Abstract
Conserved tryptophan-187 facilitates homodimerization of the influenza A virus NS1 protein effector domain. We generated a mutant influenza virus strain expressing NS1-W187R to destabilize this self-interaction. NS1-W187R protein exhibited lower double-stranded RNA (dsRNA)-binding activity, showed a temporal redistribution during infection, and was minimally compromised for interferon antagonism. The mutant virus replicated similarly to the wild type in vitro, but it was slightly attenuated for replication in mice, causing notably reduced morbidity and mortality. These data suggest biological relevance for the W187-mediated homotypic interaction of NS1.
TEXT
Influenza A virus NS1 is a multifunctional protein consisting primarily of a double-stranded RNA (dsRNA)-binding domain (RBD) and effector domain (ED) (10). NS1 acts at multiple levels to antagonize host interferon (IFN) responses, most notably by inhibiting the RIG-I/TRIM25 signaling axis (7), inhibiting posttranscriptional processing of host-cell mRNAs (including IFN-β) (19), and directly limiting function of the dsRNA-activated antiviral effectors protein kinase R (PKR) and 2′-5′-oligo(A) synthetase (2′-5′-OAS) (2, 14, 18). The mechanisms underpinning NS1 multifunctionality are not fully understood, although multiple NS1 quaternary conformations promoted by weak, transient, homotypic interactions may provide one structural route to achieve and regulate such extensive functionality (12). Here, we extend earlier studies that focused on biochemical and structural characterization of the major homotypic conformer of the NS1 ED, namely, a weak helix-helix dimer mediated by the highly conserved tryptophan-187 (W187) (1, 9, 12, 23, 24) (Fig. 1A). We provide evidence that ability to form this dimer is important for both the dsRNA-binding activity of full-length NS1 and its temporal distribution within the infected cell. We also report that the ability of NS1 to form a W187-mediated oligomer plays a role in virus replication and virulence in vivo.
Fig 1.

(A) Structural representation of the NS1 effector domain homodimer. The two monomers are colored red and yellow. W187, which reciprocally packs into a hydrophobic pocket on the neighboring monomer, is highlighted. The image was generated with PyMol (5) using Protein Data Bank (PDB) identification no. 3D6R (9). (B) Generation of WT and NS1-W187R viruses. Shown are the nucleotide sequence and corresponding amino acids (aa) for NS1 and NEP/NS2 in the region of NS1 residue 187 (PR8 strain). (C) NS1 binding to synthetic dsRNA. Lysates from 293T cells transfected with the indicated V5-tagged PR8 NS1 construct were precipitated with poly(I·C)-Sepharose (pI:C) or Sepharose only (−). Following SDS-PAGE, NS1-V5 proteins were detected by Western blotting using an anti-V5 (α-V5) antibody. Molecular mass markers (kDa) are indicated to the right.
In the context of an influenza A virus genome, tryptophan to arginine is the only amino acid substitution that can be made at position 187 of NS1 (W187R) without altering the overlapping NEP/NS2 open reading frame (ORF) (Fig. 1B). This specific mutation in the A/Udorn/72 (Ud) NS1 has been shown to abrogate ED dimerization (1). Here, we used the mouse-lethal A/Puerto Rico 8/34 (PR8) strain both to allow in vivo experiments and to negate the impact of mutation of W187 on the cellular pre-mRNA processing inhibition ability of NS1, which is inherently lacking in the NS1 of PR8 and many other strains (11, 13, 21). Consistent with previous studies implicating the NS1 ED helix-helix dimer in RBD function (1, 12), poly(I·C) pulldown assays confirmed that the W187R mutation reduces dsRNA binding by NS1 (Fig. 1C). As controls, wild-type (WT) NS1 bound efficiently to poly(I·C), while no binding was observed for the dsRNA-binding-incompetent R38A mutant (12, 22). Although we noted that the effect of W187R on poly(I·C) binding was not as striking as that of a previously reported mutation (W187A) ((12; data not shown), our observation confirms that ED dimerization contributes to the overall affinity of full-length NS1 for dsRNA, which probably only occurs efficiently via cooperative binding of NS1 multimers (1, 3).
