Abstract
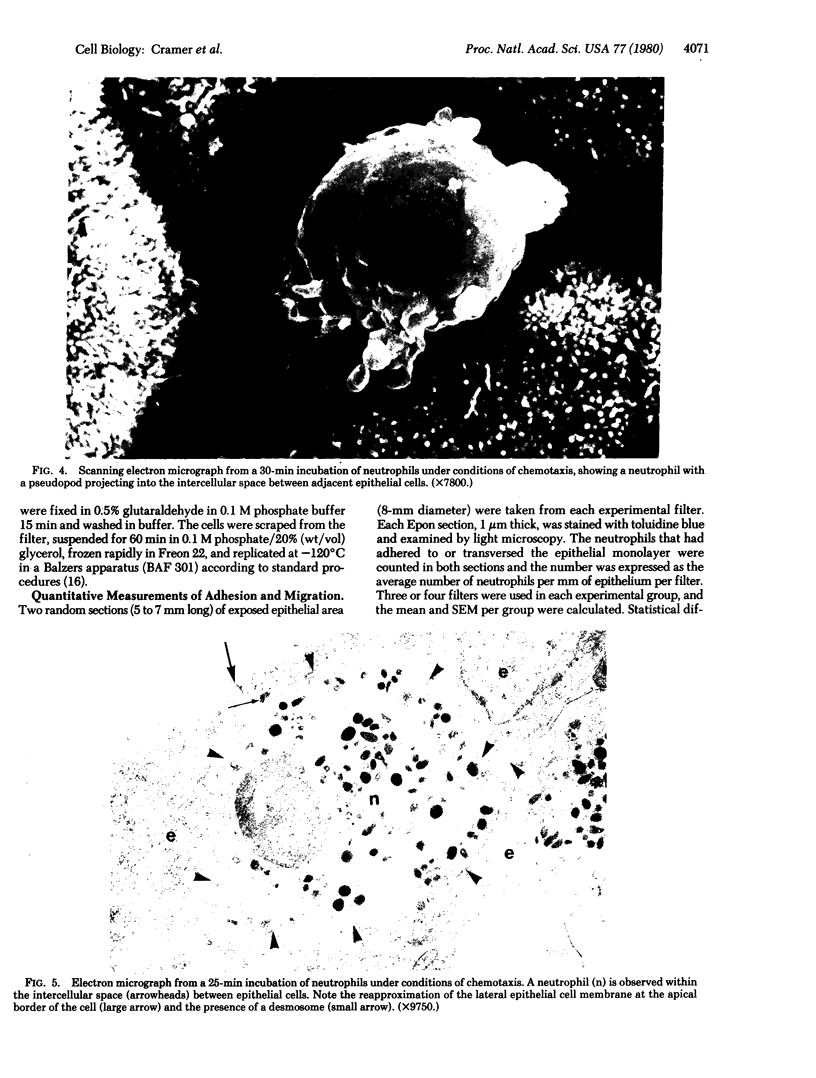
An in vitro model system for studying transepithelial migration of human neutrophils has been developed. Canine kidney epithelial cells grown on micropore filters form a confluent, polarized monolayer with an average transepithelial electrical resistance of 181 ohms.cm2. Neutrophils in a chemotactic chamber are stimulated to undergo random migration, chemokinesis, or chemotaxis through the epithelium. When stimulated by a gradient of the synthetic chemoattractant fMet-Leu-Phe, significantly more neutrophils traverse the low-resistance epithelium than do under conditions of random migration or chemokinesis. Transmission and scanning electron microscopy of this process reveal that neutrophils traverse the epithelium through the intercellular space. After leukocyte emigration, lateral epithelial cell membranes reapproximate. Neutrophils undergoing chemotaxis can also traverse the polarized epithelium from the basal epithelial surface, which suggests that the chemotactic gradient and not the apical-basal polarity of the epithelial cells determines the direction of transepithelial migration. The data further suggest that (i) the in vitro model of leukocyte transepithelial migration morphologically simulates the in vivo process, (ii) neutrophils more readily penetrate the epithelium when attracted by a chemotactic factor, and (iii) neutrophils can traverse a low-resistance epithelium in the absence of serum and connective tissue factors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. O., Anderson N. D. Lymphocyte emigration from high endothelial venules in rat lymph nodes. Immunology. 1976 Nov;31(5):731–748. [PMC free article] [PubMed] [Google Scholar]
- Atherton A., Born G. V. Quantitative investigations of the adhesiveness of circulating polymorphonuclear leucocytes to blood vessel walls. J Physiol. 1972 Apr;222(2):447–474. doi: 10.1113/jphysiol.1972.sp009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisbee C. A., Machen T. E., Bern H. A. Mouse mammary epithelial cells on floating collagen gels: transepithelial ion transport and effects of prolactin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):536–540. doi: 10.1073/pnas.76.1.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cereijido M., Robbins E. S., Dolan W. J., Rotunno C. A., Sabatini D. D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978 Jun;77(3):853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Kimball H. R. Defective granulocyte chemotaxis in the Chediak-Higashi syndrome. J Clin Invest. 1971 Dec;50(12):2645–2652. doi: 10.1172/JCI106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968 Feb 1;127(2):371–386. doi: 10.1084/jem.127.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer E. B., Gallin J. I. Localization of submembranous cations to the leading end of human neutrophils during chemotaxis. J Cell Biol. 1979 Aug;82(2):369–379. doi: 10.1083/jcb.82.