, Flag epitope-tagged IKKε (MF-IKKε) transforms HA1EM cells (4). When we assessed the levels of phosphorylated TRAF2 in HA1EM cells expressing F-IKKε or MF-IKKε, we found 2.3- and 4.8-fold increases in TRAF2 Ser11 phosphorylation, respectively (Fig. 2E). These observations confirmed that IKKε phosphorylates TRAF2 at Ser11 in vivo and suggest that this activity is important for cell transformation.
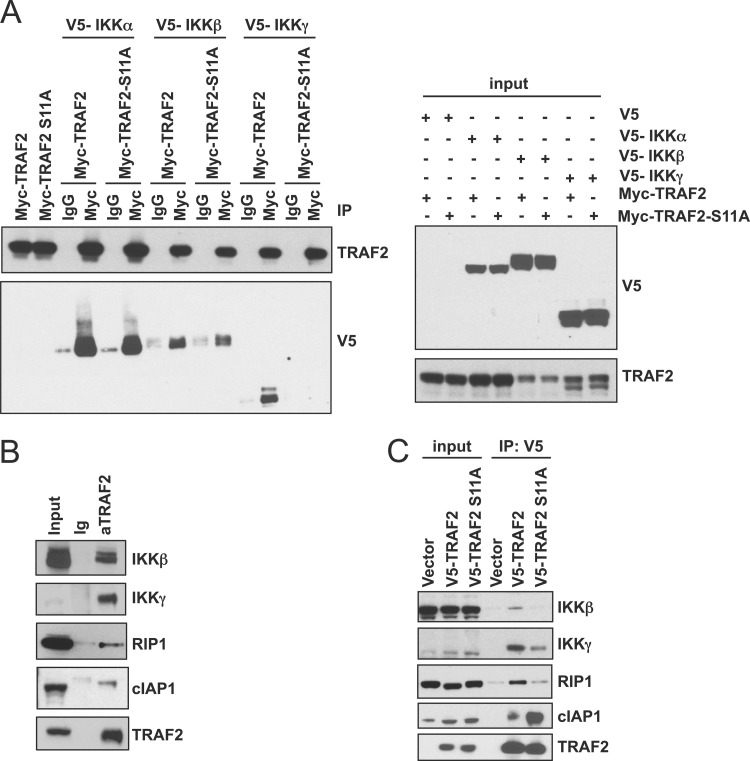
To elucidate the functional consequences of IKKε-mediated TRAF2 Ser11 phosphorylation, we assessed whether this activity modulates TRAF2 recruitment of the canonical IKK complex (IKKα, IKKβ, and IKKγ). We first assessed this interaction in HEK293T cells after overexpressing Myc-TRAF2 or Myc-TRAF2 S11A and V5-tagged forms of each component of the IKK complex, IKKα, IKKβ, and IKKγ. We isolated TRAF2 immune complexes and observed that TRAF2 interacted with V5-IKKα, IKKβ, and IKKγ. In contrast, TRAF2 S11A retained the ability to bind IKKα but failed to interact with IKKγ and exhibited decreased interactions with IKKβ (Fig. 3A). To confirm these findings in an IKKε-dependent cell line, MCF-7, we isolated TRAF2 immune complexes and found that IKKγ, IKKβ, cIAP1, and RIP1 were associated with TRAF2 (Fig. 3B). To determine whether TRAF2 phosphorylation is required for recruitment of IKKβ, IKKγ, and RIP1 in MCF-7 cells, we then isolated TRAF2 immune complexes from MCF-7 cells expressing V5-TRAF2 or V5-TRAF2 S11. We confirmed that TRAF2 associates with endogenous IKKβ, IKKγ, RIP1, and cIAP1. In contrast, we found that TRAF2 S11A failed to bind IKKβ, IKKγ, and RIP1 but showed increased affinity for cIAP1 (Fig. 3C). These observations demonstrate that IKKε-induced TRAF2 phosphorylation is necessary for the recruitment of the canonical IKK complex and RIP1 and suggest that these events facilitate downstream activation of NF-κB.
Fig 3.
TRAF2 Ser11 phosphorylation recruits the IKK complex and RIP1. (A) TRAF2 interacts with the canonical IKK complex in HEK293T cells. HEK293T cells were cotransfected as indicated with either wild-type Myc-TRAF2 or Myc-TRAF2-S11A and V5-tagged IKKα, IKKβ, and IKKγ (input, right). Myc-TRAF2 immune complexes were isolated and analyzed with antibodies specific for TRAF2 and V5 (left). (B) Endogenous interaction between of TRAF2, IKKβ, IKKγ, RIP1, and cIAP1. TRAF2 immune complexes were isolated from MCF-7 cells and analyzed with the indicated antibodies following treatment with TNF-α (20 ng/ml for 30 min). (C) TRAF2 interactions with the IKK complex, cIAP1, and RIP1 are dependent on phosphorylation. MCF-7 cells were transduced with V5-TRAF2 or V5-TRAF2 S11A. V5-TRAF2 immune complexes were isolated and analyzed by immunoblotting for endogenous IKKβ, IKKγ, RIP1, and cIAP1.
IKKε-induced TRAF2 phosphorylation promotes NF-κB activation.
