Abstract
Background
There is a heteromorphic alternative life in the brown seaweed, Saccharina japonica (Aresch.) C. E. Lane, C. Mayes et G. W. Saunders ( = Laminaria japonica Aresch.), with macroscopic monoecious sporophytes and microscopic diecious gametophytes. Female gametophytes are genetically different from males. It is very difficult to identify the parent of a sporophyte using only routine cytological techniques due to homomorphic chromosomes. A sex-specific marker is one of the best ways to make this determination.
Methodology/Principal Findings
To obtain clear images, chromosome preparation was improved using maceration enzymes and fluorochrome 4′, 6-diamidino-2-phenylindole (DAPI). The chromosome number of both male and female haploid gametophytes was 31, and there were 62 chromosomes in diploid sporophytes. Although the female chromosomes ranged from 0.77 µm to 2.61 µm in size and were larger than the corresponding ones in the males (from 0.57 µm to 2.16 µm), there was not a very large X chromosome in the females. Based on the known female-related FRML-494 marker, co-electrophoresis and Southern blot profiles demonstrated that it was inheritable and specific to female gametophytes. Using modified fluorescence in situ hybridization (FISH), this marker could be localized on one unique chromosome of the female gametophytes as well as the sporophytes, whereas no hybridization signal was detected in the male gametophytes.
Conclusions/Significance
Our data suggest that this marker was a female chromosome-specific DNA sequence. This is the first report of molecular marker localization on algal chromosomes. This research provides evidence for the benefit of using FISH for identifying molecular markers for sex identification, isolation of specific genes linked to this marker in the females, and sex determination of S. japonica gametophytes in the future.
Introduction
Saccharina japonica (Aresch.) C. E. Lane, C. Mayes et G. W. Saunders ( = Laminaria japonica Aresch.) is a brown seaweed of high economic importance especially in East Asia, such as in China, Japan and South Korea, where it has been cultivated extensively for food and industrial alginate. China is by far the largest producer, and the production of L. japonica in China in 2009 rose sharply to 4.14×109 kg wet weight [1], accounting for approximately 80% of the global production, over several decades. This has been attributed to both a fundamental understanding of its biology as well as the cultivation techniques and genetic breeding [2]–[5]. There are two heteromorphic alternative forms in S. japonica, macroscopic monoecious sporophytes (2n) and microscopic diecious gametophytes (n). The haploid female and male gametophytes differ from each other not only in morphology [6] and physiology [7], [8], but also in genetics. The female gametophytes can develop and produce eggs and even give rise to parthenogenetic sporophytes without fertilization [9]–[12] whereas the males produce sexual spermatozoids [13]. These discrepancies possibly result from the different genes or differential expression of genes between males and females. Accordingly, attempts have been made to identify sex-specific genes by construction and characterization of subtraction cDNA libraries using suppression subtractive hybridization [14], [15]. Unfortunately, no sex -specific genes were found in these studies.
At the same time, a sequence-characterized amplified region (SCAR) marker, FRML-494 (GenBank accession No. EU931619), related to the female gametophytes was developed [16] on the basis of a specific sequence (GenBank accession No. AB069714) of the Marchantia polymorpha L. Y chromosome. However, no cytological studies were conducted to further characterize the sex-specific marker. In order to utilize this putative sex-specific marker as a tool for sex identification, which otherwise was possible only through morphological identification at the early developmental stage of zoospore germination, additional studies are required to provide cytological evidence of sex linkage to this putative sex-specific marker. The objective of this study was to look into the specific relationship between the marker and female gametophytes and to determine chromosomal localization using Southern blotting and fluorescence in situ hybridization (FISH).
FISH was initially developed in the field of mammalian research [17] on the basis of the theory and protocol of in situ hybridization [18], [19]. Since the first reports of implementing the FISH technique in plant research, reported independently by Le et al. [20] and Schwarzacher et al. [21], this powerful tool has been widely used in genome analysis of higher plants (see the reviews [22]–[24]). FISH enables the physical mapping of DNA sequences on chromosomes during mitosis and meiosis. In spite of the widespread use of FISH in animals and higher plants, it has been rarely used in algae, possibly due to the difficulties in chromosomal preparation, large numbers of chromosomes and their relatively small sizes [25]. Well-spread chromosome preparation is thought to be an essential prerequisite for a successful application of FISH protocols [17], [26], [27].
The chromosome number of S. japonica has been examined by a number of researchers including Abe [28], Yabu [29], Tai and Fang [9], [30], Yabu and Yasui [31] and Zhou et al. [32], but there has not been a consistent conclusion because of the inconsistencies and difficulties in chromosome images. After comparing all the reports on kelp chromosomes, it is apparent that a sensitive staining method is needed to provide the best contrast and bright photographs because of the small sizes and nearly identical shapes of the kelp chromosomes [25], [30], [33], [34].
The interaction of 4′, 6-diamidino-2-phenylindole (DAPI) with DNA and polydeoxynucleotides has been extensively studied subsequently to this chemical being synthesized by Dann et al. [35]. It is now generally accepted that DAPI binds to DNA preferentially at AT-rich regions [36] in solution, forming highly fluorescent complexes. When excited with UV light at λ = 365 nm, the DNA-DAPI complex fluoresces a bright blue at 390 nm or >390 nm, while unbound DAPI and DAPI bound to non-DNA material may fluoresce a weak yellow [37]. DAPI, therefore, has been used as a cytochemical probe for nuclear DNA content measurement of algae [38]–[47]. However, DAPI was used in only a few reports for chromosomal observations [43], [48]. Therefore, the first step in this research was to prepare high quality kelp chromosomes stained with DAPI to better visualize them. Subsequently, the developed putative sex-specific marker FRML-494 (GenBank accession No. EU931619) from the female and male gametophytes (n = 10 each) of S. japonica [16] was mapped to the kelp chromosomes using the FISH technique. Here we present our results and experience with mapping of the putative sex-specific marker to chromosomes.
