Abstract
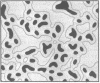
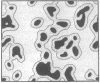
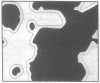
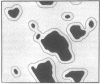
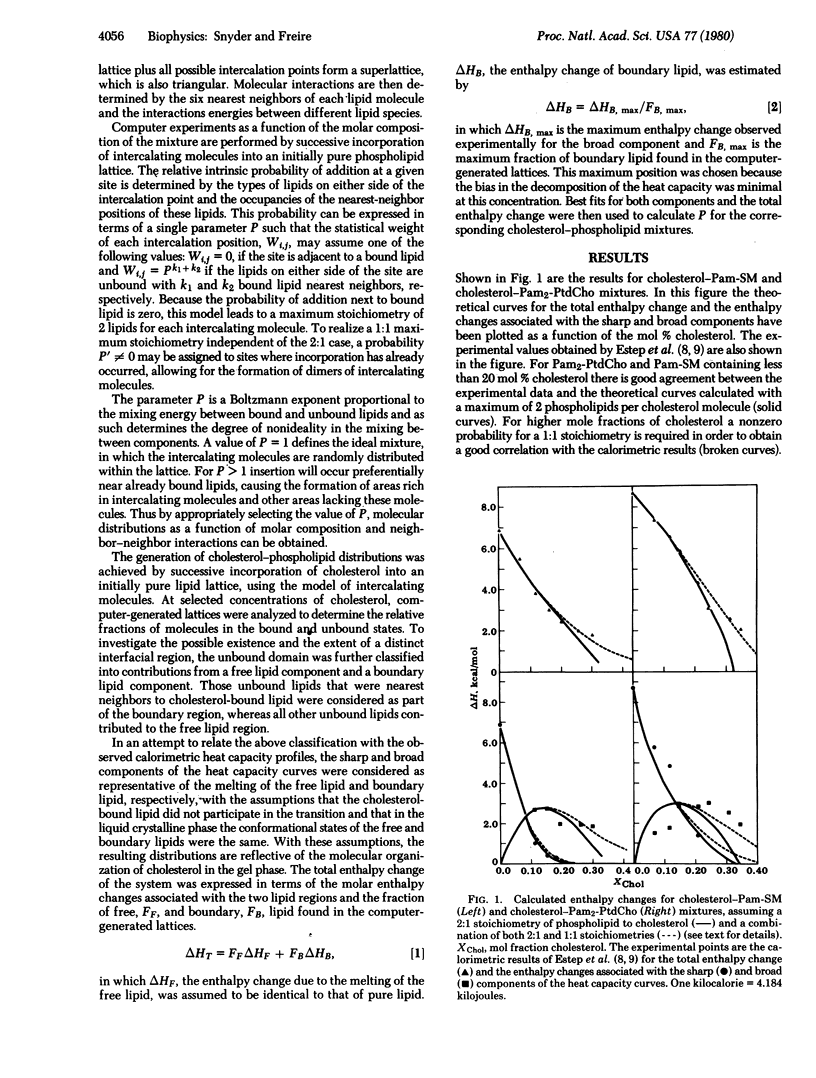
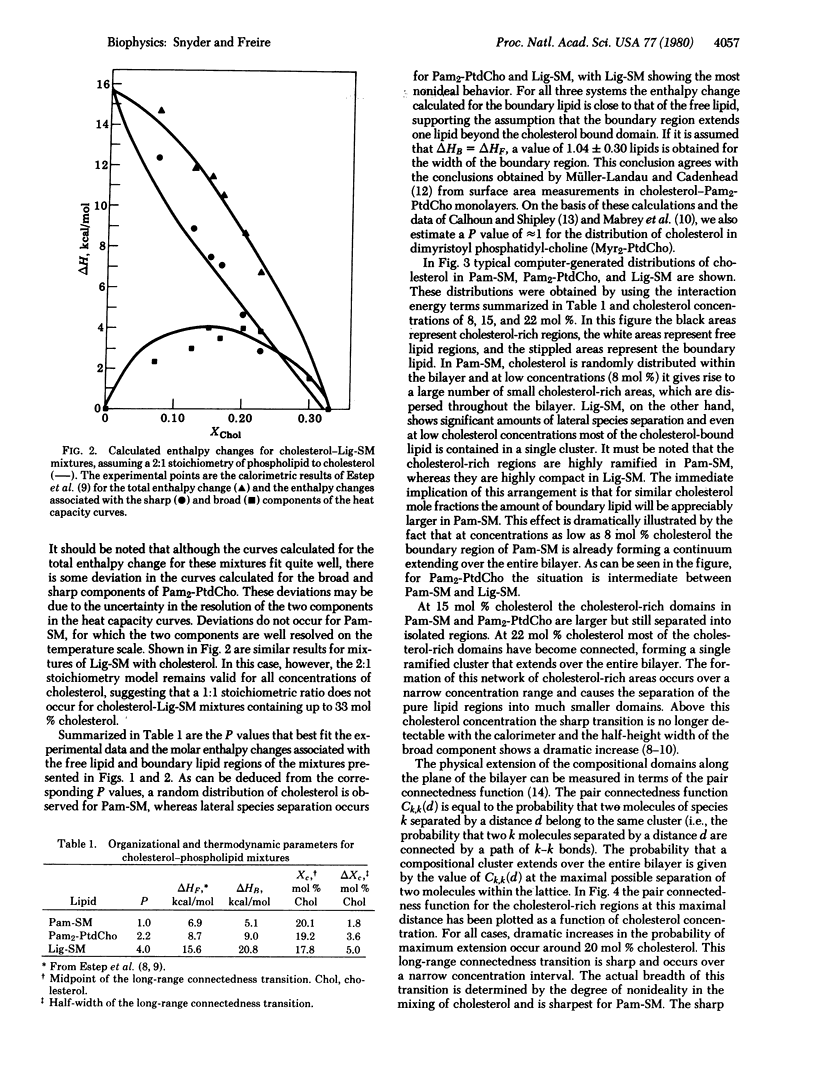
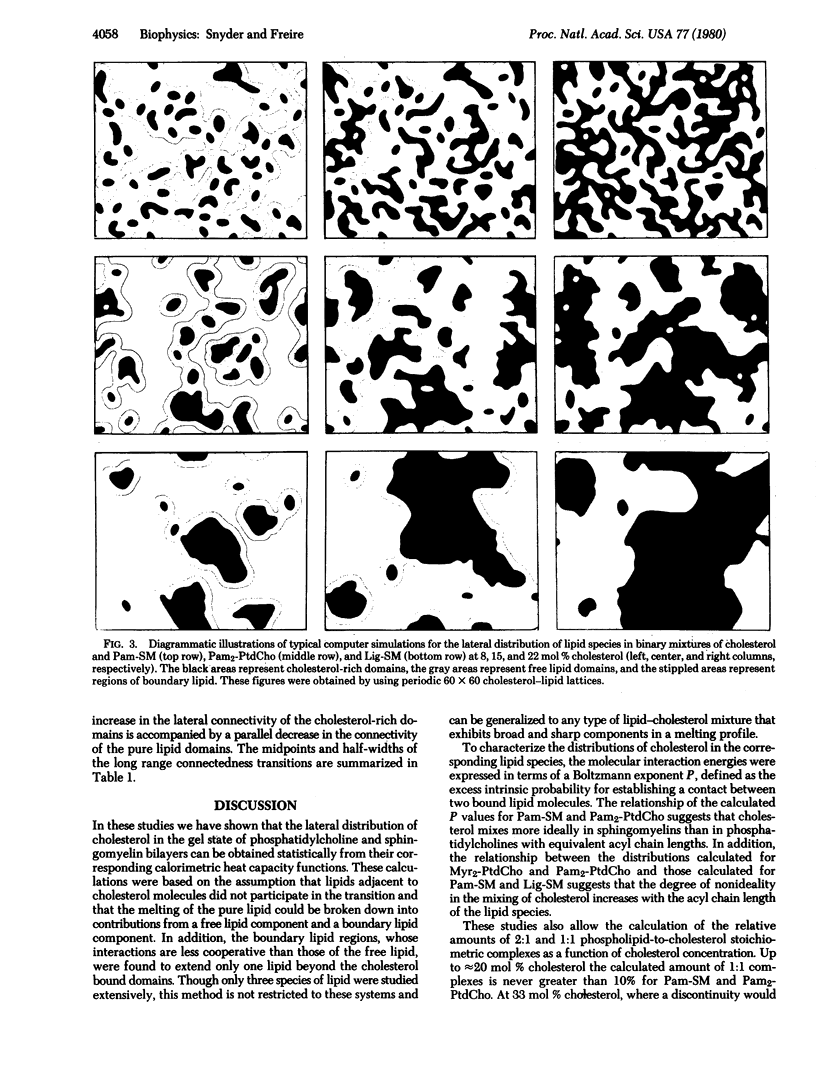
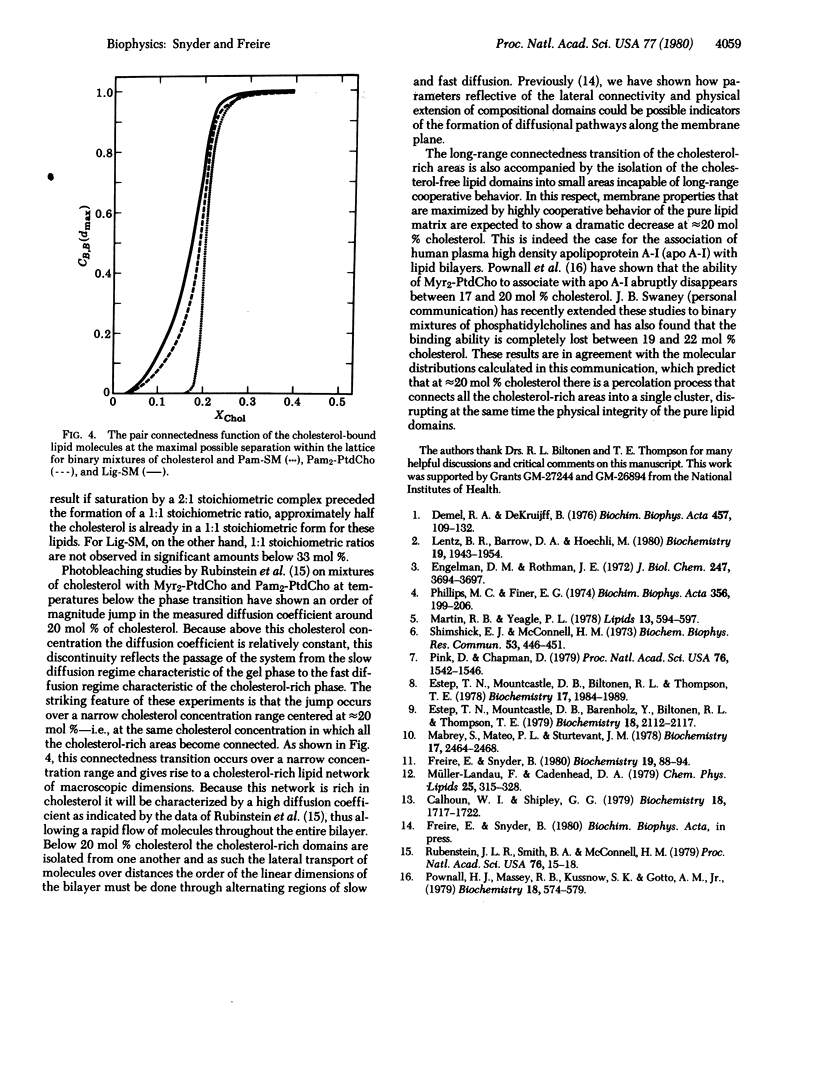
the lateral distribution of cholesterol in phospholipid bilayers has been investigated through a method of Monte Carlo calculations, using interaction energies deduced from calorimetric results for cholesterol-phospholipid mixtures. Analysis of computer-generated bilayer configurations allows calculation of the spatial localization and relative abundance of distinct regions of varying cholesterol content along the plane of the bilayer. An interfacial phospholipid region between cholesterol-bound and cholesterol-free domains is found to extend one lipid beyond the cholesterol-bound domain for mixtures of cholesterol with palmitoyl sphingomyelin, lignoceroyl sphingomyelin, and dipalmitoyl phosphatidylcholine. The results indicate that the degree of nonideality in the