Abstract
In the past 5 years, an atypical clinical outbreak of avian leukosis virus subgroup J (ALV-J), which contains a unique 205-nucleotide deletion in its 3′ untranslated region (3′UTR), has become epidemic in chickens in China. To determine the role of the 205-nucleotide deletion in the pathogenicity of ALV-J, a pair of viruses were constructed and rescued. The first virus was an ALV-J Chinese isolate (designated HLJ09SH01) containing the 205-nucleotide deletion in its 3′UTR. The second virus was a chimeric clone in which the 3′UTR contains a 205-nucleotide sequence corresponding to a region of the ALV-J prototype virus. The replication and pathogenicity of the rescued viruses (rHLJ09SH01 and rHLJ09SH01A205) were investigated. Compared to rHLJ09SH01A205, rHLJ09SH01 showed a moderate growth advantage in vitro and in vivo, in addition to exhibiting a higher oncogenicity rate and lethality rate in layers and broilers. Increased vascular endothelial growth factor A (VEGF-A) and vascular endothelial growth receptor subtype 2 (VEGFR-2) expression was induced by rHLJ09SH01 more so than by rHLJ09SH01A205 during early embryonic vascular development, but this increased expression disappeared when the expression levels were normalized to the viral levels. This finding suggests that the expression of VEGF-A and VEGFR-2 is associated with viral replication and may also represent a novel molecular mechanism underlying the oncogenic potential of ALV-J. Overall, our findings not only indicate that the unique 205-nucleotide deletion in the ALV-J genome occurred naturally in China and contributes to increased pathogenicity but also point to the possible mechanism of ALV-J-induced oncogenicity.
INTRODUCTION
Avian leukosis virus subgroup J (ALV-J) was first isolated from meat-type chickens in 1988 (54). All of the ALVs can be classified as endogenous (ALV-E) or exogenous viruses based on their mode of transmission, and exogenous ALVs have been further divided into different subgroups (A, B, C, D, and J) on the basis of host range, viral envelope interference, and cross-neutralization patterns (50, 54). ALV-J has primarily been associated with myeloid leukosis (ML) and other tumors in broiler breeders (49, 51, 52). Fadly and Smith reported that several ALV-J strains were isolated from broiler breeder and commercial broiler flocks experiencing ML in the United States in 1997 (19). In China, ALV-J-associated myeloid leukosis in chickens was first reported in 1999 (16), but ALV-J then became widespread, inducing disease in chickens from many parts of China and causing enormous economic losses. The morbidity and mortality rates for some flocks reached 60 and >20%, respectively, as ALV-J induced various tumors and production problems in commercial laying hens and local chicken breeds throughout China (9, 10, 23).
ALV-J belongs to the genus Alpharetrovirus of the Retroviridae family and contains the overall structure of a typical slow-transforming, replication-competent ALV: 5′-long terminal repeat (LTR)-leader-gag-pol-env-rTM-DR-1-E-LTR-3′. The 5′ untranslated region (UTR) contains the LTR-leader regions. The gag, pol, and env genes encode Gag (group-specific antigen), reverse transcriptase integrase, and the envelope glycoproteins, respectively (3, 53, 69). The 3′UTR, which contains rTM, DR-1, the E element, and the 3′LTR, is very important to the pathogenicity of ALV (59). The E element contributes to oncogenicity in certain genetic lines of chickens (12). The 3′LTR has been associated with the pathogenicity of ALV-J through its influence on the expression of viral genes and the host chromosomes (60). Precisely how the rTM and DR-1 components of the 3′UTR of ALV-J influence pathogenicity is currently unclear.
Oncogenicity is an important index of ALV-J pathogenicity. Most studies investigating the oncogenicity of retroviruses have focused on insertional mechanisms which cause the activation or inactivation of host genes (28, 40). However, tumor development is a multistep process that may be associated with different cellular pathways (24). Some studies have shown that the tumorigenesis mechanism induced by retroviruses involves retroviral insertion into host genes as well as retrovirus involvement in some signaling pathway (1). For example, the mouse mammary tumor virus (MMTV) can stimulate the production of the immunosuppressive cytokine IL-10 by subverting innate immune signaling (29). The studies investigating the mechanism of ALV-induced oncogenicity have also illustrated the ability of ALV to insert into host genes. For example, ALV proviral integration sites located in the telomerase reverse transcriptase region can enhance tumor progression (71), but whether signal transduction pathways are involved in ALV-induced tumorigenesis is currently unclear. The vascular endothelial growth factor pathway is an important pathway for tumorigenesis. Increased expression of vascular endothelial growth factor A (VEGF-A), an important component of this pathway, has been shown in numerous malignant tumors (26, 37). VEGF-A stimulates endothelial cells to migrate and divide, profoundly alters their pattern of gene expression, and protects endothelial cells from apoptosis and senescence (20). Of course, VEGF-A is essential for normal developmental vasculogenesis and angiogenesis and increases vascular permeability to plasma and plasma proteins. This last function is a characteristic property of the tumor microvasculature and a critical early step in tumor stroma generation (7, 62). VEGF-A and its receptor, vascular endothelial growth factor receptor subtype 2 (VEGFR-2), are important paracrine factors involved in tumorigenesis and angiogenesis. These factors also have clinical relevance, as evidenced by their widespread overexpression in human cancer (21, 73). It is worth noting that human leukemia is associated with the overexpression of VEGF-A and VEGFR-2 (46), but whether ALV-J-induced leukemia in chickens has a connection with VEGF-A and VEGFR-2 has not been explored.
The ALV-J strains recently isolated in China exhibit a unique 205-nucleotide deletion in the 3′UTR. The emergence of the ALV-J strain harboring the 205-nucleotide deletion and the subsequent epidemic has caused great economic losses in the Chinese poultry industry. As a result, the question of whether the 205-nucleotide deletion was related to pathogenicity became an interesting one. The analysis of the relationship between the 205-nucleotide deletion in ALV-J and ALV-J's pathogenicity has been helpful in understanding the associated molecular mechanisms of disease. Reverse genetics was used to produce a wild-type virus with the 205-nucleotide deletion and a variant virus containing a chimeric 205-nucleotide sequence from the corresponding region of the prototype virus HPRS-103. We rescued the viruses and simultaneously investigated the replication and pathogenicity of the rescued viruses both in vitro and in vivo. These approaches were developed to examine the effect of the 205-nucleotide deletion in the 3′UTR of ALV-J on viral infectivity in avian vascular endothelial cells and on viral infectivity and pathogenicity in layers and broilers.
MATERIALS AND METHODS
Cells and viruses.
Chicken embryos at day 14 of embryonation were used as a source of pure vascular endothelial cells from the abdominal aorta. The vessel segments were collected under sterile conditions, and the connective tissue was trimmed away under a dissecting microscope. The vascular tissue was transferred to a 15-ml centrifuge tube containing 5 mg collagenase and 1.25 mg elastase in 10 ml of serum-free Medium 199 (30 min, 37°C). The tissue underwent four rounds of digestion. The supernatant from the first digestion was discarded. The supernatants from the subsequent three digestions were collected and centrifuged at 300 × g for 4 min, after which the pellet was transferred into 2 ml of fetal bovine serum (FBS; HyClone Laboratories Inc., South Logan, UT) to stop digestion. The pellet-containing sera from the three digestions were pooled and centrifuged as before, and the pellet was washed twice with 5 ml of complete medium (Medium 199 containing 10% FBS and 100 μg/ml penicillin and streptomycin). The final pellet was suspended in complete medium and filtered through a Nuclepore membrane (10 μm), and the cells were seeded onto 100-mm-diameter dishes. The cells grew to confluence within 36 to 48 h and exhibited the typical morphological characteristics of vascular endothelial cells; the majority (>90%) of the cells obtained and grown in this manner stained positively for vascular endothelium-specific VEGFR-2 antibodies. The vascular endothelial cells were used at passage 2. The vascular endothelial cells and DF-1 cells were maintained in Gibco Dulbecco's modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 10% FBS at 37°C under 5% CO2.
The HLJ09SH01 strain used in this study was isolated from a commercial layer chicken in Heilongjiang Province, China. This virus dramatically reduced egg production and increased tumorigenesis in the infected chicken flocks. The genomic sequence of the HLJ09SH01 has been determined and is available in GenBank (accession no. HQ634806).
DNA alignments and phylogenetic analysis.
The sequences used for the sequence alignment are summarized in Table S1 in the supplemental material. The nucleotide sequences were aligned using the Clustal W program (http://clustalw.genome.ad.jp/), MegAlign program (Lasergene, Madison, WI), and GeneDoc program (www.psc.edu/biomed/genedoc).