Having found that TRAF2 phosphorylation recruits the canonical IKK complex and RIP, we next determined whether the phosphorylation of TRAF2 by IKKε promoted NF-κB activation. Using a HEK293T cell line that stably expresses a NF-κB luciferase reporter (GloResponse NF-κB-RE-luc2P), we found that expression of either IKKε or TRAF2 induced 5-fold and 14-fold increases in NF-κB activity, respectively (Fig. 4A). In contrast, both kinase-inactive IKKε K38A and TRAF2 S11A failed to promote NF-κB activation (Fig. 4A). To evaluate the effects of IKKε- and TRAF2-induced NF-κB activation in cells that are readily transformed by IKKε, we performed NF-κB luciferase reporter experiments in HA1EM cells. Expression of either IKKε or TRAF2 alone in HA1EM cells induced a 2.7-fold increase in NF-κB activity compared to the activity measured in cells expressing a control vector (Fig. 4B). In contrast, coexpression of IKKε and wild-type TRAF2 resulted in a 7-fold increase in NF-κB activity. Since we failed to observe a similar increase in IKKε-mediated NF-κB activation by coexpressing IKKε and TRAF2 S11A, we concluded that the increased NF-κB activity was due to IKKε-mediated phosphorylation of TRAF2 at Ser11. In consonance with these findings, we found that wild-type TRAF2 induced the expression of several NF-κB target genes, including CCND1, MYC, cFLIP, COX2, cIAP1, cIAP2, MMP9, and BCL2, in IKKε-transformed human mammary epithelial cells (HMELM) cells, whereas TRAF2 S11A failed to activate these NF-κB-regulated genes (Fig. 4C). Similarly, suppression of TRAF2 in IKKε-transformed HMELM cells inhibits expression of these target genes by up to 5-fold (Fig. 4D). Furthermore, when we suppressed TRAF2 in an IKKε-dependent breast cancer cell line, ZR-75-1, we also found that NF-κB target genes, including CCND1, cIAP1, COX2, CFLIP, cIAP2, and IL-6, were downregulated by up to 7-fold (Fig. 4E). In agreement with this observation, basal NF-κB reporter activity in ZR-75-1 cells was also reduced by 72% following depletion of TRAF2 (Fig. 4F). Collectively, these results suggest that IKKε-mediated TRAF2 Ser11 phosphorylation activates NF-κB signaling.
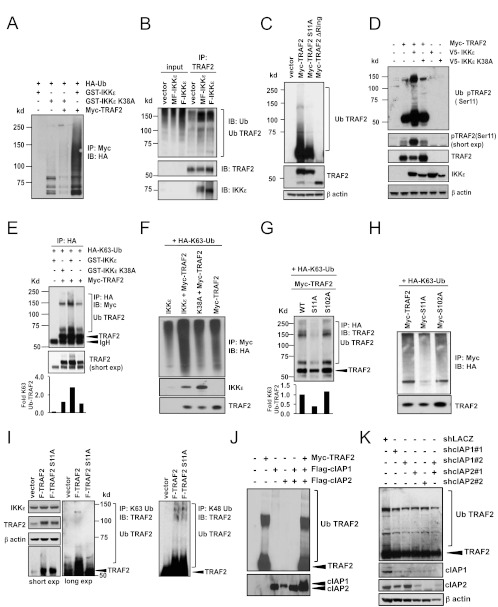
IKKε-induced TRAF2 Ser11 phosphorylation promotes TRAF2 Lys63-linked ubiquitination.
Ubiquitination plays a key role in NF-κB activation (7, 30). For example, Lys48-linked ubiquitination mediates the proteasomal degradation of IκBα that is essential for nuclear translocation of NF-κB transcription factors (19). In addition, modification of several NF-κB regulators, including TRAF6, TAB2, and TAB3, by Lys63-linked ubiquitin chains facilitates the formation of a protein platform consisting of the canonical IKKs as well as the IKKs, TAK1, RIP1, and other scaffolding molecules (7, 30). Prior work has shown that TRAF2 is modified by Lys63 ubiquitination and that these modifications regulate the recruitment of the IKK complex and downstream NF-κB activation (2, 21, 29). We reasoned that TRAF2 phosphorylation by IKKε may regulate TRAF2 ubiquitination and evaluated ubiquitination of TRAF2 in the presence and absence of IKKε. We coexpressed Myc-TRAF2, HA-ubiquitin, and either GST-IKKε or GST-IKKε K38A in HEK293T cells. From these cells, we isolated TRAF2 immune complexes and determined whether TRAF2 was ubiquitinated. We found that expression of IKKε dramatically increased TRAF2 ubiquitination in a kinase-dependent manner, suggesting that phosphorylation of TRAF2 by IKKε was required for TRAF2 ubiquitination (Fig. 5A). We made similar observations in both HMLEM and HA1EM cells stably expressing Flag-TRAF2. In both cases, when we isolated TRAF2 immune complexes, we found ubiquitin-containing species in cells overexpressing IKKε (Fig. 5B). These observations indicate that IKKε promotes TRAF2 ubiquitination in transformed cells.
Fig 5.
