Abstract
Neural stem cells (NSCs) continually generate functional neurons in the adult brain. Due to their ability to proliferate, deregulated NSCs or their progenitors have been proposed as the cells of origin for a number of primary central nervous system neoplasms, including infiltrating gliomas. The orphan nuclear receptor TLX is required for proliferation of adult NSCs, and its upregulation promotes brain tumor formation. However, it is unknown whether TLX is required for gliomagenesis. We examined the genetic interactions between TLX and several tumor suppressors, as well as the role of TLX-dependent NSCs during gliomagenesis, using mouse models. Here, we show that TLX is essential for the proliferation of adult NSCs with a single deletion of p21, p53, or Pten or combined deletion of Pten and p53. While brain tumors still form in Tlx mutant mice, these tumors are less infiltrative and rarely associate with the adult neurogenic niches, suggesting a non-stem-cell origin. Taken together, these results indicate a critical role for TLX in NSC-dependent gliomagenesis and implicate TLX as a therapeutic target to inhibit the development of NSC-derived brain tumors.
INTRODUCTION
The subgranular zone (SGZ) of the dentate gyrus (DG) and the subventricular zone (SVZ) of the lateral ventricle (LV) are the two neurogenic niches in the adult brain where neural stem cells (NSCs) reside and continually produce functional neurons (12, 24, 39). The orphan nuclear receptor TLX (also known as NR2E1) regulates proliferation in these NSCs by modulating the expression of Pten, p21, and several other genes downstream of p53 (17, 18, 26, 32, 46). However, the genetic interactions between TLX and these genes have not been examined.
The characteristic self-renewal of NSCs renders them prone to mutation, deregulated proliferation, and differentiation, all hallmarks of neoplasms in the central nervous system (CNS). Indeed, emerging evidence indicates that NSCs are the cellular origin of gliomas (1, 43, 48). Mutagenesis through viral or chemical carcinogens preferentially induces brain tumors in the vicinity of the neurogenic niche (4, 5, 14, 27). These results were further corroborated with the recent development of mouse models that are induced by mutation of the tumor suppressor p53, Pten, Nf1, Rb, or Ink4a/Arf or activation of the oncogene Ras, Akt, or Egfr (1, 11, 21, 43, 48, 49). As a key regulator of NSC proliferation, it is not surprising that TLX has been implicated in gliomagenesis. When combined with inactivation of p53 or Ink4a/Arf, ectopic expression of TLX is sufficient for glioma formation in a mouse model (18, 28). However, whether TLX is required for gliomagenesis or tumor maintenance is not known. Furthermore, the relationship between gliomagenesis and normal adult neurogenesis requires additional investigation. Here, we examined the role of TLX and TLX-dependent NSCs in a mouse model of gliomagenesis.
MATERIALS AND METHODS
Animals.
Strategies and methods for generating the following mutant mice have been described: Tlx−/− (44), Tlxflox/flox (Tlxf/f) (46), p21−/− (2), p53−/− (10), p53f/f (20), Ptenf/f (7), and hGfap-Cre (50) mice. All mice were housed under a 12-h light/dark cycle and had ad libitum access to food and water in a controlled animal facility. No significant phenotypic differences were observed between male and female mice; thus, both genders were included in the analysis. Experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern.
Gene expression analysis.
Total RNA from cultured cells or human tissues was isolated using TRIzol reagent (Invitrogen) and an RNeasy minikit (Qiagen). The Superscript III system (Invitrogen) and random primers were used to synthesize cDNA. Gene expression was analyzed using the SYBR GreenER system (Invitrogen) on a 384-well ABI 7900HT thermocycler (Applied Biosystems). Primer sequences for PCRs are available upon request. Expression of Tlx, Sox2, cd133, and Nes were also analyzed using GEO data set GDS1815 containing the global gene expression profiles of 92 human brain tumor samples (30). For RNA in situ hybridization, a cDNA fragment covering exon 2 or exons 4 to 9 of the mouse TLX gene was amplified by PCR and subcloned into a pGEM-T Easy vector. Digoxin-labeled sense or antisense riboprobes were generated by in vitro transcription with SP6 or T7 RNA polymerase (Roche). In situ hybridization was performed essentially as previously described (31).
Histology and immunohistochemistry.
Adult mice were euthanized via CO2 overdose and perfused with 1× phosphate-buffered saline (PBS) followed by ice-cold 4% paraformaldehyde (PFA) in PBS. Brains were dissected and postfixed overnight with 4% PFA at 4°C. After dehydration and paraffin embedding, the brains were sectioned at 5 μm and stained by hematoxylin and eosin (H&E). To obtain frozen sections, the brains were cryoprotected with 30% sucrose and sectioned at 40 μm with a sliding microtome. Immunostaining was performed as previously described (26).
The following primary antibodies were used: green fluorescent protein (GFP) (rabbit, 1:500 [Invitrogen]; chick, 1:1,000 [Aves Labs]), glial fibrillary acidic protein (GFAP) (mouse, 1:500 [Sigma]; guinea pig, 1:1,000 [Advanced ImmunoChemical]), 5-bromo-2-deoxyuridine (BrdU) (rat BU1/75; 1:500; Accurate Chemical), NeuN (rabbit; 1:500; Millipore), Sox2 (rabbit; 1:500; Millipore), Nes (mouse; 1:200; BD Pharmingen), Ki67 (rabbit; 1:500; Novocastra), DCX (goat; 1:150; Santa Cruz Biotechnology), Olig2 (rabbit; 1:500; Millipore), IBA1 (rabbit; 1:500; Wako), and NG2 (rabbit; 1:500; Millipore). Alexa Fluor 488-, 594-, or 647-conjugated secondary antibodies produced in goat or donkey (Invitrogen) were used for indirect fluorescence. Images were captured using a Zeiss LSM510 confocal microscope. Cells were counted using a Stereo Investigator (MBF Bioscience). Data were obtained from 12 random sections from a pool of three to five mice.
