Abstract
CD8+ T cells may contribute to vaccines for respiratory syncytial virus (RSV). Compared to CD8+ T cells responding to RSV infection, vaccine-elicited anti-RSV CD8+ T cells are less well defined. We used a peptide vaccine to test the hypothesis that vaccine-elicited RSV-specific CD8+ T cells are protective against RSV pathogenesis. BALB/c mice were treated with a mixture (previously termed TriVax) of an M282-90 peptide representing an immunodominant CD8 epitope, the Toll-like receptor (TLR) agonist poly(I·C), and a costimulatory anti-CD40 antibody. TriVax vaccination induced potent effector anti-RSV CD8+ cytotoxic T lymphocytes (CTL). Mice were challenged with RSV strain A2-line19F, a model of RSV pathogenesis leading to airway mucin expression. Mice were protected against RSV infection and against RSV-induced airway mucin expression and cellular lung inflammation when challenged 6 days after vaccination. Compared to A2-line19F infection alone, TriVax vaccination followed by challenge resulted in effector CD8+ T cells with greater cytokine expression and the more rapid appearance of RSV-specific CD8+ T cells in the lung. When challenged 42 days after TriVax vaccination, memory CD8+ T cells were elicited with RSV-specific tetramer responses equivalent to TriVax-induced effector CD8+ T cells. These memory CD8+ T cells had lower cytokine expression than effector CD8+ T cells, and protection against A2-line19F was partial during the memory phase. We found that vaccine-elicited effector anti-RSV CD8+ T cells protected mice against RSV infection and pathogenesis, and waning protection correlated with reduced CD8+ T cell cytokine expression.
INTRODUCTION
Respiratory syncytial virus (RSV) is the leading cause of viral lower respiratory tract illness in infants. RSV causes morbidity and mortality in children and the elderly, including high rates of hospitalization in infants (17, 43). Despite efforts such as inactivated, live attenuated, subunit, viral-vectored, and DNA vaccines, there is no approved RSV vaccine yet. It is well-known that anti-RSV neutralizing antibodies (Abs) are protective (50). However, inducing adequate titers of anti-RSV neutralizing antibodies at the mucosal surface by vaccination has proven elusive. It has been suggested that RSV vaccines that induce antibody and CD8+ T cells may be effective (22). Vaccines that elicit CD8+ T cell responses against cancer and viruses are being designed (14, 53). Elucidating T cell responses against RSV, our long-term goal, will advance RSV vaccines across vaccine platforms.
CD8+ T cells are central to RSV pathogenesis, but their role is still controversial. In mice, T cells mediate RSV clearance, augment weight loss, and contribute to lung pathology (11, 23). The CD8+ T cell response to RSV infection in BALB/c mice is characterized by a highly immunodominant epitope in the M2-1 protein, M282-90, followed by a subdominant epitope in the fusion (F) protein (13, 31, 34). The robust M282–90-specific CD8+ T cell response causes weight loss in RSV-infected mice and has thus been considered immunopathologic (48). On the other hand, M282–90-specific CD8+ T cells are protective in a mouse model of RSV glycoprotein (G)-primed vaccine-enhanced immunopathology (44). Importantly, there is no evidence that CD8+ T cells enhance RSV disease severity in humans (54). RSV-specific CD8+ T cells are found in the airways of infants, and the presence of CD8+ T cells correlates with convalescence, not illness (25, 35). Fatal RSV disease in infants was characterized by a lack of T cells in the lung in one study, while CD8+ T cells were noted in the lung infiltrate of a fatal RSV case in another study (28, 54). RSV inhibits T cell responses in mice and human cells. The interferon (IFN) antagonizing nonstructural proteins (NS1 and NS2) of RSV have the effect of suppressing CD8+ T cell activation and proliferation (29, 41). M282–90-specific CD8+ T cells in the lungs of BALB/c mice are relatively poor producers of gamma interferon (IFN-γ) (12). Due to viral immune modulation, phenotypic characterization of RSV-specific T cells in wild-type virus infection may underestimate the potential of these cells for vaccines.
Synthetic peptides of 8 to 10 residues representing a CD8+ T cell epitope are an attractive approach for eliciting antigen-defined, protective CD8+ T cells (18, 47). Administration of peptide in combination with a Toll-like receptor 3 (TLR3) ligand [poly(I·C)] and anti-CD40 monoclonal antibody (MAb) (termed “TriVax” by another group) results in the generation of significantly robust CD8+ T cell responses compared to other peptide vaccination strategies (3, 14). We evaluated the immunogenicity and antiviral effectiveness of TriVax vaccination in a viral model using RSV strain A2-line19F that expresses the fusion protein of the mucus-inducing RSV strain line 19 (40). Abundant mucus in the airways is a hallmark of severe RSV disease in infants (28). Infection of BALB/c mice with A2-line19F results in greater viral load, airway mucus, and lung dysfunction than laboratory A2 RSV strain, thus providing a more robust mouse model (40). In addition, we investigated whether TriVax vaccination can generate long-lasting memory CD8+ T cell responses, because vaccination with CD8+ T cell-inducing RSV recombinant vaccinia virus generated only short-lived protective immunity (15, 16). Although generation of memory T cells is a hallmark for successful vaccination, duration of RSV-specific T cell responses elicited by vaccination is largely unknown.
We hypothesized that RSV-specific CD8+ T cells elicited by TriVax ameliorate rather than contribute to RSV disease severity. We demonstrate that TriVax vaccination successfully generated large-quantity and high-quality RSV-specific CD8+ T cells at the effector phase. RSV challenge of TriVax-vaccinated mice resulted in complete clearance of viral load at the effector phase and partial clearance at the memory phase. There was no evidence of immunopathology due to TriVax vaccination as measured by lung pathology, weight loss, and expression of mucin in airways. Additionally, we found that TriVax-induced memory CD8+ T cells were protective against RSV but not to the extent of TriVax-induced effector CD8+ T cells. These results advance our knowledge of the phenotypes and potential of RSV-specific CD8+ T cells elicited by vaccination.
MATERIALS AND METHODS
Mice and virus.
Pathogen-free 6- to 8-week-old female BALB/c (H-2d) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animal procedures were conducted according to the guidelines of the Emory University Institutional Animal Care and Use Committee. RSV A2-line19 was generated as described previously (40).
Peptide, antibody, and tetramers.
Synthetic peptides representing H-2Kd-restricted CD8+ T cell epitopes M282–90 (SYIGSINNI) and F85–93 (KYKNAVTEL) were purchased from EZBiolab Inc. (Carmel, IN), and 20-mg/ml stock solutions were made in dimethyl sulfoxide (DMSO). The purity (>95%) and identity of peptides were determined by analytic high-performance liquid chromatography by the manufacturer. Rat anti-mouse CD40 monoclonal antibody (anti-CD40 MAb; clone FGK4.5) was purchased from Bio X Cell (West Lebanon, NH). Biotinylated H-2Kd-M282–90 monomer and phycoerythrin (PE)-H-2Kd-F85–93 tetramer were provided by the National Institute of Allergy and Infectious Diseases Tetramer Facility (Emory University, Atlanta, GA). M282–90 tetramers were prepared by mixing the biotinylated M282–90 monomers with streptavidin labeled with R-phycoerythrin (streptavidin-R-PE). Titration of tetramers was performed to determine optimal staining concentration.
Vaccination and infection.
