Abstract
Influenza A virus transmission by direct contact is not well characterized. Here, we describe a mouse model for investigation of factors regulating contact-dependent transmission. Strains within the H3N2 but not H1N1 subtype of influenza virus were transmissible, and reverse-engineered viruses representing hybrids of these subtypes showed that the viral hemagglutinin is a determinant of the transmissible phenotype. Transmission to contact mice occurred within the first 6 to 54 h after cohousing with directly infected index mice, and the proportion of contacts infected within this period was reduced if the index mice had been preinfected with a heterologous subtype virus. A threshold level of virus present in the saliva of the index mice was identified, above which the likelihood of transmission was greatly increased. There was no correlation with transmission and viral loads in the nose or lung. This model could be useful for preclinical evaluation of antiviral and vaccine efficacy in combating contact-dependent transmission of influenza.
INTRODUCTION
Transmission of influenza A virus from an infected individual (index) to a naïve susceptible individual (contact) occurs either via inhalation of viral particles shed from the respiratory tract in the form of large droplets (>20 μm) and fine aerosols (<5 μm) (48) or via direct contact with infectious fluids (3, 5, 43). Human studies indicate the importance of both aerosolized (4, 29, 31) and contact-dependent (2, 9) spread of virus, but it is difficult to ascertain the relative contribution of the two modes of spread as they are not mutually exclusive (48). The study of viral transmission through a human population is complex, involving viral (14, 16, 35, 45, 46, 50, 51), host (7, 10, 11, 28), social (13, 27), and environmental factors (15, 19, 22, 24, 44, 53), and animal models have been used to assess some of these parameters in a more defined system.
Ferrets have proven useful because they develop respiratory signs of influenza illness similar to those observed in humans, such as rhinorrhea, which may facilitate transmission (1, 25, 47). Like humans, ferrets spread virus via direct contact and aerosols, and this spread can be studied in isolation by housing index and contact animals in separate cages that have a shared air supply (26). In contrast, it is difficult to study contact-dependent transmission in the absence of aerosol spread, because secretions and aerosols could mediate transmission between cocaged ferrets. Guinea pigs have also been used successfully to determine some of the parameters affecting contact-dependent (24) and aerosol transmission (23). Unlike ferrets, guinea pigs do not cough or sneeze following influenza infection, and the spread of virus may be mediated via shedding of virus in exhaled breath (32), a process that may contribute to the transmission of influenza virus in humans (12). Like guinea pigs, inbred mice do not sneeze or cough in response to experimental influenza virus infection despite high titers of virus in their nasal cavities. Based on this observation, mice may be another suitable species to enable research on transmission of influenza virus. A mouse transmission model would have greater capacity, scope of reagents, and genetically defined mouse strains at one's disposal than other models that are limited in immunological reagents or, in the case of ferrets, are expensive and difficult to conduct on a large scale (49).
Numerous experiments conducted by Schulman et al. in the 1960s optimized an influenza transmission mouse model for viruses within the H2N2 subtype (37–42). However, a more recent study by Lowen et al. has found this model difficult to reproduce, because a number of strains from a variety of different subtypes (i.e., H1N1, H3N2, and H5N1) were tested and found to be nontransmissible (23). Here, we describe transmission of H3N2 viruses in mice and show this to occur exclusively by close contact and not by aerosolized spread. The use of a mouse model that can only examine contact-dependent transmission, especially for a subtype that is relevant to humans, would be of significant value in understanding transmission in close-contact exposure scenarios, for instance, those that might occur in households, schools, and workplaces. With this in mind, we have set out to investigate various factors that contribute to contact-dependent transmission between mice.
MATERIALS AND METHODS
Viruses.
Influenza viruses were propagated in 10-day-old embryonated hens' eggs at 35°C, and 3 days later the allantoic fluid was harvested and stored at −80°C. Reverse-engineered viruses were produced as previously described (17). The subtype and strains of the viruses used in this study are shown in Table 1.
Table 1.
Transmissibility of strains of influenza virus in BALB/c mice
| Virus used to infect index mouse (subtype and strain) | Growth of virus in: |
HIa | % Contact mice infectedd | |||
|---|---|---|---|---|---|---|
| Indexb |
Contactc |
|||||
| Nasal turbinates | Nasal turbinates | Trachea | Lung | |||
| H1N1 | ||||||
| A/WSN/33 | 4.13 ± 0.07 | <0.9e | <0.9 | <0.9 | <10 | 0 |
| A/PR/8/34 Mt Sinai | 4.7 ± 0.44 | <0.9 | <0.9 | <0.9 | <10 | 0 |
| A/PR/8/34 Cambridge | 5.65 ± 0.03 | <0.9 | <0.9 | <0.9 | NDf | 0 |
| A/Bellamy/42 | 2.58 ± 0.2 | <0.9 | <0.9 | <0.9 | <10 | 0 |
| H3N2 | ||||||
| X31g | 5.82 ± 0.02 | 4.24 ± 1.71 | 4.95 ± 1.92 | 4.62 ± 1.56 | 107 ± 46 | 100 |
| A/Memphis/1/71 | 4.04 ± 0.03 | <0.9 | <0.9 | <0.9 | <10 | 0 |
| A/Memphis/72 | 3.87 ± 0.23 | <0.9 | <0.9 | <0.9 | <10 | 0 |
| A/Udorn/307/72 | 5.4 ± 0.05 | 4.57 ± 0.67 | 3.36 ± 1.2 | 3.30 ± 1.47 | 512 | 100 |
| A/Port Chalmers/1/73 | 5.67 ± 0.03 | 4.82 ± 2.06 | 2.85 ± 1.29 | <0.9 | 256 | 66.6 |
| A/Victoria/3/75 | 3.94 ± 0.05 | 2.36 ± 0.72 | <0.9 | <0.9 | 256 | 66.6 |
| BJx109h | 2.27 ± 0.06 | <0.9 | <0.9 | <0.9 | <10 | 0 |
| A/Guandong/25/93 | 2.98 ± 0.16 | <0.9 | <0.9 | <0.9 | ND | 0 |
Hemagglutination inhibition (HI) assays were performed by the incubation of homologous virus with serum and the addition of chicken red blood cells. HI titers are reported as means ± SD (n = 3).
Index mice were infected via the total respiratory tract route with 104.5 PFU except for the virulent strain A/PuertoRico/8/34 (PR8), which was used at a sublethal dose of 50 PFU. Index mice were introduced to contacts, and 4 days later viral loads were measured in nasal turbinates of the index mice and are expressed as mean log10 PFU/ml ± SD (n = 4).