IKKε promotes TRAF2 Lys63-linked ubiquitination. (A) Total TRAF2 ubiquitination in HEK293T cells cotransfected as indicated with HA-Ub. Myc-TRAF2 immune complexes were isolated and analyzed by immunoblotting (IB) with an HA antibody. (B) Endogenous TRAF2 ubiquitination in HMLEM cells expressing a control vector, MF-IKKε, and F-IKKε. TRAF2 immune complexes were isolated and analyzed by immunoblotting with antibodies specific for ubiquitin, TRAF2, or IKKε. (C) Wild-type TRAF2 and TRAF2 S11A ubiquitination. HEK293T cells were transfected as indicated and analyzed by immunoblotting with a TRAF2 antibody. (D) Ubiquitination of phosphorylated TRAF2 in the presence of IKKε. HEK293T cells were cotransfected as indicated and analyzed by immunoblotting with antibodies specific for phospho-TRAF2 (Ser11) [pTRAF2 (Ser11)], TRAF2, IKKε, or β-actin. The short exposure (exp) shows pTRAF2 (Ser11). (Top) Ubiquitinated pTRAF2 (Ser11). (E and F) Lys63-linked TRAF2 ubiquitination in the presence of IKKε. HEK293T cells were cotransfected as indicated with HA-K63-Ub. (E) Immunoprecipitation was performed using an HA antibody followed by immunoblot analysis of Myc-TRAF2. The short exposure shows Myc-TRAF2 above IgH. (F) Myc-TRAF2 immune complexes were isolated and analyzed by immunoblotting with antibodies specific for HA, IKKε, andTRAF2. (G and H) Lys63-linked ubiquitination of wild-type TRAF2 and TRAF2 phosphorylation mutants. HEK293T cells were cotransfected as indicated with HA-K63-Ub. (G) HA-K63-Ub immune complexes were isolated and analyzed by immunoblotting with a TRAF2 antibody. (H) Myc immune complexes were isolated and analyzed by immunoblotting with antibodies specific for HA and TRAF2. (I) K63- and K48-linked ubiquitination of TRAF2 in IKKε-dependent cells. MCF-7 cells were transduced with F-TRAF2 or F-TRAF2 S11A (expression levels are shown in the three upper left panels). Immune complexes isolated with a K63-linked polyubiquitin-specific (left) or K48-linked polyubiquitin-specific (right) antibody were analyzed for the presence of modified TRAF2. K48-linked polyubiquitin immunoprecipitations were performed following treatment with the proteasome inhibitor MG-132 (10 μm) for 4 h. (J) In vitro TRAF2 ubiquitination in the presence and absence of cIAP1 and cIAP2. Lys63-ubiquitination assay using immunoprecipitated Myc-TRAF2, Flag-cIAP1, and Flag-cIAP2, as indicated. All reactions were performed in the presence of recombinant E1 ubiquitin-activating enzyme Ubc13 and ubiquitin and analyzed by immunoblotting with the indicated antibodies. (K) TRAF2 ubiquitination following cIAP1 and cIAP2 suppression. Ubiquitination of TRAF2 in MCF-7 cells expressing cIAP1-specific shRNA (shcIAP1#1 and shcIAP1#2) or cIAP1/cIAP2 dual-specific shRNA (shcIAP2#1 and shcIAP2#2) was analyzed by immunoblotting. Despite their nomenclature, the shcIAP2#1 and shcIAP2#2 target sequences are present in both cIAP1 and cIAP2.
To confirm that IKKε regulates TRAF2 ubiquitination specifically through phosphorylation of Ser11, we evaluated the ubiquitination status of wild-type TRAF2, TRAF2 S11A, and an ubiquitination-deficient TRAF2 mutant (TRAF2Δring). The TRAF2Δring mutant harbors an N-terminal deletion of the RING domain required for the attachment of Lys63-linked ubiquitin chains (13, 28). When we expressed wild-type Myc-TRAF2 in HEK293T cells and performed a TRAF2-specific immunoblot assay, we observed a series of high-molecular-mass species that correspond to TRAF2 ubiquitination. In contrast, Myc-TRAF2 S11A expression resulted in substantially less TRAF2 ubiquitination comparable to the level of TRAF2 ubiquitination found following Myc-TRAF2Δring expression (Fig. 5C). We confirmed these observations by assessing TRAF2 phosphorylation with a phospho-TRAF2 Ser11 antibody. Expression of Myc-TRAF2 alone resulted in low-level, constitutive TRAF2 Ser11 phosphorylation that was not ubiquitinated. In agreement with our prior findings, we found that coexpression of V5-IKKε and Myc-TRAF2 expression induced a dramatic increase in TRAF2 Ser11 phosphorylation. Moreover, the phosphorylated TRAF2 was strongly ubiquitinated in the presence of IKKε (Fig. 5D). In contrast, kinase-inactive IKKε K38A failed to promote either phosphorylation or ubiquitination of TRAF2. These findings demonstrate that Ser11 phosphorylation by IKKε is important for TRAF2 ubiquitination.
We next determined which type of ubiquitin linkages were affected by TRAF2 phosphorylation. Specifically, we coexpressed an HA-tagged ubiquitin mutant that contains only Lys63 linkages (HA-Lys63-Ub) and either wild-type IKKε or IKKε K38A in HEK293T cells, isolated HA-ubiquitin immune complexes, and determined whether TRAF2 was present. We found that the expression of wild-type but not kinase-inactive IKKε induced a 3-fold increase in Lys63-linked ubiquitination of TRAF2 (Fig. 5E). We found similar results when we performed a reciprocal immunoprecipitation by isolating Myc-TRAF2 immune complexes and looked for the presence of HA-Lys63-linked ubiquitination (Fig. 5F). These observations demonstrate that IKKε-induced ubiquitination of TRAF2 involves Lys63-linked ubiquitin and is dependent on IKKε kinase activity.
To determine whether TRAF2 Lys63-linked ubiquitination requires Ser11 phosphorylation, we introduced the Myc-TRAF2 mutants (S11A and S102A) and HA-Lys63-Ub in HEK293T cells. We then isolated HA-ubiquitin immune complexes and performed an immunoblot analysis of TRAF2. We found robust Lys63-linked ubiquitination of both wild type and TRAF2 S102A. In contrast, we failed to find evidence of Lys63-linked ubiquitination of TRAF2 S11A (Fig. 5G and H). We observed a similar result when we isolated endogenous K63-linked ubiquitin complexes and determined the presence of TRAF2 in MCF-7 cells that stably express either Flag-TRAF2 or Flag-TRAF2 S11A (Fig. 5I, left). We also evaluated whether autoubiquitination of TRAF2 or cIAP1 and cIAP2 modulates TRAF2 Lys63-linked ubiquitination. We found that recombinant TRAF2 expression sufficed to induce TRAF2 ubiquitination in an in vitro ubiquitination assay and that coexpression of TRAF2 with cIAP1 and cIAP2 resulted in increased TRAF2 ubiquitination (Fig. 5J). In consonance with these observations, we observed that suppression of cIAP1 and cIAP2 reduced TRAF2 ubiquitination in MCF-7 cells (Fig. 5K). Together, IKKε promotes TRAF2 Lys63-linked ubiquitination in a manner that is dependent on Ser11 phosphorylation and the presence of cIAP1 and cIAP2.