Materials and Methods
Alga and Cultures
The Rongfu strain of S. japonica was selected as the plant material in the present research, and its gametophyte clones germinated from zoospores were isolated according to cell sizes under a microscope and cultured under vegetative growth conditions of 30 µmol photons/m2 ·s at 17±1°C with a photoperiod of 12∶12 light∶dark (L∶D), as described previously [49]. PES medium [50] was replaced once every two weeks.
The diploid sporophytes were cultivated with the sporelings raised using the Rongfu strain gametophyte clones, as described by Li et al. [51]. The female and male gametophytes were mixed and cut into several-celled fragments with a blender and were washed thoroughly with distilled seawater. The gametophyte fragments suspended in seawater were poured into 500 mL glass beakers and kept motionless to allow the gametophytes to settle down on a palm rope substratum. When the sporelings grew to 0.8–1 cm in length, the palm ropes were taken out to the open sea. After cultivation at sea for about two months, the young sporophytes (about 1 m in length) were sampled for this study.
DNA Extraction
One gram of samples of sporophyte tissue was digested in an enzymatic solution (8 mL) containing seawater, 0.8 M mannitol, 50 mM trisodium citrate, 1% (w/v) cellulase (Sigma-Aldrich, St. Louis, MO, USA), 0.5% (w/v) macerozyme R-10 (Yakult, Tokyo, Japan), and 0.2 U/mL of the prepared abalone alginate lyase for 8 h at 14°C to eliminate polysaccharide contaminants, as described previously [52]. After digestion, unicells were filtered from the undigested debris with a 240-mesh (60 µm) nylon net and centrifuged at 3 000×g for 5 min, and then the unicell pellets were washed five times with sterilized seawater in order to remove possible contamination from other organisms.
Genomic DNA was extracted separately from the freshly harvested gametophytes and the prepared unicells from the individuals of sporophytes according to the modified cetyltrimethyl ammonium bromide (CTAB) method as described by Hu and Zhou [52].
Identification of the FRML-494 Marker in Gametophytes and Sporophytes
Polymerase chain reaction (PCR; 20 µL) solution contained 50 ng of genomic DNA as a template isolated from the female and male gametophytes and sporophytes of S. japonica, 1×reaction buffer, 2.5 mM of MgCl2, 100 µM of dNTPs, 0.2 µM of each primer and 1.0 unit of Taq DNA polymerase (TaKaRa, Dalian, China). Amplification of the genomic DNA with a pair of the reported primers P51 (forward 5′-AAGACAAGCGGGTGAACTCAGCGAGGTCT-3′, and reverse 5′-ACACTGGACATCGCATCGTCGATCAGTGT-3′) [16] was programmed using 1 cycle containing pre-denaturation at 94°C for 5 min, and 30 cycles with denaturation at 94°C for 30 s, annealing at 62°C for 45 s, and extension at 72°C for 1 min in a Mastercycler Gradient (Eppendorf, Hamburg, Germany). A final extension was performed at 72°C for 10 min. The amplified product was resolved on a 1.2% low-melting-point agarose gel for DNA recovery, cloning and sequencing. The target product was purified using a UNIQ-10 column DNA gel extraction kit (Sangon, Shanghai, China) and ligated into a pMDT-19 vector (TaKaRa, Dalian, China), and the latter was subsequently transformed into Escherichia coli JM109 competent cells (Leihao, Shanghai, China). Sequencing was performed in Sangon (Shanghai, China) using the dideoxynucleotide chain-termination method with an Applied Biosystems 3730 sequencer (Applied Biosystems, Foster City, CA, USA).
Southern Blot
Aliquots of isolated DNA (approximately 20 µg per sample) were digested to completion at 37°C for 4–6 h independently with NotI and XbaI, which could not digest the FRML-494 marker. The digested DNA samples were fractionated on a 1.0% agarose gel, blotted onto a positively charged nylon membrane (Pall, Exton, PA, USA) and hybridized with the FRML-494 marker as a probe. The probe was labeled with biotin-dUTP using a North2South Biotin Random Prime Labeling Kit (Thermo, Rockford, IL, USA). Southern blots were performed following the manufacturer's instructions. Hybridized bands were detected using a North2South Chemiluminescent Hybridization and Detection Kit (Thermo, Rockford, IL, USA) and signals were visualized by exposure to XBT-1 film (Kodak, Rochester, USA) at room temperature for 90 s.
Chromosome Preparation
The fresh gametophytes and sporophytic tissues of this kelp were separately treated with 0.02% colchicine for 8–10 h at room temperature and were washed three times in distilled seawater. The samples were then fixed in a freshly prepared Cannoy's fixative solution (100% ethanol: acetic acid, 3∶1, v/v) for 24 h [53] followed by a wash in distilled water three times. The fixed gametophytes or sporophytic tissues were digested with a gentle stir in a multi-enzyme solution I (cellulase: pectinase (Sigma-Aldrich, St. Louis, MO, USA): macerozyme R-10, 2∶1∶1, v/v) for 18 h at 37°C, and were harvested with a centrifuge at 13 000×g for 1 min. The supernatant was discarded and the pellets were re-digested in a multi-enzyme solution II (cellulase: macerozyme R-10: abalone alginate lyase, 2∶1∶2, v/v) at 37°C overnight, where the crude alginate lyase was extracted from abalone hepatopancreas as described by Hu and Zhou [52]. The samples were washed three times in 70% ethanol to remove the enzyme solution and were centrifuged at 13 000×g for 3 min to discard the ethanol. The pellets were re-suspended in acetic acid, and 6.5 µL cell suspensions were dropped on slides. The slides were screened under a phase contrast microscope for chromosomes at mitotic metaphase. The well-prepared slides were submitted to FISH detection and DAPI counterstaining.