Nasal turbinates, trachea, and lungs were collected from contact mice 4 days after cohousing with index mice, and viral loads are expressed as the mean log10 PFU/ml ± SD (n = 6).
Percentage of contact mice containing virus in nasal turbinates 4 days after cohousing (n = 6).
Detection limit of plaque assay.
ND, not determined.
Reverse-engineered virus containing A/Aichi/68 HA and NA on a background of PR8 Mt Sinai.
Reassortant containing A/Beijing/89 HA and NA on a background of PR8 Mt Sinai.
Mouse infection and transmission model.
Mouse experiments were approved by and conducted according to the guidelines of the University of Melbourne Animal Ethics Committee. Male BALB/c mice, 6 to 8 weeks old, were housed in specific-pathogen-free conditions at 21°C, 31% humidity. For each transmission experiment, two mice were infected to become the index mice, by either upper respiratory tract (URT) or total respiratory tract (TRT) routes, using direct delivery of the virus to the nares with a micropipette as described previously (33). After infection, index mice were housed separately for 6 h, unless otherwise stated, to prevent contamination of the contacts with the initial viral inoculum. Thereafter index and contact mice were cohoused; two index mice were introduced into a clean mouse box containing three naïve contact mice. Transmission to contacts was determined by measuring the infectious viral titers in the nasal turbinates, trachea, and lung or the development of an antibody response to the viral hemagglutinin (HA) by testing their sera in hemagglutination inhibition (HI) assays as described elsewhere (52). To assess contact versus aerosol transmission, index and contact mice were cohoused in a 40-cm-long by 20-cm-wide mouse transport box that was divided into two compartments by two pieces of 2-mm plastic mesh so that index and contact mice were separated by 4 cm, preventing contact between the two. The index and contact compartments had separate food and water sources.
Collection of secretions and tissues for virus quantification.
Salivation was induced by intraperitoneal injection with 200 μl of 20 μg/ml of carbamylcholine chloride (Sigma-Aldrich Pty. Ltd., Sydney, Australia), and saliva, collected with the aid of a micropipette with a plastic tip, was stored at −80°C. Blood was collected by tail bleed, and serum was harvested 12 h later and stored at −20°C. Following the euthanasia of index and contact mice at the indicated times, the nasal turbinates, trachea, and lungs were collected in 1 ml of RPMI 1640 (Sigma). Tissues were homogenized at 10,000 rpm for 30 s, and supernatants were clarified by centrifugation and stored at −80°C. Viral titers in secretions and tissues of the respiratory tract were determined by plaque assay on Madin-Darby canine kidney (MDCK) cells as previously described (33). Following 3 days of incubation, plaques were counted macroscopically to determine the PFU per sample, which was calculated from the average PFU obtained in three different dilutions of sample, each performed in duplicate.
Statistical analyses.
Statistical analysis was conducted with the software package Prism (v.5.0a) from GraphPad Software Inc. A one-way or two-way analysis of variance (ANOVA) with a 95% confidence interval was used to determine significance of data; P values shown were obtained using a Bonferroni posttest. Spearman correlation with a 95% confidence interval was used to determine if the association between two factors was significant.
RESULTS
Transmission of influenza viral strains in mice is subtype dependent.
The studies of Lowen et al. (23) and Schulman and Kilbourne (41) present contradictory findings regarding the capacity of the mouse model to assess transmission. To address this and to identify influenza viral strains that are transmissible in BALB/c mice, index mice were infected with different strains of influenza virus within the H1N1 and H3N2 subtypes and introduced to naïve contact mice. The index and contact mice were housed together for 4 days, and then respiratory tract tissues of the contacts were collected and the viral titers determined. A second group of contacts, also cohoused with the index mice for 4 days, were removed to a separate cage and bled 21 days after initial exposure to the index mice. Sera were assayed for strain-specific antibodies by inhibition of viral hemagglutination (HI) to verify the presence or absence of an established infection (Table 1).
All H1N1 strains examined were unable to transmit from index to contact mice, as demonstrated by an absence of viral replication in the nasal turbinates, trachea, and lung of all contacts and the lack of the development of an HI antibody response (Table 1). In contrast, several H3N2 strains were transmissible, as indicated by the presence of infectious virus in the respiratory tract and HI antibodies in the sera of all contacts. Notably, disease severity was not correlated with viral transmission, because disease signs and significant loss in bodyweight occurred in index mice after infection with some nontransmissible H1N1 strains, such as A/PuertoRico/8/34 virus (PR8), and transmissible H3N2 strains, such as X31 virus (Fig. 1). The majority of H3N2 transmissible viruses resembled A/Udorn/307/72 virus (Udorn) and caused no loss in bodyweight and no disease signs in index mice after infection. Despite the lack of morbidity, these transmissible H3N2 viruses replicated to high titers in the nasal turbinates of index mice (Table 1). These viral loads were not significantly higher than those of nontransmissible viruses, such as PR8, suggesting that viral growth in the nose of the index mice was not the primary determinant of subtype-specific transmission.
Fig 1.
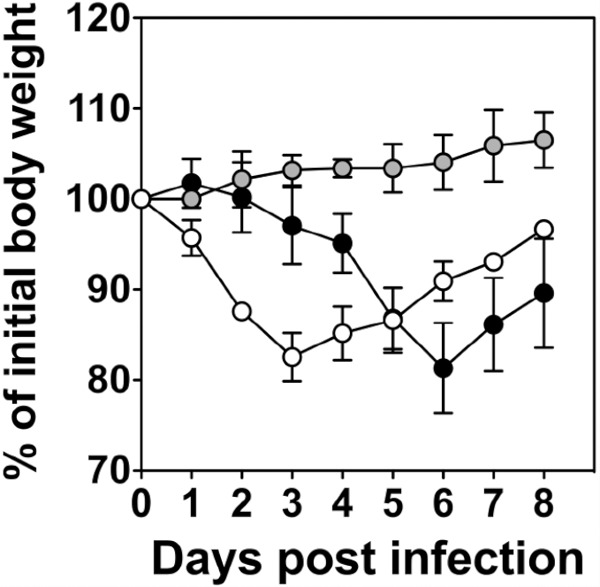
Percent weight loss of BALB/c index mice caused by infection with different strains of influenza A virus. Index mice were infected via the total respiratory tract route with 104.5 PFU of A/Udorn/307/72 (gray symbols), reverse-engineered X31 that contained A/PuertoRico/8/34 Mt Sinai (PR8) genes with the HA and NA of A/Aichi/68 (white symbols), or a sublethal dose (50 PFU) of PR8 (black symbols). Weight was measured daily and expressed as a percentage of weight at day 0. The means ± standard deviations (SD) from four index mice are shown.