Construction of the rHLJ09SH01 and rHLJ09SH01A205 infectious clones.
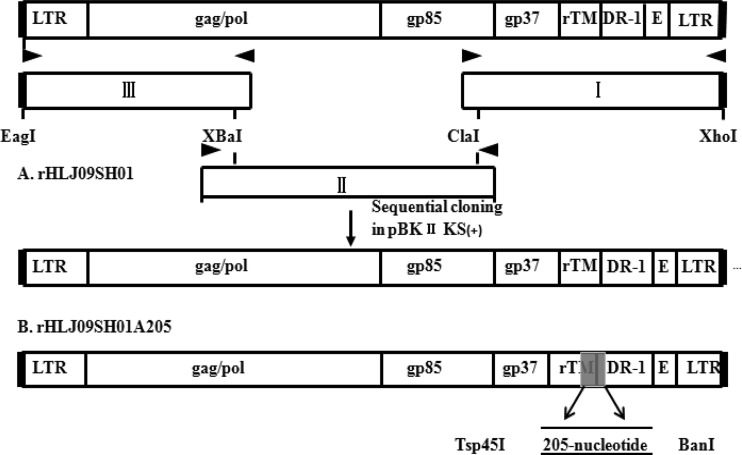
The construction strategy for the two full-length cDNA clones is illustrated in Fig. 1. Proviral DNA was extracted from HLJ09SH01-infected DF-1 cells for use as a PCR template as previously described by our laboratory (14). The HLJ09SH01 genome was cleaved into three fragments using the XbaI and ClaI restriction enzymes and amplified separately. The primer sets used to amplify fragment I were 5′-CGAGCAGCCATCGATTTCTTACTC-3′ (upstream) and 5′-CCCTCGAGTGAAGCCATCCGCTTCATGCAGGTGCTCGTAGTTGTCAGG-3′ (downstream). The primer sets used to amplify fragment II were 5′-GGCGAGGGAATGGAATCT-3′ (upstream) and 5′-GCGCCAGGAGTAAGAAATCGATG-3′ (downstream). The primer sets used to amplify fragment III were 5′-CCCGGCCGTGTAGTGTTATGCAATACTCTTATGTAACGATGAAAC-3′ (upstream) and 5′-GGCCATTTTCATGTCTAGATT-3′ (downstream). The amplicons were gel purified and subcloned into PMD-18T vectors (TaKaRa, Dalian, China) and inserted into the pBlueScript II KS(+) plasmid (TaKaRa, Dalian, China) in order by using restriction enzyme digestion. The recombinant plasmid was named pBlu-HLJ09SH01. The 205-nucleotide sequence was commercially synthesized with the Tsp45I and BanI restriction enzymes (GN_HRB00028; Sangon Biotech, Shanghai, China). The 205-nucleotide fragment was then inserted into fragment I to obtain a new fragment I. Finally, the new fragment I, fragment II, and fragment III were inserted into the pBlueScript II KS(+) plasmid in that order to obtain the pBlu-HLJ09SH01A205 recombinant plasmid.
Fig 1.
Schematic diagrams showing the construction of the rHLJ09SH01 and rHLJ09SH01A205 proviral DNA. The structure of the HLJ09SH01 proviral genome (including the LTR, gag-pol, env [gp85 and gp37], partial rTM, partial DR-1, and E element) is shown at the top. (A) The full-length HLJ09SH01 proviral genome was assembled into the pBlueScript II KS (+) plasmid from subgenomic DNA fragments (I to III) that were generated by high-fidelity PCR (not to scale) to construct the rHLJ09SH01 proviral DNA. The block arrows indicate the primers used in PCR. (B) The 205-nucleotide sequence shown in the gray box was inserted at the Tsp45I and BanI enzyme sites to construct the rHLJ09SH01A205 proviral DNA.
Viral rescue and identification.
Highly purified pBlu-HLJ09SH01 and pBlu-HLJ09SH01A205 DNA was obtained by using Qiagen Plasmid Midi kits (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The purified plasmid DNA (4 μg) from pBlu-HLJ09SH01 and pBlu-HLJ09SH01A205 was introduced into DF-1 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The culture supernatant containing the virus stocks was harvested separately 7 days later (11) and then blind passaged into secondary DF-1 cells. The rescued viruses were named rHLJ09SH01 and rHLJ09SH01A205. To determine the specificity of the rescued viruses, the secondary DF-1 cell supernatants were analyzed with an ALV group-specific antigen (p27) enzyme-linked immunosorbent assay (ELISA). An indirect immunofluorescence assay (IFA) and electron microscope identification were performed on ninth-passage infected DF-1 cells as described previously (18).
Replication kinetics of the rescued viruses.
To determine the replication kinetics of rHLJ09SH01 and rHLJ09SH01A205 in more detail, the one-step growth was analyzed. Each 60-mm-diameter plate of vascular endothelial cells or DF-1 cells (approximately 106 cells/plate) was infected with approximately 0.1 ml of 102.5/ml 50% tissue culture infectious doses (TCID50) of rHLJ09SH01 and rHLJ09SH01A205. The infected cell cultures were harvested at various time points, and the titer of infectious progeny was determined as the TCID50 per milliliter using the Reed-Muench formula directed by IFA. The mean values and standard deviations were calculated from three independent experiments.
Analysis of ALV-J p27 protein expression and reverse transcriptase (RT) activity.
The supernatants from transiently transfected DF-1 cells were harvested 72 h posttransfection, centrifuged in an Eppendorf centrifuge for 5 min at 6,000 rpm to remove floating cells, and stored at −80°C until assayed. The supernatants were infected with vascular endothelial cells. Western blot analysis was performed using a mouse anti-p27 antibody stored in our laboratory on extracts of cells using protein extraction reagent (Thermo Fisher Scientific, San Jose, CA). The RT activity was quantitated by using a colorimetric reverse transcriptase assay (Roche Applied Science, Indianapolis, IN). All of the assays were performed at the 7th day after viral infection.
Construction of subgenomic vector and detection of cytoplasmic unspliced RNA and p27 protein expression of subgenomic vector.
To generate sub-rHLJ09SH01 and sub-rHLJ09SH01A205, the 3′UTR (with and without the 205-nucleotide deletion, respectively) was inserted as XhoI/XbaI fragments into pcDNA3.1 (Invitrogen). An EcoRI/NotI fragment containing the Gag-Pol region (nucleotides 225 to 5761 for HLJ09SH01) that encompassed the 5′ and 3′ splice sites was then inserted into pcDNA3.1 containing the 3′UTR. Sub-rHLJ09SH01 and sub-rHLJ09SH01A205 were transfected into vascular endothelial cells. At 48 h posttransfection, the culture supernatants were removed and cytoplasmic RNA was harvested using a Qiagen RNeasy kit, and protein was extracted using protein extraction reagent (Thermo Fisher Scientific, San Jose, CA). RT-PCR was performed with 35 ng of the cytoplasmic RNA using the One-Step RT-PCR kit (Qiagen) to quantify the unspliced RNA. The position of RT-PCR primers is shown in Fig. 3. The following primer sequences were used: primer F, 5′-TGAAAAACAGGGACACTGATAAG-3′; primer R, 5′-AAATAATGACCACGCACACAAGTAT-3′; and probe P, 5′-6-carboxyfluorescein (FAM)-CGAAAAGTTAAACCGGACATCACCC-black hole quencher 1 (BHQ1)-3′. The amount of RNA was normalized to β-actin mRNA as previously described by our group (34). The expression of p27 was detected as described above.
Fig 3.
Deletion of 205 nucleotides in the 3′UTR contributed to the unspliced RNA export in the vascular endothelial cells. (A) The subgenomic vector (sub-rHLJ09SH01) construct. ALV-J gag-pol genes were cloned into the vector pcDNA 3.1 containing a 3′UTR with the 205-nucleotide deletion. The spliced donors (SD) and splice acceptors (SA) are indicated. (B) The subgenomic vector (sub-rHLJ09SH01A205) construct was similar to sub-rHLJ09SH01, except for the 3′UTR (sub-rHLJ09SH01A205 contained the 205 nucleotides in this region). (C) The unspliced cytoplasmic RNA was quantified using quantitative RT-PCR with primers F and R and probe P. The level of β-actin RNA was used for normalization. (D) The 205-nucleotide deletion in sub-rHLJ09SH01 increased the expression of p27 protein. Lysates from vascular endothelial cells transfected with sub-rHLJ09SH01 (lane 1) or sub-rHLJ09SH01A205 (lane 2) were incubated with mouse anti-p27 antiserum, anti-β-actin antiserum.
