Abstract
Listeria monocytogenes is an important food-borne pathogen, and its bacteriophages find many uses in detection and biocontrol of its host. The novel broad-host-range virulent phage P70 has a unique morphology with an elongated capsid. Its genome sequence was determined by a hybrid sequencing strategy employing Sanger and PacBio techniques. The P70 genome contains 67,170 bp and 119 open reading frames (ORFs). Our analyses suggest that P70 represents an archetype of virus unrelated to other known Listeria bacteriophages.
TEXT
Listeria monocytogenes is a Gram-positive food-borne pathogen that causes the rare but severe disease listeriosis, which is characterized by a high mortality rate (6, 28). Although many Listeria-specific bacteriophages have been described, only a limited number have been fully sequenced (2, 5, 15, 21, 29). Most Listeria phages are temperate siphoviruses, with B054, A511, and P100 being the only described myoviruses (2, 5, 15). Listeria can be efficiently detected and controlled by the use of highly virulent and specific bacteriophages (2, 22, 23). Bacteriophage-based biocontrol of food-borne pathogens has received increasing interest in recent years, due to its practicability, efficacy, safety, and low cost (8, 10–12). In our search for additional virulent phages useful for biocontrol, we discovered phage P70, which turned out to be a novel type of Listeria-specific bacterial virus.
Phage P70 was isolated from a grass silage sample from a dairy farm in Homburg, Switzerland. Samples taken were amplified using L. monocytogenes WSLC1001 and WSLC1042 as phage hosts, and potential phage in the resulting lysates was assessed by the soft agar overlay method (16, 27). Phage P70 was propagated on semiconfluent soft agar plates of Listeria ivanovii WSLC3009 (16). Purified phage stocks were obtained by polyethylene glycol (PEG) precipitation and density gradient centrifugation (15, 24). Host-range analysis was performed as described elsewhere (18, 19), and P70 was found to feature an unusually broad host range within the genus Listeria, infecting serovars 1/2a, 1/2b, 1/2c, 4a, 4c, 4d, 4e, 5, 6a, and 6b with similar efficiencies. A total of 18 of 29 Listeria strains tested were lysed by P70, including food isolates, reference strains, and the novel species L. marthii (9) but not L. rocourtiae (17). Consistent with other Listeria phages isolated so far, P70 does not bind to or lyse serovar 3 strains that lack the rhamnose residue in the polyribitol phosphate backbone of wall teichoic acids (7, 26).
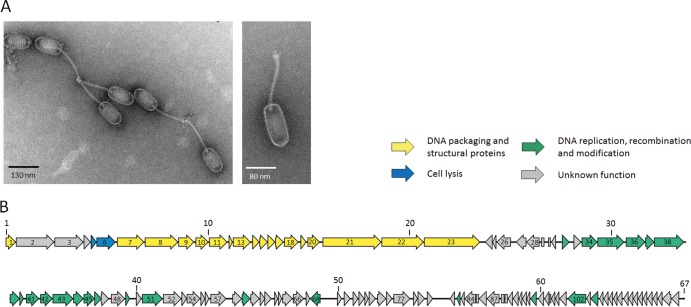
Transmission electron micrographs of P70 were obtained as described previously (15) and revealed a particle morphology previously not observed for Listeria phages, featuring a long, flexible tail connected to an elongated head (B3 morphotype [1]) with a head measuring 128 nm in length and 57 nm in diameter and a tail length of 141 nm (Fig. 1A). P70 is a member of the Siphoviridae family in the order Caudovirales.
Fig 1.
(A) Transmission electron micrographs of P70 show the elongated head and long, noncontractile tail, characteristic for the B3 morphotype of the Siphoviridae family in the order Caudovirales. Scale bars are indicated. (B) Genome map of P70. ORFs are drawn to scale and numbered consecutively. Numbers above the graph indicate nucleotide positions in kb. ORFs with an assigned function for the encoded gene product are colored, and the main functional modules are drawn in different colors.