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunkhorn P., Willis A. L. Cutaneous reactions to intradermal prostaglandins. Br J Pharmacol. 1971 Jan;41(1):49–56. doi: 10.1111/j.1476-5381.1971.tb09934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias Da Silva W., Lepow I. H. Complement as a mediator of inflammation. II. Biological properties of anaphylatoxin prepared with purified components of human complement. J Exp Med. 1967 May 1;125(5):921–946. doi: 10.1084/jem.125.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst S. A., Mills J. W. Basolateral plasma membrane localiztion of ouabain-sensitive sodium transport sites in the secretory epithelium of the avian salt gland. J Cell Biol. 1977 Oct;75(1):74–94. doi: 10.1083/jcb.75.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM H. T., LOWRY O. H., WHEELWRIGHT F., LENZ M. A., PARISH H. H., Jr Distribution of histamine among leukocytes and platelets. Blood. 1955 May;10(5):467–481. [PubMed] [Google Scholar]
- HURLEY J. V. An electron microscopic study of leucocytic emigration and vascular permeability in rat skin. Aust J Exp Biol Med Sci. 1963 Apr;41:171–186. doi: 10.1038/icb.1963.17. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Yoshinaga M., Yamamoto S. The nature of a mediator of leucocyte chemotaxis in inflammation. Antibiot Chemother (1971) 1974;19:296–332. doi: 10.1159/000395437. [DOI] [PubMed] [Google Scholar]
- Kyte J. Immunoferritin determination of the distribution of (Na+ + K+) ATPase over the plasma membranes of renal convoluted tubules. I. Distal segment. J Cell Biol. 1976 Feb;68(2):287–303. doi: 10.1083/jcb.68.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. M., Mahler S. Leukocyte emigration and migration in the vagina following mating in the rabbit. Anat Rec. 1977 Sep;189(1):45–59. doi: 10.1002/ar.1091890104. [DOI] [PubMed] [Google Scholar]
- RILEY J. F., WEST G. B. The presence of histamine in tissue mast cells. J Physiol. 1953 Jun 29;120(4):528–537. doi: 10.1113/jphysiol.1953.sp004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Wahl S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoefl G. I. The migration of lymphocytes across the vascular endothelium in lymphoid tissue. A reexamination. J Exp Med. 1972 Sep 1;136(3):568–588. doi: 10.1084/jem.136.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig L. L., Jr, Beer A. E. Transepithelial migration of leukocytes in the mammary gland of lactating rats. Biol Reprod. 1978 Jun;18(5):736–744. doi: 10.1095/biolreprod18.5.736. [DOI] [PubMed] [Google Scholar]
- Wenk E. J., Orlic D., Reith E. J., Rhodin J. A. The ultrastructure of mouse lymph node venules and the passage of lymphocytes across their walls. J Ultrastruct Res. 1974 May;47(2):214–241. doi: 10.1016/s0022-5320(74)80071-7. [DOI] [PubMed] [Google Scholar]