Mice were injected intravenously (i.v.) or intraperitoneally (i.p.) with a mixture of 200 μg peptide, 50 μg anti-CD40 MAb, and 50 μg poly(I·C) (Invivogen, San Diego, CA). As indicated, some mice were given a second identical TriVax treatment (2× TriVax) 2 weeks following the primary treatment. Mice were anesthetized by intramuscular injection of a ketamine-xylazine solution and infected intranasally with 2 × 105 PFU RSV recombinant A2-line19 (rA2-line19) in 100 μl Eagle's minimum essential medium (EMEM) at day 6 (effector phase) or day 42 (memory phase) following the last TriVax treatment.
Preparation of lung lymphocytes.
Mice were euthanized by i.p. sodium pentobarbital (8.5 mg/kg body weight) at the time points indicated. The lungs were removed from the mice and placed in complete RPMI supplemented with 10% fetal bovine serum (FBS). The lung tissues were minced and ground through a sterile mesh to obtain a single-cell suspension. Cells were layered onto Fico/Lite-LM (mouse) (Atlanta Biologicals, Lawrenceville, GA), and lung mononuclear cells were isolated by centrifugation at 2,700 rpm.
Flow cytometry.
For tetramer analysis, peripheral blood was collected from the submandibular vein, and red blood cells were lysed by treatment with ACK buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA). Freshly isolated peripheral blood lymphocytes (PBLs) in fluorescence-activated cell sorting (FACS) buffer (10 ml FBS and 0.5 g sodium azide/500 ml PBS) were incubated with H-2Kd M282–90 tetramer for 30 min at 4°C followed by surface staining for PerCP- and Cy5.5-labeled anti-CD3 (PerCP-Cy5.5-anti-CD3) and allophycocyanin (APC)- and Cy7-labeled anti-CD8 (APC-Cy7-anti-CD8). All antibodies listed were purchased from BD Biosciences (Franklin Lakes, NJ) or eBioscience (San Diego, CA). Singlet discrimination was sequentially performed by using plots for forward scatter (FSC) (forward scatter height [FSC-H] versus forward scatter width [FSC-W]) and side scatter (SSC) (SSC-W versus SSC-H), and dead cells were excluded by scatter characteristics. Flow cytometry was performed using an LSRII cytometer (BD Immunocytometry Systems). Data analysis was performed using FlowJo software (Tree Star, Ashland, OR).
ICS.
To enumerate cytokine-producing cells, intracellular cytokine staining (ICS) was performed as previously described (34). Briefly, freshly isolated lung lymphocytes (5 × 105) were left untreated or stimulated with individual peptides (1 μg/sample) for 6 h at 37°C in 5% CO2 in the presence of Golgi-plug (BD Pharmingen, San Diego, CA). Prior to surface staining, cells were preincubated with 1 μg rat anti-mouse CD16/CD32 antibody (Bio X Cell, West Lebanon, NH) for 15 min at room temperature (RT) to inhibit nonspecific binding of immunoglobulins to Fc receptors. Cell surface staining was performed, followed by ICS using Cytofix/Cytoperm (BD Pharmingen, San Diego, CA) in accordance with the manufacturer's protocol. Fluorescein isothiocyanate (FITC)-labeled anti-IFN-γ (FITC-anti-IFN-γ) (clone XMG1.2), phycoerythrin (PE)-labeled antibody against interleukin 2 (IL-2) (PE-anti-IL-2) (clone JES6-5H4), and allophycocyanin-labeled antibody against tumor necrosis factor alpha (TNF-α) (APC-anti-TNF-α) (clone MP6-XT22) along with surface markers PerCP-Cy5.5-anti-CD3 (clone 145-2C11) and APC-Cy7-anti-CD8 (clone 53-6.7) were used.
In vivo CTL assay.
In vivo cytotoxic T lymphocyte (CTL) assay was performed as described previously (5). Naïve splenocytes were unpulsed or pulsed with 1 μg M282–90 peptide for 3 h at 37°C in a humidified incubator containing 5% CO2. Splenocytes were labeled with low (0.5 μM, unpulsed) and high (5 μM, pulsed with peptide) concentrations of carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, Carlsbad, CA). Labeled splenocytes were coinjected i.v. in a 1:1 ratio (1 × 107 total cells in 100 μl of PBS) into naïve and TriVax-treated mice. Mice were euthanatized 18 h later, and CFSEHi and CFSELo cells in splenocytes were quantified by flow cytometry.
Plaque assay.
Mice were euthanized day 4 postinfection (p.i.). We used a Beadbeater (Biospec Products, Bartlesville, OK) to homogenize the lungs as described previously (51). Lung homogenates were immediately serially diluted and used to inoculate subconfluent HEp-2 cells in 24-well plates. After 1-h adsorption at room temperature on a rocking platform, the cells were overlaid with minimum essential medium (MEM) containing 10% FBS, penicillin G, streptomycin sulfate, amphotericin B solution, and 0.75% methylcellulose. After 6 days, the overlay medium was removed, and the cells were fixed with methanol. Plaques were visualized by immunodetection as previously described (37, 51).
Histopathology.
Heart-lung blocks were harvested at day 8 p.i. and fixed in 10% formalin overnight. Lungs were transferred to 70% ethanol and then embedded in paraffin blocks as described elsewhere (40, 51). Tissue sections (5 μm) were stained with hematoxylin and eosin (H&E) to assess histologic changes. Interstitial pneumonia (IP) scores were determined by a pathologist who was blinded to the experimental groups. Peribronchiolar and perivascular edema scores were also determined. Additional sections were stained with periodic acid-Schiff (PAS) stain for a measure of mucin expression. PAS-stained slides were digitally scanned using a Zeiss Mirax Midi microscope with a 20× objective having a 0.85 numerical aperture (Carl Zeiss Microimaging Inc., Thornwood, NY) (51). Each area of airway epithelium was annotated using HistoQuant software (3D Histech, Budapest, Hungary). PAS-positive areas within the airway epithelium were analyzed and identified by HistoQuant software as described previously (51).
Statistical analyses.
P values were determined by either a two-tailed t test or one-way analysis of variance (ANOVA) and Tukey multiple comparison test, using GraphPad Prism software (La Jolla, CA). Data values below limits of detection were assigned a value of half the limit of detection. Data are representative of at least three replicate experiments having consistent results. Statistical analyses of PAS staining for mucin expression in airways were performed using SAS 9.2 (Cary, NC). Statistical significance was assessed at P < 0.05. Since the outcomes were proportions ranging from 0 to 1 and nonnormal, the arcsine transformation was used to normalize the data. Normality was assessed using normal probability plots and histograms. Repeated-measures ANOVA was used to determine the effect of treatment on the outcome ratio of mask area to field area of epithelium (rMA) using the proc mixed procedure in SAS.
RESULTS
TriVax vaccination induces RSV-specific cytotoxic CD8+ T cells.