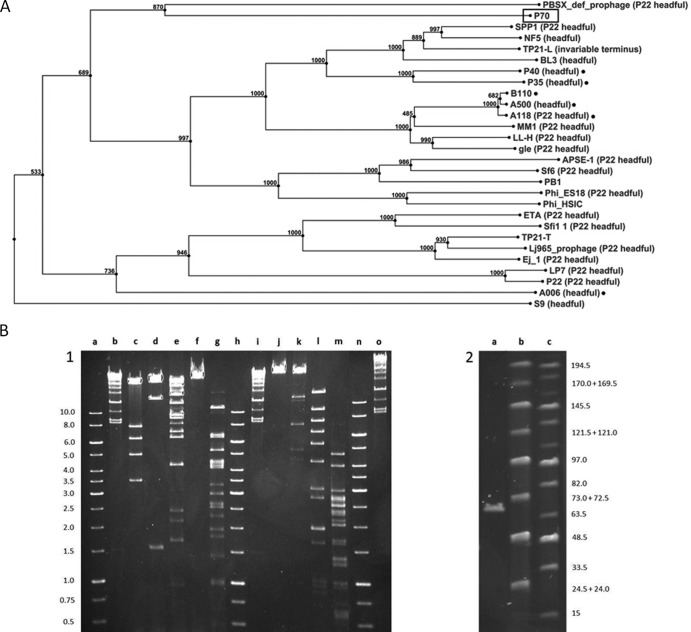
The P70 genome sequence was obtained from DNA purified by CsCl density gradient purification as previously described (15), employing a novel Sanger/PacBio hybrid sequencing strategy. A total of 643 Sanger reads (average length, 884 nucleotides [nt]) were obtained from three shotgun libraries (insert sizes of 2 kb, 1.5 kb, and 0.5 kb) and assembly of seven contigs, however, also resulting in a very high cloning bias (15a). Then, an alignment scaffold was created by sequencing of P70 DNA on a Pacific Biosciences RS device (3 SMRT cells; 2-kb library). A total of 354,960,481 prefilter bases were reduced to 70,378,003 postfilter bases (33,848 reads), with an average read length of 1,881 nt and an accuracy of 0.871. A single contig of approximately 68 kb could be assembled from these data, revealing a missing sequence of approximately 4 kb in the Sanger assembly. The frequent features of PacBio assemblies are false indels (15a) of several bases in length, which could be corrected by high sequencing coverage and combination with the Sanger reads, using the software CLC Genomics Workbench 5.1 (CLC Bio, Denmark). A hybrid Sanger/PacBio assembly produced a unit genome sequence of 67,170 bp, with a 752-fold average coverage. Ambiguities, conflicts, and few regions of lower coverage were addressed by direct Sanger sequencing using P70 DNA as template. The P70 genome features 117 open reading frames, and putative functions could be assigned to 39 gene products based on sequence homology and amino acid motif searches (Fig. 1B). The sequence has been deposited in GenBank (accession no. JX442241). Nucleotide dot plot analyses against the genomes of Listeria phages 01761, A006, A118, A500, A511, B025, B053, B054, P35, and P40 (results not shown) revealed that P70 possesses a unique genome, about 50% larger than other Listeria siphoviruses and half the size of the largest known Listeria phage genomes (5, 20). The genome is divided into several functional modules corresponding to lifestyle (5) but highly mosaic in nature and composed of more modules than any other known Listeria phage genomes (5, 20). Major structural component-encoding genes could be identified, such as the terminase subunits, portal, major capsid protein, major tail protein, head-tail connector, and a putative tail fiber. Surprisingly, the lysis cassette of P70 is located at a highly unusual position between genes encoding the large and small terminase subunits. A total of six HNH endonucleases seem to be encoded. Interestingly, P70 also specifies several functions normally associated with a temperate lifestyle, such as ORF44 (Holliday junction resolvase), ORF49 (DNA replication inhibitor), or ORF53 (C1-like repressor protein), albeit the phage is strictly virulent in its nature. It produces clear plaques on any Listeria strain, and all attempts to lysogenize host strains of different species and serovars (SV) failed (including EGD-e [SV 1/2a], WSLC1001 [SV 1/2c], WSLC1042 [SV 4b], and WSLC3009 [SV 5]). P70 appears to represent an archetype of Listeria phage, similar to P35 and P40 (5, 13), but not featuring consistent protein homologies to known phages of Listeria or other Gram-positive organisms. Further evidence for the unique nature of P70 was obtained from a phylogenetic tree of the large terminase subunit of 109 phages (3, 4) (Fig. 2A). This places P70 on a separate branch, clearly distinct from all other phages. The closest homologues are found in the PBSX-defective prophage of Bacillus subtilis (25), which supposedly employs a P22-like headful packaging mechanism. We investigated the physical genome structure of P70 by restriction profiles, Bal31 nuclease digestion (15, 21), pulsed-field gel electrophoresis (PFGE), and Sanger runoff sequencing (Fig. 2B). Both restriction profiles and Bal31 digests pointed to a genome with fixed, invariable ends (3, 14, 21). Heating of restriction digests prior to electrophoresis did not change the fragment pattern, making the presence of cohesive genome ends unlikely (data not shown) (3, 14, 21). Finally, runoff sequencing of terminal restriction fragments confirmed P70 to feature terminal repeats with lengths of 381 bp.