Neural stem cell culture and immunocytochemistry.
NSCs from 6- to 8-week-old Tlxf/f, CMV-CreER; Tlxf/f; Ptenf/f, Tlxf/f; Ptenf/f, Ptenf/f; p53f/f, or Tlxf/f; Ptenf/f; p53f/f mice were isolated and cultured in growth medium (Dulbecco's modified Eagle medium [DMEM]–F-12 medium supplemented with N2 [Invitrogen], heparin [5 μg/ml; Sigma], epidermal growth factor [20 ng/ml; Peprotech], and basic fibroblast growth factor [20 ng/ml; Peprotech]) as described previously (46). Cells on 8-well chamber slides were transduced with adenovirus expressing either GFP or Cre-IRES-GFP (GFP-Cre) for 48 h. BrdU (10 μM) was added for 4 h to label dividing cells. Cells were then fixed with 4% PFA, washed with PBS, blocked for 30 min at room temperature, and incubated overnight with primary antibodies in blocking solution at 4°C. For detection of BrdU-labeled cells, fixed cells were treated with 2 M HCl at 37°C for 30 min, washed with PBS, and incubated sequentially with primary and secondary antibodies.
Statistical analysis.
A two-tailed Student's t test with equal variance or one-way analysis of variance (ANOVA) followed by the Tukey post hoc analysis was used to determine significant differences between groups. P < 0.05 was considered significant.
RESULTS
Enriched expression of TLX in higher-grade human gliomas.
TLX, an essential factor controlling postnatal NSC proliferation, might be required for maintenance of gliomas due to the interconnection of NSCs and brain tumors. We first examined the expression of Tlx using quantitative reverse transcription-PCR (qRT-PCR) in multiple human tissue and tumor samples. While Tlx has modest expression in adult brain tissues, its expression in the fetal brain is robust (Fig. 1A). The expression pattern of human Tlx resembles that of mice: high expression in the neuroepithelium during development and enriched expression in the adult neurogenic niches (25, 32, 45). The pattern of Tlx expression lends itself to the discrimination of several types of brain tumors. While robust expression is observed in dysembryoplastic neuroepithelial tumors and a variety of high-grade gliomas, Tlx is barely detectable in many other CNS tumors, including meningioma, schwannoma, medulloblastoma, or ependymoma (Fig. 1A). Among gliomas, Tlx expression is significantly higher in more aggressive tumors (grades III and IV) than in lower-grade tumors (Fig. 1A).
Fig 1.
Enriched Tlx expression in malignant human gliomas. (A) Relative Tlx expression in human tissues by qRT-PCR analysis. Bar 1, fetal brain (n = 1); 2, adult brain (n = 1); 3, pillocytic astrocytoma (grade I; n = 15); 4, oligodendroglioma (grade II; n = 12); 5, oligodendroglioma (grade II/III; n = 4); 6, oligodendroglioma (grade III; n = 19); 7, glioblastoma (grade IV; n = 20); 8, dysembryoplastic neuroepithelial tumor (n = 8); 9, meningioma (n = 18); 10, malignant meningioma (n = 6); 11, null cell pituitary adenoma (n = 5); 12, medulloblastoma (n = 3); 13, acoustic schwannoma (n = 5); 14, papilloma (n = 1); 15, ependymoma (n = 2); 16, solitary fibrous tumor (n = 1); 17, normal colon, fat, and liver (n = 7); 18, chordoma (n = 1). The dotted line indicates normal Tlx expression in the adult brain. (B) Expression of stem cell markers in human gliomas. Data were obtained from GEO record GDS1815 by Affymetrix human genome U133A array (n = 24 for grade III and n = 68 for grade IV; *, P < 10−8; **, P < 10−12).
To confirm these findings, we further analyzed the expression of Tlx among 92 human brain samples (grade III, n = 24; grade IV, n = 68) within the global expression data set (GEO record GDS1815) (30). We found that Tlx is enriched to a greater degree in the most aggressive grade IV gliomas (glioblastoma multiforme) than in grade III (anaplastic) gliomas (Fig. 1B). This expression pattern mimics that of cd133, a marker for cancer stem cells (35, 36). It is worth noting that the expression of Nes and Sox2, two genes generally expressed in stem cells, does not correlate with the aggressiveness of gliomas. Our data indicate that Tlx serves as a biomarker for aggressive gliomas and plays a unique role in these brain tumors (18).
Deletion of either p21 or p53 is insufficient to activate Tlx−/− NSCs in vivo.
