Abstract
The crown gall teratoma tumor line BT37, incited by Agrobacterium tumefaciens strain T37, has been found to contain part of the tumor-inducing plasmid, pTi T37, of the inciting strain. This foreign DNA segment, called T-DNA, is maintained at several copies per diploid tumor cell. We have examined subcellular DNA fractions from this tumor line in an effort to determine whether T-DNA is in chloroplasts, mitochondria, or nuclei. Tumor cell chloroplast DNA exhibited EcoRI and Bst I endonuclease cleavage patterns identical to those of normal tobacco chloroplast DNA. Tumor cell mitochondrial DNA exhibited a complex Bst I cleavage pattern that did not differ detectably from that of normal tobacco mitochondrial DNA. Southern blots of tumor chloroplast and mitochondrial cleavage products did not hybridize with labeled pTi T37 DNA, whereas blots of tumor cell nuclear DNA cleavage products hybridized strongly. We conclude that T-DNA is located not in chloroplasts or mitochondria but rather in the nuclear fraction of this tumor line.
Keywords: plant tumors, teratomas, fragmentation patterns, chloroplast DNA, mitochondrial DNA
Full text
PDF


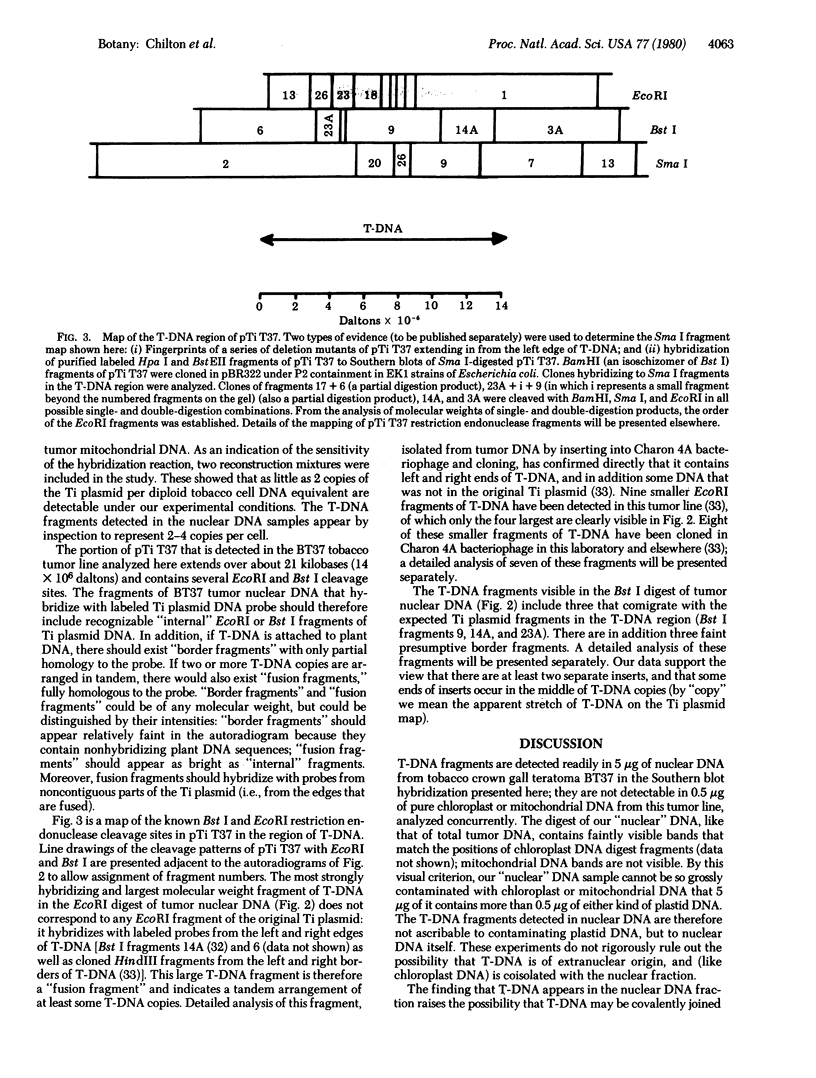

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bomhoff G., Klapwijk P. M., Kester H. C., Schilperoort R. A., Hernalsteens J. P., Schell J. Octopine and nopaline synthesis and breakdown genetically controlled by a plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1976 May 7;145(2):177–181. doi: 10.1007/BF00269591. [DOI] [PubMed] [Google Scholar]
- Braun A. C., Wood H. N. Suppression of the neoplastic state with the acquisition of specialized functions in cells, tissues, and organs of crown gall teratomas of tobacco. Proc Natl Acad Sci U S A. 1976 Feb;73(2):496–500. doi: 10.1073/pnas.73.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton M. D., Drummond M. H., Merio D. J., Sciaky D., Montoya A. L., Gordon M. P., Nester E. W. Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell. 1977 Jun;11(2):263–271. doi: 10.1016/0092-8674(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Gurley W. B., Kemp J. D., Albert M. J., Sutton D. W., Callis J. Transcription of Ti plasmid-derived sequences in three octopine-type crown gall tumor lines. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2828–2832. doi: 10.1073/pnas.76.6.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. H., Künsch U., Temperli A. Simple rapid procedures for isolation of tobacco leaf nuclei. Anal Biochem. 1972 Sep;49(1):48–57. doi: 10.1016/0003-2697(72)90241-2. [DOI] [PubMed] [Google Scholar]
- Kelly R. B., Cozzarelli N. R., Deutscher M. P., Lehman I. R., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem. 1970 Jan 10;245(1):39–45. [PubMed] [Google Scholar]