Fig 2.
(A) Snapshot of a terminase phylogeny tree of 109 TerL amino acid sequences and P70 TerL computed by using the neighbor-joining method from a ClustalW alignment. Numbers at nodes indicate bootstrap values. The P70 TerL sequence is highlighted, other Listeria phage TerL sequences are indicated by black dots. (B) Restriction profile analysis of P70 genome structure. (Panel 1) Restriction digest of purified P70 DNA with nine different restriction endonucleases. Lanes c to g and j to m: KpnI, ClaI, SwaI, SalI, EcoRI, EcoRV, PacI, NdeI, and HindIII. Lanes a, b, h, i, n, and o: 1-kb and Lambda mix markers (Fermentas). (Panel 2) PFGE run of P70 DNA (lane a). Lanes b and c: midrange PFGE markers I and II (New England BioLabs). Numbers indicate marker band sizes in kilobases.
In conclusion, the unique siphovirus P70 represents a novel species of virulent Listeria bacteriophage, featuring a distinct virion morphology, genome size, and structure unrelated to any other Listeria phage reported previously.
ACKNOWLEDGMENT
We are grateful to Steven Hagens, ETH Zurich, for help with the isolation of phage P70.
Footnotes
Published ahead of print 19 September 2012
REFERENCES
- 1. Ackermann HW. 2003. Bacteriophage observations and evolution. Res. Microbiol. 154:245–251 [DOI] [PubMed] [Google Scholar]
- 2. Carlton RM, Noordman WH, Biswas B, de Meester ED, Loessner MJ. 2005. Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul. Toxicol. Pharmacol. 43:301–312 [DOI] [PubMed] [Google Scholar]
- 3. Casjens S, Gilcrease EB. 2009. Determining DNA packaging strategy by analysis of the termini of the chromosomes in tailed-bacteriophage virions, In Clokie MRJ, Kropinski A. (ed), Bacteriophages—methods and protocols, vol 2 Molecular and applied aspects, p 91–111 Humana Press, New York, NY: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casjens SR, et al. 2005. The generalized transducing Salmonella bacteriophage ES18: complete genome sequence and DNA packaging strategy. J. Bacteriol. 187:1091–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dorscht J, et al. 2009. Comparative genome analysis of Listeria bacteriophages reveals extensive mosaicism, programmed translational frameshifting, and a novel prophage insertion site. J. Bacteriol. 191:7206–7215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farber JM, Peterkin PI. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fiedler F. 1988. Biochemistry of the cell surface of Listeria strains: a locating general view. Infection 16(Suppl 2):S92–S97 [DOI] [PubMed] [Google Scholar]
- 8. Garcia P, Martinez B, Obeso JM, Rodriguez A. 2008. Bacteriophages and their application in food safety. Lett. Appl. Microbiol. 47:479–485 [DOI] [PubMed] [Google Scholar]
- 9. Graves LM, et al. 2010. Listeria marthii sp. nov., isolated from the natural environment, Finger Lakes National Forest. Int. J. Syst. Evol. Microbiol. 60:1280–1288 [DOI] [PubMed] [Google Scholar]
- 10. Hagens S, Loessner MJ. 2007. Application of bacteriophages for detection and control of foodborne pathogens. Appl. Microbiol. Biotechnol. 76:513–519 [DOI] [PubMed] [Google Scholar]
- 11. Hagens S, Loessner MJ. 2010. Bacteriophage for biocontrol of foodborne pathogens: calculations and considerations. Curr. Pharm. Biotechnol. 11:58–68 [DOI] [PubMed] [Google Scholar]
- 12. Hagens S, Offerhaus ML. 2008. Bacteriophages—new weapons for food safety. Food Technol. 62:46–54 [Google Scholar]