To dissect the functional interplay of TLX in neurogenesis and gliomagenesis, we first examined the genetic interactions between TLX and several of its downstream targets. Previously, we and others demonstrated that the deletion of Tlx abolishes postnatal NSC proliferation in the SGZ and SVZ neurogenic niches (17, 32, 46). Tumor suppressor p21 (also known as Cdkn1a) is significantly upregulated in Tlx−/− NSCs and has a demonstrated role in maintaining stem cell quiescence (3, 15, 26, 29, 46); therefore, p21 might be a critical component mediating TLX function in NSCs. To genetically evaluate this possibility in vivo, we generated Tlx and p21 double knockout (Tlx−/−; p21−/−) mice. Germ line deletion of p21 was confirmed by PCR (Fig. 2A). Proliferating cells in adult mouse brains were identified by the incorporation of BrdU and neurogenesis identified by staining for the immature neuronal marker DCX. Interestingly, the double mutant Tlx−/−; p21−/− mice lacked proliferating NSCs and newly produced neurons in both the DG and LV, similar to Tlx−/− mice (Fig. 2C). This suggests that the upregulation of p21 is not the sole pathway mediating Tlx deletion-induced inactivation of NSCs (26).
Fig 2.
TLX is required for proliferation of stem cells with either p21 or p53 deletion. (A and B) RT-PCR analysis to confirm gene deletion (WT, wild type; Het, heterozygote; KO, knockout). A DNA ladder (marker) is also shown. (C and D) Proliferation and neurogenesis in the neurogenic zones of either wild-type or mutant cells were examined by BrdU incorporation (green signal) and staining for DCX (red signal), respectively (n = 3 to 5). NeuN (blue signal) is a marker for mature neurons. DCX is a marker for newly produced neurons.
p53, a major factor governing stem cell proliferation (19), regulates the expression of a cohort of genes involved in the cell cycle, such as p21, Btg2, Gadd45β, and Gadd45γ. These genes are upregulated in Tlx−/− NSCs (26, 46), although the expression of p53 itself is unaffected. Previously, we showed that acute p53 deletion can rescue proliferation defects in cultured Tlx−/− NSCs (26). To determine the role of p53 in adult NSCs in vivo, we generated Tlx−/−; p53−/− mutant mice by crossing double heterozygous mutants of Tlx and p53. PCR analysis confirmed the germ line deletion of p53 and Tlx (Fig. 2B). Proliferation of adult NSCs and neurogenesis were examined by BrdU labeling and DCX staining, respectively. Unexpectedly, germ line deletion of p53 is insufficient to rescue Tlx−/− NSCs in vivo, as indicated by the absence of BrdU- and DCX-labeled cells in the SGZ and SVZ (Fig. 2D). This discrepancy regarding the role of p53 in cultured Tlx−/− NSCs versus adult mice might reflect a differential response of these mutant NSCs to diverse microenvironments.
TLX is essential for the proliferation of postnatal NSCs with Pten loss.
Pten encodes a phosphatase that negatively regulates the phosphatidylinositol 3-kinase (PI3K)–AKT pathway and is frequently mutated in human gliomas (22). During neural development, the loss of Pten leads to increased cell proliferation and enhanced survival of neural progenitor cells (7). The expression of Pten is significantly upregulated upon inducible deletion of Tlx in cultured adult NSCs (Fig. 3A). This reaffirms our previous results that demonstrate TLX directly suppresses Pten expression to maintain the proliferative state of retinal progenitor cells (47). We next examined the genetic interaction between Tlx and Pten in cultured adult mouse NSCs. Cre-mediated acute deletion of Tlx causes a significant decrease in NSC proliferation. Interestingly, the impaired proliferation of Tlx−/− NSCs cannot be rescued by deletion of Pten (Fig. 3B and C).
Fig 3.
Stem cells with Pten deletion require Tlx to proliferate. (A) Tlx deletion induces Pten expression in NSCs. Adult NSCs were isolated from 2-month-old CMV-Cre-ER; Tlxf/f mice. Tamoxifen (TM) treatment induced Cre-mediated deletion of Tlx. Gene expression was examined by qRT-PCR at the indicated time points after TM treatment (n = 3; *, P < 0.05). (B) Adult NSCs were isolated from mouse brains with the indicated genotypes. Cell proliferation was examined by BrdU incorporation after transduction with adenovirus expressing either GFP or GFP-Cre (n = 3; *, P < 0.001). (C) Representative images of adenovirus-transduced NSCs. Proliferating cells were identified by BrdU staining. (D to F) Pten deletion cannot rescue proliferation defects in Tlx mutant NSCs in vivo. Mice with the indicated genotypes at 2 weeks of age were pulse-labeled with BrdU (100 mg/kg) 1 h before sacrifice. (E) BrdU+ cells within the DG were quantified. (F) Total volume of the DG was also measured. The deletion of Pten by hGfap-Cre leads to an enlarged brain and increased proliferation (n = 3 to 5 for each genotype; *, P < 0.001 compared to Tlx+/+ controls).
To further substantiate this observation in vivo, we created mutant mice containing deletions of both Tlx and Pten in NSCs using an hGfap-Cre transgenic line (since germ line deletion of Pten causes early embryonic lethality) (7). The expression of Cre in both NSCs and astrocytes starts at embryonic day 13.5 (50). Conditional deletion of Pten results in whole-brain hyperplasia and subsequent full-penetrant lethality around 2 weeks of age (Fig. 3D). Proliferation of postnatal NSCs in the DG was examined by BrdU incorporation 1 h prior to euthanization. Conditional deletion of Pten leads to a 2-fold increase of BrdU+ cells in the SGZ (Fig. 3E). However, the density of BrdU+ cells is similar to that of wild-type controls owing to an enlargement of the DG upon conditional deletion of Pten (Fig. 3F). This Pten deletion-induced increase and the basal proliferation of postnatal NSCs in the SGZ were completely abolished upon further loss of Tlx function (Fig. 3D and E). In contrast, Tlx deletion has minimal effect on brain hyperplasia, neuronal density, or proliferating cells in the cortex. Collectively, these data suggest that TLX has a more influential role in postnatal NSCs of the neurogenic niche than during neural development. This essential role of TLX cannot be replaced by the singular mutation of any tumor suppressors we examined.
