Abstract
The effects of plant hormones and sucrose (Suc) on potato (Solanum tuberosum L.) tuberization were studied using in vitro cultured single-node cuttings. Tuber-inducing (high Suc) and -noninducing (low Suc or high Suc plus gibberellin [GA]) media were tested. Tuberization frequencies, tuber widths, and stolon lengths were measured during successive stages of development. Endogenous GAs and abscisic acid (ABA) were identified and quantified by high-performance liquid chromatography and gas chromatography-mass spectrometry. Exogenous GA4/7 promoted stolon elongation and inhibited tuber formation, whereas exogenous ABA stimulated tuberization and reduced stolon length. Indoleacetic acid-containing media severely inhibited elongation of stolons and smaller sessile tubers were formed. Exogenous cytokinins did not affect stolon elongation and tuber formation. Endogenous GA1 level was high during stolon elongation and decreased when stolon tips started to swell under inducing conditions, whereas it remained high under noninducing conditions. GA1 levels were negatively correlated with Suc concentration in the medium. We conclude that GA1 is likely to be the active GA during tuber formation. Endogenous ABA levels decreased during stolon and tuber development, and ABA levels were similar under inducing and noninducing conditions. Our results indicate that GA is a dominant regulator in tuber formation: ABA stimulates tuberization by counteracting GA, and Suc regulates tuber formation by influencing GA levels.
Hormones have been suggested to play a prominent role in the control of tuberization (for review, see Ewing, 1987; Vreugdenhil and Struik, 1989). The possible role of GAs in this process was extensively studied, mainly in experiments in which this compound was applied exogenously. These experiments showed that application of GA promotes stolon elongation and inhibits tuber formation (Smith and Rappaport, 1969; Kumar and Wareing, 1972). It was also reported that a decline of GA activity in potato (Solanum tuberosum L.) plants is associated with tuberization (Okazawa, 1959, 1960; Smith and Rappaport, 1969; Pont Lezica, 1970; Railton and Wareing, 1973; Krauss and Marschner, 1982). Although the inhibiting effect of GA on potato tuberization is well documented, the interaction between GA and other regulating factors on the control of tuberization is still a matter of debate. ABA is normally regarded as a regulator that reduces GA-promoted processes in plant development. It was assumed that ABA is a promoting hormone in potato tuberization (Okazawa and Chapman, 1962; Marschner et al., 1984). However, the functions of ABA with respect to stolon elongation, tuber initiation, and tuber growth are not clear.
Most of the data concerning GA levels obtained from potato come from the determination of endogenous GA-like substances by bioassays (Okazawa, 1959, 1960; Smith and Rappaport, 1960, 1969; Boo, 1961; Rappaport and Smith, 1962; Racca and Tizio, 1968; Pont Lezica, 1970; Railton and Wareing, 1973). These reports described the determination of GA activity in leaves, roots, sprouts, and mature and resting tubers of potato. However, they did not present information about GA levels in the tuber-forming stolon, and the various types of GAs could not be identified with bioassays. In addition, the data might be unreliable because of the interference of acidic growth inhibitors (Jones et al., 1988). Because the concentration of GAs in plants can be extremely low, especially in vegetative parts, a highly sensitive detection method, combined GC-MS, is used more frequently for the precise analysis of GAs. Jones et al. (1988) applied GC-MS to determine the levels of GAs in mature tubers. They detected GA20 and GA1 in sprouts of potato tubers, but their interest and data were mainly focused on the GA-analysis technique. GA1 was detected by GC-MS in potato leaves, and higher levels were found in tall plants, as compared with dwarf plants, only the latter ones tuberizing under long-day conditions (Van den Berg et al., 1995a, 1995b). To our knowledge, there is no report of the determination of endogenous GA levels during the whole developing process of tuber formation with GC-MS.
The effects of ABA on potato tuberization have been investigated with a number of experiments. El-Antably et al. (1967) observed a stimulation of tuber formation by ABA applied to the leaves of long-day-grown potato plants. Wareing and Jennings (1980) found that ABA can replace the effect of the leaf by promoting tuberization in induced cuttings. The promoting effect of exogenous ABA was also demonstrated by the increasing numbers of tubers (Abdullah and Ahmad, 1980), the earlier initiation of tubers, and the formation of sessile tubers (Menzel, 1980). However, other reports described inhibition of tuberization by ABA, with the effect depending on concentration and variety (Palmer and Smith 1969b; Hussey and Stacey, 1984). The analysis of endogenous ABA showed an increase of ABA level under tuber-inducing conditions (Krauss and Marschner, 1982) and a reduction of ABA content when N was supplied during tuber formation (Marschner et al., 1984). Further studies are needed to clarify the conflicting data concerning the effect of ABA on tuberization.
In contrast to GA and ABA, less attention has been paid to cytokinins and IAA. Palmer and Smith (1969a) first reported an increase of the tuberization frequency on various cytokinin-containing media. Their observation was supported by further investigations of exogenous cytokinins (Kumar and Wareing, 1974; Hussey and Stacey, 1984). The level of endogenous cytokinins was high in the induced tissue (Mauk and Langille, 1978) and also during the later stage of tuber growth (Obata-Sasamoto and Suzuki, 1979). However, exogenous cytokinins may also convert a stolon into a leaf-bearing shoot (Kumar and Wareing, 1972). Harmey et al. (1966) observed that IAA treatment induced larger tubers at an earlier stage, whereas Obata-Sasamoto and Suzuki (1979) reported that the auxin content was high in the stage before tuber initiation and decreased during tuber development.
In addition to hormones, the level of sugars in the medium, notably Suc, also affects tuberization in vitro (Lawrence and Barker, 1963). However, not much is known about a possible interaction between hormonal and nutritional regulation of tuber formation.
In this study we used a well-defined tuberization system, culturing single-node cuttings in vitro. Uniform growth of stolons and tubers was obtained under tuber-inducing and -noninducing conditions by varying the level of Suc in the medium. The possible roles of GA and ABA were assessed in two ways: by applying these regulators to the culture medium and by measuring endogenous levels under inducing and noninducing conditions using GC-MS. In addition, the effects of exogenous cytokinins and auxin were determined.
MATERIALS AND METHODS
In Vitro Culture of Single-Node Cuttings
Single-node cuttings from short-day-grown potato (Solanum tuberosum L. var Bintje) plants were cultured in vitro, essentially as described by Hendriks et al. (1991). The basal medium consisted of a modified Murashige and Skoog (1962) medium with one-tenth of the standard concentration of nitrate salts (169 mg L−1 NH4NO3 and 190 mg L−1 KNO3), and was solidified with 0.8% (w/v) agar. This basal medium was supplemented with Suc or hormones as desired.