Analysis of rescued virus pathogenicity.
Fertile eggs from specific-pathogen-free (SPF) layers and commercial broilers were purchased from the Experimental Animal Center of the Harbin Veterinary Research Institute, CAAS, China, and housed in negative-pressure-filtered air isolators. The animal experiments were approved by the Animal Ethics Committee of the Institute. The SPF layers and commercial broilers were infected as 11-day-old embryos by intravenous inoculation with approximately 0.25 ml of 102.5/ml TCID50 of rHLJ09SH01 or rHLJ09SH01A205 virus stock per chicken. The control chickens were injected with uninfected tissue culture fluid. Cloacal swabs were taken from all of the embryo-infected chickens and tested for infection status with a direct p27 ELISA that analyzed the excretion of virus by the inoculated chickens. The chickens were observed for 238 days and examined postmortem for any gross or microscopic tumors. The feed was restricted for the commercial broilers by implementing alternate-day feeding after 6 weeks to limit excessive weight gain, as recommended by the breeder. The difference between rHLJ09SH01 and rHLJ09SH01A205 was identified using the primers KQF (5′-GTGACGGGAGGCTGCGAG-3′) and KQR (5′-GGTGTCATCACATTTCAAGC-3′).
Quantitative analysis of gene expression and inverse PCR.
Embryonic day (ED) 3.5 fertilized White Leghorn chicken embryos were treated with rHLJ09SH01 and rHLJ09SH01A205; the control chicken embryos were treated with an equal volume of DMEM. The membrane containing the extraembryonic vasculature was removed from the yolk sac and placed into a 1.5-ml microcentrifuge tube. The tumor and normal tissues were removed from the chickens after 238 days of feeding; this removal was followed by a postmortem analysis. Total RNA was extracted from tissues with the RNeasy minikit (Omega) according to the manufacturer's instructions. For reverse transcription, the First-Strand cDNA synthesis kit was used (Invitrogen) according to the manufacturer's instructions. The quantification of protein vascular endothelial growth factor A (VEGF-A) and vascular endothelial growth factor receptor subtype 2 (VEGFR-2) was performed using real-time PCR, chicken-specific VEGF-A and VEGFR-2 primers, and previously described methods (44). The inverse PCR was carried out as preciously described (68) using the restriction enzyme XbaI and the primer sets described in Table S2 in the supplemental material. The identity of the genes was analyzed with a BLAST (National Center for Biotechnology Information) search.
Viral load quantification assay.
A real-time RT-PCR assay was developed to detect the provirus. Primers and a probe were designed and synthesized as follows: ALV-J forward, 5′-TTTGCAGGCATTTCTGACTG-3′; ALV-J reverse, 5′-CCACGCACACAAGTATCATTTG-3′; and probe, 5′-FAM-CCTGGGAAGGTGAGCAAGAAGGA-BHQ1-3′. Viral DNA was extracted from the membrane containing the extraembryonic vasculature of the chicken embryos injected with rHLJ09SH01, rHLJ09SH01A205, and DMEM by using the AxyPrep body fluid viral DNA miniprep kit (Axygen, China) according to the manufacturer's instructions. The DNA was eluted into 50 μl of elution buffer, and 1 μl of the eluate served as the DNA template for real-time PCR assay. The real-time PCR was carried out in a total volume of 25 μl that contained 2.5 μl of 10× Ex Taq buffer, 2 μl of deoxynucleoside triphosphates (dNTPs) (2.5 mM), 3 μl of MgCl2 (25 mM), 1 μl of each primer (10 pM), 0.5 μl of probe (10 pM), 1 U of Ex Taq HS (TaKaRa, China), and 1 μl of cDNA. The cycling conditions for the real-time PCR were 95°C for 5 min, followed by 40 cycles of 95°C for 10 s and 60°C for 40 s. The reactions were performed in a Roche 480 PCR machine (Roche Applied Science, Switzerland).
Immunoassays.
The protein from the extraembryonic vasculature of the chicken embryo and tissue was isolated with the T-PER tissue protein extraction reagent (Thermo Fisher Scientific, San Jose, CA). The protein concentration was analyzed using the Bradford protein assay. VEGF and VEGFR-2 ELISAs (R&D Systems Europe Ltd., Abington, United Kingdom) were used to measure the VEGF-A and VEGFR-2 present in the embryonic chicken vasculature and tissue. Equal amounts of tissue protein lysates were used in the assays.
Statistical analysis.
The significance of the variability between the trials was analyzed using GraphPad Prism (version 5.0) software.
RESULTS
The 205-nucleotide deletion in the 3′UTR of ALV-J was isolated in China.
The 3′UTRs from 33 ALV-J isolates (including 2 classical ALV-J strains and 31 Chinese isolates) were analyzed (see Table S1 in the supplemental material). A comparison of the 3′UTR sequences revealed a gradual reduction in the Chinese ALV-J isolates and the emergence of a unique, naturally occurring 205-nucleotide deletion in the ALV-J genome in China (see Fig. S1 in the supplemental material). The 205-nucleotide deletion occurs between positions 7,051 and 7,255, an area that corresponds to the ALV-J prototype virus HPRS-103; 175 nucleotides are located in the 3′ end of the rTM region, and 30 nucleotides are located in the 5′ end of the DR-1 region. The full-length proviral HLJ09SH01 genome was 7,653 nucleotides. A comparison of the three major genes revealed that the similarity of the gag, pol, and env nucleotide sequences of the isolates was 95.7, 97.3, and 93.9%, respectively. In addition, the sequence of the HLJ09SH01 3′UTR was 78% similar to the 3′UTR of the prototype virus HPRS-103. The 3′UTR of HLJ09SH01 was the region with the lowest similarity to HPRS-103, and an analysis of its sequence revealed that HLJ09SH01 had the unique 205-nucleotide deletion in its 3′UTR (see Fig. S1).
Two ALV-J strains with and without the 205-nucleotide deletion in the 3′UTR were rescued.
Two viruses, rHLJ09SH01 (with the 205-nucleotide deletion in the 3′UTR) and rHLJ09SH01A205 (without the 205-nucleotide deletion), were rescued using reverse genetics. An IFA identification of the rescued viruses was performed; the DF-1 cells infected with the rescued viruses were stained by monoclonal antibodies specific for ALV-J gp85 (55) and exhibited green fluorescence (data not shown). Electron microscopy revealed two viruses with diameters of approximately 90 nm in the ultrathin sections of rHLJ09SH01- and rHLJ09SH01A205-infected DF-1 cells (data not shown). These data demonstrated that the rHLJ09SH01 and rHLJ09SH01A205 viruses had been rescued. To confirm the distinction between the two rescued viruses, the primer set consisting of KQF and KQR was used to amplify the cDNA extracted from the virus-containing cell supernatants. The rHLJ09SH01 and rHLJ09SH01A205 PCR products are approximately 170 and 374 bp, respectively (data not shown). To further confirm that, apart from the 205-nucleotide deletion, the other regions of rHLJ09SH01 and rHLJ09SH01A205 are completely identical, the cDNA extracted from the cell supernatants infected with rescued virus was amplified by PCR and sequenced. The results showed that there was no difference in the rHLJ09SH01 and rHLJ09SH01A205 cDNA sequences apart from the 205-nucleotide deletion (see Fig. S2 in the supplemental material).
The 205-nucleotide deletion in the 3′UTR enhanced viral replication in avian vascular endothelial cells but not in DF-1 cells.
Hemangioma is a new symptom of the Chinese ALV-J isolates that includes various vascular tumors or lesions composed of small vessels and multilayers of vascular endothelial cells (23, 31, 36). The 205-nucleotide deletion in the 3′UTR is also a unique characteristic of the ALV-J isolates in China. To explore the growth characteristics of ALV-J in vascular endothelial cells, primary avian vascular endothelial cells from chicken embryos were used to evaluate the replication of the rescued virus in vitro. The avian vascular endothelial cells were exposed to the viruses and single-step growth curves were determined. The results showed that rHLJ09SH01 has a moderate growth advantage over rHLJ09SH01A205 (Fig. 2A), suggesting that the 205-nucleotide deletion enhanced the replication of ALV-J in avian vascular endothelial cells. To further explore whether there is a special interaction between the vascular endothelial cells and the virus with the 205-nucleotide deletion, DF-1 cells, which are commonly used for growing ALV-J (61), were also used to evaluate the replication of the rescued virus in vitro. As shown in Fig. 2B, the replication of rHLJ09SH01 was similar to that of rHLJ09SH01A205, and the highest titer of rHLJ09SH01 in DF-1 cells was lower than that observed in the vascular endothelial cells. The results of viral growth curves on these two cell lines indicated that the 205-nucleotide deletion in the 3′UTR conferred a growth advantage to rHLJ09SH01 in vascular endothelial cells.