Postnatal NSCs with dual inactivation of p53 and Pten require Tlx to proliferate in the neurogenic niche.
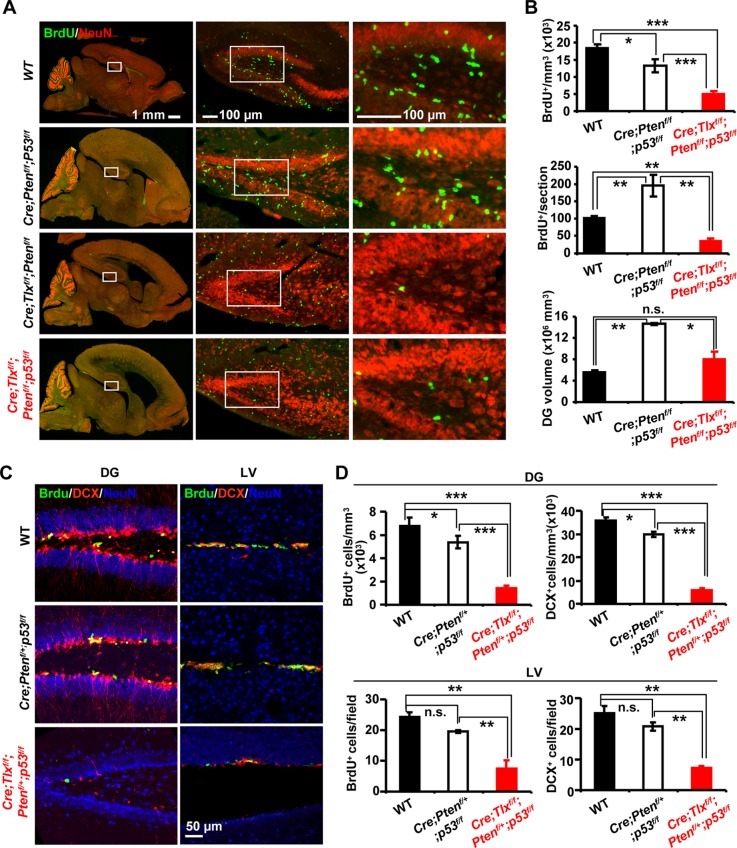
Since singular mutation of several tumor suppressors is insufficient to rescue the proliferation defect of Tlx−/− NSCs in vivo, we generated double knockouts of p53 and Pten by hGfap-Cre-mediated deletion in the nervous system. Similar to knockout of Pten, the dual inactivation of p53 and Pten results in full penetrant lethality around 2 weeks of age, accompanied by severe hyperplasia and brain enlargement (Fig. 4A). Further, BrdU labeling reveals a 2-fold increase in proliferating cells of the DG. This is consistent with the proposed role for PTEN and p53 in governance of NSC proliferation (6, 7, 23). To examine whether such an increase still requires functional TLX, we created triple mutants in the nervous system. Conditional deletion of Tlx by hGfap-Cre during later embryogenesis did not affect early postnatal lethality or DCX+ immature neurons but significantly reduced the DG enlargement caused by mutation of Pten and p53. Similarly, cell proliferation in the SVZ was not substantially affected by this later-onset hGfap-Cre-mediated deletion of Tlx (even at the time of lethality). However, the loss of Tlx completely abolished the increase of proliferating cells induced by dual inactivation of Pten and p53 in the DG (Fig. 4B).
Fig 4.
Neural stem cells with dual inactivation of Pten and p53 require Tlx to proliferate. (A) Cell proliferation within the SGZ of mice with the indicated genotypes was examined by incorporation of BrdU (100 mg/kg) administered 1 h before sacrifice. Mice were examined at 2 weeks of age. NeuN is a marker for mature neurons. Right panels are higher magnification views of boxed regions in the left panels. (B) Quantification of BrdU+ cells in the SGZ (n = 5 for WT, n = 3 for Cre; p53f/f; Ptenf/f, and n = 4 for Cre; Tlxf/f; Ptenf/f; p53f/f; *, P < 0.05; **, P < 0.001; and ***, P < 0.0001; n.s., not significant). (C and D) Two-month-old mice with the indicated genotypes were pulse-labeled with BrdU (100 mg/kg twice per day for 5 days). Adult neurogenesis was then examined by staining for BrdU+ cells and DCX+ immature neurons. NeuN is a marker for mature neurons (n = 4 for each genotype; *, P < 0.05; **, P < 0.01; and ***, P < 0.001; n.s., not significant).
TLX is required for gliomagenesis within the neurogenic niches.
p53 and PTEN are key tumor suppressors frequently mutated in human gliomas (22, 37, 48). hGfap-Cre-mediated homozygous deletion of p53 in combination with heterozygous Pten (hGfap-Cre; Ptenf/+; p53f/f) in mice is sufficient to induce acute-onset high-grade malignant glioma that phenotypically resembles human primary glioblastoma (48). We tested TLX function in this murine glioma model by generating mice harboring triple conditional alleles and an hGfap-Cre transgene (hGfap-Cre; Tlxf/f; Ptenf/+; p53f/f). Adult neurogenesis was first examined by BrdU labeling (100 mg/kg of body weight twice per day for 5 days) and DCX staining. hGfap-Cre; Ptenf/+; p53f/f mice did not exhibit a significant change in the number of either BrdU+ cells or newly generated DCX+ cells in the neurogenic niches at 2 months of age compared to wild-type controls (Fig. 4C). However, the additional deletion of Tlx (hGfap-Cre; Tlxf/f; Ptenf/+; p53f/f) led to a remarkable reduction in both NSC proliferation and adult neurogenesis in the DG and SVZ (Fig. 4D). These data further strengthen the conclusion that TLX is indispensable in maintaining adult neurogenic NSCs.