Direct contact between index and contact mice is essential for viral transmission.
To investigate the mechanisms associated with transmission in this model of H3N2 virus infection, index mice were infected with the transmissible strain Udorn and placed in a housing box separated from contacts by the presence of 2-mm mesh, providing a 4-cm separation, for a period of 4 days. In this housing scenario, the airflow between the two compartments was unrestricted, but index and contact mice did not have direct contact with each other and did not share a food and water source. We found that although high viral titers were detected in the nasal turbinates and lungs of Udorn-infected index mice, their cohoused contacts did not become infected (Fig. 2A). In contrast, cohousing index and contact mice so that they could intermingle freely over a period of 4 days resulted in high viral titers in the nasal turbinates of 100% of contacts (Fig. 2B). Viral titers in the nasal turbinates of contacts could be detected for up to 7 days after initial exposure to the index mice; however, levels of virus detected in the lung were variable and lower than those in the nose. Variation in lung viral loads in contacts could be a result of various degrees of viral progression from the nose to the lung and would be influenced by frequency of interaction between index and contact mice.
Fig 2.
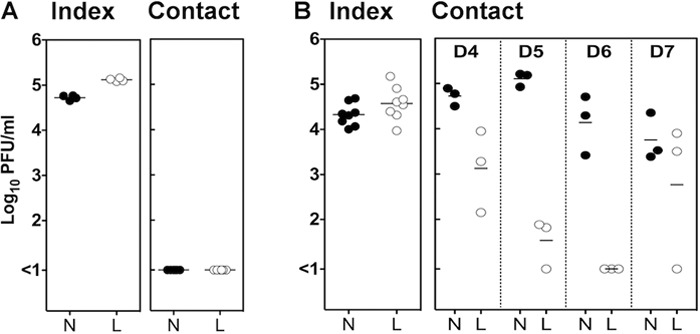
Transmission of influenza virus in mice is contact dependent. Index mice were infected via the total respiratory tract route with 104.5 PFU of Udorn, and 6 h later they were introduced to contact mice. Index and contact mice were cohoused for a period of 4 days in a mouse box that prevented direct contact (A) or allowed direct contact (B). Index mice were euthanized directly after the cohousing period, whereas contacts were euthanized 4 days (A) or 4, 5, 6, and 7 days (B) after their initial introduction to the index mice. Symbols represent viral titers in nasal turbinates (N) or lungs (L) from individual mice.
Transmission is influenced by location of virus and time after infection of index mice.
To examine when the peak of transmission took place, index mice were infected with Udorn and were introduced to contacts 6 h postinfection (Group I), 30 h postinfection (Group II), or 54 h postinfection (Group III). Mice remained together for 24 h, after which the index mice were killed and respiratory tract samples taken for assay of viral load. The contacts were left for a further 3 days before sampling of organs (Fig. 3A). In the previous experiment, reported in Table 1 and Fig. 1 and 2, index mice were exposed to virus by the TRT route, which involves delivery of 50 μl of inoculum intranasally under anesthesia to the mice, resulting in simultaneous infection of the entire respiratory tract. In this experiment, virus was delivered to mice as an upper respiratory tract (URT) infection using 10 μl of inoculum given intranasally to conscious animals (34). This mode of inoculation results in the establishment of an infection confined initially to the URT tissues, which progresses down to the lungs over time, thus allowing examination of the relationship between viral load in particular anatomical locations and transmission. Viral loads in respiratory organs of the index mice at the end of the 24-h cohousing period revealed that high titers of virus were present in the nasal turbinates in each of the groups of index mice. Viral titers in the trachea and lung were low (<2.9 log10 PFU/ml) in Group I index mice but increased by 10-fold in Group II mice and a further 10-fold in Group III mice, indicating a progression of infection from the upper to the lower respiratory tract (Fig. 3B). In contrast, there was a significant reduction (P < 0.01 by two-way ANOVA) of 1.2 logs of virus in saliva from Group III mice compared to virus in saliva from Group I and II mice.
Fig 3.
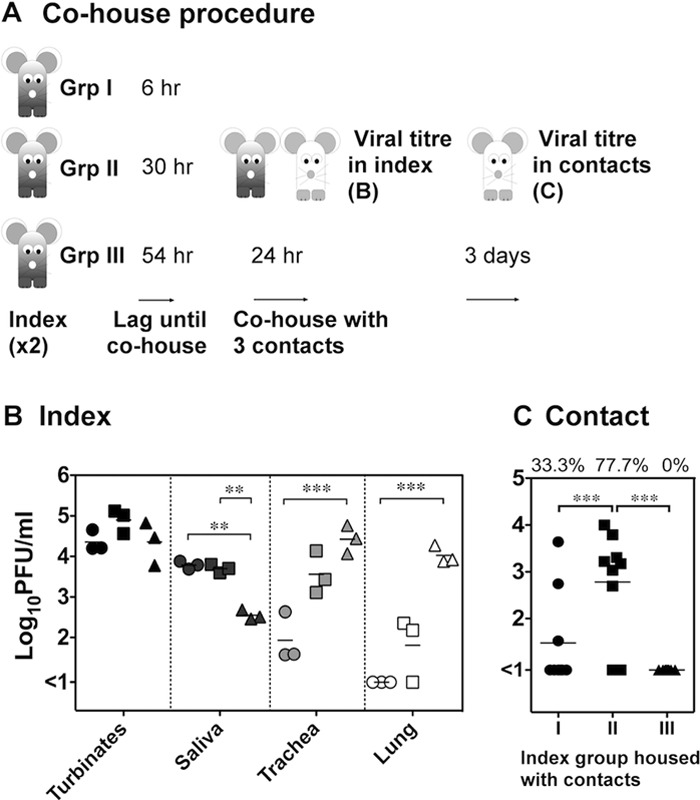
Period of transmission and its association with viral load in index mice. (A) Index mice were infected with 104.5 PFU of A/Udorn/307/72 via the upper respiratory tract, and 6 h (Group I [Grp I]), 30 h (Group II), or 54 h (Group III) later index mice were introduced to contacts. Following 24 h of cohousing, the viral load in the nasal turbinates, saliva, trachea, and lung of the index mice were measured. (B) Titers of two index mice that were cohoused in the same experiment were averaged, and the means and standard deviations of these values for three separate experiments are shown for each index group. Group I, circles; Group II, squares; Group III, triangles. (C) Three days after the cohousing period, respiratory tissues were sampled from the contacts to measure viral load. The viral titers in the nasal turbinates of each contact (n = 9) exposed to the respective index group and the percentage of contacts that became infected after cohousing (at the top) are shown. Significant differences are indicated by brackets and were determined by two-way ANOVA with a Bonferroni posttest. **, P < 0.01; ***, P < 0.001.