DAPI Counterstaining and FISH
For the FISH technique, an unlabeled 494 bp-long SCAR marker FRML-494 probe was generated by PCR with the female gametophyte genomic DNA as a template. PCR was carried out using the pair of primers P51 as mentioned above. The probe was labeled with Texas Red-5-dUTP (PerkinElmer, Boston, MA, USA) by nick translation using an ADVANCE™ Nick Translation Kit (Sigma-Aldrich, St. Louis, MO, USA) under the guidance of the manufacturer's instructions.
The FISH procedure followed Schwarzacher [26] with slight modifications. The chromosomes on slides were ultraviolet (UV) light cross-linked twice in a UV cross-linker BLX-E254 (Vilbert Lourmat, France) at 254 nm for 15 s each time, followed by the addition of 8 µL of hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl, pH 7.2, 0.01% SDS) containing 5 ng/µL of labeled FRML-494 probe. The slides were heated to 100°C in a water bath for 5 min and then transferred to a 55°C air oven for 12 h for hybridization to occur. Post-hybridization washes of the slides were carried out in a slightly more stringent solution of 2×SSC (0.3 M NaCl, 0.03 M sodium citrate, pH 7) at 42°C for 10 min to remove unbound, nonspecifically bound, or weakly hybridized probes.
The chromosomes were counterstained with 15 µL DAPI in a VectaShield mounting medium (Vector Laboratories, Burlingame, CA, USA) for 5 min incubation. Slides were stored in the dark before microscopic analysis.
Microscope Imaging and Data Analysis
Chromosome preparations were studied and photographed under an Olympus BX61 epifluorescence microscope (Tokyo, Japan) using a 360 nm filter. Hybridization signals from Texas Red were subsequently recorded on the single photograph using a 470 nm filter by the second exposure. The images were captured with an Olympus DP-71 digital camera using the DP controller program and the chromosomes were measured with Image-Pro Plus software. Chromosome sizes and localization of the FRML-494 marker were measured digitally in at least five metaphase plates, and the software Microsoft Excel was used for calculations.
The karyotypic patterns of the gametophytes were edited using Adobe Photoshop software (ver. 3.0) based on the size of chromosomes, and the color contrast and brightness uniformity were treated using this software.
Results
Specificity and Heredity of the FRML-494 Marker
A sex marker, FRML-494 (GenBank accession No. EU931619), was previously reported to be present only in the female gametophytes of S. japonica [16]. In this study, FRML-494 was detected to be present in both female gametophytes and randomly selected sporophyte individuals by crossbreeding of the female and male gametophytes, but was absent in the male gametophytes (Figure 1). Sequencing analysis showed that these existing bands were completely identical in both sequence and size, as previously reported [16]. This indicated that the FRML-494 marker related to the females was inheritable without any changes at the locus, thus being preserved as a whole in the crossbred sporophytes.
Figure 1. Electrophoresis profile of amplified products of the FRML-494 marker.

PCR amplification using the pair of primers P51 in the female (lane 1) and male (lane 2) gametophytes and the crossbred sporophytes (lanes 3–8) of Saccharina japonica (strain Rongfu) showing the FRML-494 marker (as indicated by the arrow) was only present in the female gametophytes and sporophytes. Lane M, 100 bp DNA ladder marker (Generay, Shanghai, China). Lane 0, control with H2O instead of DNA.
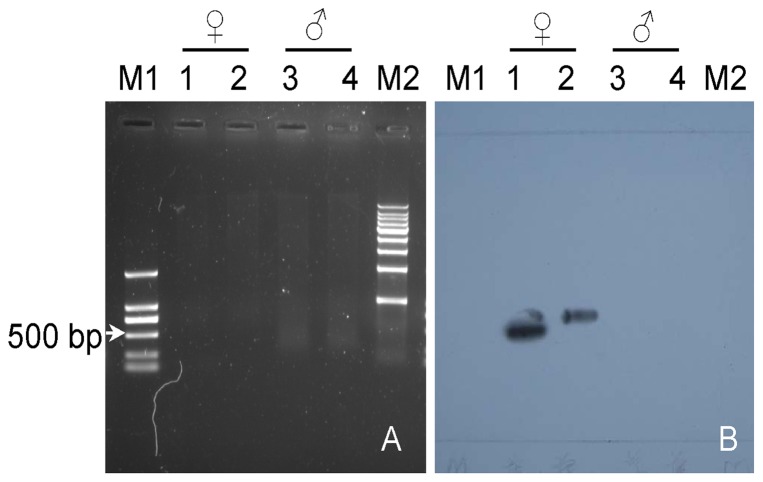
Southern blot profiling (Figure 2) showed that the labeled FRML-494 probe was able to hybridize only to DNA from the female gametophytes, but not to DNA isolated from the male gametophytes, confirming that the FRML-494 marker was specific to the female gametophytes.
Figure 2. Southern blot of the labeled FRML-494 marker.
Ethidium-bromide-stained agarose gel (A) of NotI- (lanes 1 and 3) and XbaI- (lanes 2 and 4) digested genomic DNA from the female (lanes 1 and 2) and male (lanes 3 and 4) gametophytes of S. japonica. Southern blot (B) of NotI- (lanes 1 and 3) and XbaI- (lanes 2 and 4) digested genomic DNA hybridized with the biotin-labeled FRML-494 marker. Lanes M1 and M2 show the D2000 and 1 kb DNA ladder molecular standard, respectively, (Tiangen, Beijing, China).