Consistent with previous observations (48), some mutant mice exhibited neurological symptoms at 3 months of age, including paralysis, lethargy, enlarged heads, and ataxia. Histological analysis revealed ∼33% of the moribund mice developed gliomas. In addition to gliomas, tumors under the skin were observed in ∼30% of the mice. This may reflect expression of hGfap-Cre in skin-bulge stem cells (33). In contrast to its essential role in adult neurogenesis, deletion of Tlx has minimal impact on either the overall or glioma-free survival rate of the mutant mice (Fig. 5A). Unexpectedly, histological examination indicates that deletion of Tlx dramatically shifted the locations where gliomas occur. Only 20% of the gliomas in hGfap-Cre; Tlxf/f; Ptenf/+; p53f/f mice were observed in close contact with the neurogenic niches compared to 85.7% in the control hGfap-Cre; Ptenf/+; p53f/f mice (Fig. 5B and C). Irrespective of location, these gliomas exhibit considerable hypercellularity and a predominantly astrocytic morphology. The vast majority of tumors were histologically characterized as high-grade neoplasms, manifested by the presence of mitotic activity and, in the most aggressive (grade IV) lesions, palisading necrosis and occasional foci of microvascular proliferation (Fig. 5D). Immunohistochemical analysis confirmed activation of AKT in these mutant mice, most prominently within the tumor mass (Fig. 5C). H&E staining showed hypercellularity in the neurogenic regions of tumor-bearing mice with a wild-type allele of Tlx but not in Tlx−/− mice. Markers for glia and stem cells (GFAP, Nes, and Sox2) were markedly upregulated in these tumors with a concomitant increase in cellular proliferation, as indicated by Ki67 and PCNA staining (Fig. 6E, F, G, U, V, W, Y, and AA). Proliferating cells were rarely observed in the LV and DG of Tlx mutant mice (Fig. 6J, L, Z, and AB). This suggests that Tlx−/− stem cells in the neurogenic niches are not the cellular origin for these gliomas. Interestingly, unlike the tumors in control hGfap-Cre; Ptenf/+; p53f/f mice that were diffusive, most of the gliomas in the Tlx−/− background were circumscribed with well-defined boundaries indicating a less invasive nature (Fig. 5C and 6B).
Fig 5.
Gliomagenesis within the adult neurogenic niches requires TLX. (A) Kaplan-Meier survival curves. Autopsy and histological analyses were performed at the endpoint of the tumor study. The total survival includes mice that developed gliomas and nonglioma tumors (n = 51 for Cre; Ptenf/+; p53f/f and n = 53 for Cre; Tlxf/fPtenf/+; p53f/f). The number of glioma-bearing mice was used to plot glioma-free survival (n = 14 for Cre; Ptenf/+; p53f/f and n = 20 for Cre; Tlxf/f; Ptenf/+; p53f/f). (B) Locations of gliomas within the brain parenchyma were examined by histology. +NSC indicates gliomas directly associated with the neurogenic niches. −NSC indicates gliomas not in the vicinity of the neurogenic niches. (C) Histology of the glioma-bearing mouse brains. The neurogenic regions in Tlx mutant mice lacked tumor cells (LV, lateral ventricle; DG, dentate gyrus). Tumor boundaries were circumscribed in Tlx mutant mice. Staining for pAKT showed enhanced AKT signaling within the tumor mass. Higher magnification views were taken from the boxed regions. (D) Histological features of malignant gliomas. These features include significant cellular pleomorphism (panels 1, 2, 4, 5, and 7), necrosis (panels 3, 8, and 10), pseudopalisading (panel 10), and occasional areas of microvascular proliferation (panels 4, 5, and 9). Arrows in panels 2 and 7 point to giant cells, whereas arrowheads in panels 2 and 7 point to cells with mitotic figures (also see the insets). V and mV indicate vasculature and areas with microvasculature features, respectively. N and H show areas with necrosis and hemorrhage, respectively. Scale bar, 50 μm.
Fig 6.
Immunohistological features of gliomas. (A to D) Lower magnification view of sagittal brain sections (Ctx, cortex; DG, dentate gyrus; LV, lateral ventricle). Nuclei were stained with Hoechst 33342 (Hst). (B) Unlike the tumors in hGfap-Cre; Ptenf/+; p53f/f mice that were diffusive, the tumors under the Tlx mutant background had more circumscribed boundaries. (E to AB) Confocal images of marker expression. Numerous Ki67+- or PCNA+-proliferating cells were observed in the tumor mass but not in the neurogenic regions of Tlx mutant brain (E to L and U to AB). Markers for stem cells (Sox2 and Nes), astrocytes (GFAP), oligodendrocytes (Olig2), and migrating neuroblasts (DCX) were robustly detected in the tumor mass.