- Kleckner N. Translocatable elements in procaryotes. Cell. 1977 May;11(1):11–23. doi: 10.1016/0092-8674(77)90313-0. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. The molecular size and conformation of the chloroplast DNA from higher plants. Biochim Biophys Acta. 1975 Sep 1;402(3):372–390. doi: 10.1016/0005-2787(75)90273-7. [DOI] [PubMed] [Google Scholar]
- Le Pecq J. B. Use of ethidium bromide for separation and determination of nucleic acids of various conformational forms and measurement of their associated enzymes. Methods Biochem Anal. 1971;20:41–86. doi: 10.1002/9780470110393.ch2. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo D. J., Nutter R. C., Montoya A. L., Garfinkel D. J., Drummond M. H., Chilton M. D., Gordon M. P., Nester E. W. The boundaries and copy numbers of Ti plasmid T-DNA vary in crown gall tumors. Mol Gen Genet. 1980;177(4):637–643. doi: 10.1007/BF00272674. [DOI] [PubMed] [Google Scholar]
- Montoya A. L., Chilton M. D., Gordon M. P., Sciaky D., Nester E. W. Octopine and nopaline metabolism in Agrobacterium tumefaciens and crown gall tumor cells: role of plasmid genes. J Bacteriol. 1977 Jan;129(1):101–107. doi: 10.1128/jb.129.1.101-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaky D., Montoya A. L., Chilton M. D. Fingerprints of Agrobacterium Ti plasmids. Plasmid. 1978 Feb;1(2):238–253. doi: 10.1016/0147-619x(78)90042-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sümegi J., Breedveld D., Hossenlopp P., Chambon P. A rapid procedure for purification of EcoRI endonuclease. Biochem Biophys Res Commun. 1977 May 9;76(1):78–85. doi: 10.1016/0006-291x(77)91670-9. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Turgeon R., Wood H. N., Braun A. C. Studies on the recovery of crown gall tumor cells. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3562–3564. doi: 10.1073/pnas.73.10.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Larebeke N., Engler G., Holsters M., Van den Elsacker S., Zaenen I., Schilperoort R. A., Schell J. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 1974 Nov 8;252(5479):169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Montoya A. L., Merlo D. J., Drummond M. H., Chilton M. D., Nester E. W., Gordon M. P. Foreign DNA sequences in crown gall teratomas and their fate during the loss of the tumorous traits. Mol Gen Genet. 1980;177(4):707–714. doi: 10.1007/BF00272683. [DOI] [PubMed] [Google Scholar]
- Zaenen I., Van Larebeke N., Van Montagu M., Schell J. Supercoiled circular DNA in crown-gall inducing Agrobacterium strains. J Mol Biol. 1974 Jun 15;86(1):109–127. doi: 10.1016/s0022-2836(74)80011-2. [DOI] [PubMed] [Google Scholar]