Exogenous Hormones and Suc Treatments
The hormones were applied to the medium after filter sterilization. The standard concentration of GA4/7 for a noninducing treatment was 0.5 μm. In a separate experiment, the following series of GA4/7 concentrations was added to the medium: 0, 0.01, 0.03, 0.1, and 0.3 to 1.0 μm. The exogenously added GA4/7 did not contain detectable amounts of GA1. The concentrations used for ABA, IAA, and BA in the medium were 3.8 μm (1 μg mL−1), 5.7 μm (1 μg mL−1), and 5 μm, respectively. Tuber development was also tested in hormone-free medium supplemented with Suc in various concentrations that ranged from 1 to 8% (w/v). The growth of the developing buds was observed daily by measuring the lengths of the stolons and the widths of the tubers and by determining the frequency of tuberization. The data for each culture condition are the averages collected from about 20 uniformly grown tubers or stolons.
Determination of Endogenous GA and ABA Levels
Samples were harvested at d 0, 2, 4, 5, and 10 in treatments with 1% Suc, 8% Suc, or 8% Suc plus 0.5 μm GA4/7. Whole developing buds were excised from the cuttings and analyzed. Stolon tips, generally less than 1 cm in length (including the apical region, subapical region, and young leaves), and the elongated parts of the stolons were separately analyzed at d 4 and 10. Tubers and the elongated parts of the stolons grown on tuber-inducing medium were separately analyzed at d 10. After the samples were cut they were immediately placed in vials cooled on ice. Within 45 min, the samples were frozen in liquid N2 and stored at −80°C.
For each sample, at least 0.3 g fresh weight was used. Every sample included 10 to 200 developing buds, depending on the developmental stage. The samples were homogenized in liquid N2. Then, 200 mL of 80% methanol was added together with 0.25 g of ascorbic acid to prevent oxidation reactions during the extraction. The homogenate was stirred overnight at 4°C. The insoluble material was removed first by centrifugation at 18,000g for 30 min and then by filtration with a no. 4 glass filter.
Internal standard GAs (1 mL each of 100 ng/mL [2H]GA1, [2H]GA4, and [2H]GA9 from Dr. L.N. Mander, Canberra, Australia) were added to the filtrate. Two hundred and fifty microliters of [3H]ABA (35,000 dpm) and approximately 0.1 ng of [3H]GA1, [3H]GA4, and [3H]GA9 (specific activity 3000–4000 Bq ng−1) containing 420 Bq of each GA were added, and radioactivity was checked at successive steps to monitor the loss of GAs and ABA during purification. The amount of tritiated tracer added was less than 5% of the amount in the samples, and no correction was made. The aqueous methanol was evaporated under reduced pressure at 35°C. The residue was dissolved in 15 mL of water and adjusted to pH 8.0. The sample was washed with petroleum ether (3 times 50 mL) to remove lipids, fats, pigments, etc. The aqueous sample was then poured onto a PVP column to remove acidic impurities. The sample was adjusted to pH 2.5 and partitioned against ethyl acetate.
The ethyl acetate fraction containing the free GAs and ABA was partitioned against 5% sodium bicarbonate to leave the neutral compounds in ethyl acetate. Then the sodium bicarbonate solution with the free GAs and ABA was partitioned against ethyl acetate again and dried. The residue was dissolved in 10 mL of water, adjusted to pH 8.0, and applied onto a 5-cm-long QAE-Sephadex A-25 (Pharmacia) column that had been equilibrated with 1% sodium formate at pH 8.0. Neutral impurities were washed off with water (70 mL). The GAs and ABA were eluted with 0.2 m formic acid (20 mL) and loaded onto a C18 Sep-Pak cartridge (Waters), which was prewashed with 5 mL of diethyl ether, 5 mL of methanol, and 10 mL of water. After the cartridge was washed with 2 mm acetic acid plus 1% methanol (10 mL), GAs and ABA were eluted with 5 mL of 80% methanol. The aqueous methanol solution was completely evaporated at 35°C under low pressure. Up to this stage, GAs and ABA were purified together.
HPLC was used to separate GAs and ABA into different fractions. The sample was dissolved in 1 mL of 30% methanol and injected onto a Chromsphere C18 column (Chrompack, Middleburg, The Netherlands) and eluted with a linear gradient of methanol (10–70%), containing 0.01% acetic acid, at a flow rate of 4 mL/min. The retention times of various GAs and ABA were determined by monitoring the elution of standards at 210 nm. The retention times were 19.0 to 21.0 min for GA1, 28.5 to 30.5 min for GA20, 31.5 to 33.5 min for GA4, and 34.5 to 36.5 min for GA9. The retention time of ABA was 24.0 to 26.0 min. The collected fractions were methylated with excess ethereal diazomethane and fractionated again using the same HPLC system and gradient. The retention times shifted 2 to 3 min for each fraction.
Putative methyl-GA- and methyl-ABA-containing fractions were collected. The dried fractions were trimethylsilylated with Deriva-sil (15 μL, Chrompack, Raritan, NJ) at 70°C for 10 min. Derivatized samples were analyzed using a GC-MS system (model 5970, Hewlett-Packard). Samples (4–5 μL) were injected into an Ultra-1-fused silica capillary column (Hewlett-Packard; cross-linked methyl silicone gum; 25-m × 0.2-mm × 0.33-μm film thickness) at an oven temperature of 70°C with the injector splitter closed. After 45 s the splitter was opened, and 2 min later the oven temperature was increased at a rate of 30°C min−1 to 250°C and then at a rate of 4°C min−1 to 300°C and held at that temperature for 9.5 min. The injector and the interface temperature were 250 and 290°C, respectively. After 12 min, mass spectra were acquired by scanning from 200 to 600 or by selected ion monitoring using the following ions: for GA1, ions 506 and 508; for GA4, ions 284, 286, 418, and 420; for GA9, ions 298 and 330; and for GA20, ions 418 and 420. For GA and ABA identification, the Kovats retention index values were determined with a paraffin series for the Ultra-1 column. The spectra were compared with pure standards or to published spectra (Gaskin and MacMillan, 1991). For quantification, corrected calibration curves were made for each GA by isotope-dilution analysis.