Fig 2.
Effect of the 205-nucleotide deletion on the expression of p27, reverse transcriptase (RT) activity, and the growth kinetics of the rescued viruses. (A) One-step growth curves for rHLJ09SH01 and rHLJ09SH01A205 in vascular endothelial cells. (B) One-step growth curves for rHLJ09SH01 and rHLJ09SH01A205 in DF-1 cells. (C) The 205-nucleotide deletion increased the levels of p27 protein in vascular endothelial cells. Lysates from mock-infected vascular endothelial cells (lane 1) or vascular endothelial cells infected with rHLJ09SH01 (lane 2) or rHLJ09SH01A205 (lane 3) were incubated with mouse anti-p27 antiserum, anti-β-actin antiserum. (D) The results of the RT assays performed on the virus particles isolated from the culture supernatants of vascular endothelial cells infected with each virus are shown. The growth curves were drawn by assaying the viral titers. The viral titers harvested at different intervals were calculated and expressed as TCID50 per milliliter. The standard deviations (error bars) from 3 independent experiments are shown. Significant differences between the rHLJ09SH01 and rHLJ09SH01A205 groups are indicated by asterisks (**, P < 0.01).
To further explore the mechanism of the moderate growth advantage of rHLJ09SH01 in vascular endothelial cells, we focused on the specific function of the 3′UTR with the 205-nucleotide deletion in rHLJ09SH01. The 3′UTR is thought to contain the constitutive transport element (CTE) that mediates the nuclear export of unspliced viral RNAs (41, 45). These unspliced RNAs are translated in the cytoplasm to produce the viral Gag and Gag-Pol polyproteins (57), which are associated with viral replication (66). After vascular endothelial cell infection with rHLJ09SH01 and rHLJ09SH01A205, we examined the expression of the capsid protein p27 and reverse transcriptase (RT) encoded by the gag-pol gene of rHLJ09SH01 and rHLJ09SH01A205. As shown in Fig. 2C, the Western blot analysis revealed that the 205-nucleotide deletion in rHLJ09SH01 increased the levels of p27 protein. Similar to the results observed for the p27 protein, the levels of RT activity in rHLJ09SH01 were higher than that found in rHLJ09SH01A205 (Fig. 2D). The increased expression of p27 protein and increased levels of RT activity of rHLJ09SH01 observed in vascular endothelial cells might reflect the greater CTE activity in rHLJ09SH01 (3′UTR with the 205-nucleotide deletion) compared to that in rHLJ09SH01A205 (3′UTR without 205-nucleotide deletion), but many other mechanisms during viral replication could not be removed. Therefore, we wanted to assay the 3′UTR activity irrespective of the ability of virus to replicate in vascular endothelial cells. Subgenomic vectors containing the gag-pol genes and the different 3′UTRs (with and without the 205-nucleotide deletion) were constructed (sub-rHLJ09SH01 and sub-rHLJ09SH01A205) (Fig. 3A and B). Because these vectors contain the 5′ splice donor and 3′ splice acceptor sites for gag-pol, the proteins encoded by the gag-pol genes were produced from an unspliced RNA, which is similar to that expressed by a provirus. The expression of p27 from transient transfection of these constructs could reflect changes in CTE-mediated nuclear export in retrovirus (58, 65). Sub-rHLJ09SH01 and sub-rHLJ09SH01A205 were transfected into vascular endothelial cells, and subsequently the cytoplasmic unspliced RNA and p27 expression was assayed 48 h later. As shown in the Fig. 3C and D, the cytoplasmic unspliced RNA and p27 expression of sub-rHLJ09SH01 were higher than those observed in sub-rHLJ09SH01A205 after transfection, which indicated that the 205-nucleotide deletion in the 3′UTR contributes to unspliced RNA nuclear export.
The rHLJ09SH01 strain showed a growth advantage over rHLJ09SH01A205 and increased pathogenicity in SPF layers and commercial broilers.
To evaluate the replication and pathogenicity of the rescued viruses in vivo, 11-day-old SPF layer and commercial broiler chicken embryos were inoculated with rHLJ09SH01 and rHLJ09SH01A205 through the vein in the chorioallantoic membrane.
The infection status of chickens infected as embryos was determined with p27 ELISA on cloacal swabs taken after hatching. The proportion of infected chickens can reflect viral replication in vivo (12, 32). As shown in Table 1, the SPF layers and commercial broilers infected with rHLJ09SH01 showed a higher infection proportion than the chickens infected with rHLJ09SH01A205 within the first 2 weeks after hatching. Up to 15 days after hatching, only 76.2% of the SPF layers infected with rHLJ09SH01A205 showed evidence of infection by cloacal swab ELISA, compared to 94.7% of the chickens infected with rHLJ09SH01. Only 64.2% of the commercial broilers infected with rHLJ09SH01A205 showed evidence of infection by cloacal swab ELISA, compared to 91.6% of the chickens infected with rHLJ09SH01. These data demonstrate that the 205-nucleotide deletion conferred an advantage on ALV-J replication in vivo.
Table 1.
Detection of ALV-J antigens in cloacal swab samples collected from inoculated chickens using p27 ELISA
| Day posthatch | No. of positive samples/total no. of samples (%) from: |
|||
|---|---|---|---|---|
| Commercial broiler |
SPF layer |
|||
| rHLJ09SH01 | rHLJ09SH01 A205 | rHLJ09SH01 | rHLJ09SH01 A205 | |
| 1 | 4/12 (33.3) | 3/14 (21.4) | 8/19 (42.1) | 6/21 (28.5) |
| 6 | 6/12 (50) | 5/14 (35.7) | 11/19 (57.9) | 8/21 (38) |
| 15 | 11/12 (91.6) | 9/14 (64.2) | 18/19 (94.7) | 16/21 (76.2) |
To compare the pathogenic effects of the rescued viruses, infected chickens were observed for 238 days. During this time, viral persistence, neutralizing antibody (VNAb) response, mortality, tumorigenesis, and vertical transmission in SPF layers and commercial broilers infected with rHLJ09SH01 and rHLJ09SH01A205 were studied.
No statistically significant differences in viral persistence were observed between the rHLJ09SH01 and rHLJ09SH01A205 groups, as most of the chickens were viremic by the time the study was terminated. An examination of serum samples taken from chickens 7 weeks after hatching revealed that none of the chickens infected with rHLJ09SH01 or rHLJ09SH01A205 had developed VNAb. To confirm that, apart from the 205-nucleotide deletion, the other regions of rHLJ09SH01 and rHLJ09SH01A205 were completely the same, the cDNA extracted from the blood of chickens infected with rHLJ09SH01 and rHLJ09SH01A205 was amplified by PCR and sequenced. The results showed no changes in any of the other regions of rHLJ09SH01 and rHLJ09SH01A205 (see Fig. S2 in the supplemental material).
The mortality of the chickens infected with rHLJ09SH01 increased significantly over those infected with rHLJ09SH01A205. In SPF layers, the lethality rate of rHLJ09SH01 and rHLJ09SH01A205 was 63.2 and 38.1%, respectively. In commercial broilers, the lethality rate of rHLJ09SH01 and rHLJ09SH01A205 was 75 and 50%, respectively (Table 2). The specific mortality data are shown in Fig. 4.
Table 2.
Mortality and tumorigenesis in rHLJ09SH01- and rHLJ09SH01A205-infected groupsa
| Virus | Embryonic age (days) at infection | Mortality (%) |
Details of tumors |
||||
|---|---|---|---|---|---|---|---|
| Commercial broiler | SPF layer | Commercial broiler |
SPF layer |
||||
| Incidence (%) | Type (n) | Incidence (%) | Type (n) | ||||
| rHLJ09SH01 | 11 | 9/12 (75) | 12/19 (63.2) | 10/12 (83.3) | HG (3) | 11/19 (57.9) | HG (4) |
| ML (3) | ML (3) | ||||||
| EB (3) | EB (2) | ||||||
| HG/EB (1) | ML/EB (1) | ||||||
| HG/EB (1) | |||||||
| rHLJ09SH01A205 | 11 | 7/14 (50) | 8/21 (38.1) | 8/14 (57.1) | HG (1) | 7/21 (33.3) | HG (2) |
| ML (2) | ML (2) | ||||||
| EB (4) | EB (2) | ||||||
| HG/ML (1) | ML/EB (1) | ||||||
| Uninfected control | 11 | 0/15 (0) | 0/20 (0) | 0/15 (0) | NA | 0/20 (0) | NA |
NA, not applicable; HG, hemangioma; ML, myelocytoma; EB, erythroblastosis.