Using a similar approach, we assessed Lys48-linked ubiquitination of TRAF2 in MCF7 cells stably expressing Flag-TRAF2 or Flag-TRAF2 S11A. After treatment with the proteasome inhibitor MG-132, we found comparable levels of endogenous K48-linked ubiquitination in cells expressing F-TRAF2 and F-TRAF2-S11A (Fig. 5I, right). These observations indicate that TRAF2 Ser11 phosphorylation does not substantially affect TRAF2 Lys48-linked ubiquitination.
TRAF2 is essential for IKKε-mediated NF-κB activation and cell transformation.
Since IKKε phosphorylates TRAF2 to promote its Lys63-linked ubiquitination and NF-κB activation, we investigated whether TRAF2 was essential for IKKε-induced NF-κB activation and transformation. Using two distinct TRAF2-specific shRNAs, we suppressed TRAF2 expression in HEK293T cells and two IKKε-transformed cell models, HA1EM and HMLEM cells that stably express IKKε. We found that TRAF2 suppression strongly inhibited IKKε-induced NF-κB activity (Fig. 6A) and suppressed anchorage-independent colony formation in HA1EM and HMLEM cells (Fig. 6B to D). These results indicate that TRAF2 is required for transformation driven by IKKε.
Fig 6.
TRAF2 is required for IKKε-induced NF-κB activation and transformation. (A) IKKε-induced NF-κB activity following suppression of TRAF2. HEK293T cells were cotransfected as indicated with SV40-Renilla luciferase and a NF-κB luciferase reporter. Raw light unit (RLU) activity was normalized to the activity observed with control vector. *, P = 5.2 × 10−5, calculated by a standard t test. (B) TRAF2 suppression in IKKε-transformed HA1EM and HMLEM cells. TRAF2 and IKKε immunoblots in control (vector), HA1EM-F-IKKε, and HMLEM-MF-IKKε cells following transduction with shTRAF2#1, shTRAF2#2, or control shGFP. β-Actin was analyzed as a loading control. (C) Anchorage-independent growth of IKKε-transformed HA1EM cells following TRAF2 suppression. Colony formation of HA1EM cells used in the experiment whose results are shown in panel B was analyzed after 28 days. *, P = 4.7 × 10−5, calculated by a standard t test. (D) Anchorage-independent growth of IKKε-transformed mammary cells following TRAF2 suppression. Colony formation of HMLEM cells used in the experiment whose results are shown in panel B was analyzed after 28 days. *, P = 0.0011, calculated by a standard t test. (E) TRAF2 overexpression in IKKε-transformed HA1EM cells. Immunoblot of TRAF2 and IKKε in control (vector) HA1EM or HA1EM-F-IKKε cells transduced with V5-GFP, V5-TRAF2, V5-TRAF2 S11A, or V5-TRAF2 S102A. β-Actin was used as a loading control. (F) Anchorage-independent growth of IKKε-transformed cells in the presence of TRAF2. Colony formation of cells used in the experiment whose results are shown in panel E was assayed after 28 days. *, P = 0.0045, calculated by a standard t test. The mean ± SD of three experiments is shown for all panels.
To determine whether TRAF2 Ser11 phosphorylation is necessary for IKKε-induced transformation, we generated IKKε-transformed HA1EM cells that stably express wild-type TRAF2, TRAF2 S11A, or TRAF2 S102A (Fig. 6E). As expected, we found that cells expressing F-IKKε showed robust anchorage-independent growth (Fig. 6F). However, expression of TRAF2 S11A inhibited anchorage-independent colony growth by 42%, suggesting that this mutant acts as a dominantly interfering mutant (Fig. 6F). These findings demonstrate that phosphorylation of TRAF2 is necessary for IKKε-mediated cell transformation.