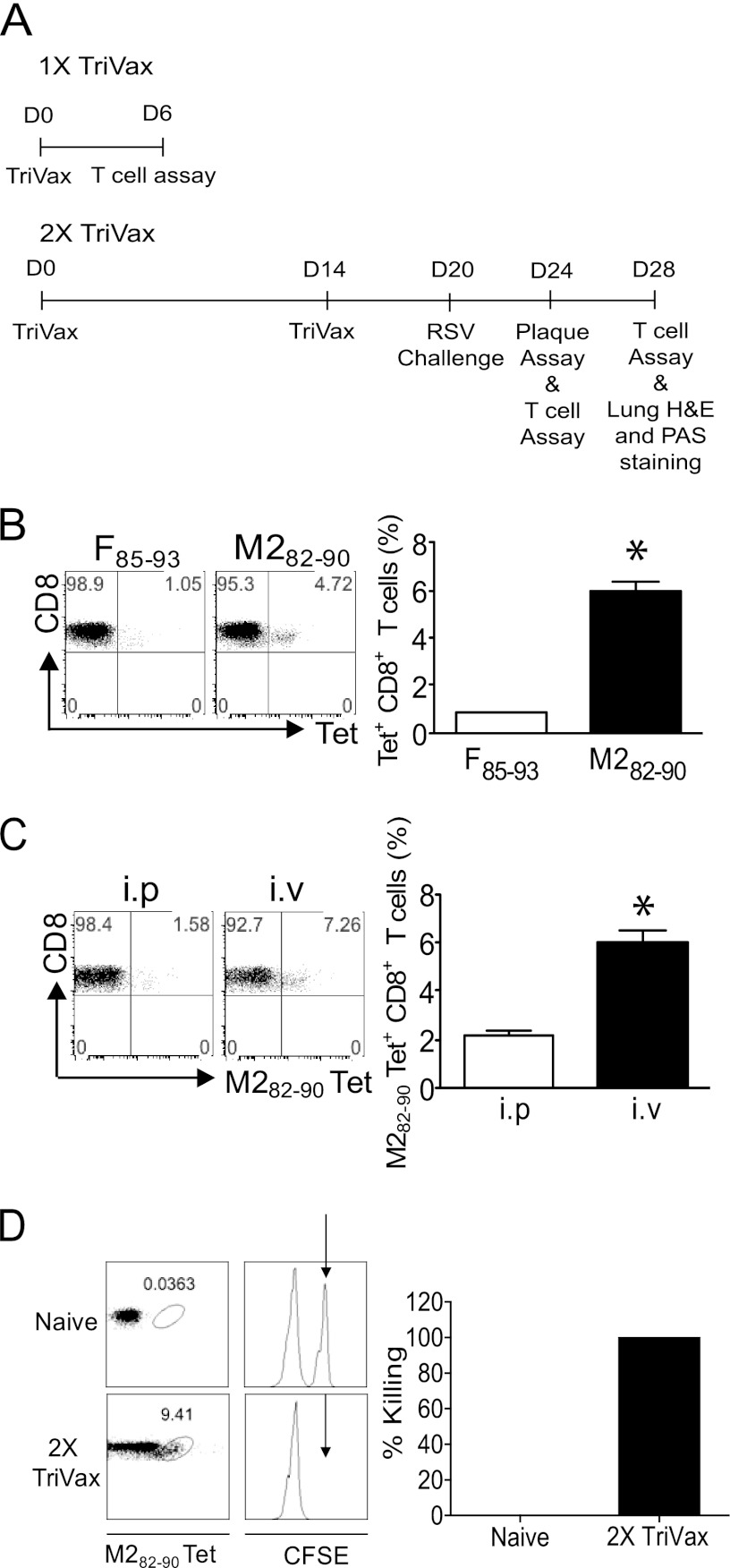
The 1X and 2X TriVax vaccination protocols are illustrated (Fig. 1A). To investigate RSV-specific CD8+ T cell immune responses induced by TriVax, we tested two RSV epitopes, M282–90 (immunodominant) and F85–93 (subdominant) (13, 31). Mice (n = 5) were vaccinated with either F85–93 TriVax or M282–90 TriVax i.v. PBLs were isolated 6 days after 1X TriVax vaccination. Tetramer staining showed that 0.8% ± 0.1% of CD3+ CD8+ T cells were F85–93 specific following vaccination with F85–93 TriVax, and 5.9% ± 1.2% of CD3+ CD8+ T cells were M282–90 specific following vaccination with M282–90 TriVax (Fig. 1B). Next, to determine the most efficient route to evaluate TriVax vaccination, mice (n = 5) were vaccinated with M282–90 1X TriVax i.p. or i.v. We observed that after 1X TriVax vaccination of the mice, the percentage of M282–90-specific CD8+ T cells generated by the i.v. route was approximately 4-fold greater than the i.p. route (Fig. 1C). The i.v. route was therefore selected for further experiments. We used an in vivo CTL assay to quantify effector cytotoxic function mediated by TriVax-induced M282–90-specific CD8+ T cells (5). M282–90 pulsed target cells (CFSEHi; Fig. 1D, arrow) were undetectable 18 h after injection into 2X TriVax-vaccinated mice. However, CFSEHi target cells were present in unvaccinated naïve mice. TriVax vaccination successfully elicited RSV-specific CTL cytotoxicity. An immunodominant epitope, M282–90, used in this study is conserved among RSV strains, including lab strains such as A2, Long, and line 19, and largely conserved among clinical isolates (46).
Fig 1.
TriVax induces robust, functional Ag-specific responses to RSV M282–90. (A) Experimental schedule for TriVax vaccination, RSV challenge, and assays. D0, day 0. (B) Enumeration of F85–93-specific and M282–90-specific CD8+ T cells. BALB/c mice (five mice per group) were vaccinated once with either F85–93 TriVax or M282–90 TriVax i.v. PBLs were isolated day 6 after vaccination. (Left) Representative dot plots show the percentage of CD3+ CD8+ T cells that were F85–93 tetramer (Tet) positive or M282–90 tetramer positive. (Right) Mean percent tetramer-positive CD8+ T cells in PBLs plus standard error of the mean (SEM) (error bar). The two values were significantly different (P < 0.05) as indicated by the asterisk. (C) BALB/c mice (five mice per group) were vaccinated once with M282–90 TriVax i.p. or i.v. PBLs were isolated day 6 after vaccination. (Left) Numbers in the upper right quadrant gate of the dot plots represent the percentage of CD3+ CD8+ cells that were tetramer positive. (Right) Percent tetramer-positive CD8+ T cells in PBLs plus SEM. The two values were significantly different (P < 0.05) as indicated by the asterisk. (D) In vivo M282–90-specific CTL activity was measured by injection of CFSE-labeled target cells at day 6 postboost (2X TriVax; n = 3). Splenocytes were isolated 18 h later, and M282–90 tetramer staining was performed. For a negative control, naïve mice (n = 3) were given CFSE-labeled target cells. CFSELo target cells, which were not pulsed with M282–90 peptide, were an internal control. The black arrows indicate M282–90-pulsed, CFSEHi target cells. Data shown represent one of three experiments with similar results.
Antiviral effect of TriVax vaccination at the effector phase.