Determination of Chromosome Number and Chromosomal Characteristics
In order to prepare high quality chromosome slides, the sporophyte tissues or filamentous gametophytes of S. japonica were pretreated with colchicine for 8–10 h and macerated with multi-enzyme solutions for one to two days. Subsequently, the well-spread slides were obtained by dropping the pretreated materials in acetic acid over the slides at approximately 30 cm in height. Clear digital images (Figure 3A, D and G) of the smeared kelp chromosomes stained with DAPI were then captured using fluorescent microscopy. Counting chromosomes after DAPI staining was more feasible since the well-spread chromosomes stained using the DAPI solution gave a stronger contrast and brighter signals (Figure 3A, D and G). In some images (e.g. Figure 3D and G), the sharp constriction where a centromere of a metaphase chromosome was located was also visible. The chromosome numbers of the haploid gametophytes were 31 no matter whether they were from females (chromosome number ranged from 25 to 31, 15 out of 30 images with 31 chromosomes) (Figure 3A) or males (chromosome number ranged from 28 to 31, 12 out of 20 images with 31 chromosomes) (Figure 3D), and those of the diploid sporophytes were 62 (chromosome number ranged from 52 to 62, 8 out of 12 images with 62 chromosomes), as illustrated in Figure 3G. It was noted that not all the observed nuclear number was 31 or 62, because there were chromosome variances at different cytological phases observed.
Figure 3. FISH of the FRML-494 marker (red) on Saccharina japonica chromosomes counterstained with DAPI (blue).
(A) Metaphase chromosomes of the female gametophytes (n = 31); (B) hybridization signal of the FRML-494 marker (red) as indicated by the arrow on the same spread as image A; the inset is the enlarged chromosome where this marker was located; (C) an interphase nucleus of the female gametophytes with hybridization signal (red) as indicated by the arrow; (D) metaphase chromosomes of the male gametophytes (n = 31); (E) an interphase nucleus of the male gametophytes without a hybridization signal; (F) an interphase nucleus of the sporophytes with a hybridization signal (red) as indicated by the arrow; (G) metaphase chromosomes of the sporophytes (2n = 62); (H) hybridization signal (red) as indicated by the arrow present on only one metaphase chromosome of the same spread as image G; the inset is the enlarged chromosome where this marker was located; and the arrowheads in images D and G show the constriction of the chromosomes.
The average absolute length (Table 1) of the chromosomes assayed in 5 images of the female slides was from 0.77 µm to 2.61 µm with 5 chromosomes longer than 2 µm and 3 shorter than 1 µm, whereas that from the males ranged from 0.57 µm to 2.16 µm with only one longer than 2 µm and 7 shorter than 1 µm. It seemed that the size of the female chromosomes was slightly larger than that of the corresponding males but there was no significant difference between their relative lengths, both of which varied approximately from 1.5% to 5.5% of the total chromosome length (Table 1). These data excluded the possibility that the female nucleus had a very large X chromosome. Apart from 3 to 6 spherical chromosomes in female gametophytes, the others were rod-like in shape (Figure 3A, D and G); such a karyogram of the gametophytes can be ranked according to their sizes (e.g. Figure S1A′, B′ and C′). Because there was not enough banding (e.g. Giemsa C- and G-banding, silver banding) information, the chromosome complement based on the image of sporophyte chromosomes (Figure 3G) could not be worked out.
Table 1. Length of chromosomes prepared from the male or female gametophytes of Saccharina japonica.
| Chromosome No. only according to the decreasing size | Length of chromosomes from the male gametophytes (means±SD, n = 5) | Length of chromosomes from the female gametophytes (means±SD, n = 5) | ||
| Absolute (µm) | Relative (% of total ones) | Absolute (µm) | Relative (% of total ones) | |
| I | 2.160±0.010 | 5.53 | 2.612±0.113 | 5.57 |
| II | 1.983±0.149 | 5.08 | 2.397±0.115 | 5.11 |
| III | 1.902±0.128 | 4.87 | 2.149±0.150 | 4.58 |
| IV | 1.792±0.140 | 4.59 | 2.109±0.159 | 4.50 |
| V | 1.724±0.162 | 4.42 | 2.014±0.178 | 4.29 |
| VI | 1.636±0.138 | 4.19 | 1.877±0.220 | 4.00 |
| VII | 1.607±0.130 | 4.12 | 1.784±0.121 | 3.80 |
| VIII | 1.481±0.140 | 3.79 | 1.751±0.151 | 3.73 |
| IX | 1.432±0.134 | 3.67 | 1.724±0.162 | 3.68 |
| X | 1.387±0.131 | 3.55 | 1.686±0.169 | 3.59 |
| XI | 1.324±0.0698 | 3.39 | 1.657±0.186 | 3.53 |
| XII | 1.316±0.0712 | 3.37 | 1.619±0.163 | 3.45 |
| XIII | 1.299±0.0580 | 3.33 | 1.588±0.194 | 3.39 |
| XIV | 1.267±0.0604 | 3.25 | 1.567±0.193 | 3.33 |
| XV | 1.222±0.0719 | 3.13 | 1.513±0.192 | 3.23 |
| XVI | 1.184±0.0489 | 3.03 | 1.463±0.220 | 3.12 |
| XVII | 1.174±0.0499 | 3.01 | 1.441±0.215 | 3.07 |
| XVIII | 1.146±0.0679 | 2.94 | 1.419±0.216 | 3.03 |
| XIX | 1.115±0.0587 | 2.86 | 1.301±0.213 | 2.77 |
| XX | 1.108±0.0584 | 2.84 | 1.289±0.201 | 2.75 |
| XXI | 1.089±0.0575 | 2.79 | 1.257±0.186 | 2.68 |
| XXII | 1.072±0.0432 | 2.75 | 1.237±0.185 | 2.64 |
| XXIII | 1.039±0.0295 | 2.66 | 1.217±0.176 | 2.59 |
| XXIV | 1.028±0.0285 | 2.63 | 1.202±0.183 | 2.56 |
| XXV | 0.951±0.0483 | 2.44 | 1.158±0.187 | 2.47 |
| XXVI | 0.927±0.0578 | 2.37 | 1.120±0.197 | 2.39 |
| XXVII | 0.867±0.0818 | 2.22 | 1.098±0.207 | 2.34 |
| XXVIII | 0.826±0.0484 | 2.12 | 1.041±0.169 | 2.22 |
| XXIX | 0.776±0.0606 | 1.99 | 0.979±0.181 | 2.09 |
| XXX | 0.638±0.0243 | 1.63 | 0.853±0.184 | 1.82 |
| XXXI | 0.567±0.0153 | 1.45 | 0.773±0.142 | 1.64 |
FISH of the FRML-494 Marker on One Unique Chromosome of the Kelp
To study the localization of the FRML-494 marker, 30 nuclei of the female samples were identified at metaphase and examined with FISH. Of these nuclei, 16 nuclei could be detected with a fluorescent signal present on one chromosome (Figure 3B). If the female interphase nuclei were examined, 25 of 30 nuclei showed a distinguishable fluorescent signal as shown in Figure 3C. On the contrary, there was no hybridization signal detected in all 20 of the examined male nuclei at metaphase (Figure 3D) or interphase (Figure 3E). These results strongly suggested that the FRML-494 marker was specifically present and localized on one chromosome of S. japonica female gametophytes without any homologous locus on the male chromosomes.