Viral replication was apparent in contact mice exposed to Group I and Group II but not Group III index mice (Fig. 3C), indicating that the index mice could transmit virus up to 54 h after they became infected. Group II index mice were capable of transmitting to a higher proportion of contacts (77.7%) than Group I index mice (33.3%), implying that the index mice were most contagious 30 to 54 h after their initial infection. Not only was the rate of transmission higher during this interval but the amount of virus transmitted, as assessed by viral loads in the nasal turbinates of the contact mice, also was significantly higher. The transmissible period ceased rapidly as virus was not detected in any contacts that were cohoused with Group III index mice, indicating that the index mice were not contagious after 54 h postinfection. This lack of transmission from Group III index mice correlated best with the drop in salivary viral loads in this group compared to Group I and II mice. This suggests that the presence of infectious virus in saliva is a major mode of contact-dependent spread. It remains unclear why Group II mice were better transmitters than Group I mice, although high titers of virus in saliva at the end of the cohousing period does not necessarily imply high titers from the beginning of the period.
The capacity of index mice to transmit virus is associated with their salivary viral load.
The association between the decrease of viral titers in saliva of Group III index mice 78 h following infection and reduced transmission to contacts implies that a certain load of virus in saliva is required to increase the likelihood that transmission will occur. To examine the relationship between viral loads in respiratory tract tissues of index mice and their capacity for transmission, index mice were infected via the total respiratory tract route with different doses of Udorn: 10, 102, 103, 103.7, or 104.5 PFU (Fig. 4A). Six hours postinfection, index mice were introduced to contact mice. The mice remained together for 24 h, and directly after cohousing, i.e., at 30 h postinfection, the nasal turbinates and saliva of the index mice were sampled and assayed for infectious virus. Four days after cohousing, the respiratory tract tissues of the contact mice were examined.
Fig 4.
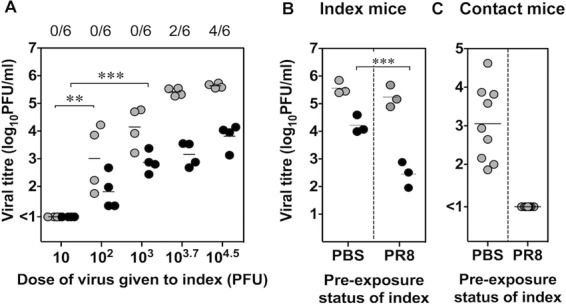
Reduced transmission correlates with lower viral loads in saliva of index mice. (A) Two index mice were infected intranasally via the total respiratory tract (TRT) route with 10, 102, 103,103.7, or 104.5 PFU of Udorn, and two pairs of index mice were each introduced to three contacts 6 h later. After 24 h of cohousing, the nasal turbinates (gray symbols) and saliva (black symbols) of the index mice were sampled, and viral loads in individual mice are expressed with a symbol. The number of contacts that became infected during cohousing, as assessed by viral loads in the nasal turbinates 3 days after introduction of the index mice, are shown at the top. (B) In a separate series of experiments, two index mice were mock infected with PBS or infected intranasally with 50 PFU of PR8 30 days prior to TRT infection with 104.5 PFU of Udorn. Six hours later, two index mice were cohoused with three contact mice for 24 h. Mean viral loads in nasal turbinates (gray) and saliva (black) of index mice are shown for three separate experiments. (C) The viral loads in nasal turbinates of individual contact mice, sampled 3 days after introduction of PBS-treated or PR8-infected index mice, are shown. The brackets indicate groups that are statistically significant by one-way (A) or two-way (B and C) ANOVA with a Bonferroni posttest. **, P < 0.01; ***, P < 0.001.
As shown in Fig. 4A, a dose of 10 PFU of Udorn was not sufficient to establish infection in the index mice due to the absence of virus detected in their nasal turbinates and saliva at 30 h after inoculation. In comparison, index mice inoculated with 102 and 103 PFU contained significantly higher viral loads in their saliva and/or nasal turbinates (P < 0.01 by one-way ANOVA), although transmission from index to contact mice did not occur at these doses (as indicated by numbers at the top of Fig. 4A). A dose of up to or >103.7 PFU was required for transmission to occur to 2/6 contacts within the first 24 h of cohousing. Index mice inoculated with 103.7 PFU seemed to contain higher viral loads in the nasal turbinates and saliva than index mice inoculated with a dose of 103 PFU; however, this trend was not statistically significant (P > 0.05 by one-way ANOVA). Although this experimental system indicated that viral dose influenced the capacity of index mice to transmit virus, these data did not elucidate whether nasal turbinates or saliva played a more important role in the process of transmission.
To further investigate the effect of reducing viral titers on transmission, index mice were given phosphate-buffered saline (PBS) intranasally or were infected with virus of a different subtype, A/PR/8/34 Mt Sinai (PR8) H1N1, 30 days prior to challenge with the transmissible strain Udorn. At 6 h postinfection, index mice were introduced to contacts. The mice remained together for 24 h, after which respiratory tissues were sampled from the index mice at 30 h postinfection (Fig. 4B). The nasal turbinates of the contacts were examined for infectious virus 4 days after the introduction of the index mice to determine the number that had become infected (Fig. 4C). Index mice that initially received PBS recorded viral titers of approximately 105.5 PFU/ml in nasal turbinates and 104.2 PFU/ml in saliva at 30 h after secondary infection with Udorn (Fig. 4B). As expected, cohousing of these index mice with contacts resulted in viral transmission to 9/9 contacts (Fig. 4C). Preinfection of index mice with PR8 resulted in a >100-fold drop in salivary viral titers of Udorn but had no significant reduction in the much higher titers in the nasal turbinates, although a slight decrease was noted (Fig. 4B). Mice preinfected with PR8 were unable to transmit Udorn virus to contacts (Fig. 4C). The fact that transmission was reduced by a significant decrease in the salivary titers of the index mice when the nasal turbinate titers remained essentially the same supports the contention that transmission is related to viral loads in saliva rather than in the nose.