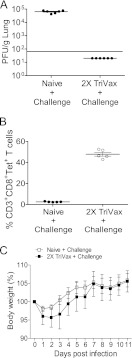
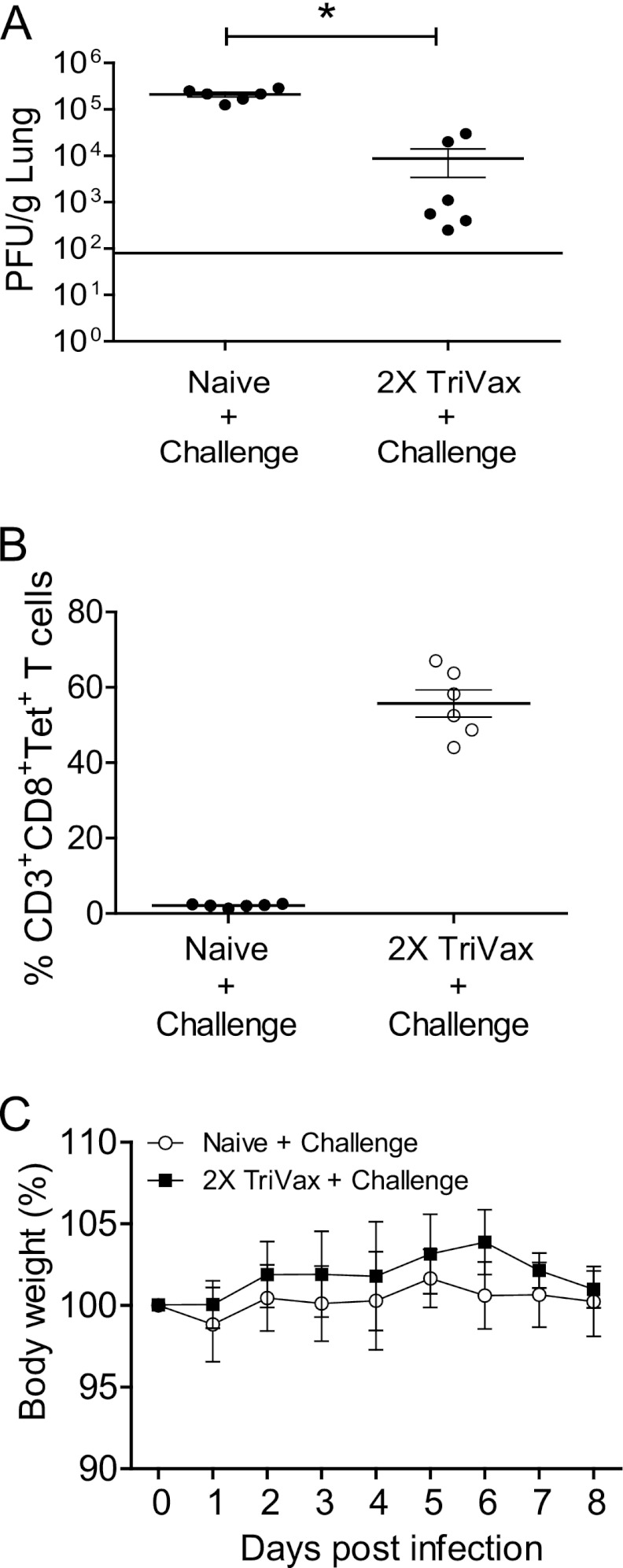
We performed RSV challenge experiments to define the antiviral potential of TriVax-induced CD8+ T cells at the effector phase. Naïve (n = 6) and 2X TriVax-vaccinated mice (n = 6) were challenged at day 6 postboost. 2X TriVax vaccination protected BALB/c mice from RSV A2-line19F infection at the effector phase (Fig. 2A). Antigen (Ag)-specific CD8+ T cells are critical for RSV clearance (39). In order to investigate whether complete protection is correlated with increased RSV-specific CD8 T cell immune responses, we measured RSV-specific CD8+ T cells infiltrating the lungs with M282–90 tetramers. Whereas we observed less than 4% tetramer-positive CD3+ CD8+ T cells in the nonvaccinated group, 50% of CD8+ T cells were tetramer positive in the 2X TriVax-vaccinated groups at day 4 p.i. (Fig. 2B). Thus, 2X TriVax vaccination resulted in the rapid appearance of M282–90-specific CD8+ T cells compared to infection. We measured weight loss during RSV infection as an indicator of RSV illness severity (23). No significant weight loss was detected in naïve or 2X TriVax-vaccinated groups over the time course (Fig. 2C). Taken together, RSV challenge during the effector phase of the response to TriVax resulted in protection against RSV infection that correlated with a rapid appearance of RSV-specific CD8+ T cells in lungs without weight loss.
Fig 2.
TriVax efficacy at the effector phase. (A) BALB/c mice were vaccinated. At day 6 following 2X TriVax vaccination, mice (n = 6) were challenged with A2-line19F. Lungs were harvested day 4 p.i., and infectious RSV was titrated by plaque assay. Each symbol represents the value for an individual mouse, and the black bar represents the mean for the group of mice. The horizontal line depicts the limit of detection. (B) Enumeration of M282–90-specific CD8+ T lymphocytes after challenge. BALB/c mice (five mice per group) were challenged with A2-line19F at day 6 postboost. Lung lymphocytes were isolated on day 4 p.i. and stained with anti-CD3, anti-CD8, and M282–90 tetramer. (C) BALB/c mice (five mice per group) were unvaccinated or vaccinated with M282–90 2X TriVax and challenged with RSV A2-line19F at day 6 postboost. Body weights were recorded daily. Data shown represent one of five experiments, all with similar results.
Altered cytokine expression of effector CD8+ T cells following TriVax and RSV challenge.
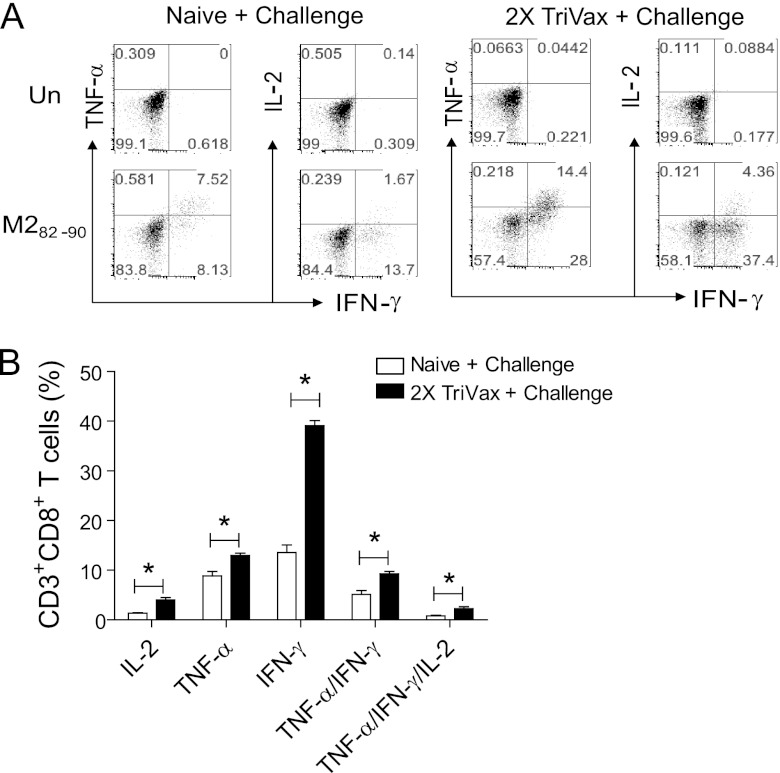
Paramyxoviruses, including RSV, impair IFN-γ production in the lungs (12, 20, 24). Only 40 to 60% of M282–90-specific CD8+ T cells produced IFN-γ following primary RSV infection (12). Studies investigating impaired IFN-γ production in the lung after RSV infection suggest that the local lung environment contributes to impaired cytokine production (20). The ratios of percent IFN-γ-expressing CD8+ T cells to percent M282–90 tetramer-positive CD8+ T cells in naïve, RSV-challenged mice were 43.4 ± 2.5 at day 4 p.i., 45.8 ± 4.1 at day 8 p.i., and 51.9 ± 2.4 at day 12 p.i. (Table 1). This result is similar to previous observations (12). Unexpectedly, 2X TriVax vaccination increased this ratio. The ratios of percent IFN-γ-expressing CD8+ T cells to percent M282–90 tetramer-positive CD8+ T cells in 2X TriVax-vaccinated, RSV-challenged mice were 82.7 ± 10.8 at day 4 p.i., 81.0 ± 4.3 at day 6 p.i., and 94.0 ± 9.6 at day 12 p.i. (Table 1). In order to investigate whether CD8+ T cells induced by 2X TriVax vaccination and challenge are polyfunctional, production of cytokines IFN-γ, TNF-α, and IL-2 were quantified by ICS. Lung lymphocytes were isolated on day 8 p.i. from naïve, challenged mice (n = 5) and from 2X TriVax-vaccinated, challenged mice (n = 5) and then restimulated in vitro in the presence or absence of peptide (M282–90). 2X TriVax vaccination and challenge resulted in higher lung CD8+ T cell IFN-γ and TNF-α production after peptide restimulation than challenge in naïve mice did (Fig. 3A and B). No detectable level of cytokine production was observed in the absence of peptide (Fig. 3A). The majority of TNF-α+ CD8+ T cells also produced IFN-γ, but not vice versa. The hierarchical order was IFN-γ > TNF-α > IL-2, similar to published data (6). Next, cytokine-producing CD8+ T cells were divided into five populations based on their production of IFN-γ, TNF-α, and IL-2 in any combination to characterize the quality of effector M282–90-specific CD8+ T cell responses elicited by 2X TriVax vaccination. There were significantly more M282–90-specific CD8+ T cells expressing TNF-α+/IFN-γ+, and TNF-α+/IFN-γ+/IL-2 in the 2X TriVax-vaccinated and challenged group compared to the naïve, challenged group (P < 0.01) (Fig. 3B). These results demonstrate that 2X TriVax vaccination and challenge resulted in greater polyfunctional CD8+ T cell responses than challenge in naïve mice. Collectively, TriVax vaccination dramatically altered the kinetics, magnitude, and quality of the CD8+ T cell response at the effector phase to RSV infection.