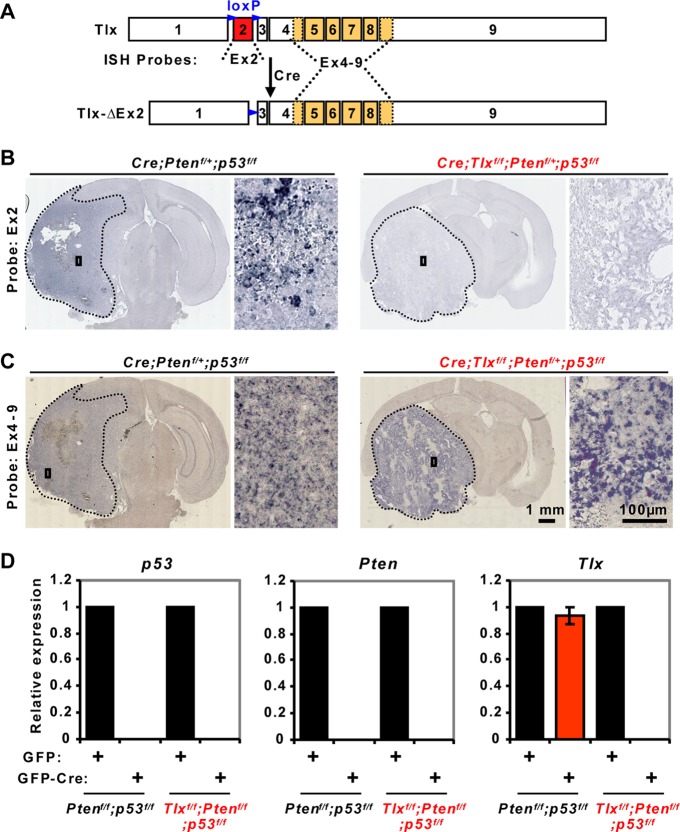
Robust expression of Tlx was identified within the tumor mass of control hGfap-Cre; Ptenf/+; p53f/f mice by RNA in situ hybridization using a probe specific for exon 2. The expression of Tlx was diminished under the Tlxf/f background, confirming Cre-mediated deletion of the floxed exon (Fig. 7A and B). Interestingly, other regions of the Tlxf/f gene were still strongly expressed when examined by a probe spanning exons 4 to 9 (Ex4 to Ex9 in Fig. 7A and C). This is consistent with our previous RT-PCR analysis (46). Enhanced Tlx expression within the tumor mass confirmed our results in human glioma samples (Fig. 1) and raises the question of whether Tlx is under the regulation of p53 and Pten. We isolated NSCs from adult mice in either the Ptenf/f; p53f/f or Tlxf/f; Ptenf/f; p53f/f background and examined gene expression after transduction of adenovirus expressing either GFP or GFP-Cre. Cre-mediated deletion of p53, Pten, or Tlx was confirmed by qPCR analysis. However, the expression of Tlx was not significantly altered by acute loss of p53 and Pten in Ptenf/f; p53f/f NSCs (Fig. 7D). This indicates that Tlx expression in brain tumors is controlled by signaling pathways associated with p53- and PTEN-dependent cellular transformation rather than by p53 and PTEN per se.
Fig 7.
Expression of Tlx in tumors or NSCs with p53 and Pten deletion. (A) Diagrams showing Tlx locus with a floxed exon 2. Ex2 and Ex4 to Ex9 indicate the positions of probes for RNA in situ hybridization (ISH). Numbers indicate exons. (B) Confirmation of Cre-mediated deletion of exon 2 of Tlx in hGfap-Cre; Tlxf/f; Ptenf/+; p53f/f mice by RNA-ISH. The tumor core is outlined. A higher magnification view of the boxed region is shown. (C) Enhanced expression of Tlx within the tumor core detected by using a probe for the 3′ region of the Tlx gene (Ex4 to Ex9). Tlx was still detectable even following Cre-mediated deletion of exon 2 in hGfap-Cre; Tlxf/f; Ptenf/+; p53f/f mice. (D) Expression of Tlx in cultured adult NSCs with either Ptenf/f; p53f/f or Tlxf/f; Ptenf/f; p53f/f. These cells were transduced with adenovirus expressing either GFP or GFP-Cre. Relative gene expression was examined by qRT-PCR (n = 3).
What is the potential cellular source for gliomas in the Tlx−/− background? In addition to NSCs in the LV and DG, hGfap-Cre-mediated recombination can be detected in GFAP+ astrocytes and NG2+ oligodendrocyte precursor cells (OPCs), but not IBA1+ microglias, throughout the brain parenchyma (Fig. 8A to C). The loss of Pten and p53 in these astrocytes or OPCs rather than in NSCs of Tlx−/− mice might serve as an alternative cellular source for gliomagenesis. The observation that TLX is not required for proliferation of postnatal OPCs or astrocytes in the brain parenchyma supports this hypothesis (Fig. 9A). Furthermore, the tumor mass is enriched with Olig2- and NG2-expressing glial cells (Fig. 6M to O and 9B), which were rarely detected in the neurogenic niches of Tlx−/− mice (Fig. 6R and T and 9B8 and B9).
Fig 8.

hGfap-Cre-mediated recombination in glial cells. (A) Recombination in oligodendrocyte precursor cells indicated by colabeling with NG2 and Cre activity-dependent reporter GFP. CC, corpus callosum; Stm, striatum; Ctx, cortex. (B) Cre-mediated recombination in Olig2+ oligodendrocytes and GFAP+ astrocytes, indicated by arrows and asterisks, respectively. (C) Lack of recombination in IBA1+ microglia. Scale bar, 20 μm.
Fig 9.