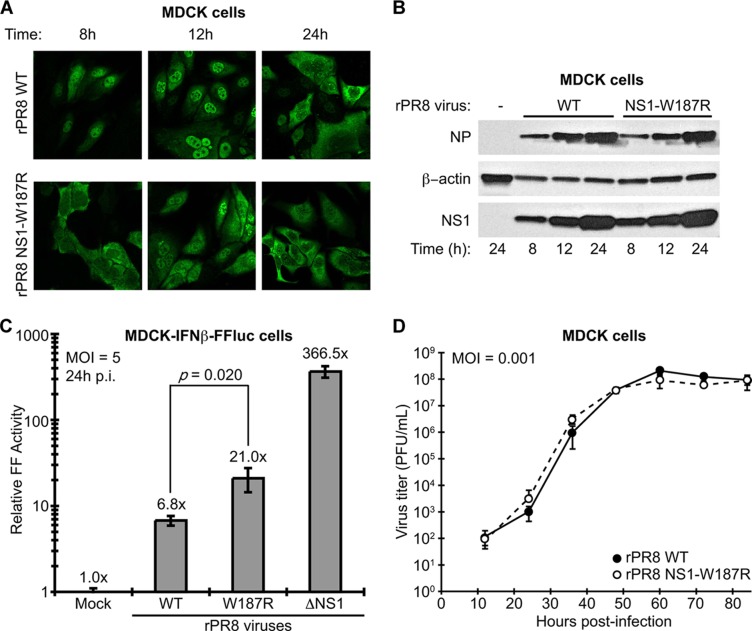
Using reverse genetics (6), we generated recombinant WT and NS1-W187R viruses. The two viruses (rPR8 WT and rPR8 NS1-W187R) (Fig. 1B) were plaque purified, and stocks were grown and titrated in MDCK cells. RNA was extracted from stock aliquots, and the NS genomic segment of each virus was sequenced to ensure integrity and the absence of additional mutations. Immunofluorescence studies of virus-infected MDCK cells revealed a distinct distribution pattern for the NS1-W187R mutant compared with WT NS1: while WT NS1 was predominantly nuclear at 8 and 12 h postinfection (88% and 94% of cells counted, respectively), NS1-W187R seemed to be partially excluded from the nucleus at 8 h (predominantly nuclear in only 4% of cells counted) and overall was more evenly distributed between the nucleus and cytoplasm at both 8 and 12 h (with 21% and 53% of cells counted at these respective time points showing similar amounts of nuclear/cytoplasmic staining) (Fig. 2A). At 24 h postinfection, the localizations of both WT NS1 and NS1-W187R were largely indistinguishable from one another, with the majority of all infected cells showing predominantly cytoplasmic NS1 staining (Fig. 2A). Western blot analysis of virus-infected MDCK cells confirmed that these observations were not due to different expression levels of the two NS1 proteins and also showed that another viral protein (NP) is similarly expressed by the two viruses (Fig. 2B), indicating that the W187R mutation does not affect any role of NS1 in regulating viral protein synthesis (16). We also assessed ability of the two viruses to repress IFN-β production during infection using an MDCK cell line stably expressing firefly luciferase (FF-Luc) under the control of the IFN-β promoter. At 16 h postinfection, both rPR8 WT and rPR8 NS1-W187R induced the IFN-β promoter to similar levels (2.5 ± 0.4-fold versus 4.4 ± 1.4-fold compared to mock infected, respectively; P = 0.095). In contrast, rPR8ΔNS1, which completely lacks the NS1 ORF (8), stimulated the reporter 300 ± 25.8-fold. At 24 h postinfection, rPR8 WT-infected samples showed an ∼7-fold induction of the reporter compared to mock infected, while infection with rPR8 NS1-W187R stimulated the reporter ∼21-fold, and rPR8ΔNS1 stimulated the reporter >365-fold (Fig. 2C). This suggests only a mild, although statistically significant (P = 0.020), impact of W187 in allowing NS1 to counteract induction of IFN-β during the later stages of influenza A virus infection, which correlates with its inefficient dsRNA-binding capability. Surprisingly, despite the striking differences in NS1 localization during infection and the small effect on IFN-β induction, in vitro growth kinetics revealed no apparent differences in replication between rPR8 WT and rPR8 NS1-W187R in MDCK cells (Fig. 2D) or human lung A549 cells (data not shown). These data indicate that disruption of the ED helix-helix dimer affects the temporal regulation of NS1 localization during infection, possibly as an indirect consequence of loss of function (e.g., dsRNA binding) or by altering the availability of the NS1 nuclear export signal (15), but does not detectably have an impact on virus replication in tissue culture.
Fig 2.
Characterization of WT and NS1-W187R viruses in vitro. (A) NS1 localization during infection. Shown is indirect immunofluorescence analysis of NS1 protein localization in MDCK cells infected for the indicated times with rPR8 WT or rPR8 NS1-W187R viruses (multiplicity of infection [MOI] of 2 PFU/cell). The primary antibody was pAb 155 (12). (B) Expression of viral NS1 and NP proteins during infection. SDS-PAGE and Western blot analysis of lysates from MDCK cells infected for the indicated times with rPR8 WT or rPR8 NS1-W187R viruses (MOI of 5 PFU/cell). NS1 was detected using pAb 155, NP was detected using monoclonal antibody (MAb) HT103 (20), and β-actin was detected using MAb A4700 (Sigma-Aldrich, St. Louis, MO). (C) Induction of IFN-β by different rPR8 mutants. MDCK-IFN-β-FF-Luc cells were infected at an MOI of 5 PFU/cell for 24 h with the indicated virus (or mock infected) prior to analysis of luciferase activity. p.i., postinfection. Bars represent mean values (n = 3), and error bars represent standard deviations (SD). (D) Multicycle growth analysis of rPR8 WT and rPR8 NS1-W187R viruses in MDCK cells. Data points show mean values (n = 3), and error bars represent SD.
