Abstract
Gammaherpesviruses, such as Epstein-Barr virus (EBV), are ubiquitous cancer-associated pathogens that interact with DNA damage response, a tumor suppressor network. Chronic gammaherpesvirus infection and pathogenesis in a DNA damage response-insufficient host are poorly understood. Ataxia-telangiectasia (A-T) is associated with insufficiency of ataxia-telangiectasia mutated (ATM), a critical DNA damage response kinase. A-T patients display a pattern of anti-EBV antibodies suggestive of poorly controlled EBV replication; however, parameters of chronic EBV infection and pathogenesis in the A-T population remain unclear. Here we demonstrate that chronic gammaherpesvirus infection is poorly controlled in an animal model of A-T. Intriguingly, in spite of a global increase in T cell activation and numbers in wild-type (wt) and ATM-deficient mice in response to mouse gammaherpesvirus 68 (MHV68) infection, the generation of an MHV68-specific immune response was altered in the absence of ATM. Our finding that ATM expression is necessary for an optimal adaptive immune response against gammaherpesvirus unveils an important connection between DNA damage response and immune control of chronic gammaherpesvirus infection, a connection that is likely to impact viral pathogenesis in an ATM-insufficient host.
INTRODUCTION
Ataxia-telangiectasia (A-T) is an autosomal recessive, multisystem disease that manifests itself in early childhood and leads to a median life expectancy of 19 to 25 years (8). The genetic lesion in A-T has been mapped to ATM, a gene that encodes a protein kinase critical for proximal steps of the DNA damage response (DDR) (29). Cancer (primarily lymphomas) and lung disease of poorly understood etiology are significant contributors to the mortality in the A-T population (40). Antibiotic treatment has a limited effect on the progression of A-T-associated lung disease, suggesting that bacterial infections are not solely responsible for the deterioration of A-T lungs (33). A-T patients exhibit heterogenous humoral and cellular immunodeficiencies, including hypogammaglobulinemia, decreased responsiveness to vaccinations, and lymphopenia (38). However, A-T patients are not predisposed to opportunistic or systemic infections, suggesting that the ATM-insufficient immune system remains competent to control many pathogens (38). Importantly, A-T patients seem to be selectively susceptible to severe herpesvirus infections. In one study, herpesviruses were the cause of 8 out of 11 severe viral infections observed in A-T patients (36). It is not known whether herpesvirus infection contributes to lung disease or cancer observed in the A-T population.
Gammaherpesviruses are cancer-associated pathogens that establish lifelong infection in a majority of the human population. Two known human gammaherpesviruses, Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV), are associated with cancer, including lymphomas (7). A-T patients have a skewed antibody response against EBV, with high titers of antibodies directed against lytic, but not latent, antigens of the virus (4, 22, 36). This antibody pattern implies uncontrolled persistent EBV replication. Unfortunately, with the exception of a single case report demonstrating a significantly increased EBV viral load (14), the parameters of EBV or other herpesvirus infection in A-T patients have not been analyzed. The high seroprevalence and exquisite species specificity of EBV and KSHV further hinder analyses of gammaherpesvirus pathogenesis in an A-T population.
ATM expression and activity are critical for the proper response to DNA damage and DNA repair. Markers of active DDR, including ATM activation, are present during lytic gammaherpesvirus infection, and gammaherpesviruses usurp several host DDR proteins to facilitate viral replication (24, 27, 28, 37, 42). Unfortunately, very little is understood about DDR-gammaherpesvirus interaction in the context of chronic infection in vivo. In this study, we used mouse gammaherpesvirus 68 (MHV68), a rodent pathogen genetically and biologically related to EBV and KSHV (13, 45, 48), to probe gammaherpesvirus-ATM interactions in vivo. MHV68 interacts with the host DDR during lytic and latent infection as evidenced by increased levels of phosphorylated H2AX, an ATM substrate, during lytic replication in primary macrophages, attenuated viral replication in primary macrophages isolated from ATM- or H2AX-deficient mice, and attenuated chronic MHV68 infection in H2AX-deficient mice (37, 42, 44). Here we show that chronic MHV68 infection was poorly controlled in ATM-deficient mice (1), a mouse model of A-T. Persistent viral replication was observed alongside an aberrant T cell response characterized by a decreased number of MHV68-specific gamma interferon (IFN-γ)-producing CD8+ T cells skewed in their reactivity to MHV68 immunodominant epitopes. The results of the presented studies have important implications for the pathogenesis of gammaherpesvirus infection in the context of A-T and other human diseases associated with mutations within the DDR network.
MATERIALS AND METHODS
Mice, virus, and infections.
Mice were bred and housed at the Medical College of Wisconsin in a specific-pathogen-free facility in accordance with all federal and institutional guidelines. 129S6/SvEvTac-Atmtm1Awb/J mice were obtained from Jackson Laboratories (Bar Harbor, ME). The ATM-deficient strain was maintained via heterozygous crosses, and wild-type (wt) and heterozygous littermates were used as controls for the ATM-deficient group. Infections were performed by intranasal inoculation at 6 weeks of age with 104 PFU of MHV68 or sterile carrier in an inoculum volume of 15 μl per mouse. All experimental manipulations of mice used in this study were approved by the institutional Animal Care and Use Committee of the Medical College of Wisconsin (AUA971). Virus passage and titer determination were performed on NIH 3T12 cells as previously described (50).
Tissue culture.
NIH 3T12 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mM l-glutamine. Mouse embryonic fibroblasts were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 25 U/ml penicillin, 25 mg/ml streptomycin, 2.6 mM l-glutamine, and nonessential amino acids.
Flow cytometry and tetramer staining.
Single-cell suspensions were prepared in fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline [PBS], 2% fetal calf serum [FCS], 0.05% sodium azide) at 1 × 107 nucleated cells/ml. A total of 1 × 106 cells were prestained with Fc block (24G2) and then incubated with an optimal amount of antibody conjugate (eFluor450, fluorescein isothiocyanate, r-phycoerythrin [PE], PE-Cy7, or allophycocyanin). Antibodies to the following molecules were purchased from eBioscience (San Diego, CA): B220/CD45R (RA3-6B2), CD80/B7.1 (16-10A1), CD86/B7.2 (GL1), CD279/PD-1 (J43), CD3e (eBio500A2), CD8a/Ly-2 (53-6.7), CD4 (RM4-5), CD44 (IM7), and CD62L/Ly-22 (MEL-14). Armenian hamster IgG (eBio299Arm; eBioscience) was used as an isotype and fluorophore-matched control for all PD-1 cell surface staining. Allophycocyanin-conjugated major histocompatibility complex (MHC) class I tetramers specific for MHV68 epitopes Db/ORF6487-495 (AGPHNDMEI) and Kb/ORF61524-531 (TSINFVKI) were obtained from the NIH Tetramer Core Facility (Emory University, Atlanta, GA). Fc receptor-blocked cells were stained with tetramers for 30 min at room temperature, followed by staining with the appropriate antibodies listed above for 15 min on ice. Data acquisition was performed on an LSR II flow cytometer (BD Biosciences, Sparks, MD) and analyzed using FlowJo software (Tree Star, Ashland, OR).
Ex vivo restimulation and intracellular cytokine staining.
Cells were restimulated with 10 ng/ml phorbol 12-myristate 13-acetate (PMA) plus 1 μg/ml ionomycin (both from Sigma-Aldrich, St. Louis, MO) or 10 μg/ml of MHV68-specfic peptides ORF6487-495 (AGPHNDMEI) plus ORF61524-531 (TSINFVKI) (Thermo Fisher Scientific, Waltham, MA) for 4 to 6 h in the presence of 10 μg/ml brefeldin A (Sigma-Aldrich, St. Louis, MO). After staining cells for surface markers, cells were fixed and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions. Next, the cells were stained with antibodies specific to IFN-γ (clone XMG1.2; Biolegend, San Diego, CA), washed, and resuspended in FACS buffer prior to analysis.
Limiting-dilution assays.
Limiting-dilution ex vivo reactivation and nested PCR analyses were performed as previously described to measure the frequency of cells reactivating MHV68 or harboring the MHV68 genome, respectively (44).
Persistent-replication assay.
Individual lungs were harvested from infected mice and collected in 1 ml of DMEM. Lungs were thawed and homogenized using sterile 1.0-mm zirconia-silica beads and a Mini-Beadbeater-8 cell disrupter (both from BioSpec Products, Inc., Bartlesville, OK) for 2 min at 4°C. Serial dilutions of lung homogenates were plated on indicator mouse embryo fibroblast (MEF) monolayers (12 replicates for each dilution) and the presence of lytic virus in each replicate scored after 14 days of culture.
Quantitative PCR (qPCR).
Individual lung homogenates were incubated overnight at 56°C in the presence of 0.25 mg/ml proteinase K (Sigma-Aldrich, St. Louis, MO), and DNA was extracted twice with phenol-chloroform and once with chloroform. DNA was precipitated with sodium acetate (NaOAc)-ethanol (EtOH) and resuspended in TE buffer (10 mM Tris-HCl [pH 8.1], 1 mM EDTA). Viral DNA was quantified using the iQ5 real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA) with gene 50 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) primers (43), and normalized values were calculated using the ΔCT method.
Statistical analyses.
All data were analyzed using GraphPad Prism software (GraphPad Software, San Diego, CA). Frequencies for limiting-dilution assays were obtained from the cell number at which 63.2% of the wells scored positive for either reactivating virus or the presence of viral genome based on the Poisson distribution. Data were subjected to nonlinear regression analysis to obtain a single-cell frequency for each limiting-dilution analysis. All P values were calculated using Student's t test. Differences were considered significant when the P value was <0.05.
RESULTS
MHV68 latency is inadequately controlled in the peritoneums of ATM-deficient mice.
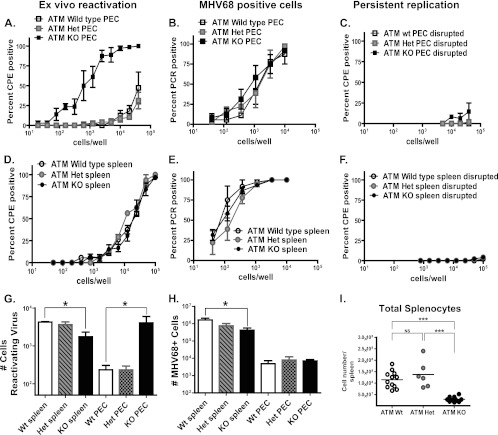
To assess parameters of chronic gammaherpesvirus infection in an ATM-deficient host, ATM-deficient mice (1) were infected with MHV68. In an immunocompetent host, MHV68 transitions from active replication to a latent state by 10 to 12 days of infection. Unlike lytic replication, latency is characterized by little if any viral gene expression and lack of infectious virion production. Poorly understood mechanisms enable a switch from latency to lytic replication (reactivation), resulting in release of infectious virions that reseed the reservoir of infected cells within the original host and allow viral transmission to a naïve host. Peak numbers of latently infected cells and MHV68 reactivation upon explantation is observed at around 16 to 18 days of infection at several anatomical sites, including the spleen and peritoneum (reviewed in reference 2). To determine whether ATM modulates establishment of MHV68 latency, ATM-deficient, heterozygous, and wild-type littermates were infected with MHV68 and MHV68 latency was assessed in splenocytes and peritoneal exudate cells (PEC) at 18 days postinfection. While the frequency and absolute numbers of MHV68 genome-positive PEC were similar in all experimental groups (Fig. 1B and H), every MHV68-positive peritoneal cell harvested from ATM-deficient mice reactivated MHV68 ex vivo, at least a 40-fold increase over the number of reactivating cells harvested from the peritoneums of ATM heterozygous and wild-type mice (1 in 1,096 PEC versus less than 1 in 40,000 PEC; P = 0.008) (Fig. 1A and G) (the percentage of reactivation could not be directly compared due to the low frequency of reactivation in wt PEC). Furthermore, low levels of lytic MHV68 were detected in ATM-deficient PEC immediately ex vivo (Fig. 1C), suggesting that persistent MHV68 replication and reactivation were not adequately controlled in the peritoneums of ATM-deficient mice.
Fig 1.
MHV68 latency is inadequately controlled in ATM-deficient mice. ATM-deficient (KO), heterozygous (Het), or wild-type mice were intranasally inoculated with 104 PFU of MHV68. Splenocytes and peritoneal exudate cells (PEC) were harvested at 18 days postinfection and pooled from 3 to 5 mice in each experimental group. (A to F) Frequencies of infected cells (B and E), ex vivo reactivation (A and D), and persistent replication (C and F) were determined using limiting-dilution assays. (G and H) Absolute numbers of reactivating cells (G) and MHV68-positive cells (H) per anatomic site were calculated using the frequencies determined in panels A, B, D, and E and absolute cell counts. (I) Numbers of splenocytes in MHV68-infected mice with the indicated genotypes at 18 days postinfection. Each symbol represents an individual spleen. For all panels, data were pooled from 3 or 4 independent experiments. *, P < 0.05; ***, P < 0.001; NS, P > 0.05.
The frequencies of MHV68 genome-positive splenocytes were similar in all experimental groups (Fig. 1E); however, the absolute number of MHV68-infected splenocytes was significantly lower in ATM-deficient mice than in wild-type controls (Fig. 1H). This was likely due to the decreased overall number of ATM-deficient splenocytes (Fig. 1I). Similar observations were made with respect to the frequency and absolute number of splenocytes reactivating MHV68 ex vivo (Fig. 1D and G) (the percentage of reactivating splenocytes ranged from 0.23% in wt mice to 0.48% of all splenocytes in ATM-deficient mice). No preformed lytic MHV68 was detected in the splenocytes harvested from any of the three experimental groups (Fig. 1F). Thus, global ATM deficiency led to poorly controlled MHV68 latency manifested as increased reactivation and persistent replication of MHV68 in the peritoneums, but not the spleens, of infected mice. Collectively, the differences in the MHV68 infection parameters between spleens and peritoneums of ATM-deficient mice could reflect cell type-specific effects of ATM in vivo, as the cell types hosting latent MHV68 differ at the two anatomic sites. Specifically, B cells represent the major cellular reservoir for MHV68 in the spleen, whereas macrophages are the predominant cell type harboring latent MHV68 in the peritoneum (41, 51, 52).
High levels of persistent MHV68 replication are found in ATM-deficient lungs.
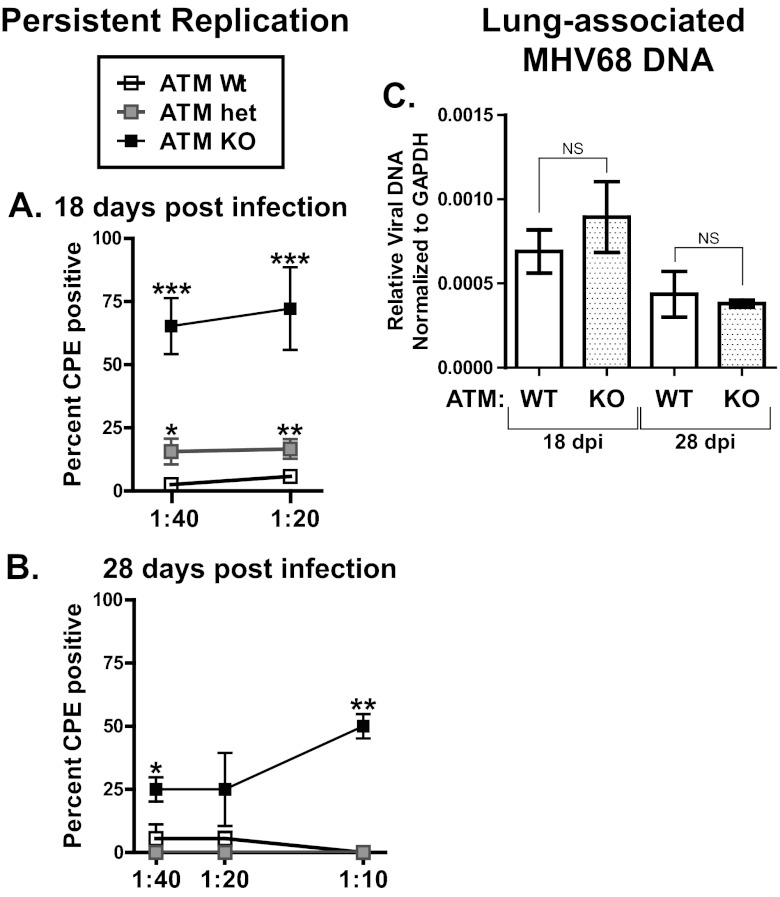
Having determined that MHV68 latency was altered in the peritoneums of ATM-deficient mice, persistent MHV68 replication was measured in lungs, another site of gammaherpesvirus latency in rodents (21). By 18 days postinfection, several cell types host latent MHV68 in the lung, with very little infectious MHV68 present in the lungs of chronically infected wild-type mice (25, 26). Importantly, persistent MHV68 replication in the lungs is associated with chronic immune stimulation and lung disease in immunocompromised mouse models (12, 34, 35). The presence of infectious MHV68, a marker of persistent viral replication, was measured in lung homogenates from chronically infected mice using a semiquantitative assay that has been optimized to provide maximum sensitivity in detecting preformed infectious virus. Consistent with previous reports, low levels of infectious MHV68 were detected in the lungs of wild-type mice at 18 days postinfection (Fig. 2A). The lungs of ATM-heterozygous mice contained a modest increase in levels of infectious MHV68. In contrast, the lungs of ATM-deficient mice exhibited a significant increase in the levels of lytic MHV68 compared to those in the wild-type and heterozygous groups (Fig. 2A). High levels of persistent MHV68 replication were present as late as 28 days postinfection in ATM-deficient mice (Fig. 2B). Similar levels of lung-associated MHV68 DNA were detected in both experimental groups at 18 and 28 days postinfection (Fig. 2C), suggesting that only a small proportion of MHV68-infected cells in the lung supported persistent viral replication. Thus, ATM was important for control of persistent MHV68 replication in the lungs of chronically infected ATM-deficient mice.
Fig 2.
Persistent MHV68 replication in ATM-deficient lungs. Lungs were harvested at 18 or 28 days postinfection (dpi) with 104 PFU MHV68. (A and B) Serial dilutions of lung homogenates were plated on indicator MEF monolayers and the presence of lytic virus scored in each replicate after 14 days of culture. Data were pooled from 10 wild-type mice, 8 ATM-heterozygous, and 6 ATM KO mice at 18 days postinfection and from 3 mice per experimental group at 28 days postinfection. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (for each indicated dilution compared to the wild-type group). (C) DNA was isolated from lung homogenates and subjected to qPCR analysis for quantification of MHV68-specific DNA. Data represent the mean and standard error from analysis of 4 mice per experimental group at 18 days postinfection and 3 mice per group at 28 days postinfection. NS, P > 0.05.
ATM-deficient T and B cells respond to MHV68 infection by increasing absolute cell numbers and upregulating activation markers.
The increased levels of MHV68 reactivation and persistent replication observed in ATM-deficient mice (Fig. 1 and 2) were in contrast to the attenuated MHV68 infection observed in mice lacking H2AX, an important ATM substrate (44). An altered immune response in the absence of ATM could account for the different MHV68 phenotypes observed in ATM- and H2AX-deficient mice. B cells and CD4+ and CD8+ T cells collaborate to attain control of chronic MHV68 infection (reviewed in reference 2). To determine whether ATM expression influenced the abundance of splenic T and B cells during chronic MHV68 infection, T and B cell numbers were measured in splenocytes harvested from ATM-deficient, heterozygous, and wild-type mice at 18 days postinfection or from mock-infected controls.
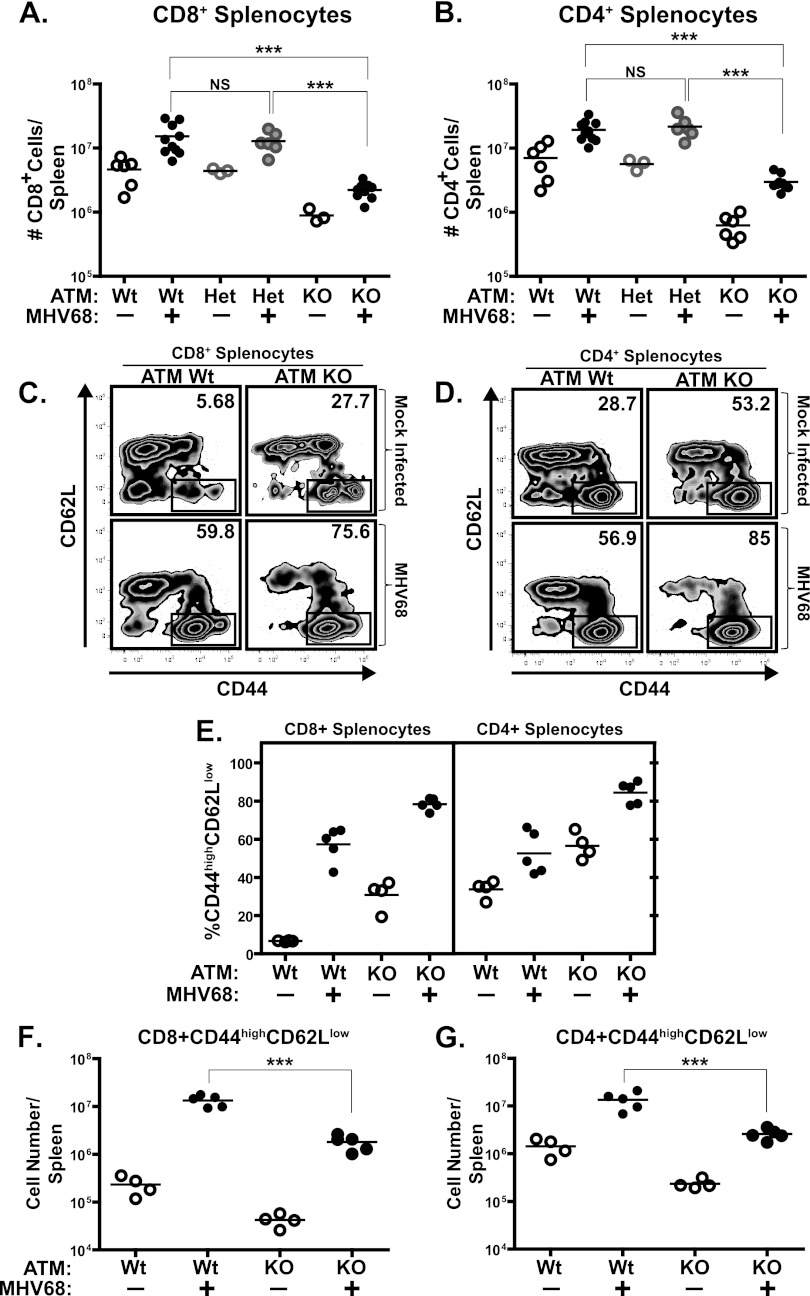
In accordance with previous reports (1), significantly lower numbers of splenic CD4+ and CD8+ T cells were observed in ATM-deficient mice than in wild-type littermates in the absence of MHV68 infection (14-fold and 7-fold decreases for the CD4+ and CD8+ populations, respectively) (Fig. 3A and B). The abundance of splenic CD4+ T cells, CD8+ T cells, and B cells and their activation status increases in MHV68-infected wild-type mice, with peak numbers observed at between 16 and 28 days postinfection (5, 39). Similar to wild-type and heterozygous controls, ATM-deficient mice displayed an increase in absolute numbers of splenic CD4+ and CD8+ T cells at 18 days postinfection, yet these numbers remained below those in infected wild-type mice (Fig. 3A and B).
Fig 3.
T cell numbers and activation status in ATM-deficient mice. ATM-deficient, heterozygous, or wild-type mice were intranasally inoculated with 104 PFU of MHV68 or carrier solution. Splenocytes were harvested at 18 days postinfection and stained with either anti-CD4 or anti-CD8 antibody (A and B) or in a tristain cocktail with the addition of anti-CD44 and anti-CD62L (C to G). Panels C and D are profiles of CD8- or CD4-gated splenocytes from individual spleens and are representative of at least 2 independent experiments (values in upper right corners indicate the percentage of CD44high CD62Llow splenocytes). Panels A, B, and E to G represent pooled data from 2 or 3 independent experiments, where floating bars represent the mean and each symbol represents an individual spleen. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, P > 0.05.
In addition to an effect on T cell development, ATM deficiency may also impair T cell activation (16). Thus, the effect of ATM deficiency on activation of CD4+ and CD8+ T cells was examined at 18 days postinfection. In the absence of infection, a higher proportion of ATM-deficient T cells exhibited markers of activation (CD44hi CD62low) (Fig. 3C to E), consistent with a marked predominance of effector memory T cells in the peripheral blood of A-T patients (17). The percentage of activated CD4+ and CD8+ T cells further increased upon MHV68 infection in both wild-type and ATM-deficient mice (Fig. 3C to E). In spite of the increased proportion of activated T cells, the absolute numbers of activated CD8+ and CD4+ splenic T cells were decreased in MHV68-infected ATM-deficient mice (Fig. 3F and G), likely due to the overall decrease in the number of ATM-deficient T cells (Fig. 3A and B).
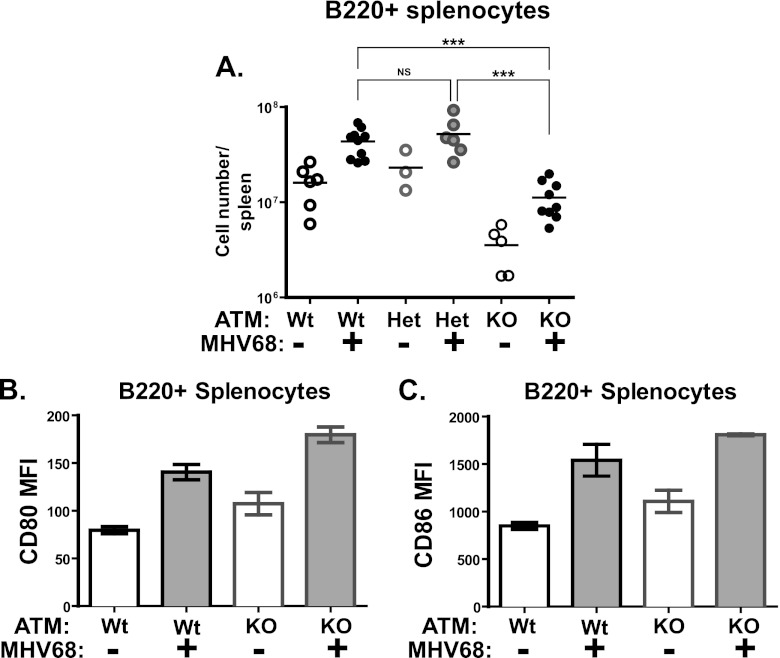
Similar to T cells, the number of splenic B cells was decreased in ATM-deficient naïve mice compared to wild-type and heterozygous controls (Fig. 4A). MHV68 infection stimulated an increase in the absolute number of B cells in mice of all three genotypes. Furthermore, MHV68-driven activation of B cells, as determined by surface expression of CD80 and CD86, was not affected by ATM deficiency (Fig. 4B and C). Thus, ATM deficiency did not abolish the ability of splenic B and T cells to respond to gammaherpesvirus infection, as judged by a relative increase in immune cell numbers and changes in activation markers.
Fig 4.
B cell numbers and activation status in ATM-deficient mice. ATM-deficient, heterozygous, or wild-type mice were intranasally inoculated with 104 PFU of MHV68 or sterile carrier solution (mock). (A) Absolute numbers of B220+ splenocytes at 18 days postinfection. Each symbol represents an individual spleen; data were pooled from 2 or 3 independent experiments. (B and C) Mean fluorescent intensity of CD80 and CD86 staining of B220+ splenocytes at 18 days postinfection. Data were pooled from 2 or 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, P > 0.05.
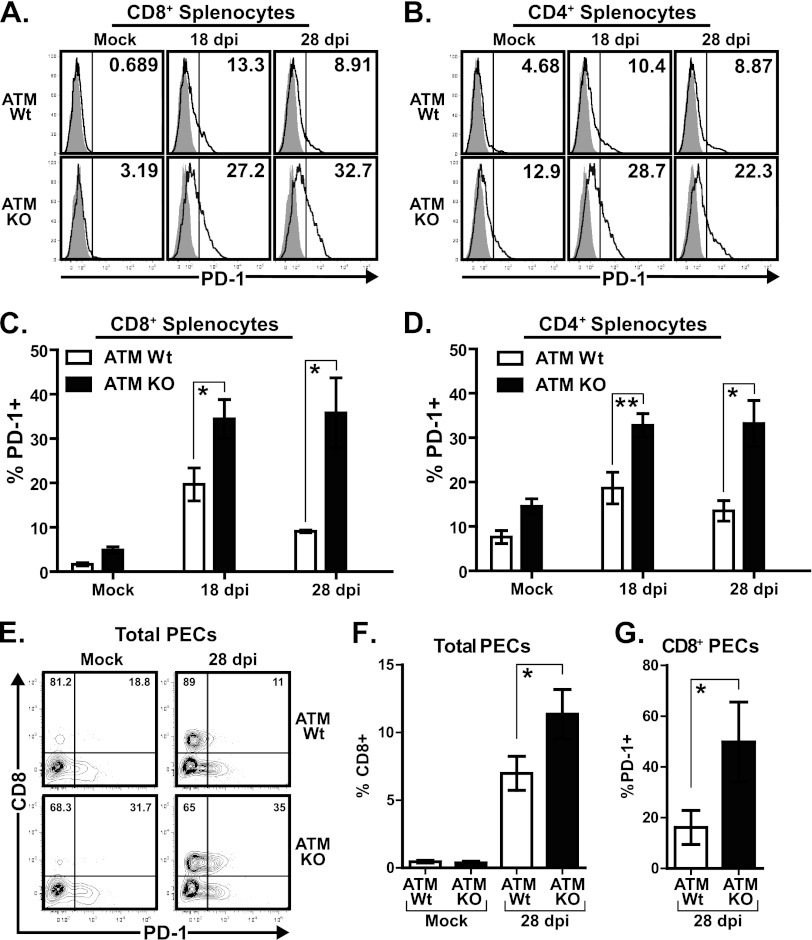
T cells of MHV68-infected ATM-deficient mice increase PD-1 expression.
The persistent MHV68 replication was likely to mediate chronic immune stimulation in ATM-deficient mice. Importantly, chronic immune stimulation can induce T cell exhaustion, characterized by a progressive decrease in T cell function concurrent with upregulation of inhibitory receptors (reviewed in reference 49). PD-1 is a prominent inhibitory receptor associated with T cell exhaustion in several chronic viral infections, including HIV, hepatitis C virus (HCV), and lymphocytic choriomeningitis virus (LCMV) infection (reviewed in reference 23). Furthermore, the PD-1 receptor is upregulated on CD8+ T cells of class II-deficient MHV68-infected mice, and PD-1 signaling contributes to uncontrolled persistent MHV68 replication in the lungs of these mice (9).
To determine whether ATM-deficient T cells increased PD-1 expression upon MHV68 infection, we assessed levels of PD-1 on CD4+ and CD8+ T cells from wild-type or ATM-deficient mice. The proportions of splenic CD8+ and CD4+ T cells with increased cell surface PD-1 levels peaked in wild-type mice at 18 days postinfection (19.7% ± 3.7% of CD8+ and 18.66% ± 3.6% of CD4+ cells) (Fig. 5A to D). Interestingly, the proportions of PD-1-expressing CD8+ and CD4+ T cells were further increased in ATM-deficient mice compared to wild-type littermates at 18 days postinfection (34.47% ± 4.3% and 32.77% ± 2.7% of CD8+ and CD4+ ATM-deficient T cells, respectively) (Fig. 5A to D).
Fig 5.
T cells of MHV68-infected ATM-deficient mice increase PD-1 expression. ATM-deficient or wild-type mice were intranasally inoculated with 104 PFU of MHV68 or carrier solution (mock). Splenocytes or peritoneal exudate cells (PEC) were harvested at 18 or 28 days postinfection (dpi), stained with anti-PD1 in combination with either anti-CD8 or CD4 antibodies, and analyzed by flow cytometry. (A and B) Representative histograms show PD-1 gating of each experimental group (shaded, isotype control; solid line, anti-PD-1). Values in the upper right corners correspond to the percentage of CD8+ or CD4+ splenocytes positive for anti-PD-1 staining as determined by the gate shown (vertical line). (C and D) Percentage of CD4- or CD8-positive cells expressing cell surface PD-1 using the gate shown in panels A and B. Data are pooled from 3 to 5 mice per group from 3 independent experiments and are shown as the mean and standard error. (E) Representative flow cytometry plots showing anti-CD8 and anti-PD-1 staining in peritoneal exudate cells (PEC), where values in the upper left and upper right quadrants correspond to the percentage of CD8+ PEC that are either negative or positive for PD-1, respectively. (F and G) Pooled data from at least 4 independent experiments (4 to 6 mice per group) showing the percentage of total PEC that are positive for CD8 (F) or the percentage of CD8+ PEC that are positive for PD-1 (G) based on the gates shown in panel E. *, P < 0.05; **, P < 0.01.
T cells are known to transiently upregulate PD-1 surface expression in response to acute viral infection, with subsequent downregulation of PD-1 upon clearance of replicating virus (53). Importantly, T cell PD-1 levels remain elevated during chronic antigen stimulation. To rule out effects of acute MHV68 replication on PD-1 expression, percentages of PD-1-positive CD8+ and CD4+ T cells were measured in wild-type and ATM-deficient mice at 28 days postinfection. As expected, the percentages of PD-1 positive CD8+ and CD4+ T cells in wild-type mice decreased between 18 and 28 days postinfection (from 19.7% ± 3.7% to 9.09% ± 0.3% and from 18.66% ± 3.6% to 13.51% ± 2.3%, respectively) (Fig. 5A to D). However, the percentage of PD-1-positive T cells remained elevated at 28 days postinfection in ATM-deficient mice (35.8% ± 7.9% CD8+ T cells and 33.19% ± 5.2% CD4+ T cells) (Fig. 5A to D).
Splenic MHV68 infection was well controlled in wild-type and ATM-deficient mice (Fig. 1D to F), in spite of significant differences in the proportions of PD-1-positive splenic CD8+ and CD4+ T cells observed between the two genotypes (Fig. 5C and D). Because MHV68 reactivation was increased in the peritoneums of ATM-deficient mice (Fig. 1A), PD-1 expression was measured in peritoneal CD8+ T cells. CD8+ T cells represented a very small proportion of the overall peritoneal exudate in uninfected mice (Fig. 5E and F). Upon MHV68 infection, a significant increase in the frequency of CD8+ T cells was observed in the peritoneums of wild-type and ATM-deficient mice (Fig. 5E and F). Similar to that observed in the spleens, the proportion of PD-1-positive peritoneal CD8+ T cells was increased in ATM-deficient mice (Fig. 5G). Thus, the population of PD-1-positive CD8+ T cells was increased in both peritoneums and spleens of ATM-deficient mice. However, this global increase in the PD-1-positive CD8+ T cell population did not correlate with the status of MHV68 infections which was well controlled in the spleens but not in the peritoneums of ATM-deficient animals (Fig. 1).
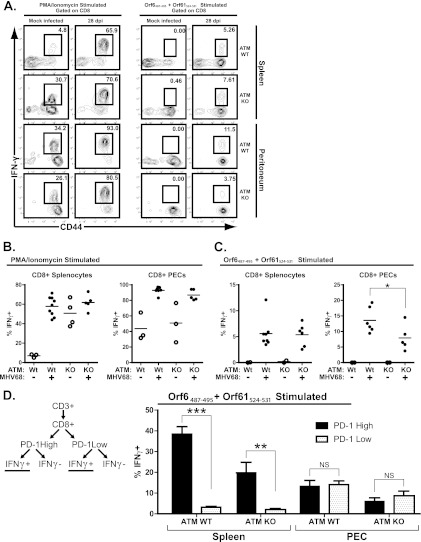
ATM-deficient mice exhibit an aberrant MHV68-specific CD8+ T cell response.
In spite of a global increase in numbers of splenic T cells in response to MHV68 infection and changes in activation markers, these responses were inadequate to control MHV68 reactivation and persistent replication in the peritoneums and lungs of ATM-deficient mice (Fig. 1 and 2). Because suppression of MHV68 reactivation and persistent replication by CD8+ T cells requires gamma interferon-dependent mechanisms (31, 47), IFN-γ responses of MHV68-specific T cells were examined. Two distinct waves of MHV68-specific CD8+ T cells have been characterized. The first wave of MHV-specific CD8 T cells (represented by the immunodominant ORF6487-495 peptide-specific response) predominates early in infection and rapidly declines following clearance of acute replication. The second wave of CD8 T cells (represented by the ORF61524-531 response) continues to expand throughout early latency in a manner that is dependent upon continued antigenic stimulation (15).
To assess the capacity for IFN-γ production by MHV68-specific CD8+ T cells, splenocytes and PEC were harvested from ATM-deficient mice or wild-type littermates that were mock infected or infected with MHV68 for 28 days. Cell suspensions were restimulated ex vivo with two representative immunodominant epitopes (ORF6487-495 and ORF61524-531 peptide) or PMA-ionomycin (positive control), followed by assessment of intracellular IFN-γ production. PMA-ionomycin stimulation of splenic CD8+ T cells elicited a higher proportion of IFN-γ-positive cells in ATM-deficient mock-infected mice than in mock-infected wild-type animals (Fig. 6A and B), consistent with the increased baseline proportion of CD62low CD44high CD8+ T cells in ATM-deficient mice (Fig. 3E). Importantly, similar proportions of CD8+ T cells upregulated IFN-γ expression in response to PMA-ionomycin in wild-type and ATM-deficient MHV68-infected mice (Fig. 6A and B), suggesting that ATM-deficient T cells from MHV68-infected animals were not refractory to further stimulation.
Fig 6.
Fewer peritoneal CD8+ T cells from infected ATM-deficient mice produce IFN-γ upon MHV68 peptide restimulation. ATM-deficient or wild-type mice were intranasally inoculated with 104 PFU of MHV68 or carrier solution (mock infected). Splenocytes or peritoneal exudate cells (PEC) were harvested at 28 days postinfection (dpi) and restimulated ex vivo with either PMA-ionomycin or ORF6487-495 plus ORF61524-531 peptide for 5 h before surface staining with anti-CD8, anti-CD44, and anti-PD-1 and intracellular staining with anti-IFN-γ for analysis by flow cytometry. (A) Representative flow cytometry plots showing CD44 and IFN-γ staining of CD8+-gated cells, where values in the upper right corners represent the percentage of CD8+ cells within the gate shown. (B and C) Combined data from at least 4 independent experiments showing the percentage of CD8+ cells positive for intracellular IFN-γ staining based on the gating strategy shown in panel A. Floating bars represent the mean, and each symbol represents an individual mouse. (D) Pooled data from at least 4 independent experiments (4 to 6 mice per experimental group) showing the mean and standard error of the percentage of CD8+ PD-1-high or CD8+ PD-1-low populations that produce IFN-γ upon ex vivo restimulation with MHV68 peptides (as determined by the gating strategy shown in the schematic). *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, P > 0.05.
As expected, specific stimulation with MHV68 immunodominant epitopes elicited very few IFN-γ-expressing CD8+ T cells in mock-infected mice (Fig. 6A and C). Similar proportions of ATM-deficient and wild-type splenic CD8+ T cells from MHV68-infected mice increased IFN-γ expression in response to stimulation with MHV68 peptides (Fig. 6A and C). In contrast, fewer peritoneal CD8+ T cells produced IFN-γ in response to MHV68 peptide stimulation in ATM-deficient mice (Fig. 6C). Thus, IFN-γ-dependent MHV68-specific CD8+ T cell responses were decreased in the peritoneums of ATM-deficient mice, consistent with inadequate control of MHV68 reactivation from PEC (Fig. 1A).
Because PD-1 expression by CD8+ T cells was elevated in MHV68-infected mice of either genotype (Fig. 5) and due to the potential inhibitory signaling mediated by this receptor, which would decrease IFN-γ production, we wanted to determine whether IFN-γ-positive CD8+ T cells responding to MHV68 peptides would be found preferentially within the PD-1-low population of CD8+ T cells. CD8+ T cells isolated from infected animals were stimulated with MHV68 peptides and stained with antibodies as indicated in Fig. 6D. To determine if PD-1 expression negatively correlates with IFN-γ production upon restimulation, CD8+ T cells were further gated into PD-1-high and -low populations and the percentage of IFN-γ-positive cells in each population was determined (Fig. 6D). Interestingly, in the spleen, a larger proportion of PD-1-high CD8+ T cells responded to MHV68 peptide stimulation regardless of the genotype. Furthermore, similar proportions of peritoneal PD-1-high and -low CD8+ T cells responded to MHV68 peptide stimulation in wild-type and ATM-deficient mice. Thus, IFN-γ-producing MHV68-specific CD8+ T cells did not preferentially associate with the PD-1-low population, indicating that PD-1 expression was unlikely to serve as a marker for a dysfunctional CD8+ T cell population in MHV68-infected wild-type and ATM-deficient mice.
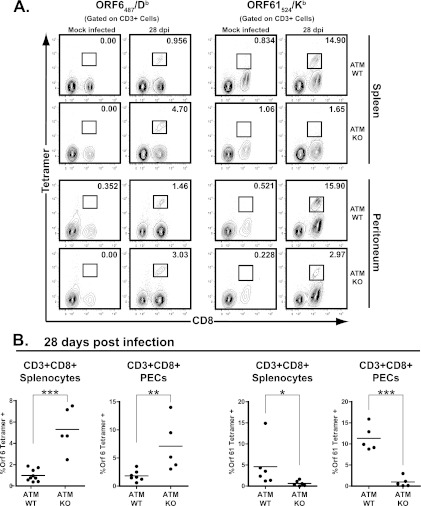
To gain further insight into the antiviral CD8+ T cell response in the context of ATM deficiency, MHC class I tetramers were used to measure the ORF6487/Db and ORF61524/Kb antigen-specific CD8+ T cell populations in MHV68-infected mice. As expected, minimal staining of splenocytes and PEC with either tetramer was observed in uninfected mice of either genotype (Fig. 7A). Surprisingly, a 2-fold increase in the proportion of MHV68 ORF6487-495 tetramer-positive CD8+ splenocytes and CD8+ PEC was observed in ATM-deficient compared to wild-type mice at 28 days postinfection (Fig. 7B). Consistent with previous reports (18), a higher proportion of CD8+ T cells were positive for MHV68 ORF61524-531 tetramer staining than for the MHV68 ORF6487-495 tetramer in wild-type mice (Fig. 7B). In contrast, a significantly lower population of CD8+ T cells was MHV68 ORF61524-531 tetramer positive in ATM-deficient mice in both the spleen and peritoneum (Fig. 7B). Thus, ATM deficiency led to a skewed CD8+ T cell response against immunodominant MHV68 epitopes. Taken together, our findings suggest that the generation of a functional MHV68-specific adaptive immune response is compromised in ATM-deficient mice.
Fig 7.
ATM-deficient mice exhibit a skewed proportion of MHV68-specific CD8+ T cells. ATM-deficient or wild-type mice were intranasally inoculated with 104 PFU of MHV68 or carrier solution (mock infected). Splenocytes or peritoneal exudate cells (PEC) were harvested at 28 days postinfection (dpi) and stained with antibodies against CD3 and CD8 in combination with either ORF6487/Db or ORF61524/Kb MHC class I tetramers for analysis by flow cytometry. (A) Representative flow cytometry plots showing anti-CD8 and MHC class I tetramer staining of CD3+-gated cells, where values in the upper right corner of each plot represent the percentage of CD3+CD8+ cells within the gate shown. (B) Pooled data from at least 4 independent experiments showing the percentage of CD3+ CD8+ cells positive for MHC class I tetramer staining based on the gating strategy shown in panel A. Floating bars represent the mean, and each symbol represents an individual mouse. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
This study presents the first analysis of a chronic virus infection in an ATM-deficient host. Specifically, we found that gammaherpesvirus latency was poorly controlled in a mouse model of ataxia-telangiectasia (A-T), a human disease associated with ATM insufficiency. Interestingly, the parameters of chronic gammaherpesvirus infection in ATM-deficient mice differed in the spleen and peritoneum, where distinct cell types support MHV68 latency (B cells and macrophages, respectively). These results suggest that ATM has cell type-specific roles in the regulation of gammaherpesvirus infection. Importantly, ATM expression was important for the generation of a robust immune response against MHV68, including the development of the proper repertoire of antiviral CD8+ T cells.
Implications for A-T and other DDR-linked diseases.
Here we demonstrate that chronic gammaherpesvirus infection was poorly controlled in a mouse model of A-T. This is in contrast to a recent study that showed comparable kinetics of LCMV clearance in ATM-deficient and wild-type mice (10), indicating that ATM deficiency does not lead to uniform susceptibility to virus infection. Thus, similar to what is observed in human A-T, ATM-deficient mice appear to be selectively susceptible to gammaherpesvirus infections. Aberrant immune responses are likely to contribute to increased susceptibility to herpesvirus infection in A-T patients. Using a mouse model of A-T, this study demonstrated skewed development of MHV68-specific CD8+ T cells under conditions of ATM deficiency. Specifically, the proportion of CD8+ T cells specific for an immunodominant ORF61524-531 epitope was significantly decreased in ATM-deficient mice at 28 days postinfection. It is not clear whether this reflects alterations in the T cell receptor (TCR) repertoire, similar to that seen in A-T patients (17), or an aberrant pattern of MHV68 gene expression in the absence of ATM. A comprehensive analysis of ATM-deficient CD8 T cell responses to multiple MHV68 epitopes is an important future direction.
Our studies also revealed that increased PD-1 levels do not correlate with decreased T cell responses and control of MHV68, indicating that PD-1 is unlikely to be a marker of T cell exhaustion in ATM-deficient mice (Fig. 6D). Because T cell activation increases expression of PD-1 and the proportion of antigen-experienced effector memory T cells is increased in A-T patients (17) and ATM-deficient mice (Fig. 3), PD-1 may simply be a marker of deregulated T cell activation in the absence of ATM. It is also possible that other inhibitory receptors, such as CTLA-4 and LAG-3, play a role in attenuated T cell responses against MHV68 infection in the absence of ATM, a possibility that will be explored in future studies.
ATM-deficient mice displayed high levels of persistent MHV68 replication in the lungs during chronic infection. High levels of persistent MHV68 replication in lungs are associated with lung disease in other immunocompromised mouse models, such as gamma interferon receptor-deficient mice (11, 12, 47). Thus, future studies are needed to determine whether persistent MHV68 replication is sufficient to induce lung pathology in ATM-deficient mice, which, unlike humans with A-T, do not spontaneously develop lung disease. While A-T patients display altered anti-EBV antibody responses suggestive of persistent viral replication (4, 22, 36), the status of EBV infection is poorly understood, and it is not known whether EBV contributes to the lung disease observed in A-T patients. The clinical finding that steroids, but not antibiotic treatment, suppress or even reverse lung deterioration in A-T patients (33, 40) suggests that chronic immune stimulation that may be driven in part by virus infections does contribute to lung disease in A-T. Future studies are needed to define the relative contribution of chronic virus infections to the development and progression of lung disease in A-T patients.
Similar to the case for humans with A-T, ATM-deficient mice spontaneously develop lymphomas, a phenotype capitalized on by many research groups in an attempt to understand lymphomagenesis in human A-T. Intriguingly, other mouse models of human cancer-associated DDR-related diseases, such as Neijmegen breakage syndrome and ataxia-telangiectasia-like disorder, do not spontaneously develop cancer (46, 54), indicating that other factors may synergize with hypomorphic DDR alleles to induce tumorigenesis in humans. Unlike animals housed in pathogen-free research facilities, humans harbor a multitude of chronic to lifelong viral infections that reset the host immune status (49). It is tempting to speculate that chronic viral infections may modify disease in DDR-insufficient hosts, who frequently display various degrees of immune deficiencies.
DDR-gammaherpesvirus interactions.
Gammaherpesviruses have a complex relationship with the host DNA damage response. Specifically, MHV68-encoded orf36 induces ATM activation and H2AX phosphorylation in infected macrophages, and the virus relies on ATM and H2AX expression to facilitate its replication in vitro (37, 42). Furthermore, efficient expression of MHV68 RTA, a critical regulator of lytic infection and reactivation, requires H2AX, an ATM substrate (37). In vivo, MHV68 chronic infection is attenuated in H2AX-deficient mice (44). Because gammaherpesvirus reactivation is important for the maintenance of the infected cell reservoir in vivo (19, 30), it is possible that MHV68 usurps DDR components to facilitate its reactivation from latency. This possibility is corroborated by the finding that MHV68 orf36, a protein kinase necessary and sufficient to activate the DDR in the context of lytic infection, is required for optimal MHV68 latency and reactivation (42, 44).
The dependence of MHV68 on the components of the DDR for efficient replication and viral gene expression contrasts with the increased reactivation and persistent replication of MHV68 in ATM-deficient mice reported in this study. Because the immune response is the single most powerful factor controlling chronic MHV68 infection in vivo, the suboptimal immune response in ATM-deficient mice may allow for increased MHV68 reactivation and persistent replication, in spite of the inability of the virus to fully utilize the host DDR to facilitate viral processes within infected cells. Because of pleiotropic ATM effects, mice with tissue-specific ATM deficiency should serve as valuable tools to dissect the extrinsic and intrinsic roles of ATM in chronic gammaherpesvirus infection.
We and others have recently shown that the physiological DDR leads to induction of antiviral type I IFN responses (6, 32), adding another important factor to the gammaherpesvirus-DDR equation. Because type I IFN suppresses MHV68 replication and reactivation in vivo (3, 20) and due to virus-driven activation of the DDR in infected cells (42), it is not surprising that MHV68 uncouples the DDR-type I IFN connection in the context of lytic infection of primary macrophages (32). Because virus-driven DDR induction is not limited to the MHV68 system, in the future it will be important to define the mechanism by which MHV68 interferes with type I IFN responses induced upon activation of the DDR.
Previous studies of DDR-virus interactions in the context of lytic infection have provided and continue to offer important insights into the regulation of virus replication by this sophisticated cellular network. In the future, it will be of great interest to extend these findings to in vivo studies, using mice insufficient in ATM and other DDR components, to determine the effects of DDR-virus interactions on viral persistence and pathogenesis, especially in the context of a DDR-insufficient host.
ACKNOWLEDGMENTS
Expert technical and managerial support was provided by Brittani Wood. We thank John Routes as well as Amy Hudson's and William Jackson's research groups for helpful discussions.
This work was supported by the Medical College of Wisconsin, Children's Research Institute (S.B.G.), and the Concern Foundation, an American Cancer Society Research Scholar Award (RSG-12-174-01-MPC), Advancing Healthier Wisconsin, and the Medical College of Wisconsin Cancer Center (V.L.T.).
Footnotes
Published ahead of print 19 September 2012
REFERENCES
- 1. Barlow C, et al. 1996. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86:159–171 [DOI] [PubMed] [Google Scholar]
- 2. Barton E, Mandal P, Speck SH. 2011. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu. Rev. Immunol. 29:351–397 [DOI] [PubMed] [Google Scholar]
- 3. Barton ES, Lutzke ML, Rochford R, Virgin HW. 2005. Alpha/beta interferons regulate murine gammaherpesvirus latent gene expression and reactivation from latency. J. Virol. 79:14149–14160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berkel AI, et al. 1979. Epstein-Barr virus-related antibody patterns in ataxia-telangiectasia. Clin. Exp. Immunol. 35:196–201 [PMC free article] [PubMed] [Google Scholar]
- 5. Brooks JW, et al. 1999. Requirement for CD40 ligand, CD4(+) T cells, and B cells in an infectious mononucleosis-like syndrome. J. Virol. 73:9650–9654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brzostek-Racine S, Gordon C, Van Scoy S, Reich NC. 2011. The DNA damage response induces IFN. J. Immunol. 187:5336–5345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cesarman E. 2011. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett. 305:163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crawford TO, Skolasky RL, Fernandez R, Rosquist KJ, Lederman HM. 2006. Survival probability in ataxia telangiectasia. Arch. Dis. Child. 91:610–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dias P, et al. 2010. CD4 T-cell help programs a change in CD8 T-cell function enabling effective long-term control of murine gammaherpesvirus 68: role of PD-1-PD-L1 interactions. J. Virol. 84:8241–8249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D'Souza AD, Parish IA, McKay SE, Kaech SM, Shadel GS. 2011. Aberrant CD8 T-cell responses and memory differentiation upon viral infection of an ataxia-telangiectasia mouse model driven by hyper-activated Akt and mTORC1 signaling. Am. J. Pathol. 178:2740–2751 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Dutia BM, Clarke CJ, Allen DJ, Nash AA. 1997. Pathological changes in the spleens of gamma interferon receptor-deficient mice infected with murine gammaherpesvirus: a role for CD8 T cells. J. Virol. 71:4278–4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ebrahimi B, Dutia BM, Brownstein DG, Nash AA. 2001. Murine gammaherpesvirus-68 infection causes multi-organ fibrosis and alters leukocyte trafficking in interferon-gamma receptor knockout mice. Am. J. Pathol. 158:2117–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Efstathiou S, et al. 1990. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J. Gen. Virol. 71:1365–1372 [DOI] [PubMed] [Google Scholar]
- 14. Folgori L, et al. 2010. Cutaneous granulomatosis and combined immunodeficiency revealing ataxia-telangiectasia: a case report. Ital. J. Pediatr. 36:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freeman ML, et al. 2010. Two kinetic patterns of epitope-specific CD8 T-cell responses following murine gammaherpesvirus 68 infection. J. Virol. 84:2881–2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia-Perez MA, et al. 2001. Novel mutations and defective protein kinase C activation of T-lymphocytes in ataxia telangiectasia. Clin. Exp. Immunol. 123:472–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giovannetti A, et al. 2002. Skewed T-cell receptor repertoire, decreased thymic output, and predominance of terminally differentiated T cells in ataxia telangiectasia. Blood 100:4082–4089 [DOI] [PubMed] [Google Scholar]
- 18. Gredmark-Russ S, Cheung EJ, Isaacson MK, Ploegh HL, Grotenbreg GM. 2008. The CD8 T-cell response against murine gammaherpesvirus 68 is directed toward a broad repertoire of epitopes from both early and late antigens. J. Virol. 82:12205–12212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoshino Y, et al. 2009. Long-term administration of valacyclovir reduces the number of Epstein-Barr virus (EBV)-infected B cells but not the number of EBV DNA copies per B cell in healthy volunteers. J. Virol. 83:11857–11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hwang S, et al. 2009. Conserved herpesviral kinase promotes viral persistence by inhibiting the IRF-3-mediated type I interferon response. Cell Host. Microbe 5:166–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hwang S, et al. 2008. Persistent gammaherpesvirus replication and dynamic interaction with the host in vivo. J. Virol. 82:12498–12509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joncas JH, Wills A, Reece E, Fox Z. 1981. Epstein-Barr virus antibodies in patients with ataxia-telangiectasia and other immunodeficiency diseases. Can. Med. Assoc. J. 125:845–849 [PMC free article] [PubMed] [Google Scholar]
- 23. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. 2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26:677–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koopal S, et al. 2007. Viral oncogene-induced DNA damage response is activated in Kaposi sarcoma tumorigenesis. PLoS Pathog. 3:e140 doi:10.1371/journal.ppat.0030140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krug LT, Collins CM, Gargano LM, Speck SH. 2009. NF-kappaB p50 plays distinct roles in the establishment and control of murine gammaherpesvirus 68 latency. J. Virol. 83:4732–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krug LT, Moser JM, Dickerson SM, Speck SH. 2007. Inhibition of NF-kB activation in vivo impairs establishment of gammaherpesvirus latency. PLoS Pathog. 3:e11 doi:10.1371/journal.ppat.0030011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kudoh A, et al. 2005. Epstein-Barr virus lytic replication elicits ATM checkpoint signal transduction while providing an S-phase-like cellular environment. J. Biol. Chem. 280:8156–8163 [DOI] [PubMed] [Google Scholar]
- 28. Kudoh A, et al. 2009. Homologous recombinational repair factors are recruited and loaded onto the viral DNA genome in Epstein-Barr virus replication compartments. J. Virol. 83:6641–6651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lavin MF. 2008. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat. Rev. Mol. Cell Biol. 9:759–769 [DOI] [PubMed] [Google Scholar]
- 30. Liang X, Collins CM, Mendel JB, Iwakoshi NN, Speck SH. 2009. Gammaherpesvirus-driven plasma cell differentiation regulates virus reactivation from latently infected B lymphocytes. PLoS Pathog. 5:e1000677 doi:10.1371/journal.ppat.1000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loh J, Thomas DA, Revell PA, Ley TJ, Virgin HW. 2004. Granzymes and caspase 3 play important roles in control of gammaherpesvirus latency. J. Virol. 78:12519–12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mboko WP, et al. 2012. Coordinate regulation of DNA damage and type I interferon responses imposes an antiviral state that attenuates mouse gammaherpesvirus type 68 replication in primary macrophages. J. Virol. 86:6899–6912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McGrath-Morrow SA, et al. 2010. Evaluation and management of pulmonary disease in ataxia-telangiectasia. Pediatr. Pulmonol. 45:847–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mora AL, et al. 2007. Control of virus reactivation arrests pulmonary herpesvirus-induced fibrosis in IFN-gamma receptor-deficient mice. Am. J. Respir. Crit. Care Med. 175:1139–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mora AL, et al. 2005. Lung infection with gamma-herpesvirus induces progressive pulmonary fibrosis in Th2-biased mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 289:L711–L721 [DOI] [PubMed] [Google Scholar]
- 36. Morio T, et al. 2009. Phenotypic variations between affected siblings with ataxia-telangiectasia: ataxia-telangiectasia in Japan. Int. J. Hematol. 90:455–462 [DOI] [PubMed] [Google Scholar]
- 37. Mounce BC, et al. 2011. Gammaherpesvirus gene expression and DNA synthesis are facilitated by viral protein kinase and histone variant H2AX. Virology 420:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nowak-Wegrzyn A, Crawford TO, Winkelstein JA, Carson KA, Lederman HM. 2004. Immunodeficiency and infections in ataxia-telangiectasia. J. Pediatr. 144:505–511 [DOI] [PubMed] [Google Scholar]
- 39. Sangster MY, et al. 2000. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J. Immunol. 164:1820–1828 [DOI] [PubMed] [Google Scholar]
- 40. Schroeder SA, Swift M, Sandoval C, Langston C. 2005. Interstitial lung disease in patients with ataxia-telangiectasia. Pediatr. Pulmonol. 39:537–543 [DOI] [PubMed] [Google Scholar]
- 41. Sunil-Chandra NP, Efstathiou S, Nash AA. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73:3275–3279 [DOI] [PubMed] [Google Scholar]
- 42. Tarakanova VL, et al. 2007. Gamma-herpesvirus kinase actively initiates a DNA damage response by inducing phosphorylation of H2AX to foster viral replication. Cell Host Microbe 1:275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tarakanova VL, Molleston JM, Goodwin M, Virgin HW., IV 2010. MHV68 complement regulatory protein facilitates MHV68 replication in primary macrophages in a complement independent manner. Virology 396:323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tarakanova VL, Stanitsa E, Leonardo SM, Bigley TM, Gauld SB. 2010. Conserved gammaherpesvirus kinase and histone variant H2AX facilitate gammaherpesvirus latency in vivo. Virol. 405:50–61 [DOI] [PubMed] [Google Scholar]
- 45. Tarakanova VL, et al. 2005. Murine gammaherpesvirus 68 infection induces lymphoproliferative disease and lymphoma in BALB β2 microglobulin deficient mice. J. Virol. 79:14668–14679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Theunissen JW, et al. 2003. Checkpoint failure and chromosomal instability without lymphomagenesis in Mre11(ATLD1/ATLD1) mice. Mol. Cell 12:1511–1523 [DOI] [PubMed] [Google Scholar]
- 47. Tibbetts SA, Van Dyk L, Speck SH, Virgin HW. 2002. Immune control of the number and reactivation phenotype of cells latently infected with a gamma-herpesvirus. J. Virol. 76:7125–7132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Virgin HW, et al. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894–5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Virgin HW, Wherry EJ, Ahmed R. 2009. Redefining chronic viral infection. Cell 138:30–50 [DOI] [PubMed] [Google Scholar]
- 50. Weck KE, Barkon ML, Yoo LI, Speck SH, Virgin HW. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J. Virol. 70:6775–6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weck KE, Kim SS, Virgin HW, Speck SH. 1999. B cells regulate murine gammaherpesvirus 68 latency. J. Virol. 73:4651–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weck KE, Kim SS, Virgin HW, Speck SH. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wherry EJ, et al. 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27:670–684 [DOI] [PubMed] [Google Scholar]
- 54. Williams BR, et al. 2002. A murine model of Nijmegen breakage syndrome. Curr. Biol. 12:648–653 [DOI] [PubMed] [Google Scholar]