A threshold level of virus in saliva is associated with an increased likelihood of transmission.
The relationship between viral titers in the saliva of index mice and transmission was further investigated. To calculate the threshold of virus titers in saliva required for transmission to occur, the percentage of infected contacts (y) was plotted as a function of the log titer of Udorn virus in saliva of the index mice (x) for each individual determination performed in this study (Fig. 5A). A similar analysis was conducted using viral loads in the nasal turbinates of index mice (Fig. 5B). Analysis revealed a significant correlation between the proportion of contacts that became infected and the viral titer in saliva of index animals (P = 0.0001 by Spearman's correlation) but not the nasal turbinates (P = 0.053). The equation defining the line of best fit (R2 = 0.5) (Fig. 5A) can be used to deduce that the likelihood of one (y = 33%) or more of three contact mice becoming infected is much greater if the titer of virus in saliva is above 3.19 log10 PFU/ml in the index mice.
Fig 5.
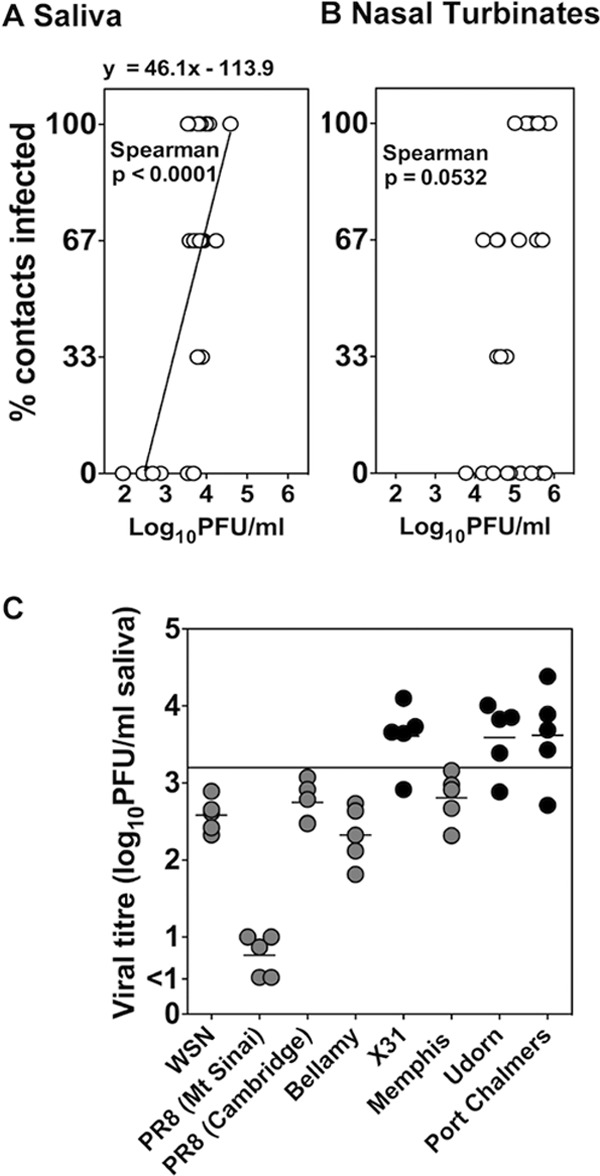
Viral titers in saliva influence transmission. For individual transmission experiments in this study, the percentage of infected contacts was plotted on the y axis against the mean viral load present in each pair of index mice on the x axis, for saliva (A) or nasal turbinates (B). Shown is the Spearman correlation for the relationship between x and y. (A) The line of best fit and the equation defining this relationship are shown. (C) Mice were infected via the upper respiratory tract with 104.5 PFU of nontransmissible (gray symbols) and transmissible (black symbols) virus strains, and 2 days later saliva samples were collected and the viral titers were determined. Viral load in saliva is plotted as a symbol for each individual mouse with the means shown. According to the equation shown in panel A, the minimum amount of virus in saliva likely to result in transmission to one of the three contact mice is indicated with a horizontal line.
To determine whether this threshold could explain the strain-specific nature of influenza virus transmission, we infected mice with nontransmissible influenza viruses represented by A/WSN/33 (WSN), PR8, A/Bellamy/42 (Bellamy), and A/Memphis/1/71 (Memphis) and transmissible strains X31, Udorn, and A/Port Chalmers/1/73 (Port Chalmers) and measured the viral titers in saliva 48 h after infection. Only transmissible strains of virus achieved an average salivary viral titer above the threshold amount of 3.19 log10 PFU/ml (Fig. 5C).
Transmission of influenza virus in the mouse model is HA dependent.
The transmissible reassortant virus X31 and the nontransmissible virus PR8 differ only in the HA and neuraminidase (NA) proteins, thus it is likely that one of these proteins influences the viral titers in saliva and therefore transmission. To explore this, reverse-engineered viruses were generated in which the HA, NA, or both HA and NA of Udorn were present on the backbone of PR8, and titers of virus in saliva 48 h postinfection for each of the viruses were assessed (Fig. 6A). PR8 and Udorn viruses that were produced through the process of reverse genetics behaved similarly to wild-type viruses, in that mice infected with Udorn but not PR8 had titers of virus in their saliva that approached the transmission threshold determined above. The inclusion of both the HA and NA of Udorn or the Udorn's HA alone onto a PR8 backbone also resulted in viruses that produced high viral loads in saliva, whereas the virus containing Udorn's NA alone produced very poor viral loads in saliva. This was despite the fact that all viruses grew to high titers in the nasal turbinates (Fig. 6B). The pattern of transmissibility of the viruses (Table 2) was as predicted from the levels of infectious virus in saliva with PR8 with Udorn's HA, but not NA, being transmissible. Together these data support the fact that transmission is determined, at least in part, by the HA expressed by the virus.
Fig 6.