To assess the contribution of NS1 ED dimerization to influenza A virus pathogenicity and replication in vivo, we intranasally infected ketamine-xylazine-anesthetized 6- to 8-week-old C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) with 32 PFU of each virus (10 mice/group) and monitored body weight changes for 14 days. All procedures were performed in accordance with the Institutional Animal Care and Use Committee guidelines of Mount Sinai School of Medicine, and animals showing more than 25% weight loss were considered to have reached the experimental endpoint and were humanely euthanized. In contrast to the results in vitro, the NS1-W187R mutation attenuated the virus in mice, resulting in only 20% mortality compared to the 100% mortality caused by WT virus (Fig. 3A). Furthermore, the rPR8 NS1-W187R virus-infected mice exhibited less morbidity (as determined by weight loss) than mice infected with WT (Fig. 3B). To assess replication, mice were intranasally infected with 1,250 PFU of each virus and lungs were excised on days 2 and 4 postinfection. Following homogenization and centrifugation (10,000 × g, 5 min, 4°C), the resulting supernatants were used to determine viral titer. As shown in Fig. 3C, rPR8 NS1-W187R replicated to titers significantly lower than those of rPR8 WT at day 2 postinfection (∼5-fold; P = 0.018). At day 4 postinfection, rPR8 NS1-W187R titers were only ∼2.5-fold lower than those of rPR8 WT (P = 0.045).
Fig 3.
Characterization of WT and NS1-W187R virus replication and pathogenicity in vivo. Six- to 8-week-old C57/BL6 mice were infected intranasally with 32 PFU of each virus (10 mice per group). Body weights were determined daily, and mice showing more than 25% weight loss were considered to have reached the experimental endpoint and were humanely euthanized. (A) Survival data; (B) mean body weights. Error bars represent SD. Statistical significance (**, P < 0.0005) was determined using Student's t test. (C) Six- to 8-week-old C57/BL6 mice were infected intranasally with 1,250 PFU of each virus. Lung titers were determined on days 2 and 4 postinfection from 3 mice per group. Bars represent mean values. P values were determined using Student's t test.
Herein, we have provided evidence to show that homotypic interaction of the influenza A virus NS1 protein ED contributes subtly to replication and virulence. Disruption of the ability to form this weak ED helix-helix dimerization interface impacts the dsRNA-binding function of the full-length protein and impairs the correct trafficking of NS1 during infection. We speculate that this aspect of transient NS1 quaternary structure (leading to NS1 multimerization and cooperative binding to dsRNA) may therefore normally function to support antagonism of host dsRNA-activated antiviral proteins, such as RIG-I, PKR, and 2′-5′-OAS. As such, we note that efficient dsRNA binding by NS1 is critical for counteracting the function of RIG-I and 2′-5′-OAS (17, 18), and it is therefore possible that W187-mediated ED dimerization contributes to antagonism of innate immunity. Indeed, rPR8 NS1-W187R virus induced slightly more IFN-β than rPR8 WT during infection. Our working model from structural data is that this NS1 ED helix-helix self-interaction is weak and transient (12), forming only to contribute to NS1 functions that require dsRNA binding, while dissociating for other NS1 functions, such as CPSF30 binding.
Although both the W187R and W187A mutations disrupt NS1 ED dimerization (1, 9, 12, 24), we observed that W187R had a weaker impact on dsRNA-binding activity than W187A (data not shown). Thus, for W187R, it may be that replacement of the homodimer-forming tryptophan with an exposed basic arginine residue partially compensates functionally for the lack of NS1 multimerization by providing an artificial dsRNA-binding site analogous to that of the lysine/arginine-rich NS1 RNA-binding domain (4). This limits phenotypic studies, as in the context of an infectious influenza A virus, arginine is the only permitted residue at position 187 that preserves integrity of the NEP/NS2 ORF. Future studies to dissect this caveat may therefore reveal a much greater biological impact of the highly conserved NS1 ED dimerization structural feature. Nevertheless, our present study provides the initial data to suggest biological relevance for the numerous structural and biophysical studies that have so far characterized NS1 ED helix-helix dimerization (1, 9, 12, 23, 24).