RESULTS
Effects of Suc and Exogenous Hormones on Tuber Formation
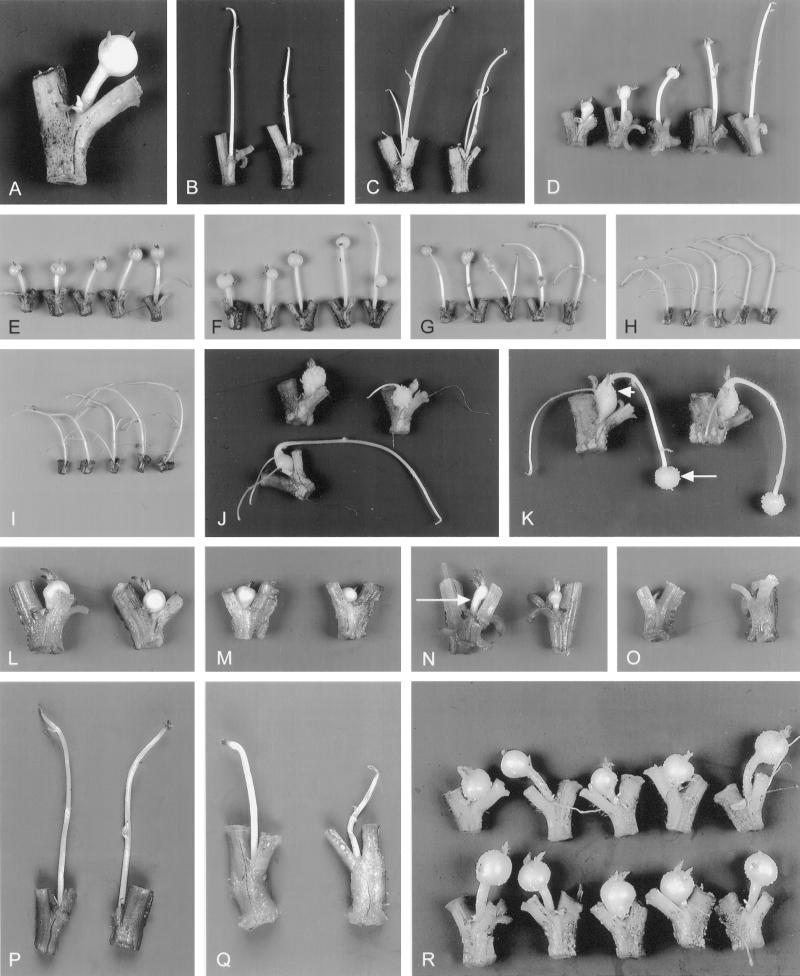
The tuber-inducing treatment (culture medium with 8% Suc) resulted in tubers, and noninducing treatments (culture medium with 1% Suc, or 8% Suc plus GA) resulted in stolon formation during the 10 d of culture (Fig. 1, A–C). Under tuber-inducing conditions, the stolons ceased growth at d 5 when tuber formation started, whereas the stolons continued to elongate under noninducing conditions.
Figure 1.
(Continued from facing page.)
Photographs showing development of single-node cuttings of potato plants grown in vitro on various media after 10 d of culture. Each photograph, except D, shows one to five representative cuttings for a culture condition. A, Tuber-inducing treatment with 8% Suc in the nutrient medium. B, Noninducing treatment with 1% Suc. C, Noninducing treatment with 8% Suc plus 0.5 μm GA4/7. D, A series of Suc concentrations: 8, 6, 4, 2, and 1% from left to right. E, 8% Suc plus 0.01 μm GA4/7. F, 8% Suc plus 0.03 μm GA4/7. G, 8% Suc plus 0.1 μm GA4/7. H, 8% Suc plus 0.3 μm GA4/7. I, 8% Suc plus 1.0 μm GA4/7. J, Formation of secondary stolons after transfer of the cuttings from 8% Suc medium to 8% Suc plus GA4/7 medium at d 5 (the bottom cutting) or at d 10 (the two top cuttings) and further culture for 5 d. K, Formation of secondary tubers by transferring cuttings first from 8% Suc medium to 8% Suc plus GA4/7 medium at d 5 and then back to 8% Suc medium at d 10 and further culturing for 5 d. The short arrow points to the first tuber and the long arrow points to a secondary one. L, 8% Suc plus ABA. M, 8% Suc plus IAA. N, 1% Suc plus ABA; arrow indicates the short stolon. O, 1% Suc plus IAA. P, 8% Suc plus GA4/7 plus ABA. Q, 8% Suc plus GA4/7 plus IAA. R, 8% Suc (top line) and 8% Suc plus BA (bottom line).
Suc
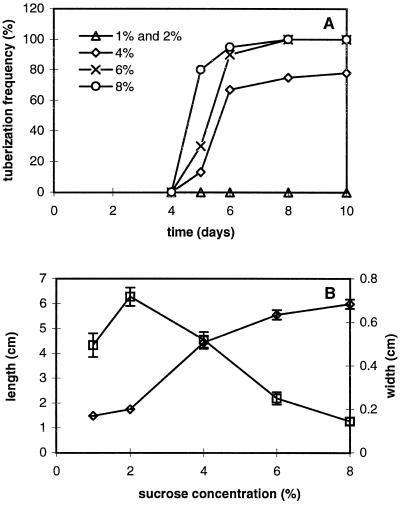
When the medium contained 2% Suc or less, no tubers were formed during the 10-d observation period. When the Suc concentration was increased beyond 2%, tuberization increased in a Suc-concentration-dependent manner (Fig. 2A). Swelling was observed at d 5 in 4, 6 and 8% Suc medium. Only 8% Suc medium resulted in a high (80%) frequency of tubers at d 5. At d 10, both 6 and 8% Suc medium resulted in 100% tuberization, whereas the 4% Suc medium gave only 75% tuberization (for overview, see Fig. 1D). The final size of the tubers in 8% Suc medium was larger than that of the tubers grown in 4 and 6% Suc medium (Fig. 2B). The final length of stolons decreased with increasing Suc level, except with 1% Suc (Figs. 1D and 2B). Stolon elongation stopped as soon as tubers were formed under 4, 6, and 8% Suc conditions (data not shown).
Figure 2.
Effects of a series of Suc concentrations on potato tuber formation in hormone-free medium. Data are based on 20 single-node cuttings for each Suc concentration. A, Tuberization frequency during 10 d of culture. B, Average stolon or tuber width (⋄) and stolon length (□) at d 10 at various Suc concentrations. Bars indicate ses when exceeding the size of the points.