To confirm whether the chromosome where the FRML-494 marker was located was the same, three different images (Figure S1) were used as examples for the chromosome rearrangement and were edited using Adobe Photoshop software. Illustration showed that the FRML-494 marker only appeared on the 10th chromosome (with a relative length from 3.56% to 3.67% in these 3 images), which was shown according to the decreasing size of the chromosomes from the female gametophytes. The shape of the chromosome where the FRML-494 marker was located and the site of the hybridization signal on this chromosome were similar as well. In addition, the FISH image (Figure 3F and H) from the diploid sporophyte nuclei showing the presence of a hybridization signal suggested that this marker was unique, too. So this FRML-494 marker was a female chromosome-specific cytogenetic DNA marker, as suggested by Jiang and Gill [23].
Discussion
In this study, for the first time, we determined the inheritance and chromosomal location of a previously reported [16] putative sex-specific marker, FRML-494, in S. japonica by FISH. In addition, we provided indisputable evidence for chromosomal number and morphological characteristics of the kelp chromosomes through improved techniques including multi-enzyme treatment and DAPI staining. The use of FISH in plants lags considerably behind its applications in cytogenetics of human and other animal systems. A major factor, as suggested by Griffor et al. [54], contributing to the difficulty in plants was obtaining mitotic and meiotic chromosomes free of cell wall material. In general, a high quality chromosome preparation should be well spread and flat, and should have plenty of chromosomes with good morphology. Therefore, high quality material from S. japonica is an essential prerequisite, as suggested by Zhang and Friebe [27], for FISH of the FRML-494 marker on kelp chromosomes.
Characteristics of S. japonica Chromosomes as Revealed by DAPI Staining
In most of the publications [29]-[31], [33], [34], [55] about chromosome preparations in the genus Laminaria or Saccharina, the samples were usually squashed without any treatment for chromosome preparation after fixation in Carnoy's solution. If the kelp gametophytes were digested with a multi-enzyme solution containing cellulase and pectinase, the preparation of chromosomes was reported to be improved for visualization [32]. Regarding the substantial existence of alginate in cell walls or middle lamella between two neighboring cells of the kelp, the crude alginase extracted from the abalone hepatopancreas was added to the multi-enzyme solution in the present research. Meanwhile, fluorochrome DAPI was used instead of the routine dyes, such as haematoxylin, orcein and carmine, for chromosome specific staining in cytological research of algae. In comparison with the published chromosome micrographs of kelp [29]–[32], the present chromosome images (Figure 3) displayed more contrast and were brighter without any noisy background, suggesting that such a chromosome preparation and staining was a successful protocol for karyotyping research of kelp. Furthermore, this result also showed that DAPI was a sensitive and effective dye for chromosome visualization in algae, such as S. japonica with small sizes and large numbers, although Lewis [25] thought the choice of stain was not critical since all the often-used dyes resulted in specific staining of chromosomes. To date, this is one of the few successful applications of DAPI in algal staining for the observations of chromosome shape and number [43], [48].
The chromosome images clearly illustrate that the chromosome numbers are 31 and 62, respectively, for the haploid (Figure 3A and D) and diploid (Figure 3G) plants of this kelp, which is in agreement with a report by Zhou et al. [32] and close to the result (haploid chromosome number was 32) by Yabu and Yasui [31], although the chromosome numbers have earlier been reported to be 22 and 44 [29], [30] for gametophytes and sporophytes, respectively. If the basic numbers of chromosomes are 8 or more in Laminariales [25], most taxa of brown algae hypothetically evolved the accompaniment of some aneuploidy from these basic numbers. In this case, the present research suggests that S. japonica, S. latissima (or Laminaria saccharina), L. digitata, L. hyperborean (Gunn.) Fosl. and L. ochroleuca Bachelot de la Pylaie are possibly at a similar evolutionary stage because they possess the same chromosome numbers in the haploid gametophytes [33], [34], although these kelps are now regarded as belonging to two different genera [2].
Most chromosomes of this kelp range from 1 µm to 2 µm in length (Table 1), so they are regarded as small chromosomes with a rigid field [56]. The ratios of the longest chromosome to the shortest are 3.81∶1 and 3.38∶1 in males and in females, respectively, showing that the kelp chromosomes belong to Type B chromosomes according to the criteria set by Stebbins (the ratio ranges from 2∶1 to 4∶1) [57]. In respect to the possible condensation of chromosomes during the preparation, the supplied relative lengths of female and male chromosomes (Table 1) demonstrate that there is no apparent distinction between males and females. Such a very large X chromosome found by Evans [33], [34] and Yasui [55] was not found to be present in the females (Figure 3A and Table 1), which is consistent with the conclusions drawn by Yabu [29], Tai and Fang [30], Yabu and Yasui [31] and Zhou et al. [32]. It seems that there is no sex chromosome in the kelp. On the contrary, the 1∶1 ratio of female to male gametophytes developed from the released meiospores [58] and all the female offspring from the parthenogenetic sporophytes [9]–[12] seemingly predict that the kelp has a sex chromosome. Therefore, the sex chromosome is supposed to be homomorphic with autosomes in both size and shape, as suggested by Fang et al. [10].