Table 1.
Kinetics of M282–90-specific CD8+ T cell responses in the lunga
| Group | Days p.i. | % Tet+ CD8+ cells | % IFN-γ+ CD8+ cells | Ratio of % IFN-γ+ cells to % Tet+ cells |
|---|---|---|---|---|
| Naïve, challenged | 4 | 1.1 ± 0.4 | 0.5 ± 0.2 | 43.4 ± 2.5b |
| 8 | 27.0 ± 3.5 | 12.4 ± 1.8 | 45.8 ± 4.1c | |
| 12 | 38.7 ± 5.0 | 20.1 ± 2.7 | 51.9 ± 2.4d | |
| 2X TriVax-vaccinated, challenged | 4 | 62.1 ± 4.8 | 51.4 ± 6.7 | 82.7 ± 10.8b |
| 8 | 54.3 ± 2.9 | 44.5 ± 2.1 | 81.0 ± 4.3c | |
| 12 | 35.6 ± 5.1 | 33.2 ± 1.7 | 94.0 ± 9.6d |
BALB/c mice were unvaccinated (naïve, challenged mice) (n = 5) or 2X TriVax vaccinated (n = 5) and challenged with A2-line19F. Lung lymphocytes were stained with M282–90 tetramer or with IFN-γ at days 4, 8, and 12 p.i. The ratio of the percentage of IFN-γ-expressing cells to the percentage of M282–90 tetramer (Tet)-positive cells was calculated. All data represent the results of one experiment. Each experiment was done three times with similar results. The means ± standard errors of the means are shown.
These two values are significantly different (P < 0.01).
These two values are significantly different (P < 0.01).
These two values are significantly different (P < 0.01).
Fig 3.
Increased cytokine production in lungs by TriVax vaccination. (A) Analysis of M282–90-reactive CD8+ T cells after challenge. BALB/c mice (five mice per group) were unvaccinated or vaccinated with M282–90 2X TriVax and challenged with RSV A2-line19F at day 6 postboost. Lung lymphocytes were isolated on day 8 p.i. and incubated in the presence (M282–90 [1 μg/sample]) or absence (un) of the M282–90 peptide. Dot plots are shown where the numbers in quadrant gates represent the percentages of CD3+ CD8+ cells that produced IL-2, TNF-α, or IFN-γ. (B) The percentage of CD3+ CD8+ cells in lung lymphocytes from naïve, challenged mice or 2X TriVax-vaccinated, challenged mice that expressed IL-2, TNF-α, IFN-γ, TNF-α+/IFN-γ+, or TNF-α+/IFN-γ+/IL-2+ in the presence of M282–90 peptide plus SEM. The cytokine profiles of these cells were determined by expressing each cytokine alone or in combination. Values that are significantly different (P < 0.05) are indicated by a bar and asterisk. Data shown represent one of three experiments with similar results.
TriVax vaccination results in decreased airway mucin expression and lung inflammation.
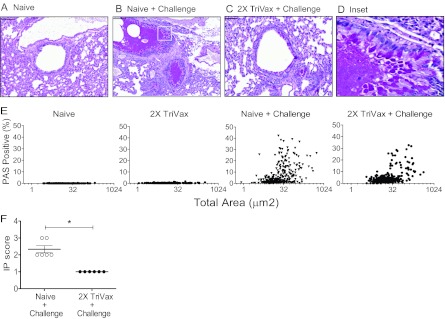
Both production of mucus and pulmonary inflammation are key features of RSV disease. Pathological change in lung airways is an important sequela of RSV infection (2, 28). We investigated whether TriVax vaccination can ameliorate RSV-induced inflammation and airway mucin expression. We quantified mucin production in the airways. The 2X TriVax-vaccinated, challenged mice had significantly less airway mucin expression than naïve, challenged mice (Fig. 4A to E). Results from the repeated-measures ANOVA indicated a significant effect (P = 0.048) of treatment: β = −0.0244 and 95% confidence interval (95% CI) of −0.0.048 to −0.0003 (Fig. 4E). Compared to naïve BALB/c mice, 2X TriVax-vaccinated, challenged mice had higher mucin production (Fig. 4E). In order to determine whether the mucin expression in the 2X TriVax-vaccinated, challenged group is elicited solely by RSV infection and not by TriVax vaccination, we also performed PAS staining in 2X TriVax-vaccinated mice (n = 6) without RSV challenge (Fig. 4E). As expected, the basal level of mucin production was detected in 2X TriVax-vaccinated mice as observed in naïve mice (Fig. 4E). In addition, the interstitial pneumonia (IP) score was determined. IP is characterized by abnormal infiltrates of inflammatory cells in the alveolar septa of the lungs (42). In mice, RSV infects alveolar epithelial cells, resulting in IP (51). The IP score was significantly higher in the naïve, challenged mice than in the 2X TriVax-vaccinated, challenged mice (Fig. 4F) (P < 0.0001). However, no significant differences in peribronchiolar and perivascular edema scores were detected between groups. Taken together, TriVax vaccination significantly reduced RSV pulmonary pathogenesis. These data correlate with reduced viral load in TriVax-vaccinated mice.
Fig 4.
Reduction of RSV-induced pulmonary mucin expression and interstitial inflammation by TriVax vaccination. Mice were unvaccinated (Naive) (n = 6) or vaccinated with 2X TriVax (n = 6). Mice were challenged with A2-line19F at day 6 postboost. Unvaccinated (Naive) (n = 6) mice and 2X TriVax-vaccinated mice without RSV challenge (n = 6) were used as control groups. Lungs were harvested on day 8 p.i. and processed for PAS staining (A to E). For PAS scores, the lung tissues were digitized, and each area of airway epithelium was annotated by hand as described in Materials and Methods. Representative airways are shown in panels A (Naive), B (Naive + Challenge), C (2X TriVax + Challenge) and D (Inset in Fig. 4B). Bars, 50 μm. (E) The percentage of each airway area that was PAS positive for the indicated groups is shown. Each symbol represents the value for one airway. There were >250 individual airways and 6 mice per group. (F) Interstitial pneumonia (IP) score ± SEM. Each symbol represents the value for an individual mouse, and the short black lines represent the mean values for the groups of mice. The values for the two groups of mice were significantly different (P < 0.005) as indicated by a bar and asterisk. Data show one of three experiments with equivalent results.