GA
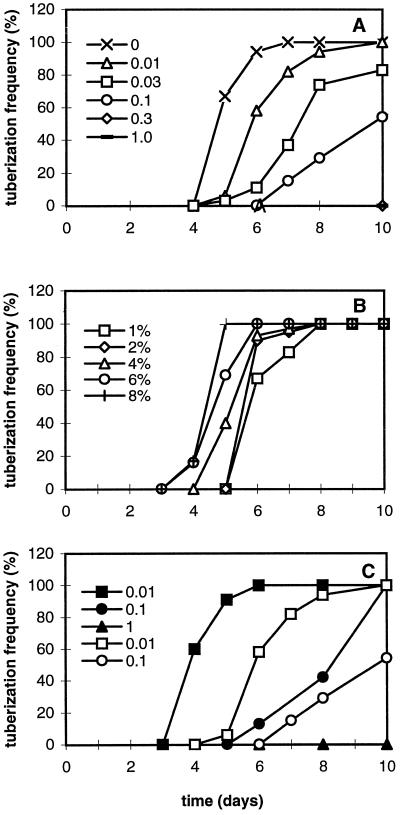
A series of GA4/7 concentrations, ranging from 0.01 to 1 μm, was applied in the 8% Suc medium to determine the influence of different GA levels on tuberization. With increasing GA4/7 concentration, tuberization was delayed, reduced, and became less synchronous (Fig. 3A). At 0.3 μm GA4/7 some tubers formed at d 14, whereas at 1 μm GA4/7 no tubers were formed at all up to d 20 (data not shown). At d 10, both tuber widths and stolon lengths were measured at various concentrations of GA4/7. With increasing concentration, tuber width decreased from 0.8 to 0.2 cm, whereas stolon length increased. Normally shaped tubers were formed in the absence of GA4/7 (Fig. 1A) or at low-GA4/7 (0.01–0.03 μm) conditions (Fig. 1, E and F). Abnormal tubers with various shapes were produced at higher-GA4/7 (0.1 and 0.3 μm) conditions, and they were much smaller (Fig. 1, G and H). Long stolons without tubers developed in the medium with 1 μm GA4/7 (Fig. 1I).
Figure 3.
A, Effects of a series of GA4/7 concentrations on tuber formation. Data are based on 20 single-node cuttings. B, Tuberization frequency on medium with 3.7 μm ABA supplemented with a series of Suc concentrations. C, Tuberization frequencies on media with 8% Suc plus 3.7 μm ABA (closed symbols) and on media with 8% Suc without ABA (open symbols), supplemented with various concentrations of GA4/7.
A transfer experiment was done to further analyze the effect of GA on stolon elongation and tuber formation. Cuttings were grown on medium with 8% Suc without GA for 5 d. When stolon tips swelled to form small tubers, the cuttings were transferred to medium with 8% Suc plus 0.5 μm GA4/7. Two days later, stolons developed from the apical region of the young tubers. The tuber size remained unchanged during further culture (Fig. 1J, bottom cutting). These cuttings with tubers and secondary stolons were transferred back to the medium with 8% Suc without GA4/7 at d 10. New tubers were formed at the stolon tips at approximately d 14 (Fig. 1K).
When a developing bud was transferred before tuber formation from 8% Suc to 8% Suc plus GA medium, i.e. before d 5, that stolon continued to elongate (data not
ABA, IAA, and BA
The effects of ABA and IAA were analyzed by adding these regulators to tuber-inducing medium (8% Suc) or to two noninducing media (1% Suc or 8% Suc plus GA). Tuber formation occurred at d 4 in both ABA- and IAA-containing inducing media, which is 1 d earlier than in the absence of ABA or IAA. From the six noninducing media tested, only the 1% Suc plus ABA condition resulted in high frequencies of tubers from d 8 onward. The final size of 8% Suc plus ABA-grown tubers was approximately the same as that of 8% Suc-grown tubers, whereas 8% Suc plus IAA-grown tubers were about 25% smaller than 8% Suc-grown tubers (Fig. 1, L and M). The tubers formed on medium with 1% Suc plus ABA were very small (Fig. 1N). Under inducing conditions, the elongation of stolons was severely inhibited by ABA and IAA treatments, leading to almost 100% sessile tubers (Fig. 1, cf. A, L, and M). In 1% Suc medium, ABA also decreased stolon length (Fig. 1N). The combination of 1% Suc and IAA completely blocked the growth of the lateral buds (Fig. 1O) and callus formed around the lower cut surface of the cuttings. Under the 8% Suc plus GA-noninducing condition, ABA did not influence stolon elongation very much (Fig. 1P), whereas IAA retarded and decreased the elongation of the lateral buds (Fig. 1Q). No clear effect of BA was observed when added either to inducing medium (Fig. 1R) or to the two noninducing media (data not shown).
The effect of ABA was studied further by treating cuttings with a series of Suc concentrations at a constant ABA level. In contrast to the experiment with various Suc concentrations without ABA (Fig. 2A), 100% tuberization occurred at all concentrations of Suc when ABA was added to the media (Fig. 3B). The inhibiting effect of GA could partly be overcome by the addition of ABA to the medium (Fig. 3C).
Determination of Endogenous GA and ABA Levels under Tuber-Inducing and -Noninducing Conditions
Biological variation and technical detection limits hamper the accurate determination of plant hormones in developing potato tubers grown in vitro. Within a series of experiments 10 to 200 developing buds had to be collected in one sample to obtain detectable quantities of hormones and to average the hormone levels in each developmental stage. Pooling of developing buds was necessary, because tubers were formed on stolons of various lengths within a given treatment. Variation in levels of endogenous hormones also occurred between series of experiments done under the same environmental conditions but at different times of the year. The variation might be due to differences in the population of cuttings at the onset of culture, although much care was taken to make the starting material as uniform as possible by culture under controlled environmental conditions. Although absolute levels of hormones differed between experiments, changes in the levels of GA and ABA showed the same trends in successive experiments; therefore, such experiments are considered successful replicates, which should not be averaged.