TRAF2 cooperates with CYLD suppression to mediate IKKε transformation.
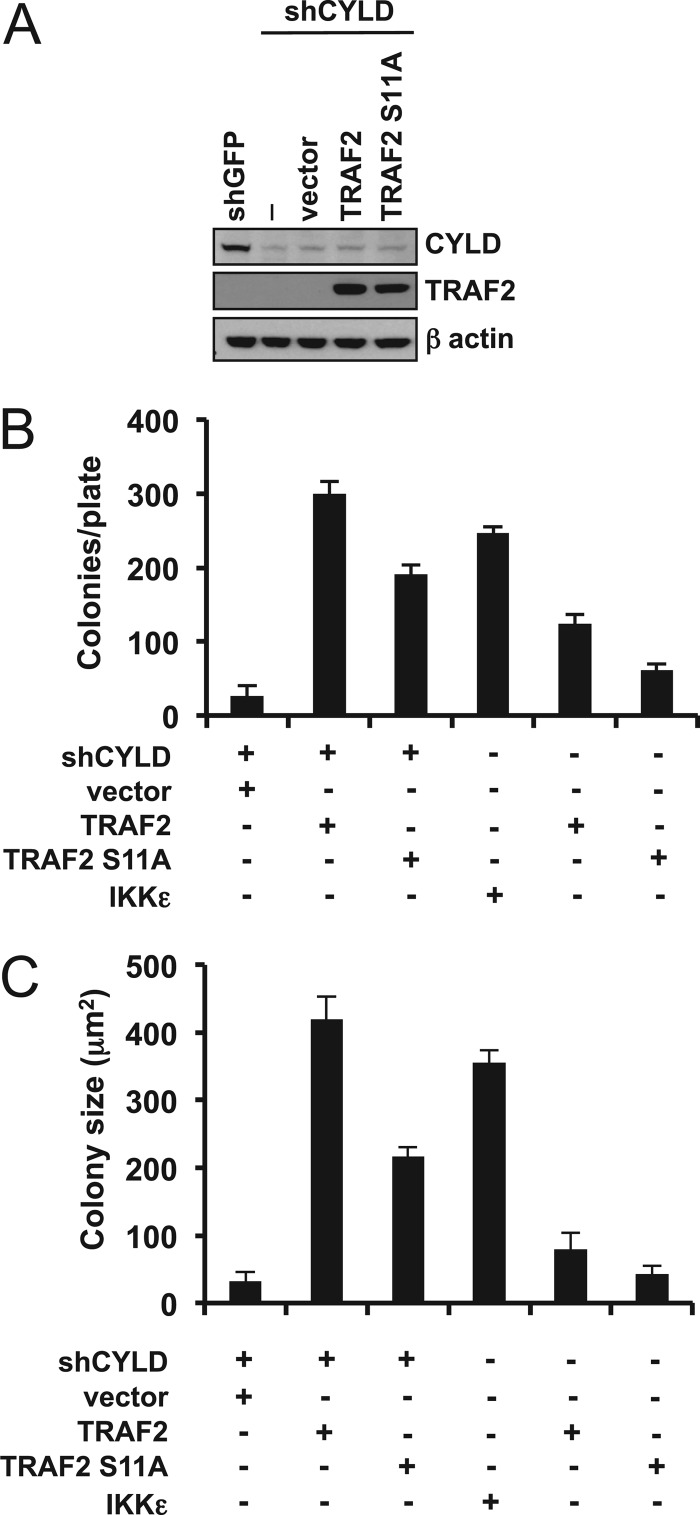
In prior work, we reported that suppression of the deubiquitinase and tumor suppressor CYLD induces anchorage-independent growth in NIH 3T3 cells. In addition, IKKε phosphorylates CYLD, and this activity is important for IKKε transformation (15). Furthermore, TRAF2 is one of several NF-κB-related effectors that are targeted by CYLD for deubiquitination (32). Since CYLD and TRAF2 are both substrates of IKKε, we determined whether TRAF2 expression and CYLD depletion cooperated to induce anchorage-independent growth. We introduced wild-type TRAF2 and TRAF2 S11A in NIH 3T3 cells expressing a CYLD-specific shRNA and found that suppression of CYLD alone promoted colony formation, as previously observed (Fig. 7A and B). Cells coexpressing wild-type TRAF2 and CYLD-specific shRNA (shCYLD) formed 11-fold more colonies that were 13-fold larger than cells transduced with the CYLD-specific shRNA alone. In contrast, TRAF2 S11A failed to fully synergize with CYLD depletion, as coexpression of TRAF2 S11A with shCYLD resulted in 36% fewer colonies and a 48% smaller colony size compared to those for cells in which wild-type TRAF2 and shCYLD were coexpressed (Fig. 7B and C). Based on these observations, we concluded that TRAF2 phosphorylation by IKKε cooperates with CYLD suppression to promote transformation.
Fig 7.
TRAF2 cooperates with suppression of CYLD to promote IKKε transformation. (A) Wild-type TRAF2, TRAF2 S11A, or control (vector) was expressed in NIH 3T3 cells expressing murine Cyld-specific shRNA (shCYLD). (B and C) Synergistic effect of CYLD suppression and TRAF2 overexpression on anchorage-independent growth. Colony formation and colony size of cells used in the experiment whose results are shown in panel A were analyzed after 28 days. The numbers of macroscopic colonies larger than 1,500 μm2 were counted (B), and colony size was measured at ×10 magnification (C). The mean ± SD of three experiments is shown.
IKKε-dependent breast cancer cells are dependent on TRAF2.
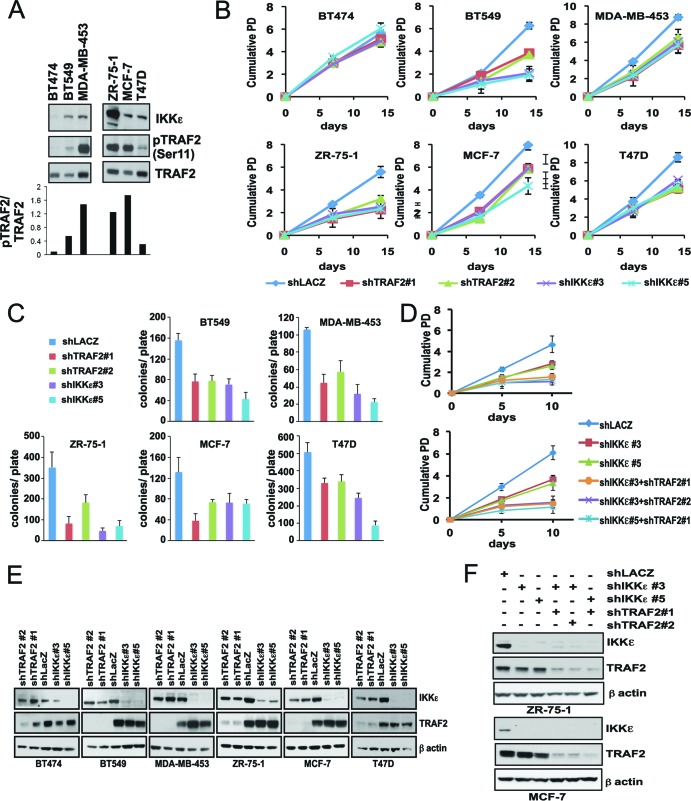
Since TRAF2 is an IKKε substrate necessary for IKKε-induced transformation, we sought to determine whether TRAF2 is essential in IKKε-dependent breast cancer cell lines. We first measured IKKε expression and TRAF2 Ser11 phosphorylation levels in several breast cancer cell lines. We identified five lines (BT549, MDA-MB-453, ZR-75-1, MCF-7, and T47D) with increased IKKε expression that also showed increased TRAF2 Ser11 phosphorylation (Fig. 8A). In accordance with our prior findings, these five cell lines exhibited a decreased proliferative capacity in a long-term proliferation assay when we suppressed IKKε with two independent IKKε-specific shRNAs (Fig. 8B and E). When we suppressed TRAF2 in these IKKε-dependent breast cancer cell lines, we observed a decrease in cell proliferation as well as anchorage-independent colony growth (Fig. 8B, C, and E). In contrast, TRAF2 depletion had no effect on the proliferation of breast cancer cell lines that do not depend on IKKε expression. Furthermore, when we suppressed both IKKε and TRAF2 in MCF-7 and ZR-75-1 cells, we found a strong effect on cell proliferation (Fig. 8D and F). Together, these observations identify TRAF2 as an IKKε substrate necessary for IKKε-induced transformation.