ACKNOWLEDGMENTS
We are grateful to Richard Cadagan and Osman Lizardo (Mount Sinai School of Medicine, New York) for excellent technical assistance.
This work was supported by NIH funding to A.G.-S. (under grants R01AI046954 and U19AI083025, and CRIP, Center for Research on Influenza Pathogenesis, an NIAID Center of Excellence for Influenza Research and Surveillance [CEIRS], contract HHSN266200700010C). Confocal laser scanning microscopy was performed at the MSSM-Microscopy Shared Resource Facility, supported with funding from a National Institutes of Health-National Cancer Institute (NIH-NCI) shared resources grant (5R24 CA095823-04), an NSF Major Research Instrumentation grant (DBI-9724504), and an NIH shared instrumentation grant (1 S10 RR0 9145-01).
Footnotes
Published ahead of print 19 September 2012
REFERENCES
- 1. Aramini JM, et al. 2011. Dimer interface of the effector domain of non-structural protein 1 from influenza A virus: an interface with multiple functions. J. Biol. Chem. 286:26050–26060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergmann M, et al. 2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 74:6203–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bornholdt ZA, Prasad BV. 2008. X-ray structure of NS1 from a highly pathogenic H5N1 influenza virus. Nature 456:985–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng A, Wong SM, Yuan YA. 2009. Structural basis for dsRNA recognition by NS1 protein of influenza A virus. Cell Res. 19:187–195 [DOI] [PubMed] [Google Scholar]
- 5. DeLano WL. 2002. The PyMOL molecular graphics system. DeLano Scientific, Palo Alto, CA [Google Scholar]
- 6. Fodor E, et al. 1999. Rescue of influenza A virus from recombinant DNA. J. Virol. 73:9679–9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gack MU, et al. 2009. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5:439–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia-Sastre A, et al. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324–330 [DOI] [PubMed] [Google Scholar]
- 9. Hale BG, Barclay WS, Randall RE, Russell RJ. 2008. Structure of an avian influenza A virus NS1 protein effector domain. Virology 378:1–5 [DOI] [PubMed] [Google Scholar]
- 10. Hale BG, Randall RE, Ortin J, Jackson D. 2008. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 89:2359–2376 [DOI] [PubMed] [Google Scholar]
- 11. Hale BG, et al. 2010. Inefficient control of host gene expression by the 2009 pandemic H1N1 influenza A virus NS1 protein. J. Virol. 84:6909–6922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kerry PS, et al. 2011. A transient homotypic interaction model for the influenza A virus NS1 protein effector domain. PLoS One 6:e17946 doi:10.1371/journal.pone.0017946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kochs G, Garcia-Sastre A, Martinez-Sobrido L. 2007. Multiple anti-interferon actions of the influenza A virus NS1 protein. J. Virol. 81:7011–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li S, Min JY, Krug RM, Sen GC. 2006. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology 349:13–21 [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Yamakita Y, Krug RM. 1998. Regulation of a nuclear export signal by an adjacent inhibitory sequence: the effector domain of the influenza virus NS1 protein. Proc. Natl. Acad. Sci. U. S. A. 95:4864–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marion RM, Aragon T, Beloso A, Nieto A, Ortin J. 1997. The N-terminal half of the influenza virus NS1 protein is sufficient for nuclear retention of mRNA and enhancement of viral mRNA translation. Nucleic Acids Res. 25:4271–4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mibayashi M, et al. 2007. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 81:514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Min JY, Krug RM. 2006. The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: inhibiting the 2′-5′ oligo (A) synthetase/RNase L pathway. Proc. Natl. Acad. Sci. U. S. A. 103:7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noah DL, Twu KY, Krug RM. 2003. Cellular antiviral responses against influenza A virus are countered at the posttranscriptional level by the viral NS1A protein via its binding to a cellular protein required for the 3′ end processing of cellular pre-mRNAS. Virology 307:386–395 [DOI] [PubMed] [Google Scholar]
- 20. O'Neill RE, Talon J, Palese P. 1998. The influenza virus NEP (NS2 protein) mediates the nuclear export of viral ribonucleoproteins. EMBO J. 17:288–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Twu KY, Kuo RL, Marklund J, Krug RM. 2007. The H5N1 influenza virus NS genes selected after 1998 enhance virus replication in mammalian cells. J. Virol. 81:8112–8121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang W, et al. 1999. RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA 5:195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xia S, Monzingo AF, Robertus JD. 2009. Structure of NS1A effector domain from the influenza A/Udorn/72 virus. Acta Crystallogr. D Biol. Crystallogr. 65:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xia S, Robertus JD. 2010. X-ray structures of NS1 effector domain mutants. Arch. Biochem. Biophys. 494:198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]