Specific Localization of the FRML-494 Marker on Kelp Chromosomes
After chromosome preparation of high quality from S. japonica, the putative sex-specific marker, FRML-494 [16], was successfully localized within the kelp chromosomes (Figures 3 and S1). Taking the Southern blot (Figure 2) and FISH images (Figure 3B and H) together, it was concluded that this marker was female-specific and its locus was on one unique chromosome of the female gametophytes, both of which implies that this marker is a female chromosome-specific DNA sequence, as suggested by Jiang and Gill [23]. The median relative size of this chromosome on which the FRML-494 marker is located (Figures 3B and S1) indicates that it is not the very large X chromosome, as observed by Evans [33], [34] and Yasui [55]. Based on the absence of the very large X chromosome in the females, as discussed above, and the specific relationship between the FRML-494 marker and the female chromosomes, it is supposed that sex determination genes possibly exist on different chromosomes; i.e., multiple sex chromosomes, rather than a large chromosome as suggested by Fang et al. [10]. Therefore, whether the chromosome on which this maker is localized is a sex chromosome needs to be answered in the future.
Using FISH of the FRML-494 marker, it was noted that not all the metaphase female nuclei showed hybridization signals, and the hybridization signal in the FISH images (Figure 3B and H) was not bright enough either. This is because the probe was only 494 bp in length, which binds less fluorescent molecules rather than longer probes. Although probes <1 kb in length and even as small as 250 bp could be visualized through a fluorescence microscope in plant [59], [60] and human [61] chromosome preparations, respectively, the region of interest on the chromosome wrapped inside the superhelical structure allowed less access, especially for the short probes, thus possibly reducing the reproducibility of FISH [23], [62]. Since the chromosomes at interphase are decondensed and therefore stretched such that the target regions are easily exposed to the probes for FISH [63], the interphase nuclei (Figure 3C, E and F) can be regarded as references. Most of the female interphase nuclei (83.3%) showed the hybridization signals (Figure 3C) but no hybridization signal was present in either 20 metaphase (Figure 3D) or 20 interphase (Figure 3E) nuclei of the male gametophytes. Taking these data and Southern blot profiles (Figure 2) together, the FISH images (Figures 3 and S1) are convincing. If a co-existing sequence, such as the 45S rRNA gene (see the review [24]), is used as a probe to hybridize both males and females, it would be more helpful to support our results, and this approach will be attempted in the future.
In brief, after multi-enzyme maceration of cell walls, the kelp chromosome preparation can meet the needs of the FISH technique. The FRML-494 marker is successfully localized on one unique female gametophyte chromosome counterstained with DAPI. If the FRML-494 marker is genetically linked to the kelp female gametophytes, using this established FISH technique can surely help not only to distinguish the females from the males of this kelp, but also to clarify whether a kelp sporophyte is produced by crossbreeding or by monogenetic reproduction, although there is no distinct variation between the female and male karyograms, as discussed above. In addition, with the help of FISH, the physical mapping can be constructed so that the genome analysis, molecular breeding research and even the cytogenetical investigation of sex differentiation in S. japonica can be fast and promoted further.
Supporting Information
FISH images and their corresponding karyograms. FISH of the labeled FRML-494 marker (red) on metaphase chromosomes of the female gametophytes counterstained with DAPI (blue). A′, B′ and C′ are the ordered chromosomes of FISH images of A, B and C, respectively, prepared with Adobe Photoshop by decreasing size in length. Arrows indicating the localization of the FRML-494 marker on one chromosome of the female gametophytes.
(TIF)
Acknowledgments
We are very grateful to Prof. F. -P. Han in the Institute of Genetics and Developmental Biology, CAS, for the generous support of FISH experiments in his laboratory, and to Prof. U. C. Lavania, Dr. L. F. Dong and Dr. X. -N. Zhang for language improvement and critical reading.
Funding Statement
This research was supported by the National Natural Sciences Foundation of China (30471328 and 31201992), the Natural Sciences Foundation of Shanghai (10ZR1413900), the National High Technology Research and Development Program of China (2012AA10A406), and the Key Discipline of Shanghai Municipal Education Commission (J50701). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.FAO (2011) 2009 FAO Yearbook. Fishery and Aquaculture Statistics. Aquaculture Production. Rome: Food and Agriculture Organization of the United Nations. 221 p
- 2. Bartsch I, Wiencke C, Bischof K, Buchholz CM, Buck BH, et al. (2008) The genus Laminaria sensu lato: recent insights and developments. Eur J Phycol 43: 1–86. [Google Scholar]
- 3. Patwary MU, van der Meer JP (1992) Genetics and breeding of cultivated seaweeds. Korean J Phycol 7 2 281–318. [Google Scholar]
- 4. Tseng CK (2001) Algal biotechnology industries and research activities in China. J Appl Phycol 13: 375–380. [Google Scholar]