Fig 4.
Analysis of the survival rates of chickens infected with rHLJ09SH01 and rHLJ09SH01A205. (A) In SPF layers, the survival rate for the chickens infected with rHLJ09SH01A205 was higher than the rate for the chickens infected with rHLJ09SH01. (B) In commercial broilers, the survival rate for the chickens infected with rHLJ09SH01A205 was higher than the survival rate for the chickens infected with rHLJ09SH01.
The tumorigenesis rate in the chickens infected with rHLJ09SH01 also increased. In SPF layers, the oncogenicity of rHLJ09SH01 and rHLJ09SH01A205 was 57.9 and 33.3%, respectively. In commercial broilers, the oncogenicity of rHLJ09SH01 and rHLJ09SH01A205 was 83.3 and 57.1%, respectively. The details regarding the types of tumors identified are shown in Table 2. The clinical manifestations of the rHLJ09SH01- and rHLJ09SH01A205-induced tumors and the histological examination of those tumors (diagnosed as an infiltration of neoplastic cells into normal tissues) are shown in Fig. 5.
Fig 5.
Clinical manifestation and histological examination of tumors from the chickens infected with rHLJ09SH01 and rHLJ09SH01A205. No. 1003 is the liver of an SPF layer infected with rHLJ09SH01, no. 413 is the liver of an SPF layer infected with rHLJ09SH01A205, no. 400 is the liver of a commercial broiler infected with rHLJ09SH01, and no. 51 is the liver of a commercial broiler infected with rHLJ09SH01A205. The red arrows indicate clinical lesions. (A) The histological examination of no. 1003. The liver shows the infiltration of myeloblasts (characterized by abundant eosinophilic cytoplasm) into the hepatic portal area. (B) The histological examination of no. 413. The liver shows a mild to moderate infiltration of erythroblasts (small hyperchromatic nuclei) mixed with some myeloblasts into the sinusoids. (C) The histological examination of no. 400. The liver shows foci of erythroblasts accompanied by the necrosis of the surrounding hepatocytes. (D) The histological examination of no. 51. The liver shows foci of erythroblasts in the portal area. All of the histological examinations used hematoxylin and eosin staining. Scale bar, 50 μm.
The vertical transmission of rHLJ09SH01 was more serious than that of rHLJ09SH01A205. Eggs were collected from infected layers to determine the vertical infection of these two viruses with an ALV p27 ELISA. Egg albumin was 100% (28/28) positive in the rHLJ09SH01 group but only 44% (15/34) positive in the rHLJ09SH01A205 group.
Overall, these data strongly suggest that the 205-nucleotide deletion in the 3′UTR conferred a pathogenic advantage on ALV-J in both SPF layers and commercial broilers.
rHLJ09SH01 induced higher expression of VEGF-A and VEGFR-2 in the extraembryonic vasculature and tumor tissues.
To explore the molecular mechanism underlying the stronger oncogenic potential of rHLJ09SH01, the membrane containing the extraembryonic vasculature of chicken embryos infected with rHLJ09SH01, rHLJ09SH01A205, or DMEM was collected on ED 19. RNA was prepared from this extraembryonic vasculature. As shown in Fig. 6A and B, the expression of VEGF-A and VEGFR-2 was highest in the extraembryonic vascular beds of the rHLJ09SH01-treated embryos, followed by the rHLJ09SH01A205-treated embryos and the control embryos. This result indicates two important conclusions. First, ALV-J could increase the expression of VEGF-A and VEGFR-2 during early embryonic vascular development, and second, rHLJ09SH01 might induce higher expression of VEGF-A and VEGFR-2. To further explore the reason for increased VEGF-A and VEGFR-2 expression in the rHLJ09SH01-infected embryonic vasculature, the viral loads of rHLJ09SH01 and rHLJ09SH01A205 in the extraembryonic vasculature of chicken embryos were determined. As shown in Fig. 6C, the viral load of rHLJ09SH01 in the extraembryonic vascular was higher than that of rHLJ09SH01A205 in the extraembryonic vascular, and this viral load was used to normalized the expression levels of VEGF-A and VEGFR-2. As shown in Fig. 6D and E, the differences in the expression of VEGF-A and VEGFR-2 lost significance after controlling for the viral load because of the enhanced replication of rHLJ09SH01. This indicated directly that the higher expression of VEGF-A and VEGFR-2 induced by rHLJ09SH01 was associated with its higher viral replication level. We also detected the expression of VEGF-A and VEGFR-2 in the extraembryonic vasculature at the protein level. The results of this experiment were similar to the results found at the mRNA level, as the expression of VEGF-A and VEGFR-2 at the protein level was highest in the rHLJ09SH01-treated embryos, followed by that of the rHLJ09SH01A205-treated embryos and the control embryos (Fig. 6F and G).
Fig 6.
Comparison of VEGF-A and VEGFR-2 expression in the extraembryonic vascular tissue of chick embryos infected with rHLJ09SH01 and rHLJ09SH01A205. (A) rHLJ09SH01 induced higher VEGF-A mRNA levels than rHLJ09SH01A205. (B) rHLJ09SH01 induced higher VEGFR-2 mRNA levels than rHLJ09SH01A205. (C) The higher viral load of rHLJ09SH01 in the extraembryonic vascular tissue from rHLJ09SH01-treated chick embryos compared to that of rHLJ09SH01A205 in the extraembryonic vascular tissue from rHLJ09SH01A205-treated chick embryos. (D) The levels of VEGF-A induced by rHLJ09SH01 do not differ significantly from the levels induced by rHLJ09SH01A205 when the expression of VEGF-A is normalized to the viral load. (E) The levels of VEGFR-2 induced by rHLJ09SH01 do not differ significantly from the levels induced by rHLJ09SH01A205 when the expression of VEGFR-2 is normalized to the viral load. (F) The levels of VEGF-A protein were measured by ELISA. (G) The levels of VEGFR-2 protein were measured by ELISA. The between-group differences were analyzed with a two-tailed unpaired t test. The median value for each experimental group is indicated by a horizontal bar. Significant differences between the rHLJ09SH01 and rHLJ09SH01A205 groups are indicated by asterisks (**, P < 0.01).
To further confirm that VEGF-A and VEGFR-2 are associated with ALV-J-induced tumorigenesis, we tested the mRNA and protein levels of VEGF-A and VEGFR-2 in the tumor tissues. As shown in Fig. 7, the expression of VEGF-A and VEGFR-2 in the tumor tissues was significantly higher than the expression of these proteins in the control tissues. This strongly supported a relationship between the increased expression of VEGF-A and VEGFR-2 and ALV-J-induced tumors. To explore whether higher expression of VEGF-A and VEGFR-2 is linked to ALV-J integration sites in VEGF-A and VEGFR-2, inverse PCR was used to identify the proviral integration sites in chicken embryos infected with the ALV-J virus and tumor DNA. None of the viral integration sites identified by inverse PCR were found to correspond to the VEGF-A and VEGFR-2 genes, illustrating that the ALV-J-induced expression of VEGF-A and VEGFR-2 does not result from the integration of the viral genome directly into these genes.
Fig 7.
Expression of VEGF-A and VEGFR-2 is upregulated in ALV-J-induced tumors. (A) The relative mRNA levels of VEGF-A in tumor tissues from virus-infected animals and normal tissues from uninfected animals. (B) The relative mRNA levels of VEGFR-2 in tumor tissues from virus-infected animals and normal tissues from uninfected animals. (C) The levels of VEGF-A were quantified by ELISA. (D) The levels of VEGFR-2 were quantified by ELISA. The results are representative of 3 independent experiments, each performed in triplicate. The error bars represent the standard deviations from triplicate experiments. Significant differences between the mock-infected tissues and tumor tissues are indicated by asterisks (*, P < 0.05).
DISCUSSION
Since ALV-J emerged in the 1980s, it has become highly prevalent and caused serious problems in chickens (13). While the western world was successful in eradicating ALV-J from breeding flocks, the virus has gained a stronger foothold in China since 2008 (48). To explore the possible causes of this phenomenon, the molecular epidemiology of the 3′UTR of the Chinese ALV-J isolates was investigated. A sequence analysis revealed the presence of a unique 205-nucleotide deletion in the Chinese ALV-J isolates. The data presented here provide the first evidence that this 205-nucleotide deletion favors viral replication and enhances pathogenicity in layers and broilers.