mixing of cholesterol is dependent on fatty acid chain length and that cholesterol mixes more ideally in sphingomyelins than in phosphatidylcholines of equal chain length. It is found that at approximately 20 mol % cholesterol the cholesterol-rich areas suddenly become connected, forming a network that extends over the entire bilayer. This change in the lateral connectivity of the cholesterol-rich domains occurs over a narrow concentration interval and is presumably responsible for the abrupt change in the lateral diffusion coefficient observed at this concentration.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calhoun W. I., Shipley G. G. Sphingomyelin--lecithin bilayers and their interaction with cholesterol. Biochemistry. 1979 May 1;18(9):1717–1722. doi: 10.1021/bi00576a013. [DOI] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Rothman J. E. The planar organization of lecithin-cholesterol bilayers. J Biol Chem. 1972 Jun 10;247(11):3694–3697. [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Barenholz Y., Biltonen R. L., Thompson T. E. Thermal behavior of synthetic sphingomyelin-cholesterol dispersions. Biochemistry. 1979 May 15;18(10):2112–2117. doi: 10.1021/bi00577a042. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Biltonen R. L., Thompson T. E. Studies on the anomalous thermotropic behavior of aqueous dispersions of dipalmitoylphosphatidylcholine-cholesterol mixtures. Biochemistry. 1978 May 16;17(10):1984–1989. doi: 10.1021/bi00603a029. [DOI] [PubMed] [Google Scholar]
- Freire E., Snyder B. Estimation of the lateral distribution of molecules in two-component lipid bilayers. Biochemistry. 1980 Jan 8;19(1):88–94. doi: 10.1021/bi00542a014. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Barrow D. A., Hoechli M. Cholesterol-phosphatidylcholine interactions in multilamellar vesicles. Biochemistry. 1980 Apr 29;19(9):1943–1954. doi: 10.1021/bi00550a034. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Mateo P. L., Sturtevant J. M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978 Jun 13;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- Martin R. B., Yeagle P. L. Models for lipid organization in cholesterol-phospholipid bilayers including cholesterol dimer formation. Lipids. 1978 Sep;13(9):594–597. doi: 10.1007/BF02535821. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Finer E. G. The stoichiometry and dynamics of lecithin-cholesterol clusters in bilayer membranes. Biochim Biophys Acta. 1974 Jul 31;356(2):199–206. doi: 10.1016/0005-2736(74)90283-1. [DOI] [PubMed] [Google Scholar]
- Pink D. A., Chapman D. Protein-lipid interactions in bilayer membranes: a lattice model. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1542–1546. doi: 10.1073/pnas.76.4.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pownall H. J., Massey J. B., Kusserow S. K., Gotto A. M., Jr Kinetics of lipid--protein interactions: effect of cholesterol on the association of human plasma high-density apolipoprotein A-I with L-alpha-dimyristoylphosphatidylcholine. Biochemistry. 1979 Feb 20;18(4):574–579. doi: 10.1021/bi00571a004. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separations in binary mixtures of cholesterol and phospholipids. Biochem Biophys Res Commun. 1973 Jul 17;53(2):446–451. doi: 10.1016/0006-291x(73)90682-7. [DOI] [PubMed] [Google Scholar]