Massive amplification of NG2+ glial cells within the tumor mass. (A) Deletion of Tlx has no effect on proliferation of oligodendrocyte precursors. Proliferating cells in 2-month-old mice were labeled by BrdU incorporation (100 mg/kg twice per day for 5 days), whereas oligodendrocytes and astrocytes were identified by Olig2 and glutamine synthetase (GS) staining, respectively. Arrows point to colabeled proliferating oligodendrocytes. It should be noted that astrocytes rarely divide under normal conditions. CC, corpus callosum; Stm, striatum. Scale bar, 20 μm. (B) Immunohistochemistry analysis of NG2+ cells within the indicated brain regions. B4, B6, and B7 are regions containing tumor cells. The dentate gyrus (DG) and the lateral ventricle (LV) are outlined (GCL, granule cell layer; HL, hilus; CTX, cortex). Scale bar, 20 μm.
DISCUSSION
The orphan nuclear receptor TLX is required to maintain adult neurogenesis under normal conditions (17, 26, 32, 46). In this study, we demonstrated that TLX is also essential for the proliferation of stem cells with individual deletion of p21, p53, or Pten or combined deletion of Pten and p53. In addition to these tumor suppressors, TLX regulates the expression of genes involved in multiple pathways, such as cell cycle, DNA replication, cell adhesion, and mitogen-activated protein kinase signaling (18, 26, 41, 46). This pleiotropic effect of TLX on gene expression might contribute to its dominant role in maintaining adult NSC proliferation. It should be noted that cultured Tlx−/− NSCs respond differently to the loss of p53 than counterparts in adult mouse brains (26). This highlights the importance of the cellular microenvironment in controlling NSC behavior.
Overexpression of TLX in NSCs or glial cells is sufficient to sensitize them to tumorigenesis under either a p53−/− or Ink4a/Arf−/− genetic background (18, 28). This implicates TLX as a potential therapeutic target against gliomas (18). Our mouse genetic analysis supports this notion, demonstrating that the deletion of Tlx significantly impedes gliomagenesis within the adult neurogenic niches where NSCs reside, while gliomas can still form in other regions of the brain. One prominent histological feature of gliomas in the Tlx−/− background was diminished infiltrative capacity, as indicated by distinctly circumscribed boundaries between tumors and adjacent tissues. As to the cellular origin of Tlx−/− gliomas, we suspect that proliferating glial cells in brain parenchyma are the most likely candidates based on recent studies showing that proliferative OPCs or reactive astrocytes with genetic mutations might also serve as the precursors for gliomagenesis (8, 9, 13, 16, 38, 40). These results highlight the heterogeneous nature of the cellular origin for gliomas and have important implications for devising therapeutic strategies against malignant brain tumors.
Tlx is robustly expressed in aggressive brain tumors (Fig. 1 and 7), lending to its use as a diagnostic biomarker (18, 28, 30, 34, 42). Future experiments are warranted to tease out the signaling pathways that lead to enhanced Tlx expression in gliomas. These pathways might serve as intervening points to prevent the formation of certain brain tumors. Additional studies are necessary to investigate whether TLX or TLX-dependent NSCs have a role in the progression and/or maintenance of gliomas, particularly under conditions where aggressive gliomas are derived from or associate with NSCs. These future studies will firmly establish whether TLX could serve as a therapeutic target in curbing certain malignant gliomas.
ACKNOWLEDGMENTS
We thank Derek Smith and Jenny Hsieh for critical reading of the manuscript and members of the Zhang laboratory for technical assistance and discussion.
C.-L.Z. is a W. W. Caruth, Jr., Scholar in Biomedical Research. This work was supported by Whitehall Foundation award 2009-12-05, Welch Foundation award I-1724, Ellison Medical Foundation award AG-NS-0753-11, and NIH grants 1DP2OD006484 and R01NS070981 (to C.-L.Z.).
Footnotes
Published ahead of print 1 October 2012
REFERENCES
- 1.Alcantara Llaguno S, et al. 2009. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell 15:45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brugarolas J, et al. 1995. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377:552–557 [DOI] [PubMed] [Google Scholar]
- 3.Cheng T, et al. 2000. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287:1804–1808 [DOI] [PubMed] [Google Scholar]
- 4.Copeland DD, Bigner DD. 1977. The role of the subependymal plate in avian sarcoma virus brain tumor induction: comparison of incipient tumors in neonatal and adult rats. Acta Neuropathol. 38:1–6 [DOI] [PubMed] [Google Scholar]
- 5.Copeland DD, Vogel FS, Bigner DD. 1975. The induction of intractranial neoplasms by the inoculation of avian sarcoma virus in perinatal and adult rats. J. Neuropathol. Exp. Neurol. 34:340–358 [DOI] [PubMed] [Google Scholar]
- 6.Gil-Perotin S, et al. 2006. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J. Neurosci. 26:1107–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groszer M, et al. 2001. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science 294:2186–2189 [DOI] [PubMed] [Google Scholar]
- 8.Hambardzumyan D, Cheng YK, Haeno H, Holland EC, Michor F. 2011. The probable cell of origin of NF1- and PDGF-driven glioblastomas. PLoS One 6:e24454 doi:10.1371/journal.pone.0024454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hambardzumyan D, Parada LF, Holland EC, Charest A. 2011. Genetic modeling of gliomas in mice: new tools to tackle old problems. Glia 59:1155–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacks T, et al. 1994. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4:1–7 [DOI] [PubMed] [Google Scholar]