Monitoring the sequence changes in the 3′UTR of ALV is very important for clarifying the pathogenic changes of this virus, because this region contains regulatory sequences that influence viral gene expression and are important in viral replication and pathogenicity (60). A 205-nucleotide deletion was observed in this region; this deletion contains 175 nucleotides at the 3′ end of the rTM region and 30 nucleotides at the 5′ end of the DR-1 region. Both rTM and DR-1 regions are additional genes within the ALV-J 3′UTR that have not previously been reported in other subgroups of ALVs (2). rTM is a partially duplicated copy of the transmembrane-encoding portion of the env gene (4). DR-1 was exclusively identified in sarcoma viruses and ALV-J (2), and this region was involved in the posttranscriptional processing of RSV (41). Although the 205-nucleotide deletion spans two regions of ALV-J, we consider the deletion effect of the entire region as a whole because the 205-nucleotide deletion naturally occurs in the ALV-J genomics. Furthermore, this characteristic deletion in the Chinese ALV-J isolates supports the idea that the 3′UTR of ALV-J is prone to substantial mutations other than point mutations, thus allowing for the relatively rapid evolution of the virus (72). This in turn suggests that the 205-nucleotide deletion in the 3′UTR of ALV-J is the result of the natural evolution of the ALV-J genome.
While the DF-1 cell line is the common substrate for exploring the replication of ALV in vitro (39), vascular endothelial cells may be more suitable for exploring the replication of the Chinese ALV-J isolates because ALV-J can induce hemangiomas, which include various vascular tumors or lesions composed of small vessels and multiple layers of vascular endothelial cells (23, 31, 36). Thus, this study used both DF-1 and avian vascular endothelial cells to explore viral replication in vitro. It is very interesting that rHLJ09SH01 replicated more quickly than rHLJ09SH01A205 in avian vascular endothelial cells but not in DF-1 cells (Fig. 2A and B). ALV is known to be a simple retrovirus that requires a cis-acting element for nuclear export (33). Furthermore, the 3′UTR is considered to contain the constitutive transport element (CTE) that mediates the export of unspliced RNA from the nucleus to the cytoplasm to produce the viral Gag and Gag-Pol polyproteins (45, 57). The naturally occurring 205-nucleotide deletion in the 3′UTR may readjust the structure of 3′UTR and even form a more effective CTE that facilitates the transport of unspliced RNA in vascular endothelial cells (Fig. 3C and D). The replication advantage of rHLJ09SH01 in vascular endothelial cells suggests a special interaction between ALV-J and vascular endothelial cells. This interaction may reflect the presence of a vascular endothelial cell-specific nuclear export factor that can directly or indirectly interact with the new ALV-J CTE to facilitate the export of unspliced RNA from the nucleus to the cytoplasm and eventually contribute to viral replication.
To explore the influence of the 205-nucleotide deletion in ALV-J on pathogenicity, we systematically analyzed the replication and pathogenicity of rHLJ09SH01 and rHLJ09SH01A205 in SPF layers and commercial broilers. In this study, infecting the embryos in ovo resulted in tolerized viremic chickens with no neutralizing antibodies (47). Thus, the failure to detect neutralizing antibodies for either virus in these chickens 7 weeks after hatching was not unexpected. Cloacal swabs from the hatched chickens were tested for the p27 antigen to evaluate the success of the ALV-J infection process; generally, the proportion of infected chickens reflects the replication of virus in vivo (12, 32). The cloacal swabs collected 1, 6, and 15 days after hatching showed that infection with rHLJ09SH01 resulted in a higher proportion of p27 ELISA-positive SPF layers and commercial broilers than infection with rHLJ09SH01A205, indicating that the 205-nucleotide deletion confers a replication advantage on ALV-J in vivo (Table 1). This may contribute to higher pathogenicity of rHLJ09SH01, because the high rate of viral growth may outcompete the antiviral responses of the infected host, leaving the infected host vulnerable to further attack (27). Additionally, one hypothesis suggests a link between in vivo replication and pathogenicity, proposing that a virus with higher pathogenicity needs to replicate more quickly in vivo because a host infected with a more pathogenic virus may ultimately be less able to support viral reproduction (17). Here, the in vivo replication advantage of rHLJ09SH01 over rHLJ09SH01A205 led to lower survival rates for the SPF layers and commercial broilers infected with rHLJ09SH01 (Fig. 4), supporting the proposed hypothesis. Although a correlation between pathogenicity and effective viral replication has also been observed in other reports, such as the study that linked the replication of Newcastle disease virus with its pathogenicity (15) and the study that showed the increased pathogenicity of avian influenza viruses with efficient viral replication (64), the molecular mechanism mediating the relationship between the replication of a virus and its pathogenicity is not fully understood. It is conceivable that higher levels of viral genome synthesis lead to more virus production, which may overwhelm the host immune response and enhance pathogenicity (15).
Chick embryos are important for exploring the mechanisms of tumorigenesis (5, 56), because they possess a mechanism for vasculature formation that is similar to the angiogenesis that plays a vital role in tumorigenesis. Angiogenesis is necessary for tumor progression and is driven by molecular interactions between tumor cells and neighboring vascular endothelial cells (13, 30). The angiogenesis process occurring in the Chinese ALV-J-induced tumors should be observed more closely, because ALV-J-induced hemangiomas are a new feature of ALV-J infection in China (31). Furthermore, VEGF-A, a potent inducer of angiogenesis, and VEGFR-2 both play crucial roles in angiogenesis (67). Much research has shown that high levels of VEGF-A and VEGFR-2 induce tumor formation through specific signaling pathways (8, 42). To explore the possible molecular mechanism underlying the increased oncogenicity of rHLJ09SH01, ED 3.5 chick embryos were inoculated with rHLJ09SH01 and rHLJ09SH01A205 through the yolk sac (where erythropoiesis in the chicken embryo begins) (70). The results showed that rHLJ09SH01 induced higher levels of VEGF-A and VEGFR-2 than rHLJ09SH01A205 during embryonic vascular development, but this advantage was eliminated when the data were controlled for the number of viral copies (Fig. 6A to E), suggesting that the increased expression of VEGF-A and VEGFR-2 is associated with the increased replication of ALV-J. Furthermore, VEGF-A and VEGFR-2 have been considered positive regulators of tumor development that promote tumorigenesis (38, 43). Studies have described a threshold level of proteins for tumorigenesis, which means that the expression of a protein needs to be over the threshold before promoting tumorigenesis (35). In this study, the increased replication of rHLJ09SH01 increased the expression of VEGF-A and VEGFR-2, indicating an increased opportunity for rHLJ09SH01 to push the levels of VEGF-A and VEGFR-2 over the threshold for promoting tumorigenesis. This finding may not only explain the higher oncogenicity of rHLJ09SH01 compared to rHLJ09SH01A205 but also suggests a novel molecular mechanism underlying the oncogenic properties of ALV-J. The results from the inverse PCR showed no viral integration sites in VEGF-A and VEGFR-2, suggesting that the increased expression of VEGF-A and VEGFR-2 is not due to the direct integration of ALV-J into these genes. Thus, this finding could result from the ALV-J-mediated activation of the relevant VEGF signaling pathways to indirectly increase the expression of VEGF-A and VEGFR-2. While increased VEGF-A and VEGFR-2 expression has been demonstrated to contribute to tumor formation, most of these tumors occur in mammals (6, 25). To our knowledge, this is the first study to show that the upregulation of VEGF-A and VEGFR-2 in the chicken embryo and tumor tissues (Fig. 6A, B, F and G and 7) may be the mechanism for avian tumor induction by ALV-J.
Compared to the patterns in Europe and the United States, the enhanced pathogenicity of ALV-J-induced disease in China was surprising and generated much attention worldwide (9, 22, 23, 48, 63). In this study, molecular epidemiological research allowed us to hypothesize that the 205-nucleotide deletion was related to the increased pathogenicity of ALV-J in China. Using reverse genetics and animal experiments, we report the first evidence supporting the hypothesis that the 205-nucleotide deletion in the 3′UTR occurred naturally and contributes to the increased pathogenicity of ALV-J not only in layers but also in broilers. In addition, the upregulation of VEGF-A and VEGFR-2 was shown to be a possible mechanism for ALV-J oncogenicity. This study not only identified the pathogenic determinants of ALV-J but also explored the possible mechanisms of pathogenicity, generating data that will benefit the efforts to control the diseases caused by ALV-J.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Xijun He (Harbin Veterinary Research Institute, CAAS, China) for critical analysis of the pathological results. We also warmly thank Changjiang Weng and Xiaojun Wang (Harbin Veterinary Research Institute, CAAS, China) for helpful discussions, and we express our gratitude to the group of Xiaojun Wang for assistance with the construction of the subgenomic vector.