GA
Starting with 1 g fresh weight, GA1, GA4, GA9, and GA20 were detected in the samples of two independent experiments. To our knowledge, this is the first time that GA4 and GA9 have been detected in potato tissue. The numerical values for these GAs were as follows: for GA4, M+418(20), 289(29), 284(100), 225(75), and 224(85); for GA9, M+330(15), 298(100), 270(68), 243(46), and 227(42). Table I shows the levels of the four GAs detected in the developing buds of the first experiment. Three stages were analyzed under both tuber-inducing and -noninducing conditions: resting buds at d 0, developing buds at d 5, and mature stolons with or without tubers at d 10. The level of GA20 was always much lower than that of the other GAs. It was even undetectable in the samples from the 8% Suc condition. GA4 and GA9 levels were low and did not show obvious changes during development and between the treatments. The level of GA1 was always the highest and it varied significantly in different treatments and between sampling times. Therefore, it was analyzed in detail in a subsequent experiment.
Table I.
Contents of GA1, GA20, GA4, GA9, and ABA in axillary buds of single-node cuttings of potato grown in vitro on various media
| Medium | Day | Structure | GA1 | GA20 | GA4 | GA9 | ABA |
|---|---|---|---|---|---|---|---|
| ng g−1 | |||||||
| — | 0 | Resting bud | 56.6 | 0.6 | 15.0 | 13.5 | 457 |
| 8% Suc | 5 | Stolon + tuber | 24.3 | n.d.a | 10.3 | 8.9 | 163 |
| 10 | Stolon + tuber | 12.8 | n.d. | 6.6 | 5.6 | 58 | |
| 1% Suc | 5 | Stolon | 58.7 | 1.0 | 12.7 | 12.1 | 183 |
| 10 | Stolon | 36.4 | 0.7 | 16.5 | 15.8 | 80 | |
| 8% Suc + GA4/7 | 5 | Stolon | 45.1 | 0.9 | 23.5 | 18.0 | 158 |
| 10 | Stolon | 24.4 | 1.4 | 13.6 | 14.0 | 34 | |
n.d., Not detectable.
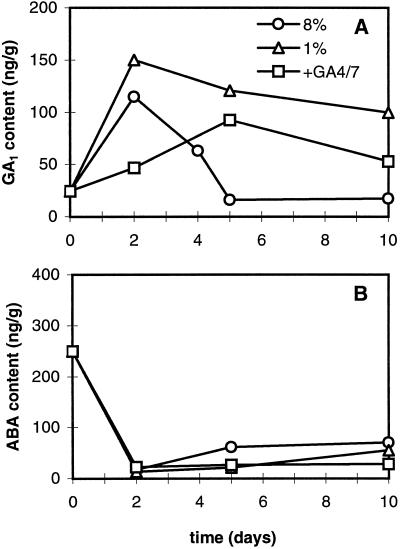
The same qualitative differences in GA1 levels were observed in the second series of experiments. At d 5 and 10, GA1 levels were highest in stolons grown at 1% Suc and 3- to 6-fold lower in tubers developing on 8% Suc medium (Fig. 4A). Comparing tuber-inducing (8% Suc) and -noninducing (1% Suc) conditions, it appeared that the level of GA1 increased more than 5-fold during the first 2 d of culture in both treatments (Fig. 4A). This coincided with the onset of stolon formation. The level remained high under noninducing conditions, whereas it sharply decreased in the inducing treatment at d 4, i.e. 1 d before visible swelling. It decreased further at d 5 and remained low thereafter. In another noninducing treatment (8% plus GA4/7), the endogenous level of GA1 increased gradually until d 5 and then slightly decreased, reaching an intermediate level at d 10.
Figure 4.
Endogenous contents of GA1 and ABA during the development of axillary buds of potato under one tuber-inducing condition (medium with 8% Suc) and two noninducing conditions (media with 1% Suc or 8% Suc plus 0.5 μm GA4/7). Data were obtained from 10 to 200 cuttings per sample. A, GA1 content. B, ABA content.
Comparing the two experiments, a similar pattern of the changing GA1 levels was obtained, although the absolute levels varied between the two experiments.
ABA
Endogenous ABA levels were determined in resting buds at d 0 and in stolons or tubers at d 5 and 10 in the first experiment (Table I) and at d 0, 2, 5, and 10 in a second series of sampling (Fig. 4B). The endogenous ABA level was highest in the resting bud, and it decreased during bud development, irrespective of the culture conditions and the type of organ formed. Although the trend of decrease was noticed in both experiments, the initial concentration of ABA was higher in the first series of experiments, but at d 10 it had decreased to the same level as found in the second experiment.
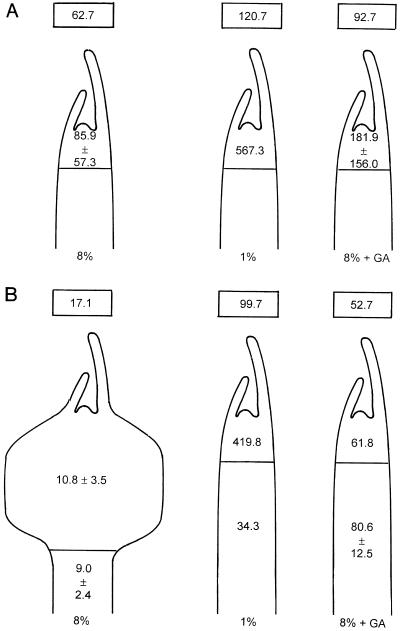
Localization of GA1 Content in Developing Stolons and Tubers
In previous research, we observed that longitudinal cell divisions resulting in swelling occur in the subapical region of stolons under the inducing condition. Under the noninducing conditions, the cell divisions in this area were transverse, leading to further elongation of the stolon. Therefore, this apical area of the stolon, including the subapical part, was analyzed separately to test whether GA was uniformly distributed over the stolon or localized in specific areas.
At d 4, which was 1 d before visible swelling, the GA1 level in the stolon tip for all three culture conditions (8% Suc, 1% Suc, and 8% Suc plus GA) was much higher than the average GA1 level in the whole stolon (Fig. 5A). Comparing the three conditions, the stolon tips contained high GA1 levels in the 1% Suc- and 8% Suc plus GA4/7-noninducing conditions and a low GA1 level in the inducing condition (8% Suc). The latter one would start swelling 1 d later. At d 10, tubers and stolons in the 8% Suc-inducing condition both had much lower levels of GA1 than those grown in the two noninducing conditions (Fig. 5B). Stolons grown in the 1% Suc-noninducing condition showed a high level of GA1 in the tip and a relatively low GA1 level in the elongated part of the stolon. In the 8% Suc plus GA4/7 treatment, the stolon tip had almost the same GA1 level as found in the elongated part of the stolon.
Figure 5.