- 11.Jacques TS, et al. 2010. Combinations of genetic mutations in the adult neural stem cell compartment determine brain tumour phenotypes. EMBO J. 29:222–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kriegstein A, Alvarez-Buylla A. 2009. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32:149–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lathia JD, Venere M, Rao MS, Rich JN. 2011. Seeing is believing: are cancer stem cells the Loch Ness monster of tumor biology? Stem Cell Rev. 7:227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leonard JR, D'Sa C, Klocke BJ, Roth KA. 2001. Neural precursor cell apoptosis and glial tumorigenesis following transplacental ethyl-nitrosourea exposure. Oncogene 20:8281–8286 [DOI] [PubMed] [Google Scholar]
- 15.Li W, et al. 2008. Nuclear receptor TLX regulates cell cycle progression in neural stem cells of the developing brain. Mol. Endocrinol. 22:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C, et al. 2011. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell 146:209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu HK, et al. 2008. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev. 22:2473–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu HK, et al. 2010. The nuclear receptor tailless induces long-term neural stem cell expansion and brain tumor initiation. Genes Dev. 24:683–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, et al. 2009. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell 4:37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. 2000. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 14:994–1004 10783170 [PMC free article] [PubMed] [Google Scholar]
- 21.Marumoto T, et al. 2009. Development of a novel mouse glioma model using lentiviral vectors. Nat. Med. 15:110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLendon R, et al. 2008. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meletis K, et al. 2006. p53 suppresses the self-renewal of adult neural stem cells. Development 133:363–369 [DOI] [PubMed] [Google Scholar]
- 24.Ming GL, Song H. 2011. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monaghan AP, Grau E, Bock D, Schutz G. 1995. The mouse homolog of the orphan nuclear receptor tailless is expressed in the developing forebrain. Development 121:839–853 [DOI] [PubMed] [Google Scholar]
- 26.Niu W, Zou Y, Shen C, Zhang CL. 2011. Activation of postnatal neural stem cells requires nuclear receptor TLX. J. Neurosci. 31:13816–13828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oda H, et al. 1997. Loss of p53 is an early event in induction of brain tumors in mice by transplacental carcinogen exposure. Cancer Res. 57:646–650 [PubMed] [Google Scholar]
- 28.Park HJ, et al. 2010. The neural stem cell fate determinant TLX promotes tumorigenesis and genesis of cells resembling glioma stem cells. Mol. Cells 30:403–408 [DOI] [PubMed] [Google Scholar]
- 29.Perucca P, et al. 2009. Loss of p21 CDKN1A impairs entry to quiescence and activates a DNA damage response in normal fibroblasts induced to quiescence. Cell Cycle 8:105–114 [DOI] [PubMed] [Google Scholar]
- 30.Phillips HS, et al. 2006. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9:157–173 [DOI] [PubMed] [Google Scholar]
- 31.Qin S, Liu M, Niu W, Zhang CL. 2011. Dysregulation of Kruppel-like factor 4 during brain development leads to hydrocephalus in mice. Proc. Natl. Acad. Sci. U. S. A. 108:21117–21121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Y, et al. 2004. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature 427:78–83 [DOI] [PubMed] [Google Scholar]
- 33.Silvis MR, et al. 2011. Alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci. Signal. 4:ra33 doi:10.1126/scisignal.2001823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sim FJ, et al. 2006. Neurocytoma is a tumor of adult neuronal progenitor cells. J. Neurosci. 26:12544–12555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh SK, Clarke ID, Hide T, Dirks PB. 2004. Cancer stem cells in nervous system tumors. Oncogene 23:7267–7273 [DOI] [PubMed] [Google Scholar]
- 36.Singh SK, et al. 2003. Identification of a cancer stem cell in human brain tumors. Cancer Res. 63:5821–5828 [PubMed] [Google Scholar]
- 37.Stiles CD, Rowitch DH. 2008. Glioma stem cells: a midterm exam. Neuron 58:832–846 [DOI] [PubMed] [Google Scholar]
- 38.Sugiarto S, et al. 2011. Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer Cell 20:328–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suh H, Deng W, Gage FH. 2009. Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 25:253–275 [DOI] [PubMed] [Google Scholar]
- 40.Sukhdeo K, Hambardzumyan D, Rich JN. 2011. Glioma development: where did it all go wrong? Cell 146:187–188 [DOI] [PubMed] [Google Scholar]
- 41.Sun G, Yu RT, Evans RM, Shi Y. 2007. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc. Natl. Acad. Sci. U. S. A. 104:15282–15287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor MD, et al. 2005. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell 8:323–335 [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, et al. 2009. Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer Cell 15:514–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu RT, et al. 2000. The orphan nuclear receptor Tlx regulates Pax2 and is essential for vision. Proc. Natl. Acad. Sci. U. S. A. 97:2621–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu RT, McKeown M, Evans RM, Umesono K. 1994. Relationship between Drosophila gap gene tailless and a vertebrate nuclear receptor Tlx. Nature 370:375–379 [DOI] [PubMed] [Google Scholar]
- 46.Zhang CL, Zou Y, He W, Gage FH, Evans RM. 2008. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature 451:1004–1007 [DOI] [PubMed] [Google Scholar]
- 47.Zhang CL, Zou Y, Yu RT, Gage FH, Evans RM. 2006. Nuclear receptor TLX prevents retinal dystrophy and recruits the corepressor atrophin1. Genes Dev. 20:1308–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng H, et al. 2008. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature 455:1129–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y, et al. 2005. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell 8:119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhuo L, et al. 2001. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31:85–94 [DOI] [PubMed] [Google Scholar]