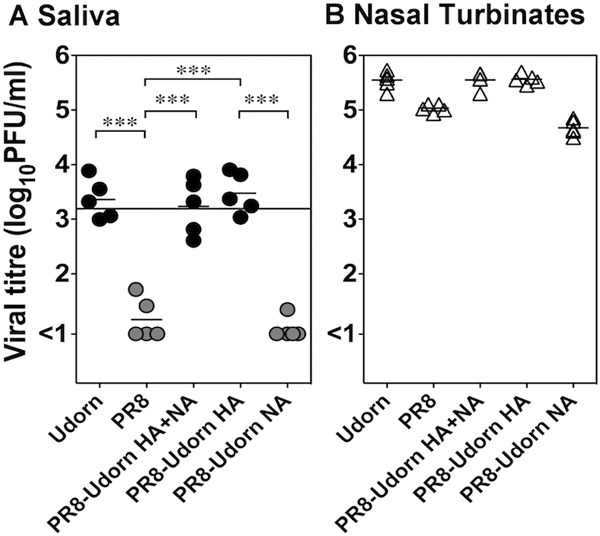
Viral loads in saliva are higher after infection with viruses containing the HA of Udorn. Mice were infected via the total respiratory tract route with 103 PFU of the reverse-engineered viruses PR8, Udorn, PR8-Udorn HA, PR8-Udorn NA, and PR8-Udorn HA + NA. PR8-Udorn HA + NA, virus containing all genes from PR8 except for the HA and NA genes of Udorn; PR8-Udorn HA, virus containing all genes from PR8 except for the HA gene of Udorn; PR8-Udorn NA, virus containing all genes from PR8 except for the NA gene of Udorn. Forty-eight h after infection, the viral titers of transmissible strains (black symbols) and nontransmissible strains (gray symbols) in saliva (A) and the nasal turbinates (B) were determined. Viral titers are shown for each individual mouse, and the mean for each group is indicated. The minimum amount of virus in saliva likely to result in transmission to one of the three contact mice (Fig. 5A) is indicated by a horizontal line. The brackets indicate groups that are statistically significant by one-way ANOVA with Bonferroni multiple-comparison tests. ***, P < 0.001.
Table 2.
Transmissibility is dependent upon the type of hemagglutinin expressed by the virus
| Virus used to infect indexa | Gene constellationb |
Growth in contact micec |
HId | % Infectede | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PB2 | PB1 | PA | HA | NP | NA | M | NS | Nasal Turbinates | Trachea | Lung | |||
| Udorn | 3.96 ± 0.8 | 2.9 ± 0.18 | 1.32 ± 1.46 | 640 | 100 | ||||||||
| PR8 | <0.9f | <0.9 | <0.9 | <10 | 0 | ||||||||
| PR8-Udorn HA + NAg | 4.8 ± 0.52 | 3.13 ± 0.35 | <0.9 | 320 | 100 | ||||||||
| PR8-Udorn HA | 3.95 ± 0.76 | 3.32 ± 1.10 | <0.9 | 1280 | 100 | ||||||||
| PR8-Udorn NA | <0.9 | <0.9 | <0.9 | <10 | 0 | ||||||||
Index mice were infected via the total respiratory tract route with 1,000 PFU of reverse-engineered viruses and cohoused with contact mice.
Reverse-engineered viruses contained different combinations of A/Udorn/307/72 (Udorn) genes (gray) and A/PuetoRico/8/34 Mt Sinai lineage (PR8) genes (white). Shading indicates the presence of the gene.
Four days after cohousing, the viral loads were measured in nasal turbinates, trachea, and lung of contacts and are expressed as mean log10 PFU/ml ± SD (n = 6).
Hemagglutination-inhibiting (HI) antibody titers of contacts are reported as means ± SD (n = 3).
Percentage of contacts containing virus in nasal turbinates (n = 6).
Detection limit of the plaque assay.
PR8-Udorn HA + NA, virus containing all genes from PR8 except for the HA and NA genes of Udorn; PR8-Udorn HA, virus containing all genes from PR8 except for the HA gene of Udorn; PR8-Udorn NA, virus containing all genes from PR8 except for the NA gene of Udorn.
DISCUSSION
In this study, we have characterized a mouse model to study transmission of influenza A virus and investigated the factors that influence transmissibility of different strains from infected index mice to naïve contacts. Contrary to the pioneering mouse transmission experiments conducted by Schulman and Kilbourne, who showed transmission occurred through aerosolized droplet nuclei (40, 41), our findings indicated that transmission required direct contact between index and contact mice. The key difference between these two transmission models was that, in Schulman's study, the index mice were infected by placing them in a chamber flooded with aerosolized viral inoculum from a nebulizer. This procedure would have coated the entire animal with viral particles that may have contributed to the aerosolized modes of transmission seen in that model. Furthermore, the Schulman model utilized experimental conditions that may have enhanced droplet transmission, such as housing index and contact mice in an enclosed chamber (41). In contrast, the experiments in our study utilized intranasal infection of the index mice and were conducted with open cages at a constant room temperature of 21°C and 31% humidity. In the absence of aerosols, however, contact-dependent transmission may still occur, for example, viral spread at high temperatures occurred only when guinea pigs had direct contact with the index animals (24).
We found that transmission of influenza A virus to contact mice occurred only when they were cohoused with index mice during the first 54 h after experimental infection; thereafter the proportion of infected contacts decreased dramatically. This supports previous studies that showed the peak period of transmission occurred between 24 and 48 h after experimental infection (41). A review of human experimental studies has likewise shown that day 2 is the peak period of viral shedding (6). The decline in viral spread observed in the mouse model is not unlike the serial interval of transmission of H1N1 in humans where transmission events decrease 3 days after symptom onset in the index (10). Whether the ability of humans to transmit virus wanes entirely in the later periods of infection, like the mouse model, is unknown, yet it is an important question to answer, as it can inform quarantine measures for future pandemics.
Transmission of influenza A virus in the present study was found to be strain specific, as most H3N2 but not H1N1 strains examined were able to transmit. A similar observation was also reported by Schulman (38), who demonstrated that A/Jap/305/57 (H2N2) transmitted to 60% of contacts and RI5+ (H2N2) transmitted to 30% of contacts, while PR8 transmitted to only 5% of contacts. While it was postulated that this was attributed to the presence of greater amounts of aerosolized virus in the chambers housing index mice and contacts (38), we have found that a contact-dependent transmission event is more likely to occur if >3.19 log10 PFU/ml of virus is present in the saliva of infected index mice. The requirement of high viral loads in saliva of index mice for transmission to contact mice suggests that saliva is facilitating viral spread. Transfer of virus in saliva could involve physical interaction between index and contact mice, although survival of virus deposited on bedding, food, or water via salivation also could contribute to transmission. The mode of contact spread was examined by placing naïve mice in cages that had previously housed infected mice for 2 days, which is the peak period of viral shedding and the time when transmission is observed (Fig. 3). The absence of any detectable virus in these contact mice at day 4 postexposure (data not shown) indicated that transmission involved physical interaction between index and contact mice rather than spread via contaminated fomites.