Localization of GA1 in various regions of developing stolons of potato cuttings cultured under tuber-inducing (8% Suc) and -noninducing conditions (1% Suc and 8% Suc plus GA4/7) at d 4 (A) and at d 10 (B). The data inside of the drawings indicate GA1 concentrations (ng g−1) in those regions. The data in the boxes above the drawings are derived from Figure 4 and represent GA1 levels (ng g−1) in the whole developing stolon. Data are averages of duplicate measurements on samples from a single series of experiments, unless the amount of tissue was insufficient for replication.
DISCUSSION
GAs
Although several reports have been published concerning the quantitative analysis of endogenous GAs in potato plants by bioassays (Okazawa, 1959, 1960; Racca and Tizio, 1968; Smith and Rappaport, 1969; Pont Lezica, 1970; Railton and Wareing, 1973; Kumar and Wareing, 1974; Krauss and Marschner, 1982) and more recently by chemical methods (Jones et al., 1988), our study is the first one to our knowledge to apply analysis with GC-MS to quantify endogenous GAs during various stages of stolon elongation and tuber formation under tuber-inducing and non-tuber-inducing conditions. GA1, GA20, GA4, and GA9 were detected in small tissue samples. GA4 and GA9 concentrations did not change significantly during the development of stolons and tubers, whereas the content of GA20 was too low to observe possible changes. The significant variation of the GA1 level during the development of stolons under non-tuber-inducing conditions and tubers under tuber-inducing conditions supports the view that GA1 is the biologically active GA in this process (Phinney and Spray, 1982). The GA1 content was high during the elongation of stolons and became very low during the development of tubers. More clearly, the obvious decrease of GA1 content occurring between d 2 and 4, which is well before visible swelling under the inducing condition, strongly suggests a regulating role of GA1 on tuber formation. The continuously low level of GA1 during the whole process of tuber enlargement also suggests that tuber growth is only permitted at low levels of GA1. Induction and growth of tubers might occur only when the GA1 content is below a critical level.
It could be argued that tuberization causes the change in GA levels rather than vice versa. However, the timing of both processes favors a prime role for GA. Moreover, studies with GA-synthesis inhibitors (Perl et al., 1991; Vreugdenhil et al., 1994), as well as analysis of GA-deficient lines (Van den Berg et al., 1995b), also point to a crucial role of GA in regulating tuber formation.
The prominent role of endogenous GA on tuberization was supported further by observations of the effects of GA4/7 in the medium on the development of the axillary buds of the cuttings. Increasing concentrations of exogenous GA4/7 progressively inhibited tuber initiation, reduced the final size of the tubers, and stimulated stolon elongation.
In a previous study we observed that, depending on the presence or absence of GA in the medium, cell division in the subapical region of developing stolons is either transverse, resulting in further elongation of the stolon, or longitudinal, leading to tuber formation (Xu et al., 1998). Therefore, we hypothesized that this region is especially sensitive to GA. This idea is supported by the results of the transfer experiments (Fig. 1, J and K): applying or removing GA switches elongation of the stolons on and off by acting on the apical meristematic region, leaving the more basal parts of the developing axillary bud unaltered. To further test this hypothesis we also analyzed GA1 levels in various parts of developing buds separately. The GA level was much higher in the stolon tip than in the basal part of the stolon; it was also higher in the young stolon tip than in the mature stolon tip, whereas it was extremely low in the swelling tuber. The changes in GA1 levels, as induced by culture conditions, were much more extreme in the apical part than in the rest of the stolon. As stated by Brent Loy (1977), GA promotes cell division and cell elongation in the subapical meristem. High GA levels might keep the transversal cortical microtubular cytoskeleton stable so that cells in the subapical region divide transversally, and cell elongation will hence result in stolon elongation. Low GA levels cause reorientation of cortical microtubules to longitudinal or oblique directions and then allow the cells in the subapical region to enlarge and divide longitudinally, leading to the swelling of the tuber (Shibaoka, 1993; Sanz et al., 1996).
ABA
Similar to effects of ABA described for whole plants (Menzel, 1980), we also observed a stimulation of tuber formation in vitro. Tuber formation in ABA-containing 8% Suc medium started earlier than the tuber formation in ABA-free 8% Suc medium, and sessile tubers or tubers on very short stolons were formed. Furthermore, the stimulating effect of ABA on tuberization was concluded from the fact that application of ABA in the 1% Suc medium significantly promoted the initiation and the frequency of tuber formation. Also, addition of ABA to 8% Suc medium supplemented with a series of concentrations of GAs showed antagonism between GA and ABA. The morphology of the tubers formed in the presence of ABA always resembled “normal” in vitro tubers, and no signs of abnormalities, as observed in the presence of ethylene, were seen (Catchpole and Hillman, 1969). Our results support the view that exogenously applied ABA promotes tuber formation in potatoes (Okazawa and Chapman, 1962; El-Antably et al., 1967; Abdullah and Ahmad, 1980; Menzel, 1980; Wareing and Jennings, 1980) and that it is an inhibitor of stolon elongation (Palmer and Smith, 1969b; Marschner et al., 1984). However, the effects of applied ABA are dependent on variety, concentration, and interaction with cytokinins (Palmer and Smith, 1969b; Hussey and Stacey, 1984).
Analysis of endogenous ABA levels showed that the levels decreased during the first 2 d of culture for all three treatments (8% Suc, 1% Suc, and 8% Suc plus GA) and remained low from d 5 to 10. No differences were detected among the three treatments. Hence, we conclude that ABA is not likely to be the main regulator of tuber formation. The effect of exogenous ABA, as observed here and in other experiments (Krauss and Marschner, 1982), is proposed to be due to an antagonistic effect between ABA and GA. Alternatively, exogenously applied ABA might stimulate tuber formation by inhibiting stolon elongation, a prerequisite for tuber formation (Vreugdenhil and Struik, 1989).
Cytokinins and IAA
The hormones cytokinin and IAA were tested under both tuber-inducing and -noninducing conditions. Cytokinins are considered to be tuber-inducing factors, according to the promoting effect of exogenous cytokinins (Palmer and Smith, 1969a; Kumar and Wareing, 1974; Hussey and Stacey, 1984) and the high level of endogenous cytokinins in induced tissue (Mauk and Langille, 1978; Obata-Sasamoto and Suzuki, 1979). However, in the experimental system we used, no significant effects of cytokinins on morphogenesis were detected under inducing or noninducing conditions. This implies that cytokinins are not a limiting factor for tuber formation in the present model system. A similar conclusion, with cytokinins being less important in regulating tuber formation than GA, can be deduced from Dimalla and van Staden (1977).