Fig 8.
TRAF2 is essential in IKKε-dependent breast cancer cells. (A) Levels of IKKε, phospho-TRAF2 (Ser11), and total TRAF2 in breast cancer cell lines. Immunoblotting was performed in the indicated breast cancer cell lines. (B) Long-term proliferative capacity of breast cancer cell lines following IKKε or TRAF2 suppression. Population doubling (PD) of breast cancer cell lines after transduction with control shRNA (shLacZ), shTRAF2#1, shTRAF2#2, shIKKε#3, or shIKKε#5. (C) Anchorage-independent growth of breast cancer cell lines following IKKε and TRAF2 suppression. Cells used in the experiment whose results are shown in panel B were assayed for anchorage-independent growth for 21 days.
TRAF2 is essential for IKKε-dependent tumorigenicity.
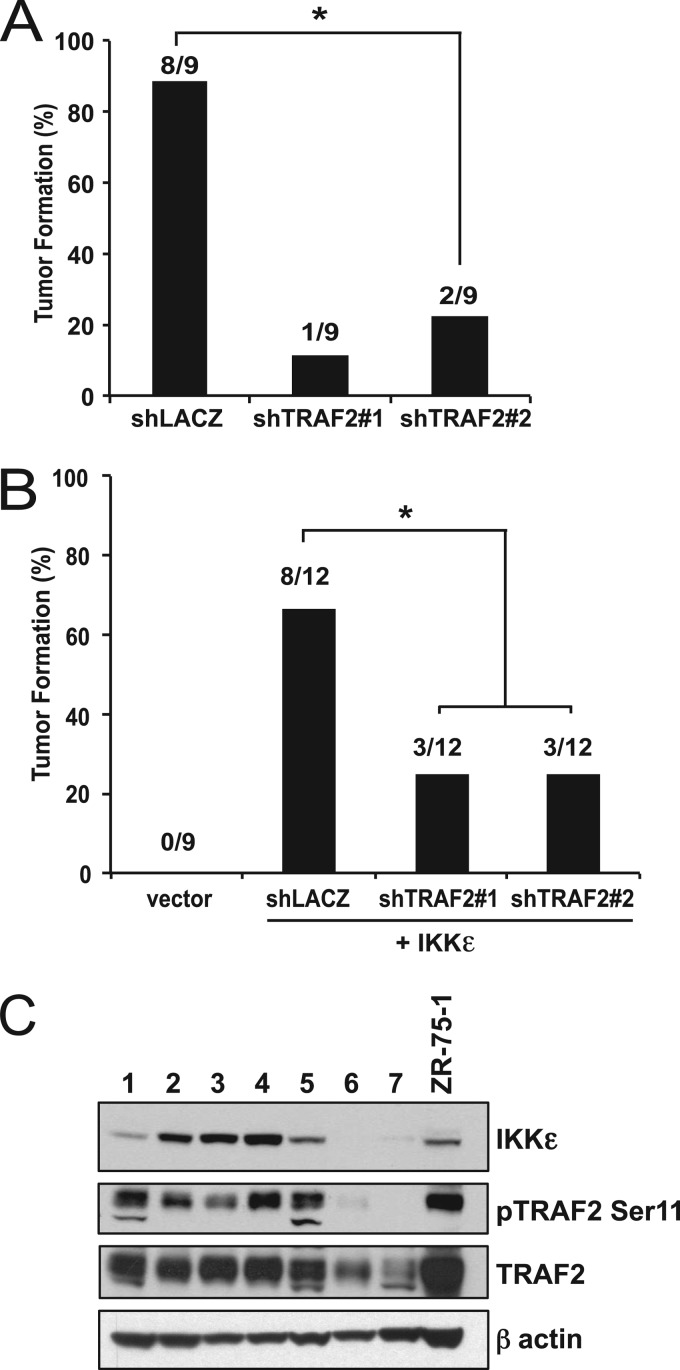
To evaluate whether IKKε-dependent cancer cells require TRAF2 expression for tumorigenesis in vivo, we performed tumorigenesis experiments in immunocompromised mice using ZR-75-1 and HA1EM cells in which we suppressed TRAF2 with two distinct TRAF2-specific shRNAs. When we compared tumor formation in ZR-75-1 breast cancer cells expressing a control vector or either of the TRAF2-specific shRNAs, we found that suppression of TRAF2 decreased tumor formation by 78% (shTRAF2#1) and 67% (shTRAF2#2). We found similar results using IKKε-transformed HA1EM cells, where we found that suppression of TRAF2 also decreased tumor formation (Fig. 9B).
Fig 9.