This study was funded by the National Natural Science Foundation of China (31072146), the earmarked fund for the Modern Agro-industry Technology Research System (no. nycytx-42-G3-01), the Harbin Programs for Science and Technology Development (no. 2010AA6AN034), and the National Natural Science Foundation of China (31201923).
Footnotes
Published ahead of print 19 September 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Acha-Orbea H. 1997. Retrovirus-host interactions. The mouse mammary tumor virus model. Medicina 57(Suppl. 2):43–52 [PubMed] [Google Scholar]
- 2. Bai J, Howes K, Payne LN, Skinner MA. 1995. Sequence of host-range determinants in the env gene of a full-length, infectious proviral clone of exogenous avian leukosis virus HPRS-103 confirms that it represents a new subgroup (designated J). J. Gen. Virol. 76(Pt 1):181–187 [DOI] [PubMed] [Google Scholar]
- 3. Bai J, Howes K, Payne LN, Skinner MA. 1998. Sequence analysis of an infectious proviral clone of HPRS-103 shows that it represents a new retrovirus envelope subgroup (designated J). Avian Pathol. 27:S92–S93 [Google Scholar]
- 4. Benson SJ, Ruis BL, Garbers AL, Fadly AM, Conklin KF. 1998. Independent isolates of the emerging subgroup J avian leukosis virus derive from a common ancestor. J. Virol. 72:10301–10304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bower RK. 1962. A quantitation of the influence of the chick embryo genotype on tumor production by Rous sarcoma virus on the chorioallantoic membrane. Virology 18:372–377 [DOI] [PubMed] [Google Scholar]
- 6. Cao X, Geradts J, Dewhirst MW, Lo HW. 2012. Upregulation of VEGF-A and CD24 gene expression by the tGLI1 transcription factor contributes to the aggressive behavior of breast cancer cells. Oncogene 31:104–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carmeliet P, et al. 1996. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380:435–439 [DOI] [PubMed] [Google Scholar]
- 8. Catalano A, Romano M, Martinotti S, Procopio A. 2002. Enhanced expression of vascular endothelial growth factor (VEGF) plays a critical role in the tumor progression potential induced by simian virus 40 large T antigen. Oncogene 21:2896–2900 [DOI] [PubMed] [Google Scholar]
- 9. Cheng Z, Liu J, Cui Z, Zhang L. 2010. Tumors associated with avian leukosis virus subgroup J in layer hens during 2007 to 2009 in China. J. Vet. Med. Sci. 72:1027–1033 [DOI] [PubMed] [Google Scholar]
- 10. Cheng ZQ, Zhang L, Liu SD, Zhang LJ, Cui ZZ. 2005. Emerging of avian leukosis virus subgroup J in a flock of Chinese local breed. Wei Sheng Wu Xue Bao 45:584–587 [PubMed] [Google Scholar]
- 11. Chesters PM, et al. 2002. The viral envelope is a major determinant for the induction of lymphoid and myeloid tumours by avian leukosis virus subgroups A and J, respectively. J. Gen. Virol. 83:2553–2561 [DOI] [PubMed] [Google Scholar]
- 12. Chesters PM, Smith LP, Nair V. 2006. E (XSR) element contributes to the oncogenicity of avian leukosis virus (subgroup J). J. Gen. Virol. 87:2685–2692 [DOI] [PubMed] [Google Scholar]
- 13. Cheung AK, et al. 2011. Cysteine-rich intestinal protein 2 (CRIP2) acts as a repressor of NF-kappaB-mediated proangiogenic cytokine transcription to suppress tumorigenesis and angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 108:8390–8395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng X, et al. 2010. Development of a loop-mediated isothermal amplification method for rapid detection of reticuloendotheliosis virus. J. Virol. Methods 168:82–86 [DOI] [PubMed] [Google Scholar]
- 15. Dortmans JC, Rottier PJ, Koch G, Peeters BP. 2010. The viral replication complex is associated with the virulence of Newcastle disease virus. J. Virol. 84:10113–10120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Du Y, Cui Z, Qin A. 1999. Subgroup J avian leukosis viruses in China. China Poult. Sci. 3:1–4 [Google Scholar]
- 17. Ebert D, Bull JJ. 2003. Challenging the trade-off model for the evolution of virulence: is virulence management feasible? Trends Microbiol. 11:15–20 [DOI] [PubMed] [Google Scholar]
- 18. Fadly AM. 2000. Isolation and identification of avian leukosis viruses: a review. Avian Pathol. 29:529–535 [DOI] [PubMed] [Google Scholar]
- 19. Fadly AM, Smith EJ. 1999. Isolation and some characteristics of a subgroup J-like avian leukosis virus associated with myeloid leukosis in meat-type chickens in the United States. Avian Dis. 43:391–400 [PubMed] [Google Scholar]
- 20. Ferrara N. 1999. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 77:527–543 [DOI] [PubMed] [Google Scholar]
- 21. Gannon G, et al. 2002. Overexpression of vascular endothelial growth factor-A165 enhances tumor angiogenesis but not metastasis during beta-cell carcinogenesis. Cancer Res. 62:603–608 [PubMed] [Google Scholar]
- 22. Gao Y, et al. 2011. Molecular epidemiology of avian leukosis virus subgroup J in layer flocks in China. J. Clin. Microbiol. 50:953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao YL, et al. 2010. Avian leukosis virus subgroup J in layer chickens, China. Emerg. Infect. Dis. 16:1637–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia MI, Kaserman J, Chung YH, Jung JU, Lee SH. 2007. Herpesvirus saimiri STP-A oncoprotein utilizes Src family protein tyrosine kinase and tumor necrosis factor receptor-associated factors to elicit cellular signal transduction. J. Virol. 81:2663–2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. George ML, et al. 2001. VEGF-A, VEGF-C, and VEGF-D in colorectal cancer progression. Neoplasia 3:420–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giles FJ. 2001. The vascular endothelial growth factor (VEGF) signaling pathway: a therapeutic target in patients with hematologic malignancies. Oncologist 6(Suppl. 5):32–39 [DOI] [PubMed] [Google Scholar]
- 27. Grimm D, et al. 2007. Replication fitness determines high virulence of influenza A virus in mice carrying functional Mx1 resistance gene. Proc. Natl. Acad. Sci. U. S. A. 104:6806–6811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ihle JN, et al. 1989. Activation of the c-H-ras proto-oncogene by retrovirus insertion and chromosomal rearrangement in a Moloney leukemia virus-induced T-cell leukemia. J. Virol. 63:2959–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jude BA, et al. 2003. Subversion of the innate immune system by a retrovirus. Nat. Immunol. 4:573–578 [DOI] [PubMed] [Google Scholar]
- 30. Kawasaki H, et al. 2001. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer 91:2026–2032 [DOI] [PubMed] [Google Scholar]
- 31. Lai H, et al. 2011. Isolation and characterization of emerging subgroup J avian leukosis virus associated with hemangioma in egg-type chickens. Vet. Microbiol. 151:275–283 [DOI] [PubMed] [Google Scholar]
- 32. Landman WJ, et al. 2002. Effect of an in ovo infection with a Dutch avian leukosis virus subgroup J isolate on the growth and immunological performance of SPF broiler chickens. Avian Pathol. 31:59–72 [DOI] [PubMed] [Google Scholar]
- 33. LeBlanc JJ, Uddowla S, Abraham B, Clatterbuck S, Beemon KL. 2007. Tap and Dbp5, but not Gag, are involved in DR-mediated nuclear export of unspliced Rous sarcoma virus RNA. Virology 363:376–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li K, et al. 2012. Development of TaqMan real-time PCR assay for detection and quantitation of reticuloendotheliosis virus. J. Virol. Methods 179:402–408 [DOI] [PubMed] [Google Scholar]
- 35. Li Q, Ishikawa TO, Oshima M, Taketo MM. 2005. The threshold level of adenomatous polyposis coli protein for mouse intestinal tumorigenesis. Cancer Res. 65:8622–8627 [DOI] [PubMed] [Google Scholar]
- 36. Liekens S, Verbeken E, Vandeputte M, De Clercq E, Neyts J. 1999. A novel animal model for hemangiomas: inhibition of hemangioma development by the angiogenesis inhibitor TNP-470. Cancer Res. 59:2376–2383 [PubMed] [Google Scholar]