The application of IAA in the tuber-inducing medium led to earlier tuber initiation, as also mentioned by Harmey et al. (1966). Contrary to their observation, slightly smaller tubers were formed, which were all sessile. In the presence of GA, addition of IAA resulted in much shorter stolons than found under GA conditions alone. The growth of the lateral buds of the cuttings was totally blocked with the application of IAA in 1% Suc medium. Thus, it seems that IAA has a significant negative effect on elongation under both tuber-inducing and -noninducing conditions. We assume that this inhibition of tuber formation is due to the well-known effect of stimulation of ethylene production by IAA, which in turn reduces stolon elongation (Vreugdenhil and van Dijk, 1989). IAA would, therefore, indirectly favor tuber formation by blocking stolon elongation and counteracting effects of endogenous GA.
Interaction between Suc and Hormones
Similar to the results obtained by Lawrence and Barker (1963), we found no tuber formation with low Suc concentrations (0–2%), slow development of tubers with 4 to 6% Suc, and rapid initiation and growth of tubers with 8% Suc. It is clear that, by comparing the experiments with a series of Suc concentrations and a series of GA concentrations (Figs. 1, D and E–I, 2A, and 3A), a gradual decrease of stolon length and an increase of tuberization were correlated with both the increase of Suc concentration and the decrease of GA concentration in the medium. Therefore, we assume that either GA regulates endogenous Suc levels or vice versa. A recent study showed that the application of GA to 8% Suc medium did not significantly alter endogenous Suc levels in early stages of tuberization (D. Vreugdenhil, unpublished data). In the present study the determination of the endogenous GA contents revealed that the GA level was much higher in the noninducing condition with 1% Suc than in the tuber-inducing condition with 8% Suc. The difference occurred during stolon elongation and tuber formation. It indicates that the endogenous GA level was highly dependent on Suc concentrations. Park (1990) reported that Suc induced the expression of tuber-specific genes and that the sensitivity toward Suc was modulated by GA. The interaction between Suc and GA in tuber formation was also studied by Mares et al. (1981). They detected an increasing level of reducing sugars with the application of GA. It was suggested that GAs may reduce the starch-synthesizing capacity by a marked reduction in the activity of ADP-Glc-pyrophosphorylase.
Contrary to GA, the endogenous ABA levels did not vary under both 8% and 1% Suc conditions. This is in agreement with the report by Van den Berg et al. (1991), in which it was pointed out that changes in carbohydrate levels were not triggered by a change in ABA levels. These data support the hypothesis that Suc acts as a regulator by influencing endogenous GA levels during tuber formation.
In conclusion, it is clear that GA is a dominant regulator, responding to the environmental conditions and controlling all steps of tuber formation, as a stimulus in stolon elongation and an inhibitor in both tuber initiation and growth. Other plant hormones are assumed to be involved in the regulation of tuber formation as well; however, their effects seem to depend on the final GA content in the tissues. ABA functions as an antagonist of GAs, and Suc regulates tuber formation by changing the GA level of the developing stolons and tubers.
ACKNOWLEDGMENTS
We thank Wilma Pons-Drexhage and Jan Vos for their assistance with the in vitro cultures, Henk Kieft for technical assistance with the morphological investigations, and Sijbout Massalt and Allex Haasdijk for photography and art work.
Footnotes
This work was supported by grants from the Royal Dutch Academy of Sciences and from the Graduate School Experimental Plant Sciences to X.X.
LITERATURE CITED
- Abdullah ZN, Ahmad R. Effect of ABA and GA3 on tuberization and some chemical constituents of potato. Plant Cell Physiol. 1980;21:1343–1346. [Google Scholar]
- Boo L. The effect of gibberellic acid on the inhibitor β complex in resting potato. Physiol Plant. 1961;14:676–681. [Google Scholar]
- Brent Loy J (1977) Hormonal regulation of cell division in the primary elongation meristems of shoots. In TL Rost, EM Gifford Jr, eds, Mechanisms and Control of Cell Division. Dowden, Hutchinson, and Ross, Inc., Stroudsburg, PA, pp 92–110
- Catchpole AH, Hillman J. Effect of ethylene on tuber initiation in Solanum tuberosum L. Nature. 1969;223:1387. [Google Scholar]
- Dimalla GG, Van Staden J. Effects of ethylene on the endogenous cytokinin and gibberellin levels in tuberizing potatoes. Plant Physiol. 1977;60:218–221. doi: 10.1104/pp.60.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Antably HMM, Wareing PF, Hillman J. Some physiological responses to d,l-abscisin (dormin) Planta. 1967;73:74–90. doi: 10.1007/BF00419842. [DOI] [PubMed] [Google Scholar]
- Ewing EE. The role of hormones in potato (Solanum tuberosum L.) tuberization. In: Davies PJ, editor. Plant Hormones and Their Role in Plant Growth and Development. Boston: Martinus Nijhoff Publishers; 1987. pp. 515–538. [Google Scholar]
- Gaskin P, MacMillan J (1991) GC-MS of the gibberellins and Related Compounds: Methodology and a Library of Spectra. Cantock's Enterprises, Bristol, UK
- Harmey MA, Crowley MP, Clinch PEM. The effect of growth regulators on tuberization of cultured stem pieces of Solanum tuberosum. Eur Potato J. 1966;9:146–151. [Google Scholar]
- Hendriks T, Vreugdenhil D, Stiekema WJ. Patatin and four serine proteinase inhibitor genes are differentially expressed during potato tuber development. Plant Mol Biol. 1991;17:385–394. doi: 10.1007/BF00040633. [DOI] [PubMed] [Google Scholar]
- Hussey G, Stacey NJ. Factors affecting the formation of in vitro tubers of potato (Solanum tuberosum L.) Ann Bot. 1984;53:565–578. [Google Scholar]
- Jones MG, Horgan R, Hall MA. Endogenous gibberellins in the potato (Solanum tuberosum) Phytochemistry. 1988;27:7–10. [Google Scholar]