- 13. Hodgson DA. 2000. Generalized transduction of serotype 1/2 and serotype 4b strains of Listeria monocytogenes. Mol. Microbiol. 35:312–323 [DOI] [PubMed] [Google Scholar]
- 14. Kim KP, et al. 2012. Inducible Clostridium perfringens bacteriophages ΦS9 and ΦS63: Different genome structures and a fully functional sigK intervening element. Bacteriophage 2:89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klumpp J, et al. 2008. The terminally redundant, nonpermuted genome of Listeria bacteriophage A511: a model for the SPO1-like myoviruses of Gram-positive bacteria. J. Bacteriol. 190:5753–5765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a. Klumpp J, Fouts D, Sozhamannan S. Next generation sequencing technologies and the changing landscape of phage genomics. Bacteriophage, in press. doi:10.4161/bact.22111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kropinski AM, Mazzocco A, Waddell TE, Lingohr E, Johnson RP. 2009. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 501:69–76 [DOI] [PubMed] [Google Scholar]
- 17. Leclercq A, et al. 2010. Listeria rocourtiae sp. nov. Int. J. Syst. Evol. Microbiol. 60:2210–2214 [DOI] [PubMed] [Google Scholar]
- 18. Loessner MJ. 1991. Improved procedure for bacteriophage typing of Listeria strains and evaluation of new phages. Appl. Environ. Microbiol. 57:882–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loessner MJ, Busse M. 1990. Bacteriophage typing of Listeria species. Appl. Environ. Microbiol. 56:1912–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loessner MJ, Calendar R. 2005. The Listeria bacteriophages, p 593–601 The bacteriophages. Oxford University Press, New York, NY [Google Scholar]
- 21. Loessner MJ, Inman RB, Lauer P, Calendar R. 2000. Complete nucleotide sequence, molecular analysis and genome structure of bacteriophage A118 of Listeria monocytogenes: implications for phage evolution. Mol. Microbiol. 35:324–340 [DOI] [PubMed] [Google Scholar]
- 22. Loessner MJ, Rees CE, Stewart GS, Scherer S. 1996. Construction of luciferase reporter bacteriophage A511::luxAB for rapid and sensitive detection of viable Listeria cells. Appl. Environ. Microbiol. 62:1133–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loessner MJ, Rudolf M, Scherer S. 1997. Evaluation of luciferase reporter bacteriophage A511::luxAB for detection of Listeria monocytogenes in contaminated foods. Appl. Environ. Microbiol. 63:2961–2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 25. Siegel EC, Marmur J. 1969. Temperature-sensitive induction of bacteriophage in Bacillus subtilis 168. J. Virol. 4:610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Uchikawa K, Sekikawa I, Azuma I. 1986. Structural studies on teichoic acids in cell walls of several serotypes of Listeria monocytogenes. J. Biochem. 99:315–327 [DOI] [PubMed] [Google Scholar]
- 27. Van Twest R, Kropinski AM. 2009. Bacteriophage enrichment from water and soil. Methods Mol. Biol. 501:15–21 [DOI] [PubMed] [Google Scholar]
- 28. Vazquez-Boland JA, et al. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zimmer M, Sattelberger E, Inman RB, Calendar R, Loessner MJ. 2003. Genome and proteome of Listeria monocytogenes phage PSA: an unusual case for programmed + 1 translational frameshifting in structural protein synthesis. Mol. Microbiol. 50:303–317 [DOI] [PubMed] [Google Scholar]