- 37. Liu W, et al. 2011. Tumor-derived vascular endothelial growth factor (VEGF)-a facilitates tumor metastasis through the VEGF-VEGFR1 signaling pathway. Int. J. Oncol. 39:1213–1220 [DOI] [PubMed] [Google Scholar]
- 38. Lorenzon E, et al. 2012. MULTIMERIN2 impairs tumor angiogenesis and growth by interfering with VEGF-A/VEGFR2 pathway. Oncogene 31:3136–3147 [DOI] [PubMed] [Google Scholar]
- 39. Maas R, van Zoelen D, Oei H, Claassen I. 2006. Replacement of primary chicken embryonic fibroblasts (CEF) by the DF-1 cell line for detection of avian leucosis viruses. Biologicals 34:177–181 [DOI] [PubMed] [Google Scholar]
- 40. Nagayama J, et al. 2001. Retrovirus insertion and transcriptional activation of the multidrug-resistance gene in leukemias treated by a chemotherapeutic agent in vivo. Blood 97:759–766 [DOI] [PubMed] [Google Scholar]
- 41. Ogert RA, Beemon KL. 1998. Mutational analysis of the Rous sarcoma virus DR posttranscriptional control element. J. Virol. 72:3407–3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohtani K, et al. 2008. High expression of GADD-45alpha and VEGF induced tumor recurrence via upregulation of IL-2 after photodynamic therapy using NPe6. Int. J. Oncol. 32:397–403 [PubMed] [Google Scholar]
- 43. Oka N, et al. 2007. VEGF promotes tumorigenesis and angiogenesis of human glioblastoma stem cells. Biochem. Biophys. Res. Commun. 360:553–559 [DOI] [PubMed] [Google Scholar]
- 44. Oosterbaan AM, Steegers EA, Ursem NT. 2012. The effects of homocysteine and folic acid on angiogenesis and VEGF expression during chicken vascular development. Microvasc. Res. 83:98–104 [DOI] [PubMed] [Google Scholar]
- 45. Paca RE, Ogert RA, Hibbert CS, Izaurralde E, Beemon KL. 2000. Rous sarcoma virus DR posttranscriptional elements use a novel RNA export pathway. J. Virol. 74:9507–9514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Padro T, et al. 2002. Overexpression of vascular endothelial growth factor (VEGF) and its cellular receptor KDR (VEGFR-2) in the bone marrow of patients with acute myeloid leukemia. Leukemia 16:1302–1310 [DOI] [PubMed] [Google Scholar]
- 47. Pandiri AR, et al. 2008. Distribution of viral antigen gp85 and provirus in various tissues from commercial meat-type and experimental White Leghorn Line 0 chickens with different subgroup J avian leukosis virus infection profiles. Avian Pathol. 37:7–13 [DOI] [PubMed] [Google Scholar]
- 48. Payne LN, Nair V. 2012. The long view: 40 years of avian leukosis research. Avian Pathol. 41:11–19 [DOI] [PubMed] [Google Scholar]
- 49. Payne LN. 1998. HPRS-103: a retro virus strikes back. The emergence of subgroup J avian leukosis virus. Avian Pathol. 27:S36–S45 [Google Scholar]
- 50. Payne LN, et al. 1991. A novel subgroup of exogenous avian leukosis virus in chickens. J. Gen. Virol. 72(Pt 4):801–807 [DOI] [PubMed] [Google Scholar]
- 51. Payne LN, Gillespie AM, Howes K. 1991. Induction of myeloid leukosis and other tumours with the HPRS-103 strain of ALV. Vet. Rec. 129:447–448 [DOI] [PubMed] [Google Scholar]
- 52. Payne LN, Gillespie AM, Howes K. 1992. Myeloid leukaemogenicity and transmission of the HPRS-103 strain of avian leukosis virus. Leukemia 6:1167–1176 [PubMed] [Google Scholar]
- 53. Payne LN, Gillespie AM, Howes K. 1993. Unsuitability of chicken sera for detection of exogenous ALV by the group-specific antigen ELISA. Vet. Rec. 132:555–557 [DOI] [PubMed] [Google Scholar]
- 54. Payne LN, Howes K, Gillespie AM, Smith LM. 1992. Host range of Rous sarcoma virus pseudotype RSV(HPRS-103) in 12 avian species: support for a new avian retrovirus envelope subgroup, designated J. J. Gen. Virol. 73(Pt 11):2995–2997 [DOI] [PubMed] [Google Scholar]
- 55. Qin A, Lee LF, Fadly A, Hunt H, Cui Z. 2001. Development and characterization of monoclonal antibodies to subgroup J avian leukosis virus. Avian Dis. 45:938–945 [PubMed] [Google Scholar]
- 56. Quigley JP, Armstrong PB. 1998. Tumor cell intravasation alu-cidated: the chick embryo opens the window. Cell 94:281–284 [DOI] [PubMed] [Google Scholar]
- 57. Rabson AB, Graves BJ. 1997. Synthesis and processing of viral RNA. In Coffin JM, Hughes SH, Varmus HE. (ed), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- 58. Rizvi TA, et al. 2009. Role of a heterologous retroviral transport element in the development of genetic complementation assay for mouse mammary tumor virus (MMTV) replication. Virology 385:464–472 [DOI] [PubMed] [Google Scholar]
- 59. Robinson HL, Blais BM, Tsichlis PN, Coffin JM. 1982. At least two regions of the viral genome determine the oncogenic potential of avian leukosis viruses. Proc. Natl. Acad. Sci. U. S. A. 79:1225–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ruddell A. 1995. Transcription regulatory elements of the avian retroviral long terminal repeat. Virology 206:1–7 [DOI] [PubMed] [Google Scholar]
- 61. Sacco MA, Howes K, Smith LP, Nair VK. 2004. Assessing the roles of endogenous retrovirus EAV-HP in avian leukosis virus subgroup J emergence and tolerance. J. Virol. 78:10525–10535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Senger DR, et al. 1983. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219:983–985 [DOI] [PubMed] [Google Scholar]
- 63. Sun H, et al. 2010. Haemangiomas, leiomyosarcoma and myeloma caused by subgroup J avian leukosis virus in a commercial layer flock. Acta Vet. Hung. 58:441–451 [DOI] [PubMed] [Google Scholar]
- 64. Suzuki K, et al. 2009. Association of increased pathogenicity of Asian H5N1 highly pathogenic avian influenza viruses in chickens with highly efficient viral replication accompanied by early destruction of innate immune responses. J. Virol. 83:7475–7486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Swanson CM, Puffer BA, Ahmad KM, Doms RW, Malim MH. 2004. Retroviral mRNA nuclear export elements regulate protein function and virion assembly. EMBO J. 23:2632–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Swiersy A, Wiek C, Reh J, Zentgraf H, Lindemann D. 2011. Orthoretroviral-like prototype foamy virus Gag-Pol expression is compatible with viral replication. Retrovirology 8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Takahashi S. 2011. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol. Pharm. Bull. 34:1785–1788 [DOI] [PubMed] [Google Scholar]
- 68. Tsuei DJ, et al. 1994. Inverse polymerase chain reaction for cloning cellular sequences adjacent to integrated hepatitis B virus DNA in hepatocellular carcinomas. J. Virol. Methods 49:269–284 [DOI] [PubMed] [Google Scholar]
- 69. Tsukamoto K, Kono Y, Arai K, Kitahara H, Takahashi K. 1985. An enzyme-linked immunosorbent assay for detection of antibodies to exogenous avian leukosis virus. Avian Dis. 29:1118–1129 [PubMed] [Google Scholar]
- 70. Wilt FH. 1974. The beginnings of erythropoiesis in the yolk sac of the chick embryo. Ann. N. Y. Acad. Sci. 241:99–112 [DOI] [PubMed] [Google Scholar]
- 71. Yang F, Xian RR, Li Y, Polony TS, Beemon KL. 2007. Telomerase reverse transcriptase expression elevated by avian leukosis virus integration in B cell lymphomas. Proc. Natl. Acad. Sci. U. S. A. 104:18952–18957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zavala G, Cheng S, Jackwood MW. 2007. Molecular epidemiology of avian leukosis virus subgroup J and evolutionary history of its 3′ untranslated region. Avian Dis. 51:942–953 [DOI] [PubMed] [Google Scholar]
- 73. Zhou YH, Tan F, Hess KR, Yung WK. 2003. The expression of PAX6, PTEN, vascular endothelial growth factor, and epidermal growth factor receptor in gliomas: relationship to tumor grade and survival. Clin. Cancer Res. 9:3369–3375 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.