- 5. Wang C, Fan XL, Wang GC, Niu JF, Zhou BC (2011) Differential expression of Rubisco in sporophytes and gametophytes of some marine macroalgae. PLoS ONE 6 1 e16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yabu H (1964) Early development of several species of Laminariales in Hokkaido. Mem Fac Fish Hokkaido Univ 12: 1–72. [Google Scholar]
- 7. Hsiao SIC, Druehl LD (1971) Environmental control of gametogenesis in Laminaria saccharina. I. The effects of light and culture media. Can J Bot 49: 1503–1508. [Google Scholar]
- 8. Hsiao SIC, Druehl LD (1973) Environmental control of gametogenesis in Laminaria saccharina. II. Correlation of nitrate and phosphate concentrations with gametogenesis and selected metabolites. Can J Bot 51: 829–839. [Google Scholar]
- 9. Tai CH, Fang TC (1976) Some preliminary observations on the parthenogenesis in Laminaria japonica Aresch. Acta Genet Sinica 3: 32–38. [Google Scholar]
- 10. Fang TC, Tai CH, Ou YL, Tcuei CC, Chen TC (1978) Some genetic observations on the monoploid breeding of Laminaria japonica . Sci China 21: 401–408. [Google Scholar]
- 11. Motomura T (1991) Immunofluorescence microscopy of fertilization and parthenogenesis in Laminaria angustata (Phaeophyta). J Phycol 27: 248–257. [Google Scholar]
- 12. Lewis RJ, Jiang BY, Neushul M, Fei XG (1993) Haploid parthenogenetic sporophytes of Laminaria japonica (Phaeophyceae). J Phycol 29: 363–369. [Google Scholar]
- 13. Motomura T (1990) Ultrastructure of fertilization in Laminaria angustata (Phaeophyta, Laminariales) with emphasis on the behavior of centrioles, mitochondria and chloroplasts of the sperm. J Phycol 26: 80–89. [Google Scholar]
- 14. Shi X-Z, Bi Y-H, Zhou Z-G (2005) Cloning and screening of differentially expressed genes from the male gametophytes of Laminaria japonica Aresch. J Fish China 29: 666–669. [Google Scholar]
- 15. Lu G-Q, Ouyang L-L, Zhou Z-G (2009) Bioinformatic analysis of a suppression subtractive cDNA library from Laminaria japonica male gametophytes. J Fish Sci China 16: 221–229. [Google Scholar]
- 16. Liu Y-S, Li L-H, Wu W-K, Zhou Z-G (2009) A SCAR molecular marker specifically related to the female gametophytes of Saccharina (Laminaria) japonica (Phaeophyta). J Phycol 45: 894–897. [DOI] [PubMed] [Google Scholar]
- 17. Pinkel D, Straume T, Gray JW (1986) Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci USA 83: 2934–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gall JG, Pardue ML (1969) Formation and detection of RNA-DNA hybrid molecules in cytological preparations. Proc Natl Acad Sci USA 63: 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. John HA, Birnstiel ML, Jones KW (1969) RNA-DNA hybrids at the cytological level. Nature 223: 582–587. [DOI] [PubMed] [Google Scholar]
- 20. Le HT, Armstrong KC, Miki B (1989) Detection of rye DNA in wheat-rye hybrids and wheat translocation stocks using total genomic DNA as a probe. Plant Mol Biol Rep 7: 150–158. [Google Scholar]
- 21. Schwarzacher T, Leitch AR, Bennett MD, Heslop-Harrison JS (1989) In situ localization of parental genomes in a wide hybrid. Ann Bot 64: 315–324. [Google Scholar]
- 22. Lavania UC (1998) Fluorescence in situ hybridization in genome, chromosome and gene identification in plants. Curr Sci 74: 126–133. [Google Scholar]
- 23. Jiang J, Gill BS (2006) Current status and the future of fluorescence in situ hybridization (FISH) in plant genome research. Genome 49: 1057–1068. [DOI] [PubMed] [Google Scholar]
- 24. Schwarzacher T, Heslop-Harrison JS (2011) Organisation of the plant genome in chromosomes. Plant J 66: 18–33. [DOI] [PubMed] [Google Scholar]
- 25. Lewis RJ (1996) Chromosomes of the brown algae. Phycologia 35: 19–40. [Google Scholar]
- 26. Schwarzacher T (2008) Fluorescent in situ hybridization to detect transgene integration into plant genomes. Methods Mol Biol 478: 227–246. [DOI] [PubMed] [Google Scholar]
- 27.Zhang P, Friebe B (2009) FISH on plant chromosomes. In: Liehr T, editor, Fluorescence in situ Hybridization (FISH)-Application Guide. Berlin Heidelberg: Springer-Verlag. pp. 365–394.
- 28. Abe K (1939) Mitosen in sporangium von Laminaria japonica Areschoug. Sci Rep Tohoku Imp Univ Biol 14: 327–329. [Google Scholar]
- 29. Yabu H (1973) Alternation of chromosomes in the life history of Laminaria japonica Aresch. Bull Fac Fish Hokkaido Univ 23 4 171–176. [Google Scholar]
- 30. Tai CH, Fang TC (1977) The chromosomes of Laminaria japonica Aresch. Acta Genet Sinica 4: 325–328. [Google Scholar]
- 31. Yabu H, Yasui H (1991) Chromosome number in four species of Laminaria (Phaeophyta). Jpn J Phycol 39: 185–187. [Google Scholar]
- 32. Zhou LR, Dai JX, Shen SD (2004) An improved chromosome preparation from male gametophyte of Laminaria japonica (Heterokontophyta). Hydrobiologia 512: 141–144. [Google Scholar]
- 33. Evans LV (1963) A large chromosome in the laminarian nucleus. Nature 198: 215.13928445 [Google Scholar]
- 34. Evans LV (1965) Cytological studies in the Laminariales. Ann Bot 29: 541–562. [Google Scholar]
- 35. Dann O, Bergen G, Demant E, Volz G (1971) Trypanocide diamidine des 2-phenyl-benzofurans, 2-phenyl-indens und 2-phenyl-indols. Liebigs Ann Chem 749: 68–89. [Google Scholar]
- 36. Lin MS, Comings DE, Alfi OS (1977) Optical studies of the interaction of 4′-6-diamidino-2-phenylindole with DNA and metaphase chromosomes. Chromosoma 60: 15–25. [DOI] [PubMed] [Google Scholar]