Only those viral strains that reached >3.19 log10 PFU/ml in saliva of the index mice were transmissible to contacts, and the failure to reach this threshold was observed for all H1N1 viruses tested despite high growth in the nasal turbinates. Even after infection with a transmissible strain, this threshold amount was not present in each individual mouse; however, this may be a result of fluctuations in viral titer during the 24-h cohousing period above or below the viral titer measured at the end of the cohousing period.
Furthermore, when the viral loads in saliva of index mice were reduced, by either decreasing viral dose or via preinfection of a heterologous subtype, the transmission to contacts was reduced. This highlights the importance of vaccines that induce mucosal immunity such as secretory IgA antibodies (8), as the presence of IgA in oral fluids could clear virus from saliva and reduce contact-dependent transmission. In humans, shedding of influenza A virus is typically measured by quantifying viral load in nasal washes and recall of preexisting immune responses, such as antibody or cytotoxic-T lymphocyte (CTL) activity, which are associated with reduced virus shedding in nasal washes (20, 30). However, considering that coughing is a more frequent symptom of influenza illness in humans than sneezing (12), sampling procedures other than collecting nasal wash, such as throat swabs, may shed light on the relationship between viral shedding and transmission. While recent studies have begun to investigate aerosols exhaled into the environment through coughing (21), little is known about infectious virus titers in saliva during human influenza infection, although viral RNA has been detected in saliva and this has been put forward as a convenient specimen to sample for diagnostic purposes (36).
Through the use of reverse genetics, we found that the replacement of the HA gene in the nontransmissible strain PR8 with that from Udorn resulted in a virus with the ability to transmit. While it has been shown in a variety of ferret models that the relationship between HA and transmission is due to adaptation to recognize specific sialic acid receptors in the respiratory tract of the host (35, 50), it is unclear how this would apply in our mouse model, as PR8 is not efficiently transmitted but is well adapted to the sialic acids present in the respiratory tract of mice, those linked to galactose in an α2,3 orientation (18). Furthermore, introduction of mutations that enhance recognition of α2,3-linked sialic acids, for example, mutation of residue 226 from L to Q in the H3 receptor binding pocket of H3N2 viruses, have not enhanced transmission in this mouse model (data not shown).
Collectively, through the use of transmissible and nontransmissible strains, our findings show that high viral loads in the saliva of index mice correlate with contact-dependent transmission of influenza A virus. While strains within the H1N1 subtype are unable to transmit in this model, numerous strains within the H3N2 subtype are transmissible, and this model could be utilized in pilot studies to investigate the effectiveness of therapeutic interventions that aim to reduce contact-dependent transmission of H3N2 influenza A virus.
ACKNOWLEDGMENTS
This work was supported by project grant 509281 and program grant 567122 from the National Health and Medical Research Council of Australia.
We thank Brendon Chua for critical review of the manuscript.
Footnotes
Published ahead of print 5 September 2012
REFERENCES
- 1. Andrewes C, Glover R. 1941. Spread of infection from the respiratory tract of the ferret. 1. Transmission of influenza A virus. Br. Exp. Pathol. 22:91–97 [Google Scholar]
- 2. Awofeso N, et al. 2001. Influenza outbreak in a correctional facility. Aust. NZ J. Public Health 25:443–446 [PubMed] [Google Scholar]
- 3. Bean B, et al. 1982. Survival of influenza-viruses on environmental surfaces. J. Infect. Dis. 146:47–51 [DOI] [PubMed] [Google Scholar]
- 4. Blachere F, et al. 2009. Measurement of airborne influenza virus in a hospital emergency department. Clin. Infect. Dis. 48:438–440 [DOI] [PubMed] [Google Scholar]
- 5. Bridges CB, Kuehnert MJ, Hall CB. 2003. Transmission of influenza: implications for control in health care settings. Clin. Infect. Dis. 37:1094–1101 [DOI] [PubMed] [Google Scholar]
- 6. Carrat F, et al. 2008. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am. J. Epidemiol. 167:775–785 [DOI] [PubMed] [Google Scholar]
- 7. Cauchemez S, et al. 2009. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N. Engl. J. Med. 361:2619–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cox R, Brokstad K, Ogra P. 2004. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand. J. Immunol. 59:1–15 [DOI] [PubMed] [Google Scholar]
- 9. Cunney R, Bialachowski A, Thornley D, Smaill F, Pennie R. 2000. An outbreak of influenza A in a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 21:449–454 [DOI] [PubMed] [Google Scholar]
- 10. Donnelly CA, et al. 2011. Serial intervals and the temporal distribution of secondary infections within households of 2009 pandemic influenza A (H1N1): implications for influenza control recommendations. Clin. Infect. Dis. 52:S123–S130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Epstein SL. 2006. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: an experiment of nature. J. Infect. Dis. 193:49–53 [DOI] [PubMed] [Google Scholar]
- 12. Fabian P, et al. 2008. Influenza virus in human exhaled breath: an observational study. PLoS One 3:e2691 doi:10.1371/journal.pone.0002691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. France A, et al. 2010. Household transmission of 2009 influenza A (H1N1) virus after a school-based outbreak in New York City, April–May 2009. J. Infect. Dis. 201:984–992 [DOI] [PubMed] [Google Scholar]
- 14. Gao Y, et al. 2009. Identification of amino acids in HA and PB2 critical for the transmission of H5N1 avian influenza viruses in a mammalian host. PLoS Pathog. 5:e1000709 doi:10.1371/journal.ppat.1000709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanley B, Borup B. 2010. Aerosol influenza transmission risk contours: a study of humid tropics versus winter temperate zone. Virol. J. 7:98–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herlocher ML, et al. 2001. Ferrets as a transmission model for influenza: sequence changes in HA1 of type A (H3N2) virus. J. Infect. Dis. 184:542–546 [DOI] [PubMed] [Google Scholar]
- 17. Hoffmann E, Krauss S, Perez D, Webby R, Webster RG. 2002. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine 20:3165–3170 [DOI] [PubMed] [Google Scholar]
- 18. Ibricevic A, et al. 2006. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J. Virol. 80:7469–7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, et al. 2007. Role of ventilation in airborne transmission of infectious agents in the built environment–a multidisciplinary systematic review. Indoor Air 17:2–18 [DOI] [PubMed] [Google Scholar]