Evaluation of memory CD8+ T cell responses induced by TriVax immunization.
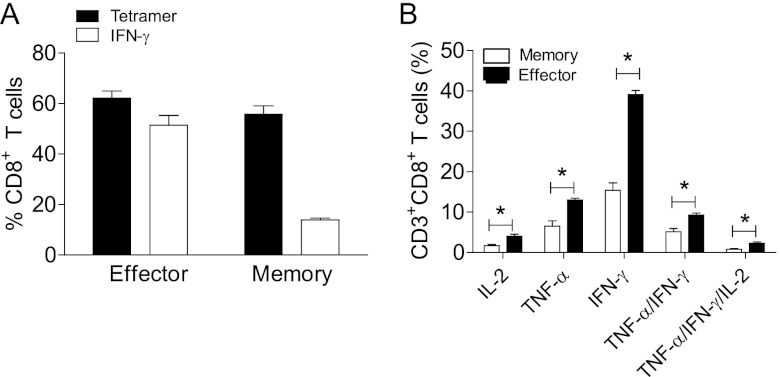
The ability to develop memory T cells after vaccination is essential for protective vaccination against infectious disease and sustenance of the adaptive immune responses. Thus, we investigated the efficacy of TriVax vaccination against RSV A2-line19F at the memory phase. Protection by 2X TriVax was significant but partial at the memory phase (Fig. 5A). The level of protection afforded by 2X TriVax at the memory phase was lower than that at the effector phase (compare Fig. 2A and 5A). On the basis of those results, we initially hypothesized that the number of M282–90 tetramer-positive CD8+ T cells at the memory phase would be lower than that in the effector phase after challenge. Unexpectedly, 40 to 60% tetramer-positive CD3+ CD8+ T cells were observed in 2X TriVax-vaccinated, challenged mice at the memory phase (Fig. 5B). There was no difference in the frequency of tetramer-positive CD8+ T cells between the effector and memory phases of 2X TriVax-vaccinated and challenged mice (compare Fig. 2B and 5B). No weight loss was detected in both groups at the memory phase (Fig. 5C). We then hypothesized that there is some defect in cytokine production by Ag-specific CD8+ T cells at the memory phase following 2X TriVax vaccination and challenge. We measured cytokine production at day 4 p.i. by ICS. First, we compared the ratio of percent IFN-γ-expressing to percent tetramer-positive CD8+ T cells at the effector phase to the memory phase. As shown in Fig. 6A, 62.1% of CD8+ T cells were tetramer positive (Tet+) and 52.5% of CD8+ T cells were IFN-γ+ during the effector phase, and the ratio of Tet+ to IFN-γ+ was therefore 84%. In contrast, during the memory phase, 55.8% of CD8+ T cells were Tet+ but only 13.9% of CD8+ T cells were IFN-γ+ (25% ratio of Tet+ to IFN-γ+). We measured IFN-γ, TNF-α, IL-2, TNF-α+/IFN-γ+, and TNF-α+/IFN-γ+/IL-2 expression to characterize the quality of M282–90 specific CD8+ T cell responses elicited by 2X TriVax vaccination at the memory phase. We thus observed significantly lower lung CD8+ T cell cytokine expression at the memory phase compared to the effector phase following 2X TriVax vaccination and RSV challenge (Fig. 6B). These data suggest that waning protection against RSV infection at the memory phase following 2X TriVax vaccination is due to the generation of memory CD8+ T cells that express relatively low levels of antiviral cytokines.
Fig 5.
TriVax efficacy at the memory phase. (A) Viral load. At day 42 following 2X TriVax vaccination, mice (n = 6) were challenged with A2-line19F. Lungs were harvested on day 4 p.i., and infectious RSV was titrated by plaque assay. The horizontal line depicts the limit of detection. (B) Enumeration of M282–90-specific CD8+ T lymphocytes after challenge. BALB/c mice (n = 6) were challenged with A2-line19F at day 42 postboost. Lung lymphocytes were isolated on day 4 p.i. and stained with anti-CD3, anti-CD8, and M282–90 tetramer. (C) Weight loss. BALB/c mice (five mice per group) were unvaccinated or vaccinated with M282–90 2X TriVax and challenged with RSV A2-line19F at day 42 postboost. Body weights were recorded daily. Data shown represent one of three experiments with similar results.
Fig 6.
Comparison of cytokine production at the effector phase and the memory phase. (A) Ratio of percent IFN-γ-expressing CD8+ T cells to percent tetramer-positive CD8+ T cells. BALB/c mice (five mice per group) were vaccinated with M282–90 2X TriVax and challenged with RSV A2-line19F at day 6 postboost (effector phase) or at day 42 postboost (memory phase). Lung lymphocytes were isolated on day 4 p.i. and incubated in the presence of M282–90 peptide (1 μg/sample) for ICS assay. Tetramer staining was performed separately from ICS staining. (B) Cytokine profiles of effector and memory CD8+ T cells. The percentage of CD3+ CD8+ cells in lung lymphocytes from TriVax-vaccinated, challenged mice that expressed IL-2, TNF-α, IFN-γ, TNF-α IFN-γ, or TNF-α IFN-γ IL-2 in the presence or absence of M282–90 peptide plus SEM. The cytokine profiles of these cells were determined by expressing each cytokine alone or in combination. Values that are significantly different (P < 0.05) are indicated by a bar and asterisk. Data show one of three experiments with equivalent results.
DISCUSSION
In this study, we demonstrated that a peptide vaccination strategy (TriVax) consisting of a comixture of an RSV M282–90 peptide, the TLR3 agonist poly(I·C), and a costimulatory anti-CD40 MAb generated high-quality anti-RSV cytotoxic T lymphocytes (CTL) and protected against RSV A2-line19F strain infection and RSV disease parameters in BALB/c mice. A higher percentage of M282–90-specific CD8+ T cells induced by 2X TriVax vaccination and RSV infection expressed IFN-γ compared to CD8+ T cells induced by no vaccination and RSV infection. Thus, TriVax vaccination induced higher-quality CD8+ T cells than primary infection. TriVax vaccination protected against RSV-induced airway mucin expression and cellular lung inflammation using the mucus-inducing RSV challenge strain A2-line19F (40). TriVax peptide vaccination has been tested in model antigen systems and tumor models, mostly using peptides representing CD8+ T cell epitopes (3, 5, 14). To our knowledge, this study represents the first evidence that the TriVax peptide/TLR/anti-CD40 vaccination approach elicits robust, protective CD8+ T cell responses in an infectious challenge model.
Mice are semipermissive for RSV infection, and RSV pathogenesis in mice depends on the mouse strain and virus strain (40). In this study, we used strain A2-line19F as a challenge virus due to higher viral load and greater airway mucin induction than laboratory RSV strains (40). We used BALB/c mice because this mouse strain is relatively more permissive to RSV among inbred mouse strains, and several groups have used BALB/c mice to study T cell responses to RSV (12, 19). Using M282–90 peptide TriVax vaccination in BALB/c mice in the current study enabled us to stringently evaluate the immunopathologic consequences of robust, dominant vaccine-elicited CD8+ T cells. We showed that RSV-induced airway mucin production is significantly reduced in TriVax-vaccinated mice, and we speculate that TriVax vaccination did not cause immunopathology because TriVax-induced CD8+ T cells highly expressed IFN-γ. It was previously shown that exogenous IFN-γ expression in the lungs of BALB/c mice protects against RSV infection (32). Also, infants with mild RSV disease had higher RSV-specific IFN-γ recall responses than infants with moderate or severe RSV disease (33). Furthermore, IFN-γ inhibits the production of mucus, which is one of the important pathological features of allergic airway inflammation (38). Our study provides additional evidence that IFN-γ plays a protective role in RSV disease.