- 37. Porter KG, Feig YS (1980) The use of DAPI for identifying and counting aquatic microfloral. Limnol Oceanogr 25: 943–948. [Google Scholar]
- 38. Shihira-Ishikawa I (1984) Chromosome behavior in the primary nucleus of Acetabularia calyculus as revealed by epifluorescent microscopy. Protoplasma 122: 27–34. [Google Scholar]
- 39. Imai I (1989) Cyst formation of the noxious red tide flagellate Chattonella marina (Raphidophyceae) in culture. Mar Biol 103: 235–239. [Google Scholar]
- 40. Garman GD, Pillai MC, Goff LJ, Cherr GN (1994) Nuclear events during early development in gametophytes of Macrocystis pyrifera, and the temporal effects of a marine contaminant. Mar Biol 121: 355–362. [Google Scholar]
- 41. Kapraun DF, Bailey JC, Dutcher JA (1994) Nuclear genome characterization of the carrageenophyte Hypnea musciformis (Rhodophyta). J Appl Phycol 6: 7–12. [Google Scholar]
- 42. Sheath RG, Whittick A (1995) The unique gonimoblast propagules of Batrachospermum breutelii (Batrachospermales, Rhodophyta). Phycologia 34: 33–38. [Google Scholar]
- 43. Soyer-Gobillard M-O, Ausseil J, Géraud M-L (1996) Nuclear and cytoplasmic actin in dinoflagellates. Biol Cell 87: 17–35. [PubMed] [Google Scholar]
- 44. Barbier M, Géraud M-L, Nicolas G, Soyer-Gobillard M-O (1998) Colocalization of the cyclin B homologue P56 and β-tubulin during the cell cycle in a unicellular eucaryote dinoflagellate. Biol Cell 90: 63–76. [DOI] [PubMed] [Google Scholar]
- 45. Kapraun DF (2005) Nuclear DNA content estimates in multicellular green, red and brown algae: phylogenetic considerations. Ann Bot 95: 7–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kapraun DF (2007) Nuclear DNA content estimates in green algal lineages: Chlorophyta and Streptophyta. Ann Bot 99: 677–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Antonia RSM, Amelia GG, Noemi SS (2011) Nuclear content estimates suggest a synapomorphy between Dictyota and six other genera of the Dictyotales (Phaeophyceae). Cryptogamie Algol 32: 205–219. [Google Scholar]
- 48. Novaczek I, Bird CJ, McLachlan JL (1986) Culture and field study of Stilophora rhizodes (Phaeophyceae, Chordariales) from Nova Scotia, Canada. Br Phycol J 21: 407–416. [Google Scholar]
- 49. Zhou Z-G, Wu C-Y (1998) Clone culture of Laminaria japonica and induction of its sporophytes. Chin J Biotechnol 14: 109–111.10196635 [Google Scholar]
- 50. Starr RC, Zeikus JA (1993) UTEX-The culture collection of algae at the University of Texas at Austin. J Phycol 29 Suppl 1–106. [Google Scholar]
- 51. Li D-P, Zhou Z-G, Liu H-H, Wu C-Y (1999) A new method of Laminaria japonica strain selection and sporeling raising by the use of gametophyte clones. Hydrobiologia 398/399: 473–476. [Google Scholar]
- 52. Hu Y-J, Zhou Z-G (2001) Extraction of RAPD-friendly DNA from Laminaria japonica (Phaeophyta) after enzymatic dissociation of the frozen sporophyte tissue. J Appl Phycol 13: 415–422. [Google Scholar]
- 53. Schweizer D (1976) Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma 58: 307–324. [DOI] [PubMed] [Google Scholar]
- 54. Griffor MC, Vodkin LO, Singh RJ, Hymowitz T (1991) Fluorescent in situ hybridization to soybean metaphase chromosomes. Plant Mol Biol 17: 101–109. [DOI] [PubMed] [Google Scholar]
- 55. Yasui H (1992) Chromosome numbers and a sex chromosome of Laminaria yendoana Miyabe (Phaeophyta). Nippon Suisan Gakkaishi 58: 1385. [Google Scholar]
- 56. Lima-De-Faria A (1980) Classification of genes, rearrangements and chromosomes according to the chromosome field. Hereditas 93: 1–46. [DOI] [PubMed] [Google Scholar]
- 57.Stebbins GL (1971) Chromosal Evolution in Higher Plants. London: Edward Arnold Ltd. 216 p.
- 58. Schreiber E (1931) Untersuchungen über parthenogenesis, geschlechtsbestimmung und bastardierungsvermögen bei laminarien. Planta 12: 331–353. [Google Scholar]
- 59. Khrustaleva LI, Kik C (1996) Localization of single-copy T-DNA insertion in transgenic shallots (Allium cepa) by using ultra-sensitive FISH with tyramide signal amplification. Plant J 25: 699–707. [DOI] [PubMed] [Google Scholar]
- 60. Stephens JL, Brown SE, Lapitan NLV, Knudson DL (2004) Physical mapping of barley genes using an ultrasensitive fluorescence in situ hybridization technique. Genome 47: 179–189. [DOI] [PubMed] [Google Scholar]
- 61. Richard F, Vogt N, Muleris M, Malfoy B, Dutrillaux B (1994) Increased FISH efficiency using APC probes generated by direct incorporation of labeled nucleotides by PCR. Cytogenet Cell Genet 65: 169–171. [DOI] [PubMed] [Google Scholar]
- 62. Trask BJ, Massa H, Kenwrickt S, Gitschiert J (1991) Mapping of human chromosome Xq28 by two-color fluorescence in situ hybridization of DNA sequences to interphase cell nuclei. Am J Hum Genet 48: 1–15. [PMC free article] [PubMed] [Google Scholar]
- 63. Jiang J, Hulbert SH, Gill BS, Ward DC (1996) Interphase fluorescence in situ hybridization mapping: a physical mapping strategy for plant species with large complex genomes. Mol Gen Genet 252: 497–502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FISH images and their corresponding karyograms. FISH of the labeled FRML-494 marker (red) on metaphase chromosomes of the female gametophytes counterstained with DAPI (blue). A′, B′ and C′ are the ordered chromosomes of FISH images of A, B and C, respectively, prepared with Adobe Photoshop by decreasing size in length. Arrows indicating the localization of the FRML-494 marker on one chromosome of the female gametophytes.
(TIF)