- Krauss A, Marschner H. Influence of nitrogen nutrition, daylength, and temperature on contents of gibberellic and abscisic acid and on tuberization in potato plants. Potato Res. 1982;25:13–21. [Google Scholar]
- Kumar D, Wareing PF. Factors controlling stolon development in the potato plant. New Phytol. 1972;71:639–648. [Google Scholar]
- Kumar D, Wareing PF. Studies on tuberization of Solanum andigena. II. Growth hormones and tuberization. New Phytol. 1974;73:833–840. [Google Scholar]
- Lawrence CH, Barker WG. A study of tuberization in the potato (Solanum tuberosum) Am Pot J. 1963;40:349–356. [Google Scholar]
- Mares DJ, Marschner H, Krauss A. Effect of gibberellic acid and carbohydrate metabolism of developing tubers of potato (Solanum tuberosum) Physiol Plant. 1981;52:267–274. [Google Scholar]
- Marschner H, Sattelmacher B, Bangerth F. Growth rate of potato tubers and endogenous contents of indolylacetic acid and abscisic acid. Physiol Plant. 1984;60:16–20. [Google Scholar]
- Mauk CS, Langille AR. Physiology of tuberization in Solanum tuberosum L. Plant Physiol. 1978;62:438–442. doi: 10.1104/pp.62.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel BM. Tuberization in potato (Solanum tuberosum) cultivar Sebago at high temperatures: responses to gibberellin and growth inhibitors. Ann Bot. 1980;46:259–266. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio-assay with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Obata-Sasamoto H, Suzuki H. Activities of enzymes relating to starch synthesis and endogenous levels of growth regulators in potato stolon tips during tuberization. Physiol Plant. 1979;45:320–324. [Google Scholar]
- Okazawa Y. Studies on the occurrence of natural gibberellin and its effect on the tuber formation of potato plants. Proc Crop Sci Soc Jpn. 1959;28:129–133. [Google Scholar]
- Okazawa Y. Studies on the relation between the tuber formation of potato and its natural gibberellin content. Proc Crop Sci Soc Jpn. 1960;29:121–124. [Google Scholar]
- Okazawa Y, Chapman HW. Regulation of tuber formation in the potato plant. Physiol Plant. 1962;15:413–419. [Google Scholar]
- Palmer CE, Smith OE. Cytokinins and tuber initiation in the potato Solanum tuberosum L. Nature. 1969a;221:279–280. [Google Scholar]
- Palmer CE, Smith OE. Effect of abscisic acid on elongation and kinetin-induced tuberization of isolated stolons of Solanum tuberosum L. Plant Cell Physiol. 1969b;10:657–664. [Google Scholar]
- Park WD (1990) Molecular approaches to tuberization in potato. In ME Vayda, WD Park, eds, The Molecular and Cellular Biology of the Potato. C.A.B. International, Wallingford, CT, pp 43–56
- Perl A, Aviv D, Willmitzer L, Galun E. In vitro tuberization in transgenic potatoes harboring β-glucuronidase linked to a patatin promoter: effects of sucrose levels and photoperiods. Plant Sci. 1991;73:87–95. [Google Scholar]
- Phinney BO, Spray CR. Chemical genetics and the gibberellin pathway in Zea mays. In: Wareing PF, editor. Plant Growth Substances. London: Academic Press; 1982. pp. 101–110. [Google Scholar]
- Pont Lezica RF. Evolution des substances de type gibberellins chez la pomme de terre pendant la tuberisation, en relation avec la lingueur du jour et la temperature. Potato Res. 1970;13:323–331. [Google Scholar]
- Racca RW, Tizio R. A preliminary study of changes in the content of gibberellin-like substances in the potato plant in relation to the tuberization mechanism. Eur Potato J. 1968;11:213–220. [Google Scholar]
- Railton ID, Wareing PF. Effects of daylength on endogenous gibberellins in leaves of Solanum andigena. Physiol Plant. 1973;28:88–94. doi: 10.1007/BF00386032. [DOI] [PubMed] [Google Scholar]
- Rappaport LS, Smith OE (1962) Gibberellins in the rest period of the potato tuber. In Eigenschaften und Wirkungen der Gibberellin. Springer-Verlag, Berlin, pp 37–45
- Sanz MJ, Mingo-Castel A, van Lammeren AAM, Vreugdenhil D. Changes in the microtubular cytoskeleton precede in vitro tuber formation in potato. Protoplasma. 1996;191:46–54. [Google Scholar]
- Shibaoka H. Regulation by gibberellins of the orientation of cortical microtubules in plant cells. Aust J Plant Physiol. 1993;20:461–470. [Google Scholar]
- Smith OE, Rappaport L (1960) Endogenous gibberellins in resting and sprouting potato tubers. In Advances in Chemistry, series 28. American Chemical Society, Washington, DC, pp 42–48
- Smith OE, Rappaport L. Gibberellins, inhibitors, and tuber formation in the potato (Solanum tuberosum) Am Potato J. 1969;46:185–191. [Google Scholar]
- Van den Berg JH, Davies PJ, Ewing EE, Halinska A. Metabolism of gibberellin A12 and A12-aldehyde and the identification of endogenous gibberellins in potato (Solanum tuberosum ssp. Andigena) shoots. J Plant Physiol. 1995a;146:459–466. [Google Scholar]
- Van den Berg JH, Simko I, Davies PJ, Ewing EE, Halinska A. Morphology and [14C]gibberellin A12 metabolism in wild-type and dwarf Solanum tuberosum ssp. Andigena grown under long and short photoperiods. J Plant Physiol. 1995b;146:467–473. [Google Scholar]
- Van den Berg JH, Vreugdenhil D, Ludford PM, Hillman LL, Ewing EE. Changes in starch, sugar and abscisic acid contents associated with second growth in tubers of potato (Solanum tuberosum L.) one-leaf cuttings. J Plant Physiol. 1991;139:86–89. [Google Scholar]
- Vreugdenhil D, Bindels P, Reinhoud P, Klocek J, Hendriks T. Use of the growth retardant tetcyclasis for potato tuber formation in vitro. Plant Growth Regul. 1994;14:257–265. [Google Scholar]
- Vreugdenhil D, Struik PC. An integrated view of the hormonal regulation of tuber formation in potato (Solanum tuberosum) Physiol Plant. 1989;75:525–531. [Google Scholar]
- Vreugdenhil D, van Dijk W. Effects of ethylene on the tuberization of potato (Solanum tuberosum) cuttings. Plant Growth Regul. 1989;8:31–39. [Google Scholar]
- Wareing PF, Jennings AMV. The hormonal control of tuberisation in potato. In: Skoog F, editor. Plant Growth Substances. Berlin: Springer-Verlag; 1980. pp. 293–300. [Google Scholar]
- Xu X, Vreugdenhil D, van Lammeren AAM. Cell division and cell enlargement during potato tuber formation: a comparison of in vitro and in vivo tuber development. J Exp Bot. 1998;49:573–582. [Google Scholar]