As the functional capacities for multiple cytokine expression of virus-specific CD8+ T cells is an important factor for virus control, we hypothesize that the generation of functionally high-quality CD8+ T cells is crucial to determine protective versus pathological roles of CD8+ T cells (8). In order to define the function of CD8+ T cell responses elicited by TriVax, we measured a common set of cytokines, IFN-γ, TNF-α, and IL-2, via polychromatic ICS assay. We found that complete protection against RSV correlated with the rapid appearance of triple positive (IFN-γ TNF-α IL-2) RSV-specific CD8+ T cells.
Several studies have shown that virus infection alters functionality of CD8+ T cells in the lungs (20, 24). Although this phenomenon has been observed in multiple viral models, the underlying mechanisms are still unknown. The impaired function of CD8+ T cells in the lung during virus infection has been proposed to be the consequence of virus-induced changes in the lung environment (24). Thus, it appears that T cell suppressive environments are present in local lung tissue. Interestingly, Fulton et al. reported that impaired cytokine production was rescued by exogenous peptide-pulsed antigen-presenting cells (APCs) (20). This result suggested the possibility that APCs in the lungs were not providing sufficient activation signal to T cells for cytokine production. In our model during the effector phase following TriVax vaccination, it seems that the combination of TLR signaling and CD40-CD40L (CD40 ligand) interaction acts like an exogenous APC to reverse a suppressive environment in the lung (7, 26). Clearly, additional mechanistic studies are required to elucidate the underlying mechanism of how TriVax vaccination rescued impaired cytokine production induced by RSV infection.
The generation of robust and long-lasting CD8+ T cell responses against RSV may enhance the development of a successful vaccine. Ag-specific CD8+ T cells persist 35 days in the respiratory tracts of mice following influenza virus infection (55). However, RSV-specific CD8+ T cells are short-lived in mice following primary infection, suggesting protective CD8+ T cell-mediated immunity wanes relatively quickly (15, 30). As we demonstrated, TriVax vaccination successfully generated RSV-specific memory CD8+ T cells in lungs in terms of tetramer-positive responses. Indeed, RSV-specific tetramer responses elicited by TriVax at the memory phase were equivalent to those at the effector phase. However, memory CD8+ T cells elicited by TriVax peptide vaccination were moderately antiviral. Compared to CD8+ T cells during the effector phase, pulmonary memory CD8+ T cells during the memory phase following TriVax vaccination and RSV challenge produced less IFN-γ, TNF-α, IL-2, TNF-α+/IFN-γ+, and TNF-α+/IFN-γ+/IL-2.
Currently, it is unclear why memory CD8+ T cells generated by TriVax produced lower cytokine levels. This could be why memory CD8+ T cells are less effective in protection compared to effector CD8+ T cells in our model. The purpose of anti-CD40 is to provide a strong costimulatory signal to APCs to facilitate T cell-APC interaction by mimicking CD40-CD40L interaction. CD40-CD40L interaction is as an important component of generating memory CD8+ T cells (10). Although TriVax components contain anti-CD40 that could resemble CD4 help function, it is possible that the effect was not sufficient to generate highly effective memory CD8+ T cells. CD8+ T cells generated in the absence of CD4+ T cell help show impaired protective function and loss of proliferative capacity (27, 49, 52). In contrast, CD4+ T cell help is not crucial for maintaining memory CD8+ T cells in some experimental models (1, 36). The role of CD4+ T cell help in the maintenance of CD8+ memory T cells in RSV models is currently unknown, and we are working on this using modified TriVax vaccination strategies.
Although T cells responding to RSV correlate with protection, induction of neutralizing antibody is a major goal of RSV vaccination (11, 35, 37, 45, 55). Neutralizing antibodies (nAb) can prevent RSV infection, but no vaccine has elicited these sufficiently (22, 45). The most effective strategy to provide robust protection against reinfection and disease may be the combination of long-lived antibody and T cell immunity (9). Different combinations of adjuvants with RSV B and T cell antigens may be an alternative approach to induce B cell immunity in addition to T cell immunity. The NS1 protein of RSV inhibits CD8+ and CD4+ T cell proliferation (41). Inhibition of CD4+ T cell responses by RSV infection may blunt CD4+ T cell help to B cells and CD8+ T cells. Therefore, elucidation of mechanisms to stimulate CD4+ T cell responses for RSV immunity may contribute to protection via CTL and/or Ab.
In summary, RSV-specific CD8+ T cells have the potential to protect against RSV-induced lung disease, but mechanisms and limits of cell-mediated immunity (CMI) to RSV are incompletely defined. TriVax peptide vaccination is a useful tool to define RSV-specific T cells induced by a model Ag-specific T cell vaccine. In fact, identification and characterization of RSV epitopes in humans is an expanding area of research to design effective RSV vaccines (4). The N protein has been considered a major target for memory CD8+ T cell responses (21). Here, we challenged mice with RSV during the acute phase as well as the memory phase of the T cell response. Further studies will be required to define the central and effector memory phenotypes and functions of TriVax-induced RSV-specific CD8+ T cells in BALB/c mice. Our findings support the notion that generation of high-quality antiviral CD8+ T cells will contribute to RSV vaccines.
ACKNOWLEDGMENTS
We thank the Emory Children's Pediatric Research Center flow cytometry core and biostatistics core, which are supported by Children's Healthcare of Atlanta (CHOA). We thank Carla Shoffeitt (CHOA) for histology technical assistance and Murali Kaja (Emory University) for critical review of the manuscript. We also thank the NIAID Tetramer Facility for providing valuable monomers.
This work was supported by the following grants: NIH 1R01AI087798, NIH 1U19AI095227, an Emory University Research Committee and Atlanta Clinical & Translational Science Institute Pilot Grant, and a Georgia Research Alliance (GRA) Next Generation Vaccines and Therapeutics Collaborative Planning Grant (M. L. Moore), as well as an Emory Egleston Children Research Center (EECRC) grant and an American Federation of Aging Research (AFAR) grant (S. Lee).
Footnotes
Published ahead of print 26 September 2012
REFERENCES
- 1. Agnellini P, et al. 2008. Kinetic and mechanistic requirements for helping CD8 T cells. J. Immunol. 180:1517–1525 [DOI] [PubMed] [Google Scholar]
- 2. Aherne W, Bird T, Court SD, Gardner PS, McQuillin J. 1970. Pathological changes in virus infections of the lower respiratory tract in children. J. Clin. Pathol. 23:7–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahonen CL, et al. 2004. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J. Exp. Med. 199:775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson R, Huang Y, Langley JM. 2010. Prospects for defined epitope vaccines for respiratory syncytial virus. Future Microbiol. 5:585–602 [DOI] [PubMed] [Google Scholar]
- 5. Assudani D, Cho HI, DeVito N, Bradley N, Celis E. 2008. In vivo expansion, persistence, and function of peptide vaccine-induced CD8 T cells occur independently of CD4 T cells. Cancer Res. 68:9892–9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belz GT, Xie W, Doherty PC. 2001. Diversity of epitope and cytokine profiles for primary and secondary influenza A virus-specific CD8+ T cell responses. J. Immunol. 166:4627–4633 [DOI] [PubMed] [Google Scholar]
- 7. Bennett SR, et al. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393:478–480 [DOI] [PubMed] [Google Scholar]
- 8. Betts MR, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bevan MJ. 2011. Understand memory, design better vaccines. Nat. Immunol. 12:463–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borrow P, et al. 1998. CD40 ligand-mediated interactions are involved in the generation of memory CD8(+) cytotoxic T lymphocytes (CTL) but are not required for the maintenance of CTL memory following virus infection. J. Virol. 72:7440–7449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cannon MJ, Openshaw PJ, Askonas BA. 1988. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 168:1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang J, Braciale TJ. 2002. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat. Med. 8:54–60 [DOI] [PubMed] [Google Scholar]
- 13. Chang J, Srikiatkhachorn A, Braciale TJ. 2001. Visualization and characterization of respiratory syncytial virus F-specific CD8(+) T cells during experimental virus infection. J. Immunol. 167:4254–4260 [DOI] [PubMed] [Google Scholar]
- 14. Cho HI, Celis E. 2009. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res. 69:9012–9019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Connors M, Collins PL, Firestone CY, Murphy BR. 1991. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J. Virol. 65:1634–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Connors M, et al. 1992. Resistance to respiratory syncytial virus (RSV) challenge induced by infection with a vaccinia virus recombinant expressing the RSV M2 protein (Vac-M2) is mediated by CD8+ T cells, while that induced by Vac-F or Vac-G recombinants is mediated by antibodies. J. Virol. 66:1277–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352:1749–1759 [DOI] [PubMed] [Google Scholar]
- 18. Firbas C, et al. 2006. Immunogenicity and safety of a novel therapeutic hepatitis C virus (HCV) peptide vaccine: a randomized, placebo controlled trial for dose optimization in 128 healthy subjects. Vaccine 24:4343–4353 [DOI] [PubMed] [Google Scholar]
- 19. Fulton RB, Meyerholz DK, Varga SM. 2010. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J. Immunol. 185:2382–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fulton RB, Olson MR, Varga SM. 2008. Regulation of cytokine production by virus-specific CD8 T cells in the lungs. J. Virol. 82:7799–7811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goulder PJ, Lechner F, Klenerman P, McIntosh K, Walker BD. 2000. Characterization of a novel respiratory syncytial virus-specific human cytotoxic T-lymphocyte epitope. J. Virol. 74:7694–7697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graham BS. 2011. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol. Rev. 239:149–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Graham BS, Bunton LA, Wright PF, Karzon DT. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Invest. 88:1026–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gray PM, Arimilli S, Palmer EM, Parks GD, Alexander-Miller MA. 2005. Altered function in CD8+ T cells following paramyxovirus infection of the respiratory tract. J. Virol. 79:3339–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heidema J, et al. 2007. CD8+ T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J. Immunol. 179:8410–8417 [DOI] [PubMed] [Google Scholar]
- 26. Iwasaki A, Medzhitov R. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995 [DOI] [PubMed] [Google Scholar]
- 27. Janssen EM, et al. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852–856 [DOI] [PubMed] [Google Scholar]
- 28. Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. 2007. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 20:108–119 [DOI] [PubMed] [Google Scholar]
- 29. Kotelkin A, et al. 2006. The NS2 protein of human respiratory syncytial virus suppresses the cytotoxic T-cell response as a consequence of suppressing the type I interferon response. J. Virol. 80:5958–5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kulkarni AB, et al. 1995. Cytotoxic T cells specific for a single peptide on the M2 protein of respiratory syncytial virus are the sole mediators of resistance induced by immunization with M2 encoded by a recombinant vaccinia virus. J. Virol. 69:1261–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kulkarni AB, Morse HC, III, Bennink JR, Yewdell JW, Murphy BR. 1993. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ cytotoxic T cells. J. Virol. 67:4086–4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kumar M, Behera AK, Matsuse H, Lockey RF, Mohapatra SS. 1999. Intranasal IFN-gamma gene transfer protects BALB/c mice against respiratory syncytial virus infection. Vaccine 18:558–567 [DOI] [PubMed] [Google Scholar]
- 33. Lee FE, et al. 2007. Human infant respiratory syncytial virus (RSV)-specific type 1 and 2 cytokine responses ex vivo during primary RSV infection. J. Infect. Dis. 195:1779–1788 [DOI] [PubMed] [Google Scholar]
- 34. Lee S, Miller SA, Wright DW, Rock MT, Crowe JE., Jr 2007. Tissue-specific regulation of CD8+ T-lymphocyte immunodominance in respiratory syncytial virus infection. J. Virol. 81:2349–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lukens MV, et al. 2010. A systemic neutrophil response precedes robust CD8(+) T-cell activation during natural respiratory syncytial virus infection in infants. J. Virol. 84:2374–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marzo AL, et al. 2004. Fully functional memory CD8 T cells in the absence of CD4 T cells. J. Immunol. 173:969–975 [DOI] [PubMed] [Google Scholar]
- 37. Miller AL, Bowlin TL, Lukacs NW. 2004. Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J. Infect. Dis. 189:1419–1430 [DOI] [PubMed] [Google Scholar]
- 38. Mitchell C, Provost K, Niu N, Homer R, Cohn L. 2011. IFN-gamma acts on the airway epithelium to inhibit local and systemic pathology in allergic airway disease. J. Immunol. 187:3815–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mok H, Lee S, Wright DW, Crowe JE., Jr 2008. Enhancement of the CD8+ T cell response to a subdominant epitope of respiratory syncytial virus by deletion of an immunodominant epitope. Vaccine 26:4775–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moore ML, et al. 2009. A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. J. Virol. 83:4185–4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Munir S, et al. 2011. Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response. PLoS Pathog. 7:e1001336 doi:10.1371/journal.ppat.1001336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Myers JL, Veal CF, Jr, Shin MS, Katzenstein AL. 1987. Respiratory bronchiolitis causing interstitial lung disease. A clinicopathologic study of six cases. Am. Rev. Respir. Dis. 135:880–884 [DOI] [PubMed] [Google Scholar]
- 43. Nair H, et al. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olson MR, Varga SM. 2007. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J. Immunol. 179:5415–5424 [DOI] [PubMed] [Google Scholar]
- 45. Piedra PA, Jewell AM, Cron SG, Atmar RL, Glezen WP. 2003. Correlates of immunity to respiratory syncytial virus (RSV)-associated hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 21:3479–3482 [DOI] [PubMed] [Google Scholar]
- 46. Rebuffo-Scheer C, et al. 2011. Whole genome sequencing and evolutionary analysis of human respiratory syncytial virus A and B from Milwaukee, WI 1998–2010. PLoS One 6:e25468 doi:10.1371/journal.pone.0025468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosenberg SA, et al. 1998. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat. Med. 4:321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ruckwardt TJ, et al. 2010. Responses against a subdominant CD8+ T cell epitope protect against immunopathology caused by a dominant epitope. J. Immunol. 185:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shedlock DJ, Shen H. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337–339 [DOI] [PubMed] [Google Scholar]
- 50. Simoes EA, et al. 2007. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J. Pediatr. 151:34–42.e31 [DOI] [PubMed] [Google Scholar]
- 51. Stokes KL, et al. 2011. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J. Virol. 85:5782–5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sun JC, Bevan MJ. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Uchida T. 2011. Development of a cytotoxic T-lymphocyte-based, broadly protective influenza vaccine. Microbiol. Immunol. 55:19–27 [DOI] [PubMed] [Google Scholar]
- 54. Welliver TP, et al. 2007. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 195:1126–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wiley JA, Hogan RJ, Woodland DL, Harmsen AG. 2001. Antigen-specific CD8(+) T cells persist in the upper respiratory tract following influenza virus infection. J. Immunol. 167:3293–3299 [DOI] [PubMed] [Google Scholar]