- 20. Liang S, Mozdzanowska K, Palladino G, Gerhard W. 1994. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J. Immunol. 152:1653–1661 [PubMed] [Google Scholar]
- 21. Lindsley WG, et al. 2010. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One 5:e15100 doi:10.1371/journal.pone.0015100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lowen AC, Mubareka S, Steel J, Palese P. 2007. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 3:1470–1476 doi:10.1371/journal.ppat.0030151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lowen AC, Mubareka S, Tumpey TM, Garcia-Sastre A, Palese P. 2006. The guinea pig as a transmission model for human influenza viruses. Proc. Natl. Acad. Sci. U. S. A. 103:9988–9992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lowen AC, Steel J, Mubareka S, Palese P. 2008. High temperature blocks aerosol but not contact transmission of influenza virus. J. Virol. 82:5650–5652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maines T, et al. 2012. Local innate immune responses and influenza virus transmission and virulence in ferrets. J. Infect. Dis. 205:474–485 [DOI] [PubMed] [Google Scholar]
- 26. Maines TR, et al. 2006. Lack of transmission of H5N1 avian–human reassortant influenza viruses in a ferret model. Proc. Natl. Acad. Sci. U. S. A. 103:12121–12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marathe A, Lewis B, Chen J, Eubank S. 2011. Sensitivity of household transmission to household contact structure and size. PLoS One 6:e22461 doi:10.1371/journal.pone.0022461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mathews JD, Chesson JM, McCaw JM, McVernon J. 2009. Understanding influenza transmission, immunity and pandemic threats. Influenza Other Respir. Vir. 3:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McLean RL. 1961. The effect of ultraviolet radiation upon the transmission of epidemic influenza in long-term hospital patients. Am. Rev. Respir. Dis. 83:36–38 [Google Scholar]
- 30. McMichael A, Gotch F, Noble G, Beare P. 1983. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 309:13–17 [DOI] [PubMed] [Google Scholar]
- 31. Moser M, et al. 1979. An outbreak on influenza aboard a commercial airliner. Am. J. Epidemiol. 110:1–6 [DOI] [PubMed] [Google Scholar]
- 32. Mubareka S, et al. 2009. Transmission of influenza virus via aerosols and fomites in the guinea pig model. J. Infect. Dis. 199:858–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ng WC, Wong V, Muller B, Rawlin G, Brown LE. 2010. Prevention and treatment of influenza with hyperimmune bovine colostrum antibody. PLoS One 5:e13622 doi:10.1371/journal.pone.0013622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Novak M, Moldoveanu Z, Schafer DP, Mestecky J, Compans RW. 1993. Murine model for evaluation of protective immunity to influenza virus. Vaccine 11:55–60 [DOI] [PubMed] [Google Scholar]
- 35. Pappas C, et al. 2010. Receptor specificity and transmission of H2N2 subtype viruses isolated from the pandemic of 1957. PLoS One 5:e11158 doi:10.1371/journal.pone.0011158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robinson JL, Lee BE, Kothapalli S, Craig WR, Fox J. 2008. Use of throat swab or saliva specimens for detection of respiratory viruses in children. Clin. Infect. Dis. 46:e61–e64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schulman JL. 1967. Experimental transmission of influenza virus infection in mice. III. Differing effects of immunity induced by infection and by inactivated influenza virus vaccine on transmission of infection. J. Exp. Med. 125:467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schulman JL. 1967. Experimental transmission of influenza virus infection in mice. IV. Relationship of transmissibility of different strains of virus and recovery of airborne virus in the environment of infector mice. J. Exp. Med. 125:479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schulman JL. 1968. The use of an animal model to study transmission of influenza virus infection. Am. J. Pub. Health Nations Health 58:2092–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schulman JL, Kilbourne ED. 1962. Airborne transmission of influenza virus infection in mice. Nature 195:1129–1130 [DOI] [PubMed] [Google Scholar]
- 41. Schulman JL, Kilbourne ED. 1963. Experimental transmission of influenza virus infection in mice. I. The period of transmissibility. J. Exp. Med. 118:257–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schulman JL, Kilbourne ED. 1963. Experimental transmission of influenza virus infection in mice. II. Some factors affecting the incidence of transmitted infection. J. Exp. Med. 118:267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schurmann W, Eggers H. 1983. Antiviral activity of an alcoholic hand disinfectant-comparison of the in vitro suspension test with in vivo experiments on hands, and on individual fingertips. Antivir. Res. 3:25–41 [DOI] [PubMed] [Google Scholar]
- 44. Shaman J, Kohn M. 2009. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc. Natl. Acad. Sci. U. S. A. 106:3243–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sorrell EM, Wan H, Araya Y, Song H, Perez DR. 2009. Minimal molecular constraints for respiratory droplet transmission of an avian–human H9N2 influenza A virus. Proc. Natl. Acad. Sci. U. S. A. 106:7565–7570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Steel J, Lowen AC, Mubareka S, Palese P. 2009. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog. 5:e1000252 doi:10.1371/journal.ppat.1000252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sweet C, Fenton RJ, Price GE. (ed). 1999. The ferret as an animal model of influenza virus infection. Academic Press, London, United Kingdom [Google Scholar]
- 48. Tellier R. 2009. Aerosol transmission of influenza A virus: a review of new studies. J. R. Soc. Interface 6:S783–S790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tripp R, Tompkins SM. 2009. Animal models for evaluation of influenza vaccines. Curr. Top. Microbiol. Immunol. 333:397–412 [DOI] [PubMed] [Google Scholar]
- 50. Tumpey TM, et al. 2007. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science 315:655–659 [DOI] [PubMed] [Google Scholar]
- 51. Van Hoeven N, et al. 2009. Human HA and polymerase subunit PB2 proteins confer transmission of an avian influenza virus through the air. Proc. Natl. Acad. Sci. U. S. A. 106:3366–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. WHO 2011. Manual for the laboratory diagnosis and virological surveillance of influenza. WHO Press, World Health Organization, Geneva, Switzerland [Google Scholar]
- 53. Xie X, Li Y, Chwang A, Ho P, Seto W. 2007. How far droplets can move in indoor environments–revisiting the Wells evaporation-falling curve. Indoor Air 17:211–225 [DOI] [PubMed] [Google Scholar]