TRAF2 is essential in IKKε-dependent breast cancer cells in vivo. (A) Tumorigenesis of ZR-75-1 cells following suppression of TRAF2. *, P = 0.0152, calculated by a Fisher exact test. (B) Tumorigenesis of HA1EM cells transduced with either vector or MF-IKKε following suppression of TRAF2, as indicated. *, P = 0.0089, calculated by a Fisher exact test. (C) TRAF2 Ser11 phosphorylation and IKKε expression in primary breast carcinomas harboring IKKε overexpression. Immunoblot analysis of lysates from primary breast carcinoma samples (1 to 7) and the ZR-75-1 breast cancer cell line was performed using the indicated antibodies.
To confirm that TRAF2 Ser11 phosphorylation occurs in human breast cancers, we interrogated IKKε in a panel of primary breast cancers and found 5 of 7 specimens that expressed levels of IKKε, comparable to the results for ZR-75-1 cells (Fig. 9C). When we assessed the same samples for phosphorylation of TRAF2 using the Ser11 phosphospecific antibody, we found that only those samples that overexpressed IKKε exhibited TRAF2 Ser11 (Fig. 9B). As we previously reported (4), this pattern of expression did not correlate with the expression status of estrogen receptor (ER), progesterone receptor (PR), or HER2 in the tumors (Table 1). These observations provide evidence that overexpression of IKKε observed in a subset of breast cancers leads to phosphorylation of TRAF2 at Ser11.
Table 1.
Annotation of breast cancer markers in patient samples
| Sample no. | Marker expressiona |
||
|---|---|---|---|
| PR | ER | HER2 | |
| 1 | + | + | + |
| 2 | + | + | ++ |
| 3 | − | − | ++ |
| 4 | − | − | ++ |
| 5 | − | − | − |
| 6 | + | + | − |
| 7 | + | + | − |
Expression status of progesterone receptor (PR), estrogen receptor (ER), and HER2 in patient breast carcinoma samples analyzed for TRAF2 Ser11 phosphorylation and IKKε expression.
DISCUSSION
IKKε phosphorylates and regulates TRAF2.
TRAF2 is an adaptor molecule that mediates a series of ubiquitination events that facilitate NF-κB activation (2) and is itself modified by ubiquitination (7). Recent work indicates that TRAF2 is phosphorylated at various serine residues important for NF-κB activation (3, 21, 31, 35). In particular, TRAF2 Ser11 phosphorylation has been previously reported, and this activity occurs constitutively in some cancer cell lines (3). Here, we demonstrate that IKKε phosphorylates TRAF2 both in vitro and in vivo. We performed a mutational analysis of five TRAF2 serine residues that matched the IKKε recognition motif and identified Ser11 as the residue on TRAF2 that is phosphorylated by IKKε. This activity was necessary for recruitment of the canonical IKK complex, TRAF2 Lys63-linked ubiquitination, NF-κB activation, and subsequent transformation. Indeed, these observations delineate one path by which the noncanonical IKKε leads to activation of canonical NF-κB signaling.
Phosphorylation of TRAF2 at Ser11 is required for TRAF2 Lys63-linked but not Lys48-linked ubiquitination. Phosphorylation and subsequent Lys63-linked ubiquitination of TRAF2 at serine 11 are necessary for IKKε-mediated NF-κB activation and transformation. Since cIAP1 regulates TRAF2 stability, the interaction of IKKε and cIAP1 may be involved in the regulation of TRAF2 stability. cIAP1 plays multiple roles in promoting TRAF2 ubiquitination, attenuating caspase activation and apoptosis, and promoting NF-κB activation (12, 33, 34). Thus, dynamic regulation of TRAF2 ubiquitination through cIAP1 may also be modulated by IKKε.
IKKε regulates both TRAF2 and CYLD.
We previously demonstrated that IKKε regulates the activity of CYLD, a tumor suppressor and inhibitor of NF-κB signaling (15). The observations presented herein indicate that the phosphorylation of CYLD and TRAF2 by IKKε coordinates these NF-κB regulators to activate NF-κB signaling in the context of cell transformation. Specifically, IKKε inactivates CYLD and facilitates recruitment of the canonical IKK complex through TRAF2 phosphorylation. Since TRAF2 is also directly inactivated by CYLD through deubiquitination (27), it is not surprising that regulation of these two IKKε substrates synergizes to induce NF-κB signaling and transformation.
We found that suppression of CYLD and expression of wild-type TRAF2 cooperated to induce mammary epithelial cell transformation, likely through cooperative activation of NF-κB signaling. We note that we also observed that expression of the TRAF2 S11A mutant in cells in which we suppressed CYLD also induced increased colony formation compared to that by cells expressing a control vector. Since phosphorylation of TRAF2 by protein kinase C at Ser55 has been shown to promote TRAF2 ubiquitination and NF-κB activation (21), we conclude that other modifications of TRAF2 independent of IKKε phosphorylation may also contribute to NF-κB activation. Nonetheless, the observations presented herein provide evidence that IKKε-induced phosphorylation of TRAF2 is a major mechanism by which TRAF2 induces NF-κB signaling.
IKKε is amplified and overexpressed in 30% of breast cancers and is essential in cells that harbor IKKε copy number gain (4). We have determined that TRAF2 also plays an essential role in IKKε-driven breast cancers and provide in vivo evidence that TRAF2 phosphorylation is correlated with IKKε overexpression in primary breast carcinomas. Since IKKε-induced TRAF2 phosphorylation appears to be a key event occurring in IKKε-amplified breast cancers, the identification of these dependencies provides multiple therapeutic strategies in targeting the NF-κB pathway in breast cancer.
ACKNOWLEDGMENTS
We thank Jessica Hutti, Lew Cantley, and members of the W. C. Hahn lab and the K. Cichowski lab for thoughtful discussion, reagents, and technical assistance.
W.C.H. is a consultant for Novartis Pharmaceuticals.
This work was supported in part by R01 CA130988 (to W.C.H.), Ruth L. Kirschstein National Research Service Award F32 CA128265 (to R.R.S.), and a grant from Aid for Cancer Research (to R.R.S.).
Footnotes
Published ahead of print 24 September 2012
REFERENCES
- 1. Abbott DW, Wilkins A, Asara JM, Cantley LC. 2004. The Crohn's disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr. Biol. 14: 2217–2227 [DOI] [PubMed] [Google Scholar]
- 2. Au PY, Yeh WC. 2007. Physiological roles and mechanisms of signaling by TRAF2 and TRAF5. Adv. Exp. Med. Biol. 597: 32–47 [DOI] [PubMed] [Google Scholar]
- 3. Blackwell K, et al. 2009. TRAF2 phosphorylation modulates tumor necrosis factor alpha-induced gene expression and cell resistance to apoptosis. Mol. Cell. Biol. 29: 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boehm JS, et al. 2007. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell 129: 1065–1079 [DOI] [PubMed] [Google Scholar]
- 5. Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. 2003. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature 424: 797–801 [DOI] [PubMed] [Google Scholar]
- 6. Chau TL, et al. 2008. Are the IKKs and IKK-related kinases TBK1 and IKK-epsilon similarly activated? Trends Biochem. Sci. 33: 171–180 [DOI] [PubMed] [Google Scholar]
- 7. Chen ZJ. 2005. Ubiquitin signalling in the NF-kappaB pathway. Nat. Cell Biol. 7: 758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Compagno M, et al. 2009. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature 459: 717–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fitzgerald KA, et al. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4: 491–496 [DOI] [PubMed] [Google Scholar]
- 10. Ghosh S, Hayden MS. 2008. New regulators of NF-kappaB in inflammation. Nat. Rev. Immunol. 8: 837–848 [DOI] [PubMed] [Google Scholar]
- 11. Greten FR, et al. 2004. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118: 285–296 [DOI] [PubMed] [Google Scholar]
- 12. Gyrd-Hansen M, Meier P. 2010. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat. Rev. Cancer 10: 561–574 [DOI] [PubMed] [Google Scholar]
- 13. Habelhah H, et al. 2004. Ubiquitination and translocation of TRAF2 is required for activation of JNK but not of p38 or NF-kappaB. EMBO J. 23: 322–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hutti JE, et al. 2004. A rapid method for determining protein kinase phosphorylation specificity. Nat. Methods 1: 27–29 [DOI] [PubMed] [Google Scholar]
- 15. Hutti JE, et al. 2009. Phosphorylation of the tumor suppressor CYLD by the breast cancer oncogene IKKepsilon promotes cell transformation. Mol. Cell 34: 461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karin M. 2006. Nuclear factor-kappaB in cancer development and progression. Nature 441: 431–436 [DOI] [PubMed] [Google Scholar]
- 17. Karin M, Greten FR. 2005. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 5: 749–759 [DOI] [PubMed] [Google Scholar]
- 18. Kovalenko A, et al. 2003. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature 424: 801–805 [DOI] [PubMed] [Google Scholar]
- 19. Krappmann D, Scheidereit C. 2005. A pervasive role of ubiquitin conjugation in activation and termination of IkappaB kinase pathways. EMBO Rep. 6: 321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lenz G, et al. 2008. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 319: 1676–1679 [DOI] [PubMed] [Google Scholar]
- 21. Li S, Wang L, Dorf ME. 2009. PKC phosphorylation of TRAF2 mediates IKKalpha/beta recruitment and K63-linked polyubiquitination. Mol. Cell 33: 30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meylan E, et al. 2009. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature 462: 104–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Novak U, et al. 2009. The NF-{kappa}B negative regulator TNFAIP3 (A20) is inactivated by somatic mutations and genomic deletions in marginal zone lymphomas. Blood 113: 4918–4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perkins ND. 2007. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 8: 49–62 [DOI] [PubMed] [Google Scholar]
- 25. Pikarsky E, et al. 2004. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 431: 461–466 [DOI] [PubMed] [Google Scholar]
- 26. Pomerantz JL, Baltimore D. 1999. NF-kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 18: 6694–6704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reiley W, Zhang M, Wu X, Granger E, Sun SC. 2005. Regulation of the deubiquitinating enzyme CYLD by IkappaB kinase gamma-dependent phosphorylation. Mol. Cell. Biol. 25: 3886–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rothe M, Sarma V, Dixit VM, Goeddel DV. 1995. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science 269: 1424–1427 [DOI] [PubMed] [Google Scholar]
- 29. Shi CS, Kehrl JH. 2003. Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor-associated factor 2 (TRAF2). J. Biol. Chem. 278: 15429–15434 [DOI] [PubMed] [Google Scholar]
- 30. Sun SC. 2008. Deubiquitylation and regulation of the immune response. Nat. Rev. Immunol. 8: 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thomas GS, Zhang L, Blackwell K, Habelhah H. 2009. Phosphorylation of TRAF2 within its RING domain inhibits stress-induced cell death by promoting IKK and suppressing JNK activation. Cancer Res. 69: 3665–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trompouki E, et al. 2003. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature 424: 793–796 [DOI] [PubMed] [Google Scholar]
- 33. Vince JE, et al. 2009. TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (TNF) to efficiently activate NF-{kappa}B and to prevent TNF-induced apoptosis. J. Biol. Chem. 284: 35906–35915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu CJ, et al. 2005. TNF-alpha induced c-IAP1/TRAF2 complex translocation to a Ubc6-containing compartment and TRAF2 ubiquitination. EMBO J. 24: 1886–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang L, Blackwell K, Altaeva A, Shi Z, Habelhah H. 2011. TRAF2 phosphorylation promotes NF-{kappa}B-dependent gene expression and inhibits oxidative stress-induced cell death. Mol. Biol. Cell 22: 128–140 [DOI] [PMC free article] [PubMed] [Google Scholar]