Abstract
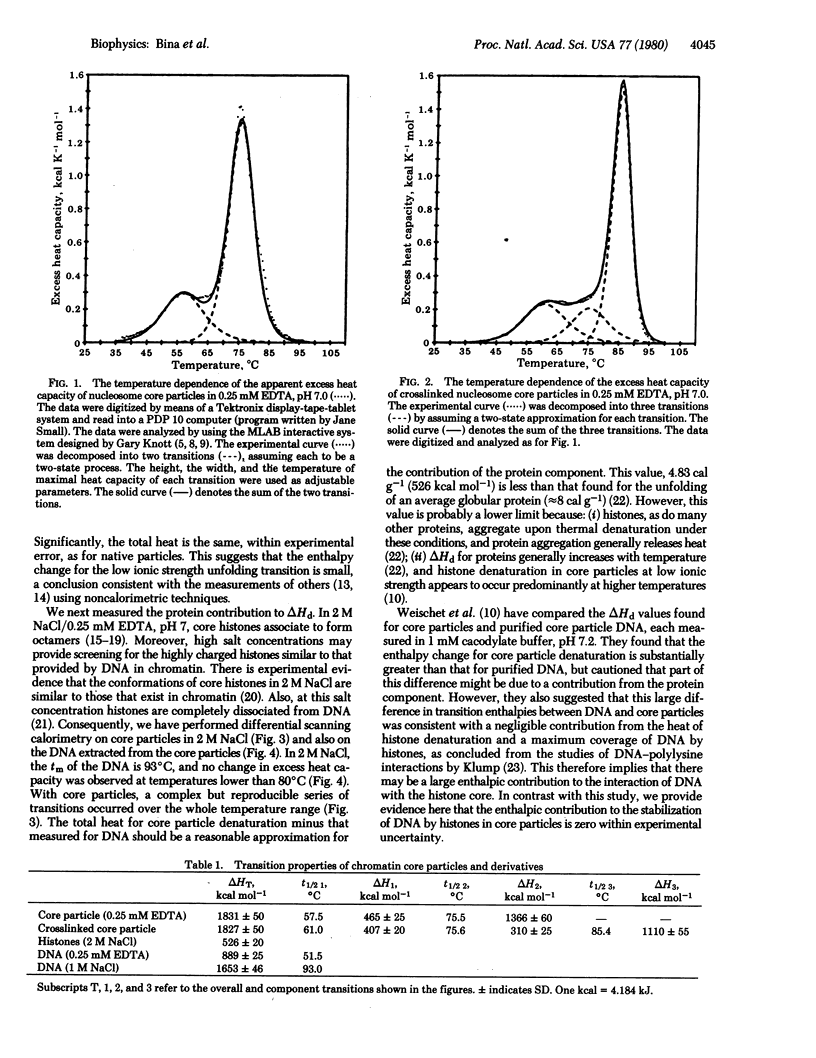
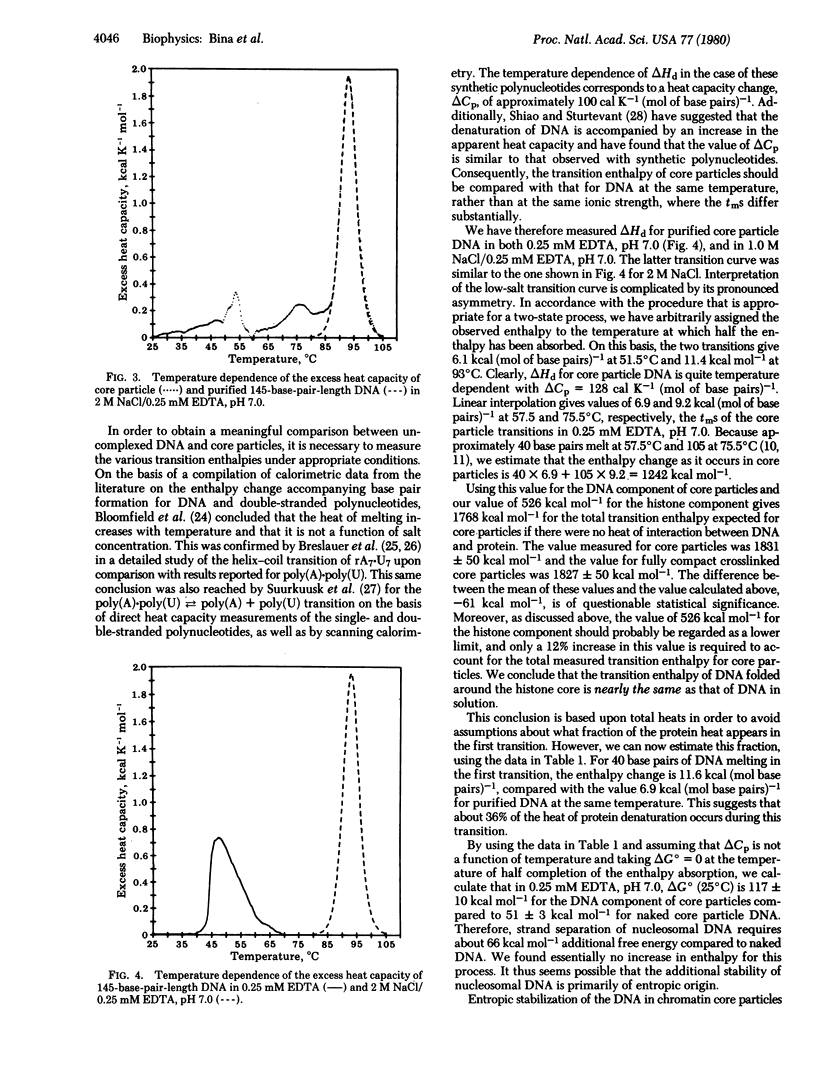
Heats of thermal denaturation of chromatin core particles and core particles with covalently crosslinked histones were measured by differential scanning calorimetry. The additional stabilization of the nucleoprotein complex by crosslinking is not reflected in the transition enthalpy. The contribution of protein denaturation to the total heat was estimated by comparison of core particles with core particle DNA in a high-salt solution. By taking into account the temperature dependence of the transition enthalpy of DNA, we conclude that the enthalpy change for denaturation of DNA in core particles is nearly the same as that for naked DNA in solution.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bina-Stein M. Folding of 140-base pair length DNA by a core of arginine-rich histones. J Biol Chem. 1978 Jul 25;253(14):5213–5219. [PubMed] [Google Scholar]
- Breslauer K. J., Bina-Stein M. Relaxation kinetics of the helix-coil transition of a self-complementary ribo-oligonucleotide: A7U7. Biophys Chem. 1977 Nov;7(3):211–216. doi: 10.1016/0301-4622(77)87024-5. [DOI] [PubMed] [Google Scholar]
- Breslauer K. J., Sturtevant J. M., Tinoco I., Jr Calorimetric and spectroscopic investigation of the helix-to-coil transition of a ribo-oligonucleotide: rA7U7. J Mol Biol. 1975 Dec 25;99(4):549–565. doi: 10.1016/s0022-2836(75)80171-9. [DOI] [PubMed] [Google Scholar]
- Chambon P. Summary: the molecular biology of the eukaryotic genome is coming of age. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1209–1234. doi: 10.1101/sqb.1978.042.01.122. [DOI] [PubMed] [Google Scholar]
- Eickbush T. H., Moudrianakis E. N. The histone core complex: an octamer assembled by two sets of protein-protein interactions. Biochemistry. 1978 Nov 14;17(23):4955–4964. doi: 10.1021/bi00616a016. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Gordon V. C., Schumaker V. N., Olins D. E., Knobler C. M., Horwitz J. The temperature and pH dependence of conformational transitions of the chromatin subunit. Nucleic Acids Res. 1979 Aug 24;6(12):3845–3858. doi: 10.1093/nar/6.12.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYMER W. C., KUFF E. L. ISOLATION OF NUCLEI FROM MAMMALIAN TISSUES THROUGH THE USE OF TRITON X-100. J Histochem Cytochem. 1964 May;12:359–363. doi: 10.1177/12.5.359. [DOI] [PubMed] [Google Scholar]
- Jorcano J. L., Ruiz-Carrillo A. H3.H4 tetramer directs DNA and core histone octamer assembly in the nucleosome core particle. Biochemistry. 1979 Mar 6;18(5):768–774. doi: 10.1021/bi00572a005. [DOI] [PubMed] [Google Scholar]
- Klump H. Calorimetric studies of the interaction between DNA and poly-L-lysine. Biophys Chem. 1976 Sep;5(3):363–367. doi: 10.1016/0301-4622(76)80048-8. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Ohlenbusch H. H., Olivera B. M., Tuan D., Davidson N. Selective dissociation of histones from calf thymus nucleoprotein. J Mol Biol. 1967 Apr 28;25(2):299–315. doi: 10.1016/0022-2836(67)90143-x. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Khechinashvili N. N. A thermodynamic approach to the problem of stabilization of globular protein structure: a calorimetric study. J Mol Biol. 1974 Jul 5;86(3):665–684. doi: 10.1016/0022-2836(74)90188-0. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Lohman M. L., De Haseth P. Ion effects on ligand-nucleic acid interactions. J Mol Biol. 1976 Oct 25;107(2):145–158. doi: 10.1016/s0022-2836(76)80023-x. [DOI] [PubMed] [Google Scholar]
- Riemer S. C., Bloomfield V. A. Packaging of DNA in bacteriophage heads: some considerations on energetics. Biopolymers. 1978 Mar;17(3):785–794. doi: 10.1002/bip.1978.360170317. [DOI] [PubMed] [Google Scholar]
- Shiao D. D., Sturtevant J. M. Heats of thermally induced helix-coil transitions of DNA in aqueous solution. Biopolymers. 1973;12(8):1829–1836. doi: 10.1002/bip.1973.360120810. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Mechanism of a reversible, thermally induced conformational change in chromatin core particles. J Biol Chem. 1979 Oct 25;254(20):10123–10127. [PubMed] [Google Scholar]
- Stein A., Bina-Stein M., Simpson R. T. Crosslinked histone octamer as a model of the nucleosome core. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2780–2784. doi: 10.1073/pnas.74.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A., Page D. Core histone associations in solutions of high salt. An osmotic pressure study. J Biol Chem. 1980 Apr 25;255(8):3629–3637. [PubMed] [Google Scholar]
- Suurkuusk J., Alvarez J., Freire E., Biltonen R. Calorimetric determination of the heat capacity changes associated with the conformational transitions of polyriboadenylic acid and polyribouridylic acid. Biopolymers. 1977 Dec;16(12):2641–2652. doi: 10.1002/bip.1977.360161206. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Prescott B., Olins D. E. Secondary structure of histones and DNA in chromatin. Science. 1977 Jul 22;197(4301):385–388. doi: 10.1126/science.560060. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Butler P. J. Characterization of the octamer of histones free in solution. J Mol Biol. 1977 Nov;116(4):769–781. doi: 10.1016/0022-2836(77)90270-4. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weischet W. O., Tatchell K., Van Holde K. E., Klump H. Thermal denaturation of nucleosomal core particles. Nucleic Acids Res. 1978 Jan;5(1):139–160. doi: 10.1093/nar/5.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. M., Dattagupta N., Hogan M., Crothers D. M. Structural changes of nucleosomes in low-salt concentrations. Biochemistry. 1979 Sep 4;18(18):3960–3965. doi: 10.1021/bi00585a018. [DOI] [PubMed